Supplemental Digital Content is available in the text.
Keywords: randomized controlled trial, sex, stroke, thrombectomy
Background and Purpose—
Previous studies have reported less favorable outcome and less effect of endovascular treatment (EVT) after ischemic stroke in women than in men. Our aim was to study the influence of sex on outcome and on the effect of EVT for ischemic stroke in recent randomized trials on EVT.
Methods—
We used data from 7 randomized controlled trials on EVT within the HERMES collaboration. The primary outcome was 90-day functional outcome (modified Rankin Scale). We compared baseline characteristics and outcomes between men and women. With ordinal logistic regression, we evaluated the association between EVT and 90-day functional outcome for men and women separately, adjusted for potential confounders. We tested for interaction between sex and EVT.
Results—
We included 1762 patients in the analyses, of whom 833 (47%) were women. Women were older (median, 70 versus 66 years; P<0.001), were smoking less often (30% versus 44%; P<0.001), and had higher collateral grades (grade 3: 46% versus 35%; P<0.001) than men. Functional independence (modified Rankin Scale score, 0–2) at 90 days was reached by 318 women (39%) and 364 men (39%). The effect of EVT on the ordinal modified Rankin Scale was similar in women (adjusted common odds ratio [acOR], 2.13; 95% CI, 1.47–3.07) and men (acOR, 2.16; 95% CI, 1.59–2.96), with a P for interaction of 0.926.
Conclusions—
Sex does not influence clinical outcome after EVT and does not modify treatment effect of EVT. Therefore, sex should not be a consideration in the selection of patients for EVT.
See related articles, p 2285, 2299, 2420
Previous studies have reported less favorable outcome1–3 and less effect of endovascular treatment (EVT)4–8 after ischemic stroke among women compared with men. EVT was proven to be safe and effective in a meta-analysis of 5 randomized controlled trials (RCTs) within the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.7 As with the results of new and upcoming clinical trials more patients seem to benefit from EVT, individualized selection of patients for EVT has become of increased importance. This is best done by combining prognostic information from multiple clinical and radiological characteristics.9 However, uncertainty remains about the size of the treatment effect in specific subgroups, such as in women. In MR CLEAN (A Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands), a significant interaction between sex and EVT was found.4 Men experienced a major benefit from EVT, whereas no significant treatment effect of EVT was found in women. The meta-analysis of HERMES did not confirm these findings but did not further elaborate on this topic, and baseline characteristics by sex were not reported.7 Since then, 2 more RCTs on EVT have been added to the HERMES collaboration data.10,11 We aimed to provide more insight on the influence of sex on outcome and on the effect of EVT for ischemic stroke in patient-pooled data of the 7 RCTs within the HERMES collaboration.
Methods
Study Population and Design
We pooled the data from 1764 participants in the 7 RCTs on EVT within the HERMES collaboration (MR CLEAN,12 ESCAPE [Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times],5 EXTEND-IA [Extending the Time for Thrombolysis in Emergency Neurological Deficits - Intra-Arterial],13 SWIFT PRIME [Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment],6 REVASCAT [Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke due to Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours of Symptom Onset],14 THRACE [Mechanical Thrombectomy After Intravenous Alteplase Versus Alteplase Alone After Stroke],10 and PISTE [Pragmatic Ischaemic Stroke Thrombectomy Evaluation]11). These RCTs compared EVT (intervention), primarily performed with stent retrievers, with standard care (control) in patients with an ischemic stroke caused by a large vessel occlusion in the anterior circulation. All participants provided informed consent according to each trial protocol, and each RCT was approved by the local ethics committee. The HERMES protocol and main outcomes have been reported previously.7,15 HERMES data are available via the VISTA-Endovascular repository.
Outcome Measures
The primary outcome was functional outcome, measured with the modified Rankin Scale (mRS) score, at 90 days. Secondary outcomes were excellent 90-day functional outcome (mRS, 0–1), 90-day functional independence (mRS, 0–2), extent of neurological deficits measured with the National Institutes of Health Stroke Scale (NIHSS) at 24 hours after randomization, successful reperfusion after EVT (modified Thrombolysis in Cerebral Infarction score ≥2B), and follow-up infarct volume (FIV) on noncontrast CT or magnetic resonance imaging at 12 hours to 2 weeks. Safety outcomes were 90-day mortality and symptomatic intracranial hemorrhage, defined according to each trial protocol.15
Statistical Analyses
Patients with missing data on sex were excluded from the analyses. We compared baseline characteristics and outcomes between men and women using descriptive statistics. With generalized linear mixed models, with trial as a random effect, we evaluated the association between EVT and primary and secondary outcomes. FIV was log transformed (log+1) because of its skewed distribution, to best satisfy the linear model. We tested for interaction between sex and treatment allocation using multiplicative interaction terms in the regression model. Regression analyses were adjusted for age, baseline NIHSS, time from onset to randomization, diabetes mellitus, prior stroke, occlusion location, intravenous tPA (tissue-type plasminogen activator) administration, and collateral grade. Missing data in these covariates were imputed with simple imputation, with the exception of collateral grade (≥10% missing data), which was imputed with single imputation with regression based on relevant covariates, trial, and mRS. Unadjusted and adjusted common odds ratios and adjusted betas are reported with 95% CIs, and all P are 2-sided. Statistical analyses were performed with SAS software, version 9.4, and R, version 3.3.
Results
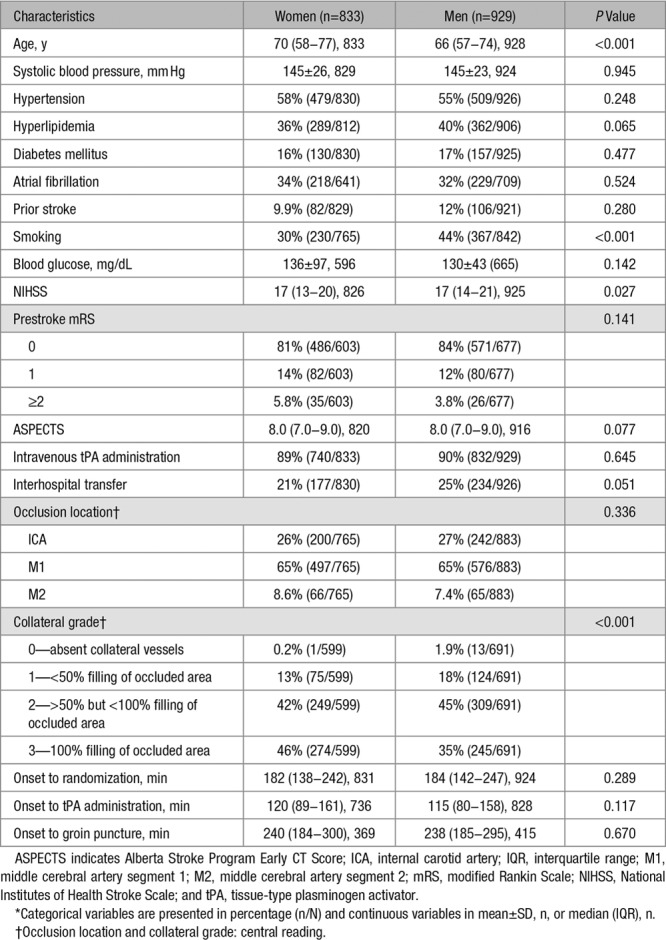
After excluding 2 patients with unknown sex, we included 1762 patients in the analyses, of whom 929 (53%) were men and 833 (47%) were women. Women were older (median, 70 versus 66 years; P<0.001), were smoking less often (30% versus 44%; P<0.001), and had higher collateral grade (grade 3: 46% versus 35%; P<0.001) than men (Table 1). There were no differences in medical history, especially atrial fibrillation, prestroke mRS, administration of tPA, and onset to randomization or groin puncture times between women and men.
Table 1.
Baseline Characteristics by Sex*
The median 90-day mRS was the same for women and men in the intervention groups, and for women and men in the control groups, with significant differences between the intervention groups versus the control groups (4.0 versus 3.0; P<0.001; Table 2). Functional independence (mRS, 0–2) at 90 days was reached by 318 women (39%) and 364 men (39%), with a similar distribution among the intervention and control groups, significantly in favor of the intervention group (Figure 1; Table 2). Mortality at 90 days was 15% for women and 16% for men. Mortality also did not differ between the intervention and control group for both sexes. There was no difference between women and men in symptomatic intracranial hemorrhage (3.7% versus 3.6%) or in successful reperfusion (75% versus 76%). FIV was smaller in women (32 mL) than in men (53 mL; Table 2). FIV was also significantly smaller in the intervention group than in the control group in both women (27 versus 40 mL; P=0.009) and men (39 versus 70 mL; P<0.001; Table 2), with an adjusted beta of −0.26 (95% CI, −0.49 to −0.02) and −0.33 (95% CI, −0.50 to −0.15; Table I in the online-only Data Supplement), respectively.
Table 2.
Outcome Measures for All Patients (Intervention+Control) by Sex, and by Sex and Treatment Allocation*
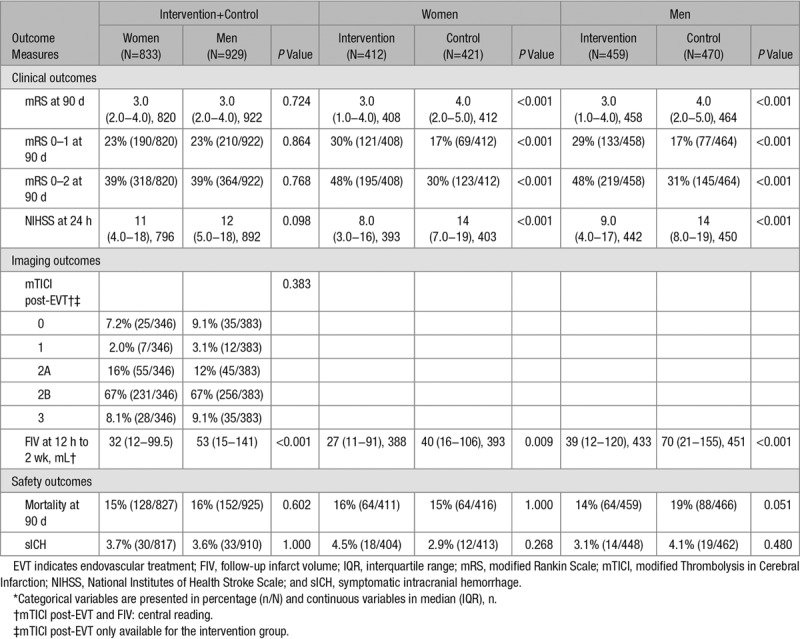
Figure 1.
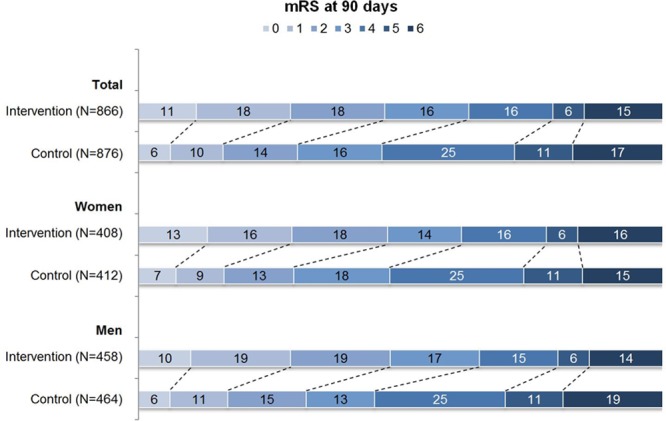
Distribution of the modified Rankin Scale (mRS) score in percentages among the intervention and control group, for all patients and by sex. mRS was missing in 5 patients of the intervention group (4 women and 1 man) and in 15 patients of the control group (9 women and 6 men).
Treatment effect of EVT on the ordinal mRS was comparable in women (adjusted common odds ratio, 2.13; 95% CI, 1.47–3.07) and men (adjusted common odds ratio, 2.16; 95% CI, 1.59–2.96) with a P for interaction of 0.926 (Figure 2). Similarly, there was no difference between women and men in the effect of any of the additional secondary outcomes (Figure 2; Table I in the online-only Data Supplement).
Figure 2.
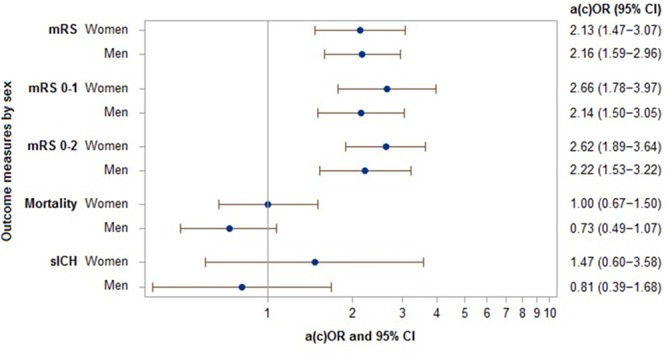
Forest plot showing adjusted treatment effect (adjusted [common] odds ratios [a(c)ORs] and 95% CI) of endovascular treatment (EVT) per outcome measure for women and men. Adjusted for age, baseline NIHSS, time from onset to randomization, diabetes mellitus, prior stroke, occlusion location, intravenous tPA administration, collateral grade, and trial (as random effect). mRS indicates modified Rankin Scale; and sICH, symptomatic intracranial hemorrhage.
Discussion
In this individual patient data meta-analysis that included 1762 patients with ischemic stroke from multiple centers in multiple countries, treatment effects of EVT on functional outcome and other clinical, imaging, and safety outcome measures were similar in women and men.
To date, one study (MR CLEAN), which was also included in the current analysis, has previously addressed the same research question.4 Contrary to the results of the present analysis, a significant interaction between sex and treatment effect in favor of men was found. Although in MR CLEAN, women had more unfavorable baseline characteristics, more serious adverse events, and higher mortality than men, a play of chance was suggested because these differences seemed to be insufficient to explain the lack of an overall treatment effect in women. A study on sex-based group differences in RCTs showed that statistically significant sex-treatment interactions are only slightly more frequent than what would be expected by chance.16 This underpins the idea that the significant sex-treatment interaction observed in MR CLEAN was indeed a play of chance. Our results, which indicate that sex does not modify treatment effect of EVT in ischemic stroke, support previous findings of ESCAPE, EXTEND-IA, and HERMES, which all performed a subgroup analysis by sex but did not report baseline characteristics and secondary analyses by sex, that there are no differences in treatment effect of EVT on functional outcome between women and men.5–7 Our results are also in line with an analysis in patients with acute basilar artery occlusion, where no significant sex differences for outcome and recanalization were observed, regardless of treatment with tPA (with or without EVT) or EVT alone.8 Also, several post hoc analyses based on pooled data from RCTs have shown that sex does not modify the treatment effect of tPA on clinical outcome.17–20
Clinical and safety outcomes also did not differ between women and men in the intervention group, which is similar to the findings of several previous studies that have assessed sex differences in functional outcome after EVT,21–23 with the exception of one study, which found that in patients treated with EVT, women were less likely to be independent at 90 days.24
Other studies, from before the implementation of EVT, have reported poorer stroke-related outcomes in women than in men, independently of treatment.1,3,25 However, in the present study, 90-day functional independence and mortality were equal among women and men, also for those in the control groups only. An explanation for the difference between previous literature and our study may be selection, visible in the somewhat different baseline characteristics of women in our study compared with previous reports. In our study, for example, occurrence of atrial fibrillation was equal between women and men, while the prevalence of ischemic stroke caused by atrial fibrillation is usually higher in women.26 Moreover, the NIHSS score at baseline was lower in women than in men and collateral grade was higher.
To the best of our knowledge, no research has been done on a possible association of sex with collateral grade, and not many studies have previously described baseline collateral grade by sex in patients eligible for EVT. The higher baseline collateral grade in women, which might be the result of selection, is in line with the findings of an analysis done in MR CLEAN.27 In IMS III (Interventional Management of Stroke III Trial), which evaluated EVT+tPA versus tPA alone, no difference in collateral grade was found between women and men.28 As a higher collateral grade is associated with smaller FIVs after EVT,29–31 the smaller FIVs in women in our study might be correlated with the higher baseline collateral grade in women as well. However, this did not impact 90-day functional outcome in women. We think that the difference in FIV and in baseline collateral grade between men and women might not have been large enough explain a possible better outcome in women. Moreover, outcome is not dependent on FIV or baseline collateral grade alone but on multiple variables (together), including age, prestroke disability, time, recanalization status, and notably the NIHSS score at 24 hours, which is a strong predictor of outcome and did not differ between women and men.
Women are often underrepresented in (stroke) clinical trials. In the present study, women, eligible for EVT, were also less often included than men (47% versus 53%), even though more women than men experience a stroke in high-income countries,25 and large vessel occlusions (in the anterior circulation) seem to occur more often in women than in men.32,33 This may be the result of selection because clinical trials tend to select younger patients with lower baseline NIHSS scores. In a nonclinical trial setting, in 2 studies performed after the HERMES main results, 52% and 56% of all patients treated with EVT were women.23,24 Moreover, women seem to be less likely to receive any acute reperfusion therapy for (ischemic) stroke.34–36 This may be caused by various factors identified as being more common in women with ischemic stroke than in men, such as older age, higher prestroke disability, living alone, and higher occurrence of aphasia and reduced level of consciousness at presentation, which are also risk factors for late arrival.1,2,37,38 These factors could reduce the use of and access to acute reperfusion therapy in women. Future studies should focus on whether there are clinically relevant sex differences in the incidence of large vessel occlusion and use of and access to EVT, which was also emphasized in a review on sex differences in ischemic stroke.39
A limitation of this study is the use of RCT data instead of data from more recent registries and surveys representing the current clinical practice. However, the use of RCT data made it possible to analyze potential differences in treatment effect of EVT between women and men and resolve the uncertainty that still existed about the treatment benefit of EVT in women. This would have been difficult, if not at all possible, if we had used observational data. Moreover, by using RCT data, we were able to control for numerous factors that were measured in a consistent, rigorous manner. Another limitation is lack of information about marital status. Marital status is known to be related to baseline sex differences, such as longer onset to randomization times (reflecting prehospital delays) in women, and could impact outcome.
We conclude that sex does not influence clinical outcome after EVT for ischemic stroke and that women and men benefit equally from EVT. Sex should, therefore, not be a consideration in the selection of patients for EVT.
Acknowledgments
We would like to thank the HERMES collaboration (Highly Effective Reperfusion Using Multiple Endovascular Devices) investigators.
Sources of Funding
The University of Calgary received an unrestricted grant from Medtronic for the HERMES collaboration (Highly Effective Reperfusion Using Multiple Endovascular Devices) initiative, which had no role in the study design, data collection, data analysis or interpretation, writing of the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosures
Dr Brown reports personal fees from the University of Calgary during the conduct of the HERMES collaboration (Highly Effective Reperfusion Using Multiple Endovascular Devices) and personal fees from Medtronic outside the submitted work. Dr Goyal reports that Medtronic provided a grant to the University of Calgary toward the HERMES collaboration and that Stryker provided a research grant to the University toward the UNMASK EVT study (Decision Making Under Uncertainty in the Management of Acute Stroke With Endovascular Thrombectomy: Applying Principles From Neuroeconomics). Dr Muir reports grants from Medtronic and Codman outside the submitted work and has an advisory relationship with Boehringer Ingelheim. Dr White has consulted for Microvention, Codman, and Stryker. Dr Dávalos reports consultancy and advisory board fees from Medtronic Neurovascular (Steering Committee STAR [Solitaire FR Thrombectomy for Acute Revascularization]) and an unrestricted grant for the REVASCAT trial (Randomized Trial of Revascularization With Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours of Symptom Onset) from Medtronic (paid to his institution). Dr Jovin has consulted for Cerenovus as Data Safety Monitoring Board/steering committee and Stryker Neurovascular as a principal investigator of the DAWN trial (DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo); received honoraria from Biogen as a consultant; holds stock or other ownership at Silk Road, Anaconda, Route 92, Corindus, Blockade Medical, and FreeOx Biotech. Dr Hill reports a research grant from Medtronic LCC paid to the University of Calgary for the HERMES collaboration. Dr Mitchell reports speaking engagements from Stryker and Microvention and honoraria from Stryker, outside the submitted work. Dr Demchuk reports honoraria from Medtronic during the conduct of the HERMES collaboration. Dr Saver has acted as a scientific consultant regarding trial design and conduct for Medtronic, Stryker, Cerenovus, and Rapid Medical and reports that the University of California has patent rights in the retrieval devices for stroke. Dr van Zwam reports speaking engagements from Stryker and Cerenovus (both paid to his institution). Dr Dippel reports research grants from the Dutch Heart Foundation, Dutch Brain Foundation, the Netherlands Organisation for Health Research and Development, Health Holland Top Sector Life Sciences and Health, and unrestricted grants from AngioCare BV, Medtronic/Covidien/EV3, MEDAC Gmbh/LAMEPRO, Penumbra, Inc, Stryker, Stryker European Operations BV, Top Medical/Concentric, and Thrombolytic Science, LLC (all paid to his institution). The other authors report no conflicts.
Footnotes
Guest Editor for this article was Natalia S. Rost, MD, MPH.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.023743.
References
- 1.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. European BIOMED Study of Stroke Care Group. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 2.Niewada M, Kobayashi A, Sandercock PA, Kamiński B, Członkowska A International Stroke Trial Collaborative Group. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–128. doi: 10.1159/000082999. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 3.Lisabeth LD, Reeves MJ, Baek J, Skolarus LE, Brown DL, Zahuranec DB, et al. Factors influencing sex differences in poststroke functional outcome. Stroke. 2015;46:860–863. doi: 10.1161/STROKEAHA.114.007985. doi: 10.1161/STROKEAHA.114.007985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ridder IR, Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Wermer MJ, et al. Is intra-arterial treatment for acute ischemic stroke less effective in women than in men? Interv Neurol. 2016;5:174–178. doi: 10.1159/000447331. doi: 10.1159/000447331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. HERMES Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 8.Arnold M, Fischer U, Compter A, Gralla J, Findling O, Mattle HP, et al. BASICS Study Group. Acute basilar artery occlusion in the basilar artery international cooperation study: does gender matter? Stroke. 2010;41:2693–2696. doi: 10.1161/STROKEAHA.110.594036. doi: 10.1161/STROKEAHA.110.594036. [DOI] [PubMed] [Google Scholar]
- 9.Venema E, Mulder MJHL, Roozenbeek B, Broderick JP, Yeatts SD, Khatri P, et al. Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. 2017;357:j1710. doi: 10.1136/bmj.j1710. doi: 10.1136/bmj.j1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. THRACE Investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 11.Muir KW, Ford GA, Messow CM, Ford I, Murray A, Clifton A, et al. PISTE Investigators. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88:38–44. doi: 10.1136/jnnp-2016-314117. doi: 10.1136/jnnp-2016-314117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 14.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 15.Campbell BCV, van Zwam WH, Goyal M, Menon BK, Dippel DWJ, Demchuk AM, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17:47–53.. doi: 10.1016/S1474-4422(17)30407-6. [DOI] [PubMed] [Google Scholar]
- 16.Wallach JD, Sullivan PG, Trepanowski JF, Steyerberg EW, Ioannidis JP. Sex based subgroup differences in randomized controlled trials: empirical evidence from cochrane meta-analyses. Bmj. 2016;355:i5826. doi: 10.1136/bmj.i5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, et al. Group ISTc. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the Third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindley RI, Wardlaw JM, Whiteley WN, Cohen G, Blackwell L, Murray GD, et al. IST-3 Collaborative Group. Alteplase for acute ischemic stroke: outcomes by clinically important subgroups in the Third International Stroke Trial. Stroke. 2015;46:746–756. doi: 10.1161/STROKEAHA.114.006573. doi: 10.1161/STROKEAHA.114.006573. [DOI] [PubMed] [Google Scholar]
- 19.Hametner C, MacIsaac RL, Kellert L, Abdul-Rahim AH, Ringleb PA, Lees KR VISTA Collaborators. Sex and stroke in thrombolyzed patients and controls. Stroke. 2017;48:367–374. doi: 10.1161/STROKEAHA.116.014323. doi: 10.1161/STROKEAHA.116.014323. [DOI] [PubMed] [Google Scholar]
- 20.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutsep HL, Hill MD. Effects of sex on mechanical embolectomy outcome. J Stroke Cerebrovasc Dis. 2012;21:240–242. doi: 10.1016/j.jstrokecerebrovasdis.2010.08.002. doi: 10.1016/j.jstrokecerebrovasdis.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Arnold M, Kappeler L, Nedeltchev K, Brekenfeld C, Fischer U, Keserue B, et al. Recanalization and outcome after intra-arterial thrombolysis in middle cerebral artery and internal carotid artery occlusion: does sex matter? Stroke. 2007;38:1281–1285. doi: 10.1161/01.STR.0000259711.13490.23. doi: 10.1161/01.STR.0000259711.13490.23. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho A, Cunha A, Gregório T, Paredes L, Costa H, Veloso M, et al. Is the efficacy of endovascular treatment for acute ischemic stroke sex-related. Interv Neurol. 2018;7:42–47. doi: 10.1159/000484098. doi: 10.1159/000484098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen TE, DeCroce-Movson E, Hemendinger M, McTaggart RA, Yaghi S, Cutting S, et al. Sex differences in 90-day outcomes after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2018;0:1–6. doi: 10.1136/neurintsurg-2018-014050. [DOI] [PubMed] [Google Scholar]
- 25.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccini JP, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation I, patients. Differences in clinical and functional outcomes of atrial fibrillation in women and men-reply. JAMA Cardiol. 2017;2:108–110. doi: 10.1001/jamacardio.2016.3711. [DOI] [PubMed] [Google Scholar]
- 27.Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al. MR CLEAN Investigators. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47:768–776. doi: 10.1161/STROKEAHA.115.011788. doi: 10.1161/STROKEAHA.115.011788. [DOI] [PubMed] [Google Scholar]
- 28.Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, et al. IMS III Investigators. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014;45:759–764. doi: 10.1161/STROKEAHA.113.004072. doi: 10.1161/STROKEAHA.113.004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elijovich L, Goyal N, Mainali S, Hoit D, Arthur AS, Whitehead M, et al. CTA collateral score predicts infarct volume and clinical outcome after endovascular therapy for acute ischemic stroke: a retrospective chart review. J Neurointerv Surg. 2016;8:559–562. doi: 10.1136/neurintsurg-2015-011731. doi: 10.1136/neurintsurg-2015-011731. [DOI] [PubMed] [Google Scholar]
- 30.Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol. 2011;32:1640–1645. doi: 10.3174/ajnr.A2564. doi: 10.3174/ajnr.A2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nambiar V, Sohn SI, Almekhlafi MA, Chang HW, Mishra S, Qazi E, et al. CTA collateral status and response to recanalization in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2014;35:884–890. doi: 10.3174/ajnr.A3817. doi: 10.3174/ajnr.A3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheitz JF, Abdul-Rahim AH, MacIsaac RL, Cooray C, Sucharew H, Kleindorfer D, et al. SITS Scientific Committee. Clinical selection strategies to identify ischemic stroke patients with large anterior vessel occlusion: results from SITS-ISTR (Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Registry). Stroke. 2017;48:290–297. doi: 10.1161/STROKEAHA.116.014431. doi: 10.1161/STROKEAHA.116.014431. [DOI] [PubMed] [Google Scholar]
- 33.Silva GS, Lima FO, Camargo EC, Smith WS, Lev MH, Harris GJ, et al. Gender differences in outcomes after ischemic stroke: role of ischemic lesion volume and intracranial large-artery occlusion. Cerebrovasc Dis. 2010;30:470–475. doi: 10.1159/000317088. doi: 10.1159/000317088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Towfighi A, Markovic D, Ovbiagele B. Sex differences in revascularization interventions after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:e347–e353. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.018. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 35.de Ridder I, Dirks M, Niessen L, Dippel D PRACTISE Investigators. Unequal access to treatment with intravenous alteplase for women with acute ischemic stroke. Stroke. 2013;44:2610–2612. doi: 10.1161/STROKEAHA.113.002263. doi: 10.1161/STROKEAHA.113.002263. [DOI] [PubMed] [Google Scholar]
- 36.Reeves M, Bhatt A, Jajou P, Brown M, Lisabeth L. Sex differences in the use of intravenous rt-PA thrombolysis treatment for acute ischemic stroke: a meta-analysis. Stroke. 2009;40:1743–1749. doi: 10.1161/STROKEAHA.108.543181. doi: 10.1161/STROKEAHA.108.543181. [DOI] [PubMed] [Google Scholar]
- 37.Reid JM, Dai D, Gubitz GJ, Kapral MK, Christian C, Phillips SJ. Gender differences in stroke examined in a 10-year cohort of patients admitted to a Canadian teaching hospital. Stroke. 2008;39:1090–1095. doi: 10.1161/STROKEAHA.107.495143. doi: 10.1161/STROKEAHA.107.495143. [DOI] [PubMed] [Google Scholar]
- 38.Reeves MJ, Prager M, Fang J, Stamplecoski M, Kapral MK. Impact of living alone on the care and outcomes of patients with acute stroke. Stroke. 2014;45:3083–3085. doi: 10.1161/STROKEAHA.114.006520. doi: 10.1161/STROKEAHA.114.006520. [DOI] [PubMed] [Google Scholar]
- 39.Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018;17:641–650. doi: 10.1016/S1474-4422(18)30201-1. doi: 10.1016/S1474-4422(18)30201-1. [DOI] [PubMed] [Google Scholar]


