Supplemental digital content is available in the text.
Key Words: 2-ARACHIDONOYLGLYCEROL, N-ARACHIDONOYLETHANOLAMINE, ACUTE, AEROBIC, PREFERRED INTENSITY, MODERATE INTENSITY
ABSTRACT
The endocannabinoid (eCB) system is implicated in the pathophysiology of depression and is responsive to acute exercise in healthy adults.
Purpose
We aimed to describe acute changes in serum eCB across a prescribed moderate (MOD) and a self-selected/preferred (PREF) intensity exercise session in women with major depressive disorder (MDD) and determine relationships between changes in eCB and mood states.
Methods
Women with MDD (n = 17) exercised in separate sessions for 20 min on a cycle ergometer at both MOD or PREF in a within-subjects design. Blood was drawn before and within 10 min after exercise. Serum concentrations of eCB (anandamide [AEA], 2-arachidonoylglycerol) and related lipids (palmitoylethanolamine, oleoylethanolamine, 2-oleoylglycerol) were quantified using stable isotope-dilution, liquid chromatography/mass spectrometry/mass spectrometry. The profile of mood states and state-trait anxiety inventory (state only) were completed before, 10 min and 30 min postexercise.
Results
Significant elevations in AEA (P = 0.013) and oleoylethanolamine (P = 0.024) occurred for MOD (moderate effect sizes: Cohen’s d = 0.58 and 0.41, respectively). Significant (P < 0.05) moderate negative associations existed between changes in AEA and mood states for MOD at 10 min (depression, confusion, fatigue, total mood disturbance [TMD] and state anxiety) and 30 min postexercise (confusion, TMD and state anxiety). Significant (P < 0.05) moderate negative associations existed between 2-arachidonoylglycerol and mood states at 10 min (depression and confusion) and 30 min postexercise (confusion and TMD). Changes in eCB or related lipids or eCB–mood relationships were not found for PREF.
Conclusion
Given the broad, moderate–strength relationships between improvements in mood states and eCB increases after MOD, it is plausible that the eCB system contributes to the mood-enhancing effects of prescribed acute exercise in MDD. Alternative mechanisms are likely involved in the positive mood state effects of preferred exercise.
Major depressive disorder (MDD) is a heterogeneous illness affecting roughly 3 in 10 people during their lifetime (1) with no identified single cause or specific biological abnormality that fully explains this pervasive disease. Exercising can improve mood states and well-being in healthy adults and in patients with MDD (2–4). Indeed, exercise training is an effective treatment for MDD (5) and appears to be as effective at treating depression as antidepressant medications and other accepted behavioral therapies (6–8). In addition to exercise’s chronic effects, a single session of exercise improves various mood states for minutes to hours in healthy adults (9–11) and in those suffering from MDD (12). However, the mechanisms by which exercise influences mood acutely or chronically are not well understood. As a result, exercise training trials in MDD are designed and powered based primarily on the probability of a significant mood or other clinical response. Given the heterogeneity and variability of depressed mood in MDD, it is plausible that treatment efficacy would be better realized if exercise interventions were designed to optimize engagement of the biological mechanisms that underlie exercise’s mood effects. The most well-known hypothesized mechanism that exercise improves mood states through release of endorphins has limited scientific support (13). Although exercise’s anti-inflammatory effects, influences on neurotransmitters and on neurotrophins have all been hypothesized to be involved in the mental health effects of exercise (14–17), limited evidence from humans evaluates the hypothesized mechanistic relationships between potential factors and the psychological effects of both acute and chronic exercise. Therefore, there is a need to continue to explore the potential mechanisms underlying the mood effects of exercise, particularly in populations suffering from mood disorders where exercise may be the most influential (18).
One system that has been implicated in the pathophysiology of MDD is the endocannabinoid (eCB) system (19,20). The two main eCB, 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamide (anandamide [AEA]) bind to the two major cannabinoid receptors (CB1 and CB2), which are found throughout the central and peripheral nervous systems. Previous reports suggest circulating eCB concentrations are lower in depressed patients than healthy adults (21,22). In addition, a genetic polymorphism in the CB1 receptor gene has been associated with an increased risk of antidepressant treatment resistance in depressed patients (23). Therefore, assessments of circulating eCB in MDD in relation to mood states may be informative for further understanding the disease pathology and treatment.
Exercise increases circulating concentrations of eCB and there is emerging support for a relationship between exercise-induced changes in circulating eCB and acute exercise-induced improvements in mood states. For example, Raichlen et al. (24) reported that exercise-induced increases in circulating eCB were associated with increases in positive affect in a sample of recreationally fit adults. Brellenthin et al. (25) found significant relationships between exercise-induced alterations in circulating eCB and improvements in mood states (i.e., decreases in feelings of depression, tension, and total mood disturbance and increases in feelings of vigor) in healthy adults with varying levels of physical activity after both preferred and prescribed moderate-intensity aerobic exercise. Further, Crombie et al. (26) reported that greater increases in circulating 2-AG were associated with decreases in negative affect after moderate aerobic exercise in adults with posttraumatic stress disorder (PTSD). It has yet to be determined what effect, if any, exercise has on circulating eCB in MDD and what role circulating eCB may play in the mood-enhancing effects of acute exercise. In addition, past research has examined relatively short-duration mood effects of exercise (10 m or less) and further assessments would be useful to determine the potential extent of the effects of eCB on variation in mood states after exercise.
Past research has found significant associations between exercise-induced eCB changes and exercise-induced mood state changes after both preferred intensity and duration (25) and moderate-intensity aerobic exercise (26) across various mood states in healthy and PTSD populations. Considering past eCB and exercise research, and the mood-enhancing potential of exercise in MDD (18,27), the present research sought to compare mood state and eCB responses to moderate and preferred-intensity aerobic exercise in MDD. Therefore, the present investigation pursued two primary research questions. First, in adults with MDD, does acute exercise alter serum levels of eCB (AEA and 2-AG) and related lipids (palmitoylethanolamine [PEA], oleoylethanolamine [OEA], and 2-oleoylglycerol [2-OG]) in response to moderate and preferred-intensity exercise? Second, are exercise-induced changes in mood states associated with exercise-induced changes in serum eCB in adults with MDD after moderate and preferred-intensity exercise?
MATERIALS AND METHODS
The present analysis details the eCB response to two acute exercise sessions in female participants with MDD. The samples used in these secondary analyses were stored samples from a larger study (n = 24) that evaluated the effect of acute exercise intensity on depressed mood (12), biological markers related to depression/exercise (28), and exercise intensity preferences (29). The larger study included five visits examining single sessions of quiet rest, or light-, moderate-, or hard-intensity exercise or a preferred-intensity exercise session that were completed by each participant in a randomized and counterbalanced order with visits at least 1 wk apart from each other. Only serum samples from the moderate and preferred exertion sessions were assessed for eCB content. The information below details the events of those two visits, complete project details are reported elsewhere (12,28,29). All procedures were approved by the local institutional review board at the University of Wisconsin-Madison and written informed consent was obtained from each participant. The described work was carried out in accordance with the Declaration of Helsinki. Samples for the present analysis come from the (a) moderate-intensity exercise session and (b) the preferred exertion session, and only from participants whose preexercise profile of mood states (POMS) depression scores were above published norms (i.e., 4+ (30)) for both sessions, and for whom preexercise and postexercise samples existed for both sessions (n = 17). As some of the participants from the larger study reported zero or low values for feelings of depressed mood preexercise for these sessions (i.e., no to minimal depressed mood), we did not include those participants due to our interest in evaluating eCB and mood relationships.
Participants
Details regarding methods, participants and exercise sessions are published elsewhere (12,28,29) and only information pertinent to the present analyses are reported here. Participants were recruited from the University of Wisconsin-Madison campus and surrounding areas. Interested potential participants underwent a phone screen to assess possible eligibility. Those who appeared eligible after the phone screen were invited to the laboratory for the first day of testing. Participants were females, age 20 to 60 yr, who self-reported a current diagnosis of MDD, were deemed safe to exercise based on the Physical Activity Readiness Questionnaire (31) and were not currently undergoing psychiatric treatment (counseling or medication) or were on a stable regimen for the 8 wk preceding their initial visit. Self-reported current diagnosis of MDD was confirmed via the Mini International Neuropsychiatric Interview (32) on each participant’s first visit. Participants taking stable doses of antidepressant medications were included if they currently met criteria for MDD and if they did not change their medication regimen during the course of the study. Participants were excluded if they self-reported being pregnant, a current smoker, currently taking opioid or analgesic medications, or abusing alcohol or other illegal drugs (including cannabis). Participants were compensated US $20 at the end of each study visit.
Procedures
Participants reported to the research laboratory on 2 d, separated by at least 1 wk. Participants first completed baseline questionnaires and then completed one of the two exercise sessions. Each session in the current analysis included 30 min of exercise, either 1) at a moderate intensity or 2) at a preferred intensity, without intensity prescription (i.e., a session where participants could change their workload freely and without restrictions). After the exercise session, participants filled out questionnaires at 10 and 30 min postexercise. Blood was drawn via standard venipuncture into serum tubes from the antecubital vein after completing baseline questionnaires (pre) and as quickly as possible after the session ended (from the opposite arm), always within 10 min (post). Each participant’s sessions were scheduled at the same time of day, with sessions lasting approximately 2 h. All sessions were performed on an electronically braked cycle ergometer (Lode Corival, Lode BV, Groningen, The Netherlands). Ventilation, oxygen consumption (V˙O2), carbon dioxide production (V˙CO2) and work rate were obtained continuously and analyzed with 15-s averaging using a metabolic cart (TrueOne, ParvoMedics, Sandy, UT) and two-way non-rebreathing valve (Hand-Rudolph, Kansas City, MO) which allowed for metabolic equivalent estimation (METs). Respiratory exchange ratio was calculated as the ratio of V˙CO2 to V˙O2. Multiple comparisons to a 3-L syringe were performed each test for gas analyzer calibration. A Polar heart rate monitor (Polar, Lake Success, NY) was used to record heart rate throughout each session.
Exercise sessions
Exercise sessions and monitoring are detailed elsewhere (12). Briefly, exercise was performed on a stationary bicycle with ratings of perceived exertion (RPE), using Borg’s 6 to 20 rating scale (33), used both to prescribe the intensity of the exercise for the moderate session and to evaluate exertion during both moderate and preferred sessions. Before the first session, participants were provided with standardized RPE instructions according to the manual (33). Participants self-reported their RPE every 5 min during exercise. For the moderate-intensity exercise session, participants were instructed to maintain a moderate intensity, which corresponds to a RPE of “13” with a verbal anchor of ‘somewhat hard.’ Workload adjustments were encouraged as necessary to maintain the prescribed intensity (i.e., if a participant self-reported a “12” RPE during the moderate session, they were encouraged to manipulate the resistance on the bicycle to achieve a “13”).
For the preferred intensity condition (as detailed in (29)), participants were instructed to choose any intensity of exercise at which they would prefer to exercise with freedom to manipulate the intensity throughout. The rest of the exercise instructions were identical to those given in the prescribed session with the sole difference being the lack of an intensity prescription (instructional sets for the two sessions are in Supplemental Digital Content 1 in (29)).
During the entirety of both sessions, the RPE scale was affixed to the wall in front of the participant to allow participants to self-monitor their exertion throughout the 20 min of exercise. Participants were instructed to maintain 60 to 70 rpm and adjust the resistance to maintain the intensity instructions for each session. Over the 5-min warm-up, participants increased their exertion to achieve the session’s intensity at the end of the 5-min period (i.e., either end the 5 min at a moderate intensity or, in the preferred condition, whatever intensity they would like to begin with). Participants then exercised for 20 min according to the session’s instructions before ending with a 5-min cool-down.
Self-report measures
Participants completed the Beck Depression Inventory-II (BDI; (34)), the 65-item POMS (35) and the 20 state items from the State-Trait Anxiety Inventory (STAI; (36)) at baseline and then completed the POMS and STAI at 10 min and 30 min postexercise. The primary outcomes for the changes in mood states across exercise were the original six POMS subscales (depression, tension, confusion, fatigue, vigor, and anger), the total mood disturbance (TMD) score from the POMS and state anxiety. The POMS subscales have been shown to have internal consistency ranging from 0.63 to 0.96 (37) (although the psychometric properties of the confusion subscale have been questioned (38,39)). In addition, the STAI also has high internal consistency ranging from 0.86 to 0.95. Mood states and state anxiety when measured via the POMS and, respectively, are responsive to acute exercise when used with “right now” instructions (used in the present study) and these measures have been widely used to measure exercise’s acute mood effects (9).
Sample collection and processing
Blood was allowed to clot for 15 min at room temperature and then centrifuged at −4°C for 10 min. Serum was then separated and immediately stored at −80°C until further processing took place. ECB extractions and sample processing was performed by the same individual and all samples were prepared within the same week (see (26) for step-by-step eCB extractions and sample processing procedures). After preparation, the concentrations of eCB (AEA and 2-AG), along with related biogenic lipids (PEA, OEA, 2-OG) were quantified using stable isotope-dilution, electrospray ionization liquid chromatography/mass spectrometry of the daughter ions (LC-ESI-MS-MS) as previously described (26). Analysis of all samples by mass spectrometry was conducted within 1 wk of processing and each participant’s samples were run together.
Data Analysis
All analyses were conducted with SPSS version 23.0 for Windows (IBM Corporation, Armonk NY). There were no missing data for any outcomes. Univariate repeated-measures ANOVA for each of the subscores and total scores from the POMS and STAI were performed to assess changes in mood states across time. Dependent-samples t-tests compared exercise-related variables between sessions. To meet the normality assumption (i.e., Shapiro–Wilk test) for parametric tests, lipid concentrations were logarithmically transformed (log10) before conducting parametric statistical analyses. Presession self-report measures, exercise variables and serum content of eCB and related lipids were compared between the sessions with paired-samples t-tests. Due to the secondary nature of these analyses, alpha was set at 0.05 for each statistical test.
Question I
Paired-sample t-tests were conducted comparing presession to postsession values for each of the circulating eCB and related lipids (i.e., AEA, 2-AG and PEA, OEA, 2-OG) for each session. Cohen’s d (40) effect sizes along with 95% confidence intervals (41) were used to quantify the magnitude of changes presession to postsession. Effect sizes were considered to be small (0.20–0.49), moderate (0.50–0.79) or large (>0.80) based upon Cohen’s cautious recommendations (40).
Question II
Due to the small cell size for each mood state-eCB correlation, nonparametric testing using Spearman’s ρ correlations assessed relationships between presession to postsession changes in circulating eCB and changes in mood states and state anxiety. Values of ρ < 0.4 were considered weak, between 0.4 and 0.7 to be moderate and greater than 0.7 to be strong.
RESULTS
Participant characteristics and exercise sessions
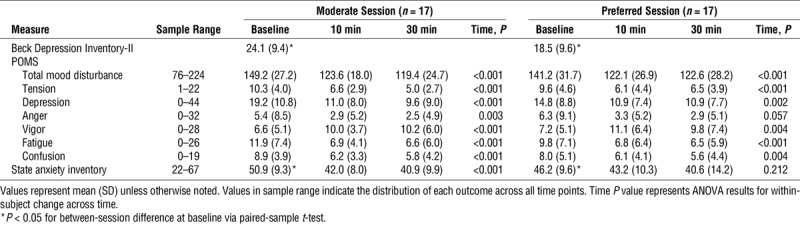
Participants were adult females (n = 17, age: 40.8 ± 14.8 yr old; mean ± SD) with a mean body mass index of 31.2 ± 8.5 kg·m−2 with 9 of 17 taking at least one antidepressant medication. Means and SD are presented in Table 1 for self-report measures. Participants self-reported having mild-to-moderate depression over the past 2 wk upon arrival for each session (BDI), with significantly lower depressive symptoms for the preferred session than for the moderate session (P = 0.028). Participants also reported significantly lower state anxiety for the preferred session (P = 0.017). On average, each subscale of the POMS as well as TMD improved across both exercise sessions (Table 1). Vigor increased while each other subscale, TMD and state anxiety decreased from baseline to both 10 min and 30 min postsession. Univariate repeated-measures ANOVA for each of the subscores and total scores from the POMS and STAI (P < 0.05) demonstrated significant improvements in all measures across time, with the exception of the STAI and anger which did not significantly improve in the preferred session only.
TABLE 1.
Baseline symptoms and mood across the exercise sessions.
Exercise sessions resulted in similar mean responses to the exercise stimuli across the sessions (all P > 0.05; Table 2). Participants selected a range of exercise intensities in the preferred condition (Fig. 1), although sessions were not significantly different from each other on average.
TABLE 2.
Exercise data summary for both sessions.
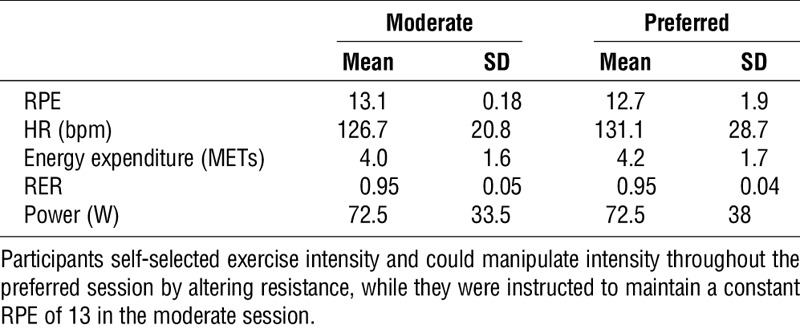
FIGURE 1.
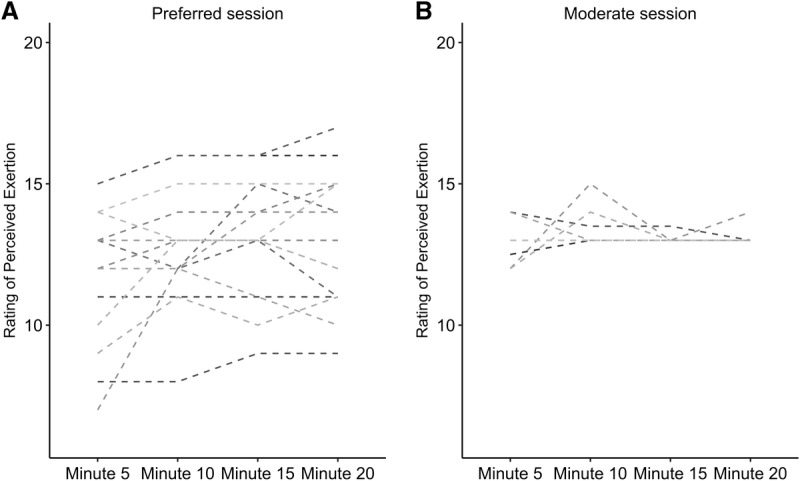
Rating of perceived exertion for each participant across 20 min of exercise in preferred (A) and moderate (B) sessions. Each line represents a single participant’s self-reported rating of perceived exertion recorded at 5-min intervals across each bout. Participants could change their exertion as they desired across the preferred session (A) while they were instructed to maintain a rating of perceived exertion of 13 in the moderate session (B).
Effects of exercise sessions on eCB and related lipids
There were no baseline differences between sessions for any of the eCB or related lipids (paired-samples t-tests; all P > 0.05). Changes in eCB and related lipids across exercise are presented in Table 3. In the moderate session, a significant increase from preexercise to postexercise was found for AEA (t (16) = −2.802, P < 0.05) and OEA (t (16) = −2.487, P < 0.05), whereas no significant differences were found for 2-AG (t (16) = 1.695), P > 0.05), PEA (t (16) = −0.006, P > 0.05), or 2-OG (t (16) = 1.747, P > 0.05). For the preferred session, no significant differences were found for AEA (t (16) = −0.963, P > 0.05), 2-AG (t (16) = 1.739, P > 0.05), OEA (t (16) = −0.605, P > 0.05), PEA (t (16) = 1.804, P > 0.05), and 2-OG (t (16) = 1.396, P > 0.05). Cohen’s d effect sizes representing the magnitude of changes in eCB and related lipids after the moderate and preferred exercise sessions are illustrated in Figure 2. In addition, effect sizes for changes in eCB and related lipids across the exercise sessions based on antidepressant usage are found in Supplemental Digital Content 1 (see Table, Supplemental Digital Content 1, Log eCB and related lipid changes based on antidepressant usage, http://links.lww.com/MSS/B582).
TABLE 3.
Changes in serum eCB and related lipid (log10 values) after moderate- and preferred-intensity exercise.
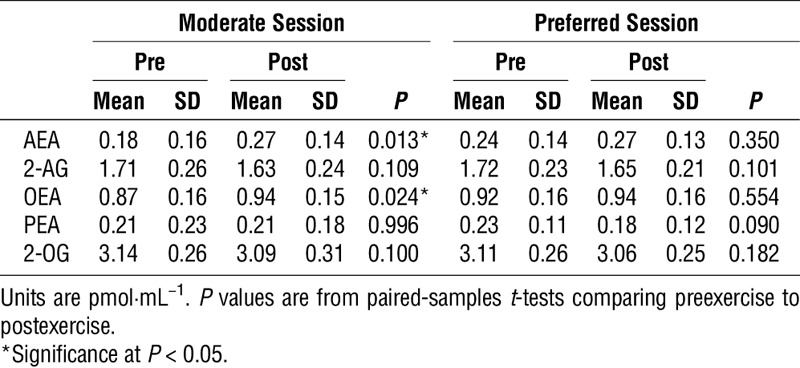
FIGURE 2.
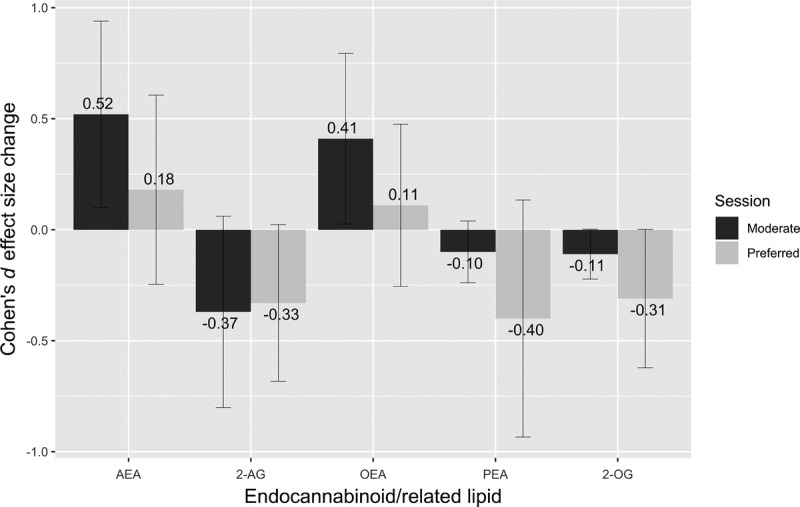
Cohen’s d effect sizes for the raw change in serum eCB and related lipids preexercise to postexercise in the moderate and preferred exercise sessions with 95% confidence intervals.
Relationships between changes in eCB and mood states
Spearman ρ correlation coefficients were calculated to examine the relationship between the percent change (pre to post) in eCB within 10 min postexercise and the change in mood states at 10 and 30 min after both the moderate and preferred sessions (Table 4).
TABLE 4.
Moderate and preferred session relationships between changes in serum eCB and changes in mood.
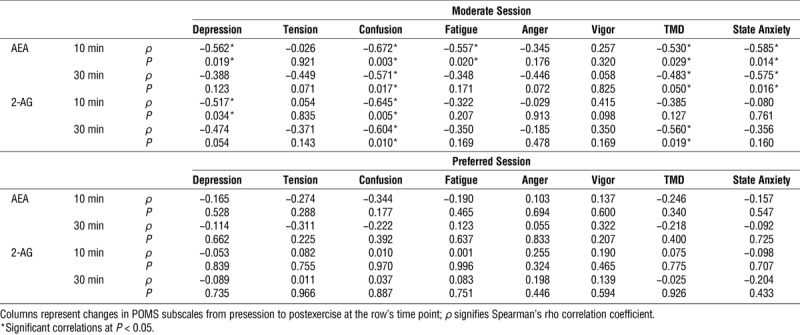
At 10 min after the moderate session, significant small-to-moderate negative correlations were found between the change in AEA and the change in feelings of depression, confusion, fatigue, TMD and state anxiety. Additionally, significant moderate negative correlations were found between the change in 2-AG across the exercise and the change in depression and confusion.
At 30 min after the moderate session, significant moderate negative correlations were found between the change in AEA across the exercise and the change in confusion, total mood disturbance, and state anxiety. Additionally, significant moderate negative correlations were found between the change in 2-AG across the exercise and the change in confusion and total mood disturbance.
In contrast to the moderate session, there were no significant correlations between the change in eCB and the change in mood states at 10 or 30 min postexercise for the preferred-intensity session.
DISCUSSION
The present results show that moderate but not preferred-intensity exercise resulted in increases in circulating levels of one eCB (AEA) and a related lipid (OEA) in women with MDD (Fig. 2). Further, changes in AEA after the moderate session were broadly related to exercise-related changes in feelings of depression, confusion, fatigue, total mood disturbance and state anxiety while changes in 2-AG were also significantly associated with changes in feelings of depressed mood, confusion and total mood disturbance up to 30 min postsession (Table 4). Significant overall changes in circulating eCB and related lipids did not occur across the preferred session (all d ≤ 0.40) and changes in mood states were not significantly related to changes in eCB for this session. Taken together, these results partially corroborate and extend previous findings of associations between exercise-induced changes in mood states and in circulating eCB after aerobic exercise in men and women with varying physical activity levels (25) and in adults with PTSD (26) to a clinically depressed female sample. The present results also extend this previous work to show that longer-duration mood changes at 10 and 30 min postexercise were weakly-to-moderately correlated to the changes in eCB within 10 min postmoderate exercise (absolute value of rho = 0.25 to 0.67). This represents a longer timeframe although a similar magnitude of association in comparison to previous work. The continuity of many of the eCB–mood relationships at both 10 and 30 min post–moderate-intensity exercise suggests that during-exercise eCB alterations may have longer-term (i.e., 30 min or longer) associations with fluctuations in mood states.
There is a growing appreciation for the influence of exercise on eCB and their potential role in exercise-related changes in mood states and affect (42). Raichlen and colleagues (24) hypothesized that the “runner’s high” (defined by Raichlen et al. as neurobiological rewards during or postexercise) may be due to eCB release during exercise, and found that increases in AEA postexercise were positively associated with increases in positive affect after exercise. Their additional research (43) suggested a potential intensity dependence of AEA changes across exercise in regular runners whereby moderate-intensity exercise yielded increases in circulating AEA, but very low and very high intensity did not. Heyman and colleagues (44) found that AEA did not increase after 60 min cycling at 55% of maximal power output, although AEA did increase after a subsequent time trial corresponding to an amount of work equal to 30 min at 75% of maximal power output and was still elevated at 15 min postexercise in trained cyclists. Our findings also indicate an increase in AEA after moderate exercise supporting the past findings of elevated serum AEA after moderate-intensity exercise.
Reports of the effects of exercise on circulating 2-AG concentrations have been less consistent. The early research in this area had not found significant elevations in 2-AG after exercise (43–46), but more recent research with larger sample sizes have reported elevations in 2-AG after exercise (25,26,47), and weak-to-moderate correlations between changes in 2-AG and improvements in mood. For example, Brellenthin et al. (25) found increases in AEA and 2-AG after moderate- and preferred-intensity treadmill exercise in healthy college-age men and women, which were associated with improvements in mood states, including depression, tension, total mood disturbance and vigor. After moderate aerobic exercise in adults with PTSD, Crombie et al. (26) found a negative correlation between the change in negative affect and 2-AG concentrations, indicating greater reductions in negative affect for individuals with a greater increase in 2-AG. Additionally, the magnitude of mean changes of AEA across moderate exercise were similar to those of Crombie et al.’s PTSD sample with both showing a roughly d = 0.50 effect size, although the present results showed a small mean decrease in 2-AG while the previously reported PTSD sample saw a small increase in 2-AG (d = 0.21). Overall, the consistency of mood–eCB relationships both in the present study at 10 and 30 min postexercise and with recent research suggests that exercise-induced eCB changes may be one mechanism through which exercise improves mood states across a variety of clinical and healthy populations.
The significant elevation (d = 0.41) of OEA in the present study after moderate exercise is consistent with previous research. Heyman and colleagues (44) found increased OEA across and exercise bout of increasing intensity and up to 15 min postexercise. Cedernaes and colleagues (47) did not find a significant immediate increase in OEA after cycling exercise at 75% of V˙O2 reserve, but did see an increase in OEA 4 h postexercise in young, healthy males. Finally, these results are also consistent with those of both Brellenthin et al. (25) and Crombie et al. (26) who found elevations in OEA after moderate exercise.
Current and past research implicate a dysfunctional eCB system in depression (19,48,49). Either genetic knockout of components of the eCB system or pharmacological antagonism leads to increased depressive-like behavior in rodents (48). In humans, depression has been linked to lower peripheral eCB content, with women with major depression having lower serum 2-AG content in one report (21), whereas a separate sample of women with major depression had reduced serum 2-AG and AEA compared with healthy controls (22). Moreover, eCB signaling can mimic many of the behavioral and biological effects of antidepressant medications (49). The present data further support an eCB–mood link as we found broad relationships between acute moderate exercise-induced changes in AEA and 2-AG with improvements in multiple mood states in women with MDD. Together, this demonstrates both that peripheral eCB content can be affected by exercise in patients with MDD and suggests a role for this system in how acute exercise improves mood state and well-being in depression.
Preferred-intensity exercise did not lead to significant elevations in either of the eCB or any of the related lipids (Table 3) despite similar mood changes to the moderate session (Table 1). Moreover, changes in mood states were not significantly related to changes in eCB (all ρ between −0.344 and 0.322; Table 4). This may be due to specific differences between the two exercise sessions. The moderate-intensity session included 20 min of steady-state exercise which was instructed to be held at a constant relative intensity (RPE of “13,” corresponding to a verbal anchor of ‘somewhat hard’), with a 5-min warm-up and a 5-min cool-down. Intensity was controlled by the participant who was instructed to manually adjust the resistance on the bicycle to keep their perceived exertion level at a “13.” In the preferred exertion session, participants had complete control over the resistance during the 20-min between the warm-up and cool-down and could exercise at whatever perceptual intensity they wanted and could adjust the intensity at any time throughout the session. The differential findings across the two sessions may be due to the intersubject variability in how participants chose to exercise during the preferred session (some chose to exercise at a lower intensity while others exercised at a harder intensity; Fig. 1) which could mask the potential effect of exercise if there is a threshold intensity necessary to elicit exercise-induced eCB changes (as suggested in (44)). However, this does not appear to be the sole explanation as neither changes in AEA or 2-AG appeared related to average RPE during the preferred session (Supplemental Digital Content 2 and 3, http://links.lww.com/MSS/B583 and http://links.lww.com/MSS/B584; figures depicting average RPE vs change in serum 2-AG and AEA, respectively). Additionally, the ability to choose the intensity may have also altered the eCB response to the exercise if this cognitive difference influenced the biological response. Finally, participants were able to manipulate their intensity during the preferred session rather than exercising at a steady-state intensity as in the moderate session. Any of these differences (i.e., variable intersubject intensity, ability to control intensity, or intra-session intensity manipulation) may have influenced the serum eCB changes, and further research is needed to determine what characteristics of exercise are important for consistently eliciting an eCB response. The presence of mood improvements in spite of the lack of significant eCB changes in the preferred session suggests that there are likely multiple biological mechanisms involved in the mood-enhancing effects of exercise.
The lack of significant overall changes in eCB and related lipids across the preferred-intensity session or relationships between exercise-induced changes in mood states and eCB are interesting in the context of Brellenthin and colleagues’ results (25). They found significant elevations in 2-AG and AEA and moderate correlations between changes in mood states and changes in eCB in response to their “preferred” exercise session. However, the “preferred” exercise sessions in each of these studies were considerably different. Brellenthin and colleagues allowed participants to manipulate both their duration of exercise (which was fixed at 20 min in the present study) and their intensity (similar to the present study) while running on a treadmill (compared with a stationary bicycle in the present study) resulting in different exercise stimuli. Additionally, the previous study assessed mood state immediately after exercise, while the present study was designed to look at slightly longer-term mood effects (i.e., 10 and 30 min postexercise) which may also explain the difference in findings. Finally, the populations were different; participants in the present study were depressed women, whereas those in the Brellenthin et al. report were healthy young adults with either low, moderate or high self-reported physical activity. Even with these differences, a significant group by time interaction in the Brellenthin report showed that AEA was increased to a greater extent after their moderate session which mirrors the results in the present sample (Fig. 2). Still, unique aspects of the population or exercise stimulus (e.g., preference, intensity, duration, mode) may be important in determining the effects of exercise sessions on eCB and future systematic research characterizing the eCB effects of both prescribed and preferred exercise are warranted.
Limitations
Presession differences in depressive symptoms between moderate and preferred sessions and/or variability in individual choices for the preferred session may have limited statistical power to find significant relationships between eCB and changes in mood states for the preferred session. Floor effects for mood states do not appear to have been a major concern with the present data as each of the POMS subscales were well above adult normative values for each of the sessions (see (30)). Although this study evaluated multiple mood states across time, future research would benefit from understanding the duration of postexercise elevations in eCB and how they relate to postexercise mood state and other symptoms of depression. As this was a secondary analysis, precise control of substances that could affect the eCB system was not performed, and this will be important in future research. Our sample of 17 women with depression with an average BMI in the obese category was small, and the lack of a control group or condition is a limitation, although the sample is not uncharacteristic of a depressed population, and the sample size is higher than, or in line with, other eCB-exercise studies. However, given the small sample size and number of mood states assessed without multiple comparison adjustments, it is possible that one or more of the eCB–mood relationships may be spurious. Nevertheless, the 12 moderate strength associations that were significant at uncorrected P < 0.05 between eCB changes and prescribed exercise-induced changes in mood states (Table 4) would indicate that larger future studies with clinical and nonclinical samples are needed. Finally, independent verification of eCB relationships to exercise-induced changes in mood states would strengthen these initial findings in depression.
CONCLUSIONS
The moderate correlations between changes in mood states after moderate-intensity exercise and changes in serum eCB content suggest alterations in eCB signaling may be one mechanism through which prescribed moderate exercise improves mood in patients with MDD. The consistency in relationships between changes in eCB and changes in mood states at both 10 min and at 30 min postexercise extends previous reports finding eCB–mood relationships with a single, immediate postexercise mood state assessment in other populations. However, exercise at a preferred intensity resulted in generally small (all d ≤ 0.40) nonsignificant mean eCB changes even in the presence of mood state improvements, suggesting that aspects of the exercise stimulus (e.g., preference, intensity, duration, mode, etc.) could be important in determining the serum eCB response to exercise in depression. There are likely multiple mechanisms involved in the mood effects of exercise. Future research designed to manipulate the mood response to exercise to evaluate its influence on eCB is encouraged to better understand this system potential related to acute exercise’s psychological effects.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the work of Lauren Schlapman, Hannah Feinstein, Shawn Tipple, Matthew Patton, Caroline Wickler and Rachel Prince for assistance in sample collection and preparation.
Financial supporters of this work included the Virginia Horne Henry Gift Fund, the University of Wisconsin-Madison Graduate School, the Wisconsin Center for Education Research, and the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin. The funding sources had no roles in the study design, collection, analysis or interpretations of the data or in the publication process. The authors have no conflicts of interest to report. The results of the present study do not constitute endorsement by ACSM. All authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan W, Goldston S. Exercise and Mental Health. Taylor & Francis; 1987. 218 p. [Google Scholar]
- 3.Morgan WP, Roberts JA, Brand FR, Feinerman AD. Psychological effect of chronic physical activity. Med Sci Sports. 1970;2(4):213–7. [PubMed] [Google Scholar]
- 4.Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18(2):189–93. [DOI] [PubMed] [Google Scholar]
- 5.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–56. [DOI] [PubMed] [Google Scholar]
- 8.Hallgren M, Helgadóttir B, Herring MP, et al. Exercise and Internet-based cognitive–behavioural therapy for depression: multicentre randomised controlled trial with 12-month follow-up. Br J Psychiatry. 2016; bjp.bp.115.177576. [DOI] [PubMed] [Google Scholar]
- 9.Yeung RR. The acute effects of exercise on mood state. J Psychosom Res. 1996;40(2):123–41. [DOI] [PubMed] [Google Scholar]
- 10.Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. Meta-analysis of acute exercise effects on state anxiety: an update of randomized controlled trials over the past 25 years. Depress Anxiety. 2015;32(8):624–34. [DOI] [PubMed] [Google Scholar]
- 11.Berger BG, Motl RW. Exercise and mood: a selective review and synthesis of research employing the profile of mood states. J Appl Sport Psychol. 2000;12(1):69–92. [Google Scholar]
- 12.Meyer J, Koltyn K, Stegner A, Kim JS, Cook D. Influence of exercise intensity for improving depressed mood in depression: a dose-response study. Behav Ther. 2016;47(4):527–37. [DOI] [PubMed] [Google Scholar]
- 13.Dishman RK, O’Connor PJ. Lessons in exercise neurobiology: the case of endorphins. Ment Health and Phys Act. 2009;2(1):4–9. [Google Scholar]
- 14.Helmich I, Latini A, Sigwalt A, et al. Neurobiological alterations induced by exercise and their impact on depressive disorders [corrected]. Clin Pract Epidemiol Ment Health. 2010;6:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rethorst CD, Toups MS, Greer TL, et al. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry. 2013;18(10):1119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavebratt C, Herring MP, Liu JJ, et al. Interleukin-6 and depressive symptom severity in response to physical exercise. Psychiatry Res. 2017;252:270–6. [DOI] [PubMed] [Google Scholar]
- 17.Dishman RK, Berthoud H-R, Booth FW, et al. Neurobiology of exercise. Obesity. 2006;14(3):345–56. [DOI] [PubMed] [Google Scholar]
- 18.Lane AM, Lovejoy DJ. The effects of exercise on mood changes: the moderating effect of depressed mood. J Sports Med Phys Fitness. 2001;41(4):539–45. [PubMed] [Google Scholar]
- 19.Hill MN, Gorzalka BB. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav Pharmacol. 2005;16(5–6):333. [DOI] [PubMed] [Google Scholar]
- 20.Huang W-J, Chen W-W, Zhang X. Endocannabinoid system: role in depression, reward and pain control (review). Mol Med Rep. 2016;14(4):2899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill MN, Miller GE, Ho W-SV, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41(2):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34(8):1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domschke K, Dannlowski U, Ohrmann P, et al. Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol. 2008;18(10):751–9. [DOI] [PubMed] [Google Scholar]
- 24.Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high. J Exp Biol. 2012;215(8):1331–6. [DOI] [PubMed] [Google Scholar]
- 25.Brellenthin AG, Crombie KM, Hillard CJ, Koltyn KF. Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med Sci Sports Exerc. 2017;49(8):1688–96. [DOI] [PubMed] [Google Scholar]
- 26.Crombie KM, Brellenthin AG, Hillard CJ, Koltyn KF. Psychobiological responses to aerobic exercise in individuals with posttraumatic stress disorder. J Trauma Stress. 2018;31(1):134–45. [DOI] [PubMed] [Google Scholar]
- 27.Mata J, Thompson RJ, Jaeggi SM, Buschkuehl M, Jonides J, Gotlib IH. Walk on the bright side: physical activity and affect in major depressive disorder. J Abnorm Psychol. 2012;121(2):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer J, Koltyn K, Stegner A, Kim J-S, Cook D. Relationships between serum BDNF and the antidepressant effect of acute exercise in depressed women. Psychoneuroendocrinology. 2016;74:286–94. [DOI] [PubMed] [Google Scholar]
- 29.Meyer JD, Ellingson LD, Koltyn KF, Stegner AJ, Kim J-S, Cook DB. Psychobiological responses to preferred and prescribed intensity exercise in major depressive disorder. Med Sci Sports Exerc. 2016;48(11):2207–15. [DOI] [PubMed] [Google Scholar]
- 30.Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55(1):79–86. [DOI] [PubMed] [Google Scholar]
- 31.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci. 1992;17(4):338–45. [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;(59 Suppl 20):22–33; quiz 34-57. [PubMed] [Google Scholar]
- 33.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. 104 p. [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Second Edition (BDI-II). San Antonio, TX: The Psychological Corporation; 1996. 38 p. [Google Scholar]
- 35.McNair DM, Lorr M, Droppleman LF. Profile of mood states: manual. San Diego. In: Calif.: Educational and Industrial Testing Services (Edits). 1971.
- 36.Spielberger C. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Counseling Psychologists Press; 1983. 36 p. [Google Scholar]
- 37.McNair DM, Lorr M, Droppleman LF. EdITS manual for the Profile of Mood States (POMS). San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 38.Norcross JC, Guadagnoli E, Prochaska JO. Factor structure of the profile of mood states (POMS): two partial replications. J Clin Psychol. 1984;40(5):1270–7. [DOI] [PubMed] [Google Scholar]
- 39.Bourgeois A, LeUnes A, Meyers M. Full-scale and short-form of the profile of mood states: a factor analytic comparison. J Sport Behav. 2010;33(4):355–76. [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic; 1988. 490 p. [Google Scholar]
- 41.Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol. 1988;41(2):257–78. [Google Scholar]
- 42.Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43(1):155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2012;113(4):869–75. [DOI] [PubMed] [Google Scholar]
- 44.Heyman E, Gamelin F-X, Goekint M, et al. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–51. [DOI] [PubMed] [Google Scholar]
- 45.Feuerecker M, Hauer D, Toth R, et al. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur J Appl Physiol. 2012;112(7):2777–81. [DOI] [PubMed] [Google Scholar]
- 46.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14(17):2209–11. [DOI] [PubMed] [Google Scholar]
- 47.Cedernaes J, Fanelli F, Fazzini A, et al. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology. 2016;74:258–68. [DOI] [PubMed] [Google Scholar]
- 48.Gorzalka BB, Hill MN. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1575–85. [DOI] [PubMed] [Google Scholar]
- 49.Hillard CJ, Liu Q. Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr Pharm Des. 2014;20(23):3795–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


