ABSTRACT
Objectives:
In this study, we hypothesized that replacing conventional milk, which contains A1 and A2 β-casein proteins, with milk that contains only A2 β-casein in the diet of dairy or milk-intolerant preschoolers (age 5 to 6 years) would result in reduced gastrointestinal symptoms associated with milk intolerance, and that this would correspond with cognitive improvements.
Methods:
This randomized, double-blind, crossover study aimed to compare the effects of 5 days’ consumption of conventional milk versus milk containing only A2 β-casein on gastrointestinal symptoms, as assessed via visual analog scales, average stool frequency and consistency, and serum inflammatory and immune biomarkers in healthy preschoolers with mild-to-moderate milk intolerance. The study also aimed to compare changes in the cognitive behavior of preschoolers, based on Subtle Cognitive Impairment Test scores.
Results:
Subjects who consumed milk containing only A2 β-casein had significantly less severe gastrointestinal symptoms as measured by visual analog scales, reduced stool frequency, and improvements in stool consistency, compared with subjects consuming conventional milk. There were significant increases from baseline in serum interleukin-4, immunoglobulins G, E, and G1, and beta-casomorphin-7 coupled to lower glutathione levels, in subjects consuming conventional milk compared with milk containing only A2 β-casein. Subtle Cognitive Impairment Test analysis showed significant improvements in test accuracy after consumption of milk containing only A2 β-casein. There were no severe adverse events related to consumption of either milk product.
Conclusions:
Replacing conventional milk with milk containing only A2 β-casein reduced gastrointestinal symptoms associated with milk intolerance in Chinese preschool children, with corresponding improvements in aspects of cognitive performance.
Keywords: beta-casomorphin-7, gastrointestinal symptoms, milk intolerance, pediatric
What Is Known/What Is New
What Is Known
Milk or dairy intolerance is more prevalent in Chinese adults and children than in their Western or European counterparts.
This undesirable physiological response is primarily attributed to genetic polymorphisms of the lactase gene leading to lower activity of gastrointestinal lactase.
What Is New
This study compares conventional milk (containing A1 and A2 β-casein) with milk containing A2 β-casein only. We report that conventional milk exacerbates the symptoms of digestive discomfort associated with lactose intolerance.
Consumption of conventional milk also led to increased serum inflammatory and immune biomarkers, and an increased cognitive test error rate compared with consumption of milk containing A2 beta-casein only.
There is increasing evidence in the literature that the effects of dairy products on gastrointestinal dysfunction may be at least partially attributed to the proteolytic release of the bioactive peptide beta-casomorphin-7 (BCM-7) from β-casein, rather than lactose intolerance (LI) (1–3). Recent studies report that consumption of A1 β-casein induces inflammation in the small intestine, which may potentiate LI symptoms by downregulating lactase enzyme expression or activity (4,5). The A2 variant of β-casein, however, has a much slower rate of proteolytic digestion; therefore, its consumption results in a nil or not physiologically relevant, much lower yield of BCM-7 (6,7).
LI is caused by a deficiency of lactase, the enzyme needed to break down lactose, and lactase deficiency is strongly associated with ethnicity (8). Studies in Chinese children revealed a prevalence of lactase deficiency of 38.5% in children aged 3 to 5 years increasing to 87% in children aged 7 to 8 years and 11 to 13 years; prevalence of LI in the same children was 12.2% in children aged 3 to 5 years, 33.1% in those aged 7 to 8 years, and 30.5% in children aged 11 to 13 years (9). Based on the results of recent studies (4,5), we hypothesized that consumption of A1 β-casein would lead to an inflammatory state in Chinese children that would exacerbate the symptoms of LI, and that switching to milk containing A2 β-casein only would reduce the symptoms of LI.
Furthermore, gastrointestinal inflammation induced by diet-derived bioactive peptides is linked to deficits in behavioral parameters such as memory, attention, and processing speed (10). Therefore, this study aimed to compare the effects of 5 days’ consumption of conventional milk (containing both A1 and A2 β-casein) versus milk containing only A2 β-casein on gastrointestinal symptoms, gut inflammation, and cognitive behavior in healthy preschoolers with mild-to-moderate milk intolerance.
METHODS
Ethical Approval
This study was conducted according to International Conference on Harmonization guidelines, Chinese Good Clinical Practice guidelines, and Chinese regulations issued by the State Food and Drug Administration. The study was registered at ClinicalTrials.gov (NCT03081845) and was reported according to CONSORT reporting guidelines (11). It was performed at 3 kindergartens in Jin Hua, China, and the institutional review board of the Shanghai Nutrition Society reviewed and approved the study protocol. A 5-day study period was based on a decision between the ethics committee and principal investigators, due to incorporation of a diet that may cause gastrointestinal discomfort in children. All parents provided signed informed consent forms before enrollment.
Study Design
This was a multicenter, double-blind, randomized, controlled, parallel, crossover study which aimed to compare the effects of milk containing A2 beta-casein only with those of conventional milk in preschoolers with mild-to-moderate LI (see Figure, Supplemental Digital Content 1, which illustrates the study design). Subjects were initially screened for clinical symptoms of LI and underwent urinary galactose tests (a positive test representing lactase deficiency) to confirm which subjects had genuine LI. If eligible, subjects entered a 10-day washout period. During phase 1, subjects were randomized and stratified by sex ratio (approximate male:female ratio 1:1) to a 5-day intervention (day 1–5) of either conventional milk or milk containing A2 beta-casein only. All subjects then entered a 9-day washout period before commencing phase 2, where subjects crossed over to the other milk product for 5 days (days 15–19).
Subjects
Subjects underwent a physical examination, and baseline characteristics were recorded. Eligible subjects completed a visual analog scales (VAS) questionnaire to assess baseline gastrointestinal symptoms.
The study inclusion criteria included children aged 5 to 6 years (age group selected to include a high proportion of lactase-deficient, LI individuals, hypothesized to be the most likely responders to switching milk products) who were nonregular milk drinkers reporting mild-to-moderate discomfort after milk consumption. Key exclusion criteria included known allergies to dairy products, severe milk intolerance, a history of fecal impaction, and having suffered from gastrointestinal disorders (eg, irritable bowel syndrome, colitis, ulcerative colitis, or celiac disease). In addition, subjects were excluded if they were currently being administered drugs for cardiovascular or metabolic disease, or were trying to lose weight by following a diet or exercise regimen or taking any appetite or weight loss drugs for the previous 3 months.
Interventions
Milks containing A1 and A2 β-casein (conventional milk) or only A2 β-casein were provided by a2 Infant Nutrition Limited (Shanghai, China). S.P.R.I.M. (S.P.R.I.M. China [Shanghai] Consulting Co, Ltd) repackaged all products into identical packaging. All products were assigned and labeled according to subject ID number. Used and unused cartons were collected at each visit to evaluate compliance and confirm that blinding was intact.
Subjects were randomized to each sequence, according to allocation numbers in sealed envelopes. Allocation was based on a computer-generated list, and subjects and investigators were blinded to intervention allocation until after statistical analysis was complete. Enrolled subjects received the product in a 1-L TetraPak and consumed 150 mL twice daily after a meal during the 5-day intervention. Subjects used a diary to record milk intake, adherence to each intervention, and adverse events. During the intervention, subjects visited the study site on the first and last day of each phase. Telephone follow-up was performed during washout. A Complete Nutritional Assessment Questionnaire was filled out at baseline and after each 5-day intervention (days 1, 5, 15, and 19) to analyze nutritional intake over 24 hours.
Study Measurements
The primary outcome of this study was the change from baseline in gastrointestinal symptoms, including bloating, abdominal pain, flatulence, and heavy or full stomach. Gastrointestinal symptoms were assessed via VAS daily during the intervention phases. VAS scores were measured for evaluation of gastrointestinal symptoms (0 = never; 1 = rarely; 2 = frequently; 3 = all the time) and were analyzed using the Generalized Estimating Equation (12). Baseline symptoms were evaluated on days 1 and 15 before milk consumption; postintervention symptoms were evaluated on days 1 to 5 and days 15 to 19 after milk consumption. A post-hoc subgroup analysis of VAS scores stratified by lactose tolerability was also performed.
Secondary outcomes of this study included the effects of conventional milk versus milk containing A2 beta-casein only on markers of gut inflammation and the analysis of stool frequency and consistency as assessed via the Bristol Stool Chart. See text, Supplemental Digital Content 2, which describes these secondary outcome methods in detail.
Another secondary outcome was analysis of the response time and error rate for a subject's information processing ability using the computer-based Subtle Cognitive Impairment Test (SCIT; NeuroTest.com) (13). The SCIT is a brief computer-based, perceptual judgment task that has been shown to be sensitive to small differences in global cognitive performance (13–15). See text, Supplemental Digital Content 2, which describes the SCIT method in detail.
Sample Size
Sample size was determined by power analysis of corresponding data points derived from a previous study in adults (4), and doubled after consideration of the lower LI incidence in Han Chinese children versus adults. The planned sample size was 80 subjects, accounting for a possible dropout rate of 10%, resulting in at least 35 subjects per study arm.
Statistical Analyses
Statistical analyses were completed using SAS version 9.3 (SAS Institute Inc, Cary, NC), with the exception of SCIT data, which were analyzed using Statistical Package for the Social Sciences version 22 (IBM Corporation, Armonk, NY). All tests of hypotheses were 2-sided, and the significance level was 0.05. For baseline and post-intervention data, continuous variables are described using mean and standard deviation (SD) for normally distributed results, whereas medians (ranges) are used for non-normal distribution outcomes. Categorical variables were described as frequency (%). The Kolmogorov-Smirnov test was used to assess the normality of blood and fecal measurements. Product differences were evaluated using 1-way analysis of variance for normally distributed outcomes, and the Kruskal-Wallis test for non-normal distribution outcomes. Analysis of SCIT variables was carried out using a linear mixed-model analysis for crossover study designs, which included the following 3 factors: phase (phase 1 and phase 2 of the crossover design); milk type (conventional milk and milk containing A2 beta-casein only); and condition (performance pre- and postconsumption of milk).
RESULTS
Subjects
This study was conducted between September and October 2016. A total of 835 preschool children aged 5 to 6 years were screened, of whom 104 were eligible, and 80 were enrolled (Fig. 1). Nineteen children were excluded because their parents did not want to sign the informed consent form (as collection of blood samples was necessary in the study); and 5 children were excluded because they arrived at the study site without having had breakfast. Of the 80 children enrolled, 68 were confirmed lactase deficient (positive urinary galactose test) and LI, and 12 were not, consistent with the lactase deficiency rates in this age group in China (9,16). There were no significant differences between study sequence groups (n = 40 in each group) (see Table, Supplemental Digital Content 3, which outlines subjects’ baseline characteristics). During phase 2, 1 subject changed school and a further 4 subjects were absent from start to finish in phase 2. Therefore, the results for these 5 subjects were excluded from the analysis.
FIGURE 1.
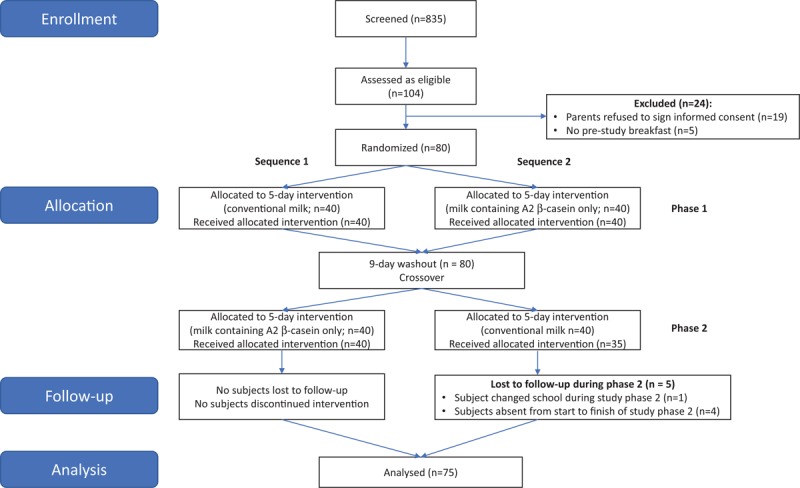
CONSORT flow diagram.
Gastrointestinal Symptoms
There were no significant differences in baseline total VAS scores between the 2 sequence groups in both phases (phase 1: P = 0.915; phase 2: P = 0.801) (see Table, Supplemental Digital Content 4, which demonstrates the mean daily total VAS scores).
Subjects consuming conventional milk, both overall (Fig. 2A) and stratified by confirmed LI (Fig. 2B and 2C), showed increased daily total VAS symptom scores throughout the intervention period. Lactose-tolerant subjects experienced more gastrointestinal symptoms when starting the intervention, and mean VAS scores gradually decreased over time. Mean daily total VAS scores for subjects consuming milk containing A2 beta-casein only were lower than scores in subjects consuming conventional milk, and remained at a similar level from baseline to intervention end. Furthermore, Generalized Estimating Equation analysis showed that subjects consuming milk containing A2 beta-casein only had significantly lower odds of gastrointestinal symptoms than subjects consuming conventional milk (see Table, Supplemental Digital Content 5, which demonstrates the gastrointestinal symptom outcomes).
FIGURE 2.
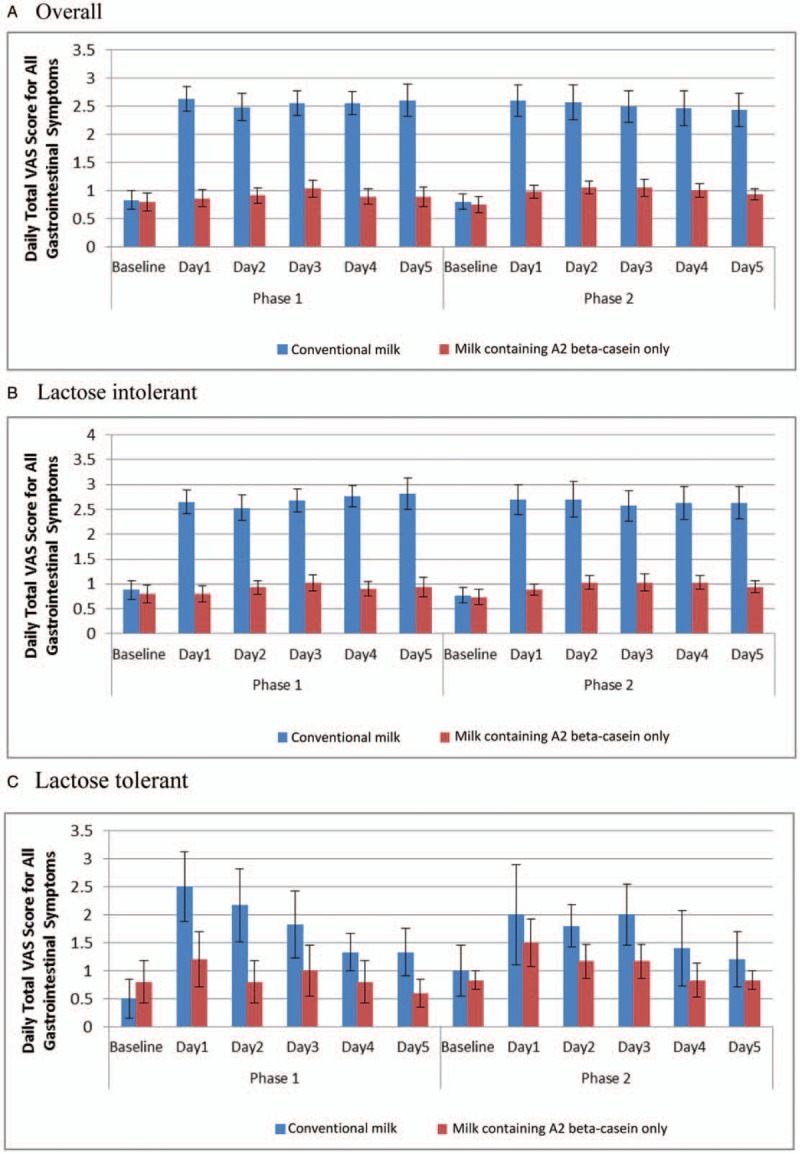
Daily total visual analog scale (VAS) score for all gastrointestinal symptoms by study product (A) (mean and standard deviation) and stratified by lactose tolerability; (B) confirmed lactose intolerant; and (C) lactose tolerant. A lower daily total VAS score is advantageous, as this indicates fewer gastrointestinal symptoms.
Figure 3A and B shows mean data (SE) by study product for 5-day average total gastrointestinal symptom VAS scores during each intervention phase for confirmed LI and lactose-tolerant subjects, respectively. When consuming conventional milk, confirmed LI subjects showed a significant increase in VAS symptom score throughout the intervention period (P < 0.0001). Conversely, lactose-tolerant subjects experienced more gastrointestinal symptoms at intervention start (P < 0.0001, P = 0.0006, and P = 0.0013 for days 1, 2, and 3, respectively); as the intervention continued, VAS scores gradually decreased. When subjects consumed milk containing A2 beta-casein only, daily total VAS score maintained a similar level to baseline in confirmed LI and lactose-tolerant subjects.
FIGURE 3.
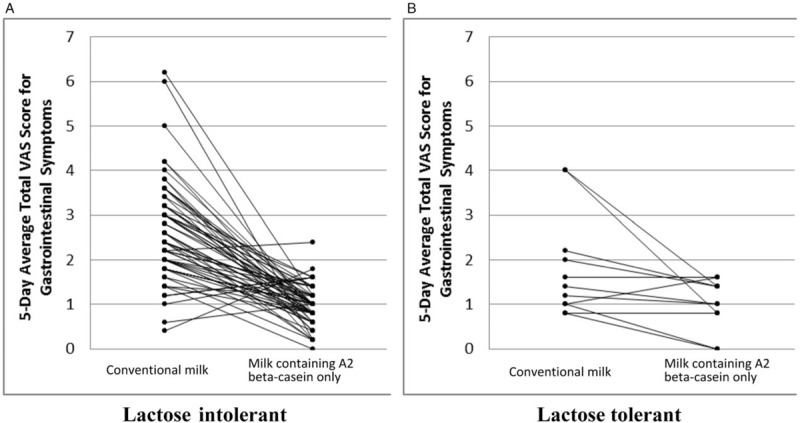
Subject profile for 5-day average total VAS scores for gastrointestinal symptoms during product intervention by study product and stratified by confirmed lactose tolerability. VAS = visual analog scale.
A significant difference in daily total VAS score was observed from baseline across the following 5 days (P < 0.0001) for all subjects, and within the confirmed LI and lactose-tolerant groups. In addition, subjects consuming different milk products showed different changes in daily total VAS scores for gastrointestinal symptoms over the intervention period (see Table, Supplemental Digital Content 6, which demonstrates the weekly total VAS scores).
Serum Immune and Inflammatory Biomarkers
At baseline, there were no significant differences between the 2 groups for all serum markers assessed (Table 1). Evaluation of within-product differences showed significant changes from baseline in serum interleukin 4 (P = 0.007), immunoglobulin G (IgG; P < 0.0001), IgG1 (P = 0.003), and IgE (P < 0.0001) for subjects consuming conventional milk by the end of study phase 1, but not for subjects consuming milk containing A2 beta-casein only (Table 1).
TABLE 1.
Summary statistics for serum and fecal markers at baseline and at the end of the 5-day intervention period of study phase 1
| Baseline | Postintervention | ||||||
| Measurement | Conventional milk (n = 40) | Milk containing A2 beta-casein only (n = 40) | P | Conventional milk (n = 40) | Milk containing A2 beta-casein only (n = 40) | P | |
| HGB, g/L | 116.3 (8.8) | 118.7 (8.7) | 0.233 | 117.0 (7.8) | 117.9 (7.8) | 0.608 | |
| IL-4, ng/mL | 0.73 (0.07) | 0.75 (0.09) | 0.280 | 0.77 (0.08) | 0.73 (0.08) | 0.025* | |
| IgG, g/L | 9.1 (1.7) | 9.7 (1.9) | 0.157 | 11.2 (2.0) | 9.2 (1.6) | <0.0001*** | |
| IgG1, g/L | 5.88 (1.62) | 6.10 (1.26) | 0.628 | 7.15 (1.36) | 6.04 (1.25) | 0.010* | |
| BCM-7, ng/mL | 2.63 (1.82–4.02) | 2.45 (1.95–4.26) | 0.173 | 3.15 (2.05–5.49) | 2.54 (1.95–4.38) | 0.004** | |
| GSH, nmol/mL | 0.90 (0.06–3.13) | 1.05 (0.30–2.37) | 0.223 | 1.44 (0.37–3.88) | 2.22 (0.68–3.88) | 0.0007*** | |
| CRP, mg/L | 0.24 (<0.158–5.23) | 0.27 (<0.158–3.12) | 0.553 | 0.23 (<0.158–3.83) | 0.16 (<0.158–4.96) | 0.668 | |
| Below detection limit | 14 (17.5%) | 16 (20.0%) | 16 (20.0%) | 20 (25.0%) | |||
| IgE, IU/mL | 68.5 (6.4–166.0) | 66.0 (1.8–161.0) | 0.935 | 90.5 (11.5–153.0) | 52.2 (16.3–160.0) | 0.036* | |
| MPO, ng/mL | Phase 1 | 0.11 (0–16.08) | 0.35 (0–14.70) | 1.77 (0–19.83) | 0.24 (0–23.16) | ||
| Phase 2 | 1.57 (0–8.36) | 0.34 (0–14.47) | 4.15 (0–14.22) | 0.98 (0–21.52) | |||
| Acetic acid (%) | Phase 1 | 0.40 (0.08) | 0.42 (0.08) | 0.40 (0.09) | 0.50 (0.09) | ||
| Phase 2 | 0.41 (0.09) | 0.39 (0.07) | 0.40 (0.07) | 0.48 (0.13) | |||
| Propanoic acid | Phase 1 | 0.16 (0.04) | 0.17 (0.04) | 0.17 (0.05) | 0.18 (0.05) | ||
| Phase 2 | 0.17 (0.05) | 0.16 (0.05) | 0.16 (0.04) | 0.17 (0.04) | |||
| Butanoic acid | Phase 1 | 0.12 (0.06) | 0.10 (0.05) | 0.10 (0.05) | 0.13 (0.04) | ||
| Phase 2 | 0.10 (0.05) | 0.10 (0.04) | 0.11 (0.04) | 0.13 (0.04) | |||
| Total SCFA (%) | Phase 1 | 0.68 (0.11) | 0.70 (0.11) | 0.67 (0.13) | 0.81 (0.11) | ||
| Phase 2 | 0.68 (0.12) | 0.65 (0.09) | 0.67 (0.09) | 0.77 (0.13) | |||
Normally distributed outcomes: HGB, IL-4, IgG, IgG1, acetic acid, propanoic acid butanoic acid, total SCFA; non-normally distributed outcomes: CRP, IgE, MPO.
Blood test outcomes are mean (SD) / median (range), frequency (%); fecal outcomes are mean (SD) / median (range) (75 subjects).
Blood laboratory testing outcomes at baseline and the end of study phase 1 were analyzed using 1-way analysis of variance. Non-normal distributed blood test outcomes were evaluated using the nonparametric Kruskal-Wallis test.
Within-product difference of post-intervention values versus baseline values was evaluated using a paired t test for normal-distributed outcomes and using Wilcoxon signed ranks test for paired non-normal outcomes.
Some serum CRP concentration were below the detection limit and the average rank was calculated. A nonparametric Kruskal-Wallis test (based on the rank statistic) was used for evaluating product difference at baseline and after intervention.
BCM-7 = beta-casomorphin 7; CRP = C-reactive protein; GSH = glutathione; HGB = hemoglobin; IgG = immunoglobulin G; IL-4 = interleukin 4; MPO = myeloperoxidase; SCFA = short-chain fatty acid
*P < 0.05; ** P < 0.01; *** P < 0.001; no symbol: P ≥ 0.05.
Subjects consuming conventional milk showed significantly increased BCM-7 (P < 0.0001), whereas subjects consuming milk containing A2 beta-casein only showed no statistically significant change (P = 0.134). Serum reduced glutathione (GSH) levels increased after consumption of both products, but greater increases were seen in the group that received milk containing A2 beta-casein only than in the group receiving conventional milk (Table 1). Note that subjects provided blood samples only at the end of phase 1; blood sampling was not performed in phase 2 owing to parents’ objections following the sampling in phase 1.
Fecal Markers
After a 5-day intervention, subjects consuming milk containing A2 beta-casein only had significantly higher mean fecal concentrations of acetic acid, butanoic acid, and total short-chain fatty acids (SCFAs) than subjects consuming conventional milk.
Stool Frequency and Consistency
Subjects who completed the sequence of milk containing A2 beta-casein only in phase 1, then conventional milk in phase 2, showed no change from baseline in daily stool frequency during phase 1; however, this increased during phase 2 (see Figure, Supplemental Digital Content 7, which illustrates stool frequencies).
Subjects who completed the sequence of conventional milk then milk containing A2 beta-casein only showed a notable increase in daily stool frequency during phase 1. On day 6, daily stool frequency decreased and returned to a similar level as that in the phase 1 washout period. During phase 2, there was no change in daily stool frequency observed (see Figure, Supplemental Digital Content 7, which illustrates stool frequencies).
Similar changes were observed in Bristol Stool score for stool consistency (see Figure, Supplemental Digital Content 8, which illustrates Bristol Stool scores). When both groups consumed conventional milk, there were notable increases in Bristol Score. During the 9-day washout period before phase 2, mean Bristol Scores in both groups were the same as baseline values in phase 1.
Subtle Cognitive Impairment Test
SCIT data are shown in Figure 4A for phase 1 and Figure 4B for phase 2 for each group. SCIT head response times were recorded after stimulus durations of 16 to 64 ms, whereas SCIT tail response times were recorded after durations of 80 to 128 ms. After consumption of the study product, SCIT-response time head and SCIT-response time tail data were significantly improved (P < 0.014 and P < 0.002, respectively) for both conventional milk and milk containing A2 beta-casein only, although there was no significant difference between milk products. SCIT-error rate head (EH, P < 0.000) and SCIT-error rate tail (ET, P < 0.000), however, showed significant improvements in overall performance between baseline and the postintervention measures after consumption of milk containing A2 beta-casein only.
FIGURE 4.
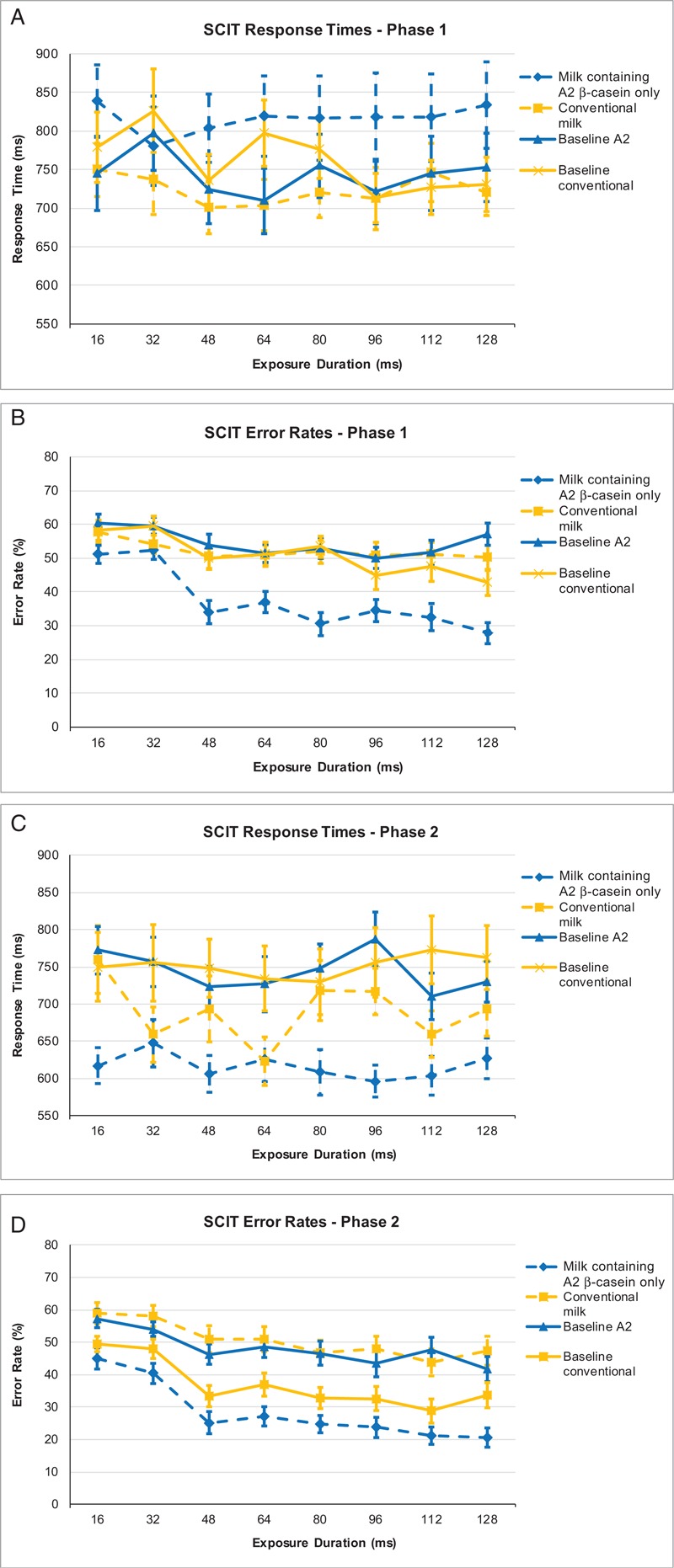
SCIT response times according to the intervention received in phase 1 (A); SCIT response times according to the intervention received in phase 2 (B); SCIT error rates according to the intervention received in phase 1 (C); SCIT error rates according to the intervention received in phase 2 (D). SCIT = Subtle Cognitive Impairment Test.
When subjects consumed conventional milk in phase 1, there was no effect on error rates (EH, P = 0.549; ET, P = 0.101), but when consuming milk containing A2 beta-casein only in phase 2, there was a significant decrease in error rates (EH and ET: P < 0.000). When subjects consumed milk containing A2 beta-casein only in phase 1, there was a significant decrease in error rate that continued through the washout period (EH and ET: P < 0.000). Error rates, however, significantly increased in phase 2 for these subjects after consumption of conventional milk (EH and ET: P < 0.000).
Adverse Events
There were a total of 15 adverse events in the study (6 with conventional milk, 5 with milk containing A2 beta-casein only, and 4 during the crossover washout period). None of these events were deemed related to the study product. The most common adverse events were upper respiratory infection (26.7%), pharyngitis (13.3%), otitis media (13.3%), eczema (13.3%), and cough (13.3%). No severe adverse events occurred.
DISCUSSION
The results of this crossover study, which incorporated numerous statistical tests, show that among preschoolers self-reporting mild-to-moderate discomfort after milk consumption, consumption of milk containing A2 beta-casein only significantly decreased gastrointestinal symptom scores compared with consumption of conventional milk. Confirmed LI preschoolers experienced a significant increase in symptoms throughout phases 1 and 2 when consuming conventional milk. In comparison, lactose-tolerant subjects experienced increased symptoms only at the start of both phases, but this gradually reduced as the intervention continued. These results indicate that there is an apparent desensitization to conventional milk containing A1 β-casein over time, which may be due to epigenetic modifications affecting gene expression after BCM-7 exposure, as previously reported (17).
In contrast, when both confirmed LI and lactose-tolerant subjects consumed milk containing A2 beta-casein only, daily total VAS scores were maintained at a level similar to baseline. Although the effects of phase and sequence on gastrointestinal symptoms were not significant, consumption of conventional milk induced a significant increase (P < 0.0001) in gastrointestinal symptoms. These results are consistent with those from a recent study in adults, which also showed that A1 β-casein was associated with increased gastrointestinal symptoms compared with consumption of milk containing A2 beta-casein only (4). Furthermore, a recently published systematic review reported the effects of A1 β-casein and BCM-7 on human health–related outcomes and found that milk containing A2 β-casein provides digestive health benefits (18).
Measures of serum biomarkers showed significant changes in the inflammatory cytokine interleukin 4, and the antibodies IgG, IgG1, and IgE, when preschoolers consumed conventional milk, but not when they consumed milk containing A2 beta-casein only. This is consistent with our previous observations showing significant differences in serum biomarkers of inflammation in Chinese adults (4). These results are also similar to those from a study by Ul Haq and colleagues, who reported that consumption of A1 β-casein induced an inflammatory response by activating the Th2 pathway in a mouse model (19). Moreover, in the same mouse model, consumption of BCM-7 induced an inflammatory immune response (20). This is consistent with the present finding that preschoolers who consumed conventional milk had a significant increase in BCM-7 (P < 0.0001), whereas preschoolers consuming milk containing A2 beta-casein only had no meaningful change in BCM-7 (P = 0.134).
Serum GSH levels increased significantly in both groups, although the group consuming milk containing A2 beta-casein only experienced significantly greater changes than the conventional-milk group. This is in line with published results showing that consumption of milk containing A2 beta-casein only is associated with a greater increase in concentration of the antioxidant GSH than consumption of conventional milk (21).
When assessing the presence of an induced inflammatory gut response based on fecal biomarkers, all fecal measurements, except myeloperoxidase concentration, followed a normal distribution at baseline levels. After 5 days’ consumption of milk containing A2 beta-casein only, there were, however, significantly higher mean fecal concentrations of acetic acid, butanoic acid, and total SCFA, when compared with consuming conventional milk. These results are consistent with previous observations in adults that conventional milk reduces SCFA levels and impairs colonic health (4). This is relevant because SCFAs are fermentation products of gut microbiota, with reported anti-inflammatory properties, able to amplify colonic cell function (22–25).
In this study, consumption of conventional milk was associated with a significantly higher stool frequency and significantly higher Bristol Stool Scale scores compared with the consumption of milk containing A2 beta-casein only. In an 8-week crossover study conducted by Ho et al (26), in which participants with LI were screened out during trial enrollment, consumption of A1 milk was shown to adversely affect gastrointestinal function, further highlighting the putative proinflammatory effects of A1 milk. Similarly, the study by Jianqin et al (4) revealed longer gastrointestinal transit times, softer stools, and a greater incidence of diarrhea, when adults consumed conventional milk. Taken together, these data suggest that exclusion of A1 β-casein may help alleviate adverse gastrointestinal symptoms.
The specific effects of BCM-7 on human cognition are largely unknown; however, elevated basal BCM-7 has been reported in infants presenting with delays in psychomotor development after consuming formula containing cow's milk (27). Furthermore, another study reported elevated levels of BCM-7 in autistic children (28). Although consumption of conventional milk had no effect on the performance of preschoolers during our analysis, consumption of milk containing A2 beta-casein only significantly improved response accuracy relative to baseline. This pattern of improved accuracy in both the head and tail error rates equates to increased efficiency of processing in both automatic and attentional processes. These data suggest that consumption of milk containing A2 beta-casein only has a positive effect not only on the gastrointestinal system, but also on cognition in preschoolers. Interestingly, our earlier study in adults showed that consumption of conventional milk was associated with small but significant increases in response time and error rate compared with consumption of milk containing A2 beta-casein only, further suggesting a beneficial effect of milk containing A2 beta-casein only on cognition and underscoring the importance and relevance of the gut-brain axis (4).
The limitations of this study include the short intervention period, although this was due to ethical considerations concerning feeding LI preschoolers lactose-containing milk; therefore, this study could only examine the acute effects of both milk products. In addition, although subjects were blinded to the product consumed, the VAS questionnaire comes with potential variance due to self-reporting.
In summary, these findings reveal an important interplay between consumption of conventional milk and LI in preschool children, with conventional milk inducing an inflammatory state that exacerbates symptoms associated with LI. This exacerbation can be reduced by removal of A1 β-casein (via replacement with A2 β-casein), with subsequent improvement in gastrointestinal symptoms and aspects of cognitive function.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Keyra Martinez Dunn, MD, and James Graham, PhD, of Edanz Medical Writing for providing medical writing support, which was funded by The A2 Milk Company. The authors would also like to acknowledge the assistance of the clinical research organization S.P.R.I.M. China (Shanghai) Consulting Co., Ltd. for conducting the clinical trial.
Footnotes
The a2 Milk Company provided funding for the study and had insight into drafting the manuscript, but the authors had full responsibility for the data generated in the study and data analysis, and decided about manuscript content. All authors had full access to all of the data generated in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Yelland is co-director of NeuroTest Pty Ltd, the company that owns and distributes the Subtle Cognitive Impairment Test. The other authors report no conflicts of interest.
REFERENCES
- 1.Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice-myths and realities. Aliment Pharmacol Ther 2008; 27:93–103. [DOI] [PubMed] [Google Scholar]
- 2.Savaiano DA, Boushey CJ, McCabe GP. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration. J Nutr 2006; 136:1107–1113. [DOI] [PubMed] [Google Scholar]
- 3.Jinsmaa Y, Yoshikawa M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides 1999; 20:957–962. [DOI] [PubMed] [Google Scholar]
- 4.Jianqin S, Leiming X, Lu X, et al. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J 2016; 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He M, Sun J, Jiang ZQ, et al. Effects of cow's milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J 2017; 16:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutrou R, Gaudichon C, Dupont D, et al. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr 2013; 97:1314–1323. [DOI] [PubMed] [Google Scholar]
- 7.Ul Haq MR, Kapila R, Kapila S. Release of beta-casomorphin-7/5 during simulated gastrointestinal digestion of milk beta-casein variants from Indian crossbred cattle (Karan Fries). Food Chem 2015; 168:70–79. [DOI] [PubMed] [Google Scholar]
- 8.Welsh JD, Poley JR, Bhatia M, et al. Intestinal disaccharidase activities in relation to age, race, and mucosal damage. Gastroenterology 1978; 75:847–855. [PubMed] [Google Scholar]
- 9.Yang Y, He M, Cui H, et al. The prevalence of lactase deficiency and lactose intolerance in Chinese children of different ages. Chin Med J (Engl) 2000; 113:1129–1132. [PubMed] [Google Scholar]
- 10.Yelland GW. Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J Gastroenterol Hepatol 2017; 32 suppl 1:90–93. [DOI] [PubMed] [Google Scholar]
- 11.CONSORT. Transparent reporting of trials. www.consort-statement.org Accessed June 4, 2019. [Google Scholar]
- 12.Hardin JW, Hilbe JM. Generalized Estimating Equations. Wiley Encyclopedia of Clinical Trials 2008:1–8. [Google Scholar]
- 13.Bruce KM, Robinson SR, Smith JA, et al. Validity of a screening tool for detecting subtle cognitive impairment in the middle-aged and elderly. Clin Interv Aging 2014; 9:2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speirs SJ, Rinehart NJ, Robinson SR, et al. Efficacy of cognitive processes in young people with high-functioning autism using a novel task. J Autism Dev Disord 2014; 44:2809–2819. [DOI] [PubMed] [Google Scholar]
- 15.Friedman TW, Robinson SR, Yelland GW. Impaired perceptual judgment at low blood alcohol concentrations. Alcohol 2011; 45:711–718. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, He M, Cui H, et al. Study on the incidence of lactose intolerance of children in China [in Chinese]. Wei Sheng Yan Jiu 1999; 28:44–46. [PubMed] [Google Scholar]
- 17.Trivedi MS, Hodgson NW, Walker SJ, et al. Epigenetic effects of casein-derived opioid peptides in SH-SY5Y human neuroblastoma cells. Nutr Metab (Lond) 2015; 12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Küllenberg de Gaudry D, Lohner S, Schmucker C, et al. Milk A1 b-casein and health-related outcomes in humans: a systematic review. Nutr Rev 2019; 77:278–306. [DOI] [PubMed] [Google Scholar]
- 19.Ul Haq MR, Kapila R, Sharma R, et al. Comparative evaluation of cow beta-casein variants (A1/A2) consumption on Th2-mediated inflammatory response in mouse gut. Eur J Nutr 2014; 53:1039–1049. [DOI] [PubMed] [Google Scholar]
- 20.Ul Haq MR, Kapila R, Saliganti V. Consumption of β-casomorphins-7/5 induce inflammatory immune response in mice gut through Th2 pathway. J Funct Foods 2014; 8:150–160. [Google Scholar]
- 21.Deth R, Clarke A, Ni J, et al. Clinical evaluation of glutathione concentrations after consumption of milk containing different subtypes of beta-casein: results from a randomized, cross-over clinical trial. Nutr J 2016; 15:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greer JB, O’Keefe SJ. Microbial induction of immunity, inflammation, and cancer. Front Physiol 2011; 1:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut 1994; 35 1 suppl:S35–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong JM, de Souza R, Kendall CW, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 2006; 40:235–243. [DOI] [PubMed] [Google Scholar]
- 25.Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des 2003; 9:347–358. [DOI] [PubMed] [Google Scholar]
- 26.Ho S, Woodford K, Kukuljan S, et al. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. Eur J Clin Nutr 2014; 68:994–1000. [DOI] [PubMed] [Google Scholar]
- 27.Kost NV, Sokolov OY, Kurasova OB, et al. Beta-casomorphins-7 in infants on different type of feeding and different levels of psychomotor development. Peptides 2009; 30:1854–1860. [DOI] [PubMed] [Google Scholar]
- 28.Sokolov O, Kost N, Andreeva O, et al. Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides 2014; 56:68–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


