Abstract
In the phase 3 LUX-Lung 8 study, the ERBB family blocker, afatinib, significantly prolonged progression-free survival and overall survival relative to erlotinib in patients with relapsed/refractory squamous cell carcinoma of the lung. We describe the case of a 53-year-old Asian male enrolled in LUX-Lung 8 who experienced long-term benefit from afatinib following failure of platinum-based chemotherapy. The patient received afatinib, and remained progression-free for 14.7 months according to investigator review. Overall survival was 17.7 months. Tolerability-guided dose adjustments helped facilitate long-term afatinib use by mitigating drug-related adverse effects. Next-generation sequencing revealed that multiple genetic aberrations were present, including epidermal growth factor receptor copy number amplification, and mutations in ERBB4, ALK, RET, and BRCA2. These findings may help to explain the enhanced response to afatinib and highlight the importance of biomarker analysis in guiding treatment decisions in patients with squamous cell carcinoma of the lung.
Keywords: afatinib, EGFR, ERBB, NSCLC, squamous cell carcinoma of the lung
Background
Squamous cell carcinoma (SqCC) of the lung is usually diagnosed when disease is already advanced [1], limiting treatment options. Further, due to the wide molecular heterogeneity of SqCC [2], most patients are not suitable for targeted therapies. As a result, systemic platinum-based chemotherapy remains the standard first-line treatment for SqCC. Recently, however, a number of novel therapies have been introduced into routine clinical practice in the first- and second-line settings, as monotherapy or in combination with chemotherapy. These include monoclonal antibodies targeting the epidermal growth factor receptor (EGFR; necitumumab), vascular endothelial growth factor (ramucirumab), or the immune checkpoint system (pembrolizumab, nivolumab, atezolizumab) [3].
Although EGFR mutations are rare in SqCC, there is biological rationale for ERBB family inhibition in this setting because EGFR overexpression occurs in 60%–80% of tumours [4], and approximately 10% of tumours demonstrate EGFR copy number alterations [5,6]. In addition, other members of the ERBB family, including HER2 and HER3 are often over-expressed [7,8], suggesting that ERBB signalling may play a key role in SqCC disease pathology. The ERBB family blocker, afatinib, is approved for the treatment of relapsed/refractory SqCC of the lung based on the results phase 3 LUX-Lung 8 study. In this study, afatinib significantly prolonged progression-free survival [PFS; median 2.4 vs. 1.9 months; hazard ratio (HR) 0.82; P = 0.043] and overall survival (OS; median 7.9 vs. 6.8 months; HR 0.81; P = 0.008) vs. erlotinib [9]. Notably, 5% of patients received long-term benefit with afatinib (remained on treatment for ≥12 months) [10].
Afatinib irreversibly inhibits signalling from all homodimers and heterodimers of the ERBB family [11], which cooperate via interconnected intracellular pathways to regulate cellular proliferation [12]. Thus, it was hypothesized that specific genetic aberrations within the ERBB family might predict the long-term response to afatinib observed in some patients [13]. Indeed, recent comprehensive biomarker analysis, including next-generation sequencing (NGS) to identify genetic abnormalities, demonstrated a trend towards improved PFS (4.9 vs. 3.0 months; HR 0.62; P = 0.06) and OS (10.6 vs. 8.1 months; HR 0.75; P = 0.21) with afatinib in patients with ERBB mutation-positive disease vs. those without [13].
In this case study, we describe the clinical and tumour molecular characteristics of a patient included in LUX-Lung 8 who remained on afatinib for over a year, with the aim of providing further insight into possible factors underlying long-term response to afatinib.
Case report
A Chinese male patient initially presented in August 2012, aged 53 years, with paroxysmal cough, a small amount of bloody phlegm, and asthma following activity. The patient, an ex-smoker with a 30-year smoking history (75 pack-years) was subsequently diagnosed with SqCC of the left lower lobe and underwent a left pneumonectomy. Pathology confirmed a moderately-differentiated SqCC of the bronchus at the root of the left lower lobe (Fig. 1a); P-T2bN3M0, R (-), stage IIIB, Eastern Cooperative Oncology Group (ECOG) performance status 1, with infiltration of bronchial wall, and hilar vascular wall invasion. Tumour size was 6.5 × 5.0 × 3.4 cm. Metastasis was detected in the subcarina (Fig. 1b), tracheal bronchus, lower pulmonary ligament and posterior vena cava, right hilar and supraclavicular lymph nodes, thus precluding radiotherapy.
Fig. 1.
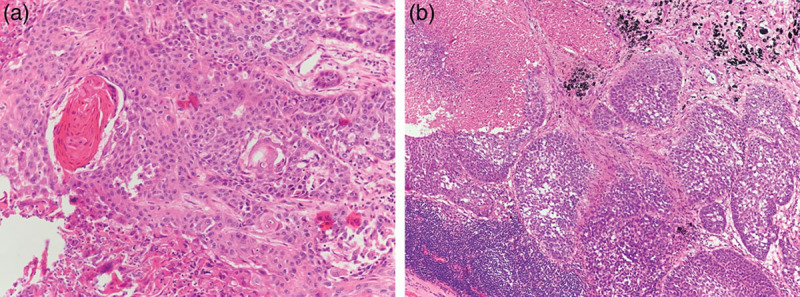
Pathological images. (a) Lung squamous cell carcinoma was identified by haematoxylin and eosin (HE) staining of primary tumour at the root of left lower lobe (magnification ×200). (b) Subcarina lymph nodes involvement was indicated by HE staining (magnification ×100).
Following surgery, the patient received four cycles of platinum-based chemotherapy (carboplatin 450 mg plus paclitaxel 300 mg for two cycles followed by carboplatin 450 mg plus paclitaxel 270 mg for the final two cycles), with a best response of stable disease recorded. Imaging conducted in April 2013 identified progressive disease, with a malignant lesion in the patient’s right lumbar lymph nodes. The presence of progressive disease after receiving four cycles of chemotherapy meant that the patient eligible for enrolment into LUX-Lung 8 [9], and he started treatment with afatinib 40 mg/day on 25 April 2013, with an ECOG performance score of 0, and normal renal and hepatic function.
Results
At the time of the first follow-up CT scan on 20 June 2013, the patient had achieved a partial response by independent review (Fig. 2). Progressive disease by investigator review was identified on 17 July 2014 with new nodules identified on the right adrenal gland (Fig. 2). PFS by investigator review was 448 days (14.7 months). Progressive disease by independent review was identified on 28 January 2014 due to progression of the lesion in the right supraclavicular lymph node. PFS by independent review was 278 days (9.1 months). Although the supraclavicular lymph node was detected at baseline by investigator review, and shrunk significantly on afatinib treatment, it was not considered a measurable lesion. Hence, afatinib treatment was continued; the patient received afatinib until 11 September 2014, a total of 504 days (16.6 months). As such, the patient was treated beyond radiological progression according to independent review; the time between independent (PFS-1) and investigator progression (PFS-2) was 170 days (5.6 months). OS was 537 days (17.7 months).
Fig. 2.
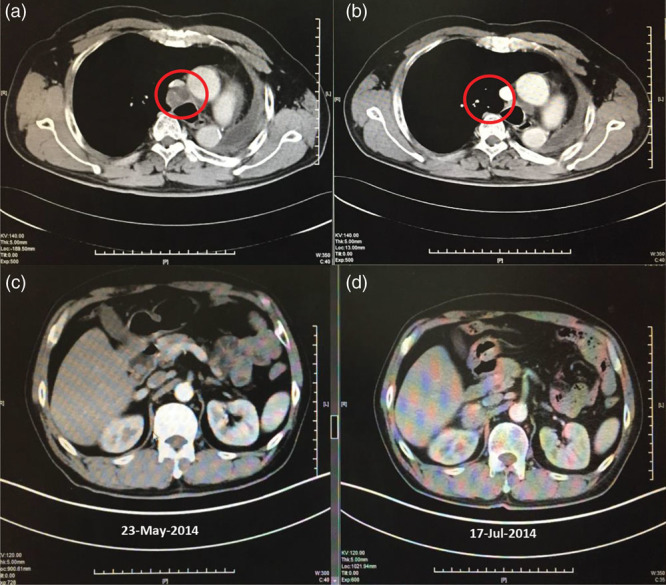
CT scans showing response to afatinib in (a) April 2013 (pre-treatment), (b) June 2013 (partial response), and (c) July 2014 (progressive disease; left panel shows same field before progression). CT, computed tomography.
Adverse events were consistent with those experienced by the wider LUX-Lung 8 population [9]. The patient experienced diarrhoea (maximum grade 2, managed with loperamide), rash (maximum grade 3), and grade 2 paronychia. He required two separate afatinib dose reductions, from 40 to 30 mg due to rash, and then from 30 to 20 mg. Adverse events decreased after dose reduction, and were primarily grade 1 or 2.
According to the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 and its lung cancer-specific module (QLQ-LC13), the patient’s global quality of life remained largely stable during treatment, declining markedly only between the last two visits. Symptom scores, including dyspnoea and overall lung cancer symptoms, were similarly stable, declining after disease progression had occurred.
Foundation Medicine FoundationOne NGS analysis conducted on archived paraffin-embedded tissue samples revealed that the patient had multiple genetic aberrations: EGFR copy number amplification (copy number = 3.68); FGFR1 copy number amplification (copy number = 3.38); a missense S408F mutation in exon 5 of ALK; a missense V130A mutation in exon 4 of BRCA2; a missense R982H mutation in exon 18 of RET; and a missense R847H mutation in exon 21 of ERBB4. Veristrat analysis, a serum protein test shown to be predictive of response to EGFR tyrosine-kinase inhibitors [14], was not conducted. EGFR expression, determined using an EGFR pharmDx immunohistochemistry kit (Dako, Glostrup, Denmark), was positive (H-score of ≥200).
Discussion
The case described above demonstrates the clinical efficacy of afatinib in a patient with advanced SqCC of the lung who relapsed following first-line chemotherapy. PFS by independent review was 9.1 months, which is higher than median PFS observed with other recently approved agents for the second-line treatment of SqCC of the lung, including nivolumab (3.5 months [15]), pembrolizumab (~3.9–4.0 months [16]), and atezolizumab (~2.8 months [17]). Furthermore, the prolonged OS in this patient (17.7 months), as well as practical benefits of afatinib, such as oral administration and its predictable and manageable tolerability profile, illustrate the feasibility of afatinib as a treatment option for patients with relapsed/refractory SqCC of the lung.
Although continued treatment with afatinib following radiological progression was not mandated in LUX-Lung 8, this patient received afatinib for 226 days following progression by independent review (the patient was progression-free for 448 days according to investigator review). Several studies have suggested that continued ErbB inhibition beyond progression in the absence of clinical deterioration may improve outcomes in patients with NSCLC [18,19] and reduce the risk of disease flare, although data are limited in patients with SqCC of the lung. Had the investigator stopped afatinib treatment at PFS-1 (when progression was detected by independent review), third-line treatment options would have been limited. Radical radiotherapy was contraindicated due to the pneumonectomy and few data exist regarding third-line treatment options in patients with SqCC of the lung. Single-agent chemotherapy (docetaxel) may have been an option, but is associated with median PFS of <4 months in this setting [16,17]. Although data are limited, no single-agent targeted agent, to date, has demonstrated superiority over docetaxel in a third-line setting [20]. Other patients may only be fit enough to receive best supportive care, although radiation therapy may be appropriate in some cases (but not in this case). Given the paucity of effective third-line treatment options, the observations in this study suggest that there may be a case for continuing second-line afatinib treatment in patients without clinical deterioration or slowly progressing tumours.
Results of NGS analysis suggest that the prolonged response to afatinib in this case may be related to the presence of multiple ERBB aberrations in tumour tissue, and highlights the value of molecular analysis to help identify patients who may derive most benefit from targeted agents like afatinib. Notably, the patient harboured a missense ERBB4 mutation. Although rare in the LUX-Lung 8 trial (present in 14 of the 245 patients treated with afatinib included in NGS analysis), ERBB4 mutations were associated with a trend towards improved OS [HR 0.22; 95% confidence interval (CI) 0.05–1.04] and PFS (HR 0.21; 95% CI 0.02–1.94) [13]. Other putative biomarkers, such as EGFR expression [21] and EGFR copy number [22], and positive Veristrat classification, which was associated with favourable survival outcomes in LUX-Lung 8 [14], may also prove useful when evaluating patient suitability for targeted therapy.
It is likely that the prolonged clinical benefit with afatinib in this patient was made possible by applying tolerability-guided dose reductions, which mitigated afatinib-related adverse events. Evidence suggests that such dose reductions do not reduce the efficacy of afatinib in patients with EGFR mutation-positive NSCLC [23]. Simple, oral dosing may also have enhanced long-term compliance.
With the ever-expanding range of second-line treatments for SqCC of the lung, identification of patients likely to derive long-term benefit from each treatment option is essential and will help facilitate a more personalized approach to patient care. Our case demonstrates that despite a poor initial prognosis, some patients, such as those with ERBB aberrations, can benefit from prolonged therapy with afatinib. In this case, rebiopsy of the supraclavicular lymph node could have revealed the resistance mechanism to afatinib, helping to drive treatment decisions.
Acknowledgements
Medical writing assistance was provided by Lynn Pritchard of GeoMed, an Ashfield company, part of UDG Healthcare plc, during the preparation of the article.
Boehringer Ingelheim Pharmaceuticals Inc. contracted and compensated writing and editorial support and was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Consent for publication: The patient provided written informed consent for participation in this study.
Availability of data and material: The datasets generated and analysed during the study are available from Hong Jian on reasonable request.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011; 32:669–692 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012; 489:519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017; 15:504–535 [DOI] [PubMed] [Google Scholar]
- 4.Hirsh V. New developments in the treatment of advanced squamous cell lung cancer: focus on afatinib. Onco Targets Ther. 2017; 10:2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, Shim HS, Park MS, Kim JH, Ha SJ, Kim SH, Cho BC. High EGFR gene copy number and skin rash as predictive markers for EGFR tyrosine kinase inhibitors in patients with advanced squamous cell lung carcinoma. Clin Cancer Res. 2012; 18:1760–1768 [DOI] [PubMed] [Google Scholar]
- 6.Chang H, Yang Y, Lee JS, Jheon SH, Kim YJ, Chung JH. Epidermal growth factor receptor gene amplification predicts worse outcome in patients with surgically resected nonadenocarcinoma lung cancer. Clin Lung Cancer. 2019; 20:7–12.e1 [DOI] [PubMed] [Google Scholar]
- 7.Ugocsai K, Mándoky L, Tiszlavicz L, Molnár J. Investigation of HER2 overexpression in non-small cell lung cancer. Anticancer Res. 2005; 25:3061–3066 [PubMed] [Google Scholar]
- 8.Yi ES, Harclerode D, Gondo M, Stephenson M, Brown RW, Younes M, Cagle PT. High c-erbb-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol. 1997; 10:142–148 [PubMed] [Google Scholar]
- 9.Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, et al. ; LUX-Lung 8 Investigators. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015; 16:897–907 [DOI] [PubMed] [Google Scholar]
- 10.Yang JCH, Goss G, Felip E, Lu S, Ardizzoni A, Gadgeel SM, et al. 102P LUX-Lung 8 phase III trial: analysis of long-term response to second-line afatinib in patients with advanced squamous cell carcinoma (SCC) of the lung. Ann Oncol. 2017; 28:mdx091.022mdx091.022 [Google Scholar]
- 11.Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible erbb family blocker. J Pharmacol Exp Ther. 2012; 343:342–350 [DOI] [PubMed] [Google Scholar]
- 12.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012; 12:553–563 [DOI] [PubMed] [Google Scholar]
- 13.Goss GD, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, et al. Association of ERBB mutations with clinical outcomes of afatinib- or erlotinib-treated patients with lung squamous cell carcinoma: secondary analysis of the LUX-lung 8 randomized clinical trial. JAMA Oncol. 2018; 4:1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadgeel S, Goss G, Soria JC, Felip E, Georgoulias V, Lu S, et al. Evaluation of the veristrat® serum protein test in patients with advanced squamous cell carcinoma of the lung treated with second-line afatinib or erlotinib in the phase III LUX-lung 8 study. Lung Cancer. 2017; 109:101–108 [DOI] [PubMed] [Google Scholar]
- 15.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–1550 [DOI] [PubMed] [Google Scholar]
- 17.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. ; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017; 389:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuler M, Yang JC, Park K, Kim JH, Bennouna J, Chen YM, et al. ; LUX-Lung 5 Investigators. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-lung 5 trial. Ann Oncol. 2016; 27:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuler M, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. First-line afatinib vs gefitinib for patients with EGFR mutation-positive NSCLC (LUX-lung 7): impact of afatinib dose adjustment and analysis of mode of initial progression for patients who continued treatment beyond progression. J Cancer Res Clin Oncol. 2019; 145:1569–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N, Guo N, Tian L, Miao Z. Systematic review and meta-analysis of third-line salvage therapy for the treatment of advanced non-small-cell lung cancer: A meta-analysis of randomized controlled trials. Oncotarget. 2018; 9:35439–35447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang H, Oh J, Zhang X, Kim YJ, Lee JH, Lee CT, et al. EGFR protein expression using a specific intracellular domain antibody and PTEN and clinical outcomes in squamous cell lung cancer patients with EGFR-tyrosine kinase inhibitor therapy. Onco Targets Ther. 2016; 9:5153–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang Y, Tang H, He J. EGFR gene copy number as a predictive/biomarker for patients with non-small-cell lung cancer receiving tyrosine kinase inhibitor treatment: a systematic review and meta-analysis. J Investig Med. 2017; 65:72–81 [DOI] [PubMed] [Google Scholar]
- 23.Yang JC, Sequist LV, Zhou C, Schuler M, Geater SL, Mok T, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-lung 3 and 6 trials. Ann Oncol. 2016; 27:2103–2110 [DOI] [PubMed] [Google Scholar]


