Abstract
Background:
Previous meta-analyses based on aggregate group-level data report antihypertensive effects of isometric resistance training (IRT). However, individual participant data meta-analyses provide more robust effect size estimates and permit examination of demographic and clinical variables on IRT effectiveness.
Methods:
We conducted a systematic search and individual participant data (IPD) analysis, using both a one-step and two-step approach, of controlled trials investigating at least 3 weeks of IRT on resting systolic, diastolic and mean arterial blood pressure.
Results:
Anonymized individual participant data were provided from 12 studies (14 intervention group comparisons) involving 326 participants (52.7% medicated for hypertension); 191 assigned to IRT and 135 controls, 25.2% of participants had diagnosed coronary artery disease. IRT intensity varied (8–30% MVC) and training duration ranged from 3 to 12 weeks. The IPD (one-step) meta-analysis showed a significant treatment effect for the exercise group participants experiencing a reduction in resting SBP of −6.22 mmHg (95% CI −7.75 to −4.68; P < 0.00001); DBP of −2.78 mmHg (95% CI −3.92 to −1.65; P = 0.002); and mean arterial blood pressure (MAP) of −4.12 mmHg (95% CI −5.39 to −2.85; P < 0.00001). The two-step approach yielded similar results for change in SBP −7.35 mmHg (−8.95 to −5.75; P < 0.00001), DBP MD −3.29 mmHg (95% CI −5.12 to −1.46; P = 0.0004) and MAP MD −4.63 mmHg (95% CI −6.18 to −3.09: P < 0.00001). Sub-analysis revealed that neither clinical, medication, nor demographic participant characteristics, or exercise program features, modified the IRT treatment effect.
Conclusion:
This individual patient analysis confirms a clinically meaningful and statistically significant effect of IRT on resting SBP, DBP and mean arterial blood pressure.
Keywords: blood pressure, hypertension, individual patient data meta-analysis, isometric exercise
BACKGROUND
Hypertension, or high blood pressure, remains a leading modifiable risk factor for the development of cardiovascular disease, disability and death around the world [1–3]. Present guidelines recommend that the first line of therapy to manage high blood pressure should be the adoption of lifestyle modifications (e.g. increased physical activity, smoking cessation, healthy dietary habits, stress management) [4–7]. In particular, considerable evidence supports the nonpharmacological antihypertensive effects of dynamic aerobic exercise, with additional potential benefit for adjunct dynamic resistance training [8]. Unfortunately, these types of exercises can be time consuming, limit compliance and adherence and may be unsuitable for patients with mobility limitations. Given that approximately 50% of people with hypertension do not have their blood pressure successfully controlled to within clinical targets [9], prevention and treatment of hypertension are now global priorities of the WHO [10]. Taken together, there is an urgent need to implement effective interventions to prevent or better manage high blood pressure.
In 2013, the American Heart Association reported that there was emerging evidence supporting the use of isometric resistance training (IRT) for blood pressure management (Class IIB, Level of Evidence C) [11]. This mode of exercise, performed using hand or leg dynamometry, demonstrates reductions in resting blood pressure in small prospective trials of normotensive and hypertensive participants [12,13]. These trials stimulated interest as IRT can be performed easily at home and requires a smaller investment of time from participants (approximately 12–40 min per week) [14]. The overall benefit of IRT has been confirmed by recent meta-analyses [8,15–18], which report SBP and DBP reductions between 5–10 and 4–6 mmHg, respectively. This collective work contributed to the recent inclusion of IRT as a formal recommendation put forth by the American College of Cardiology and the American Heart Association in their newly released 2017 Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults (listed under ‘Best Nonpharmacologic Interventions for Prevention and Treatment of Hypertension’) [6].
Although there is evidence to suggest a greater training response in those with higher pre-IRT blood pressure [19], the available data has not consistently supported overwhelmingly a larger hypotensive response for patients with hypertension [15]. In fact, sub-group analysis from a meta-analysis detected smaller reductions in SBP, in medicated people with hypertension compared with normotensive participants completing IRT [15]. It has been hypothesized that certain classes of antihypertensive medications may overlap with the mechanisms responsible for IRT adaptations resulting in smaller responses in medicated participants with hypertension [14]. Unfortunately, exploring the relationships between reductions in resting blood pressure after IRT and baseline participant characteristics has not been possible with the small sample sizes of prior prospective trials or meta-analyses based on aggregate data. Overcoming this limitation, meta-analyses based on individual participant data (IPD) are considered to be more stringent in reducing bias and can improve the quality of evidence [20–22]. IPD also allows associations of demographic, clinical, medicinal and IRT variables with changes in blood pressure to be identified. Previous meta-analyses have been unable to make these associations that are vital for the transition of IRT into clinical practice, however, several confounding factors may also influence change in blood pressure including body mass [23], age [24] and sex [25].
The primary study aim was to carry out an IPD meta-analysis to examine the efficacy of IRT in managing resting blood pressure. The primary objective was to quantify the change in resting SBP, DBP and mean arterial pressure (MAP) following more than 3 weeks of IRT. The secondary objective was to explore relationships between baseline characteristics [medication usage, age, sex, BMI, and coronary artery disease (CAD) diagnosis] and the magnitude of changes in resting blood pressure after IRT.
METHODS
This study was conducted and reported in accordance with current IPD guidance and the Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data (PRISMA IPD) statement [26]. The study was registered with PROSPERO (CRD42018109167).
Search methods for identification of studies
Trials for inclusion in the IRT IPD meta-analysis were identified from our recent meta-analysis [15] and by more recent searches of PubMed, Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, EMBASE, MEDLINE, CINAHL; from 1966 up until 1 July 2018. Conference Proceedings were searched on Web of Science. Trial registers (Controlled-trials.com and Clinicaltrials.gov) and reference lists of all eligible trials and identified systematic reviews were also checked. No language limitations were imposed. The PubMed search strategy, used for all databases, is available in supplementary files. We included randomized, controlled trials, but also nonrandomized, controlled trials because of the difficulty with concealment in exercise trials. With respect to the latter, this difficulty is known to compromise randomization and in some previous systematic reviews and meta-analyses, the effects of exercise have been no different when nonrandomized trials have been included, compared with only randomized controlled trials [27], moreover by including nonrandomized controlled trials we were able to maximize the data sample.
Eligibility criteria for studies
We included studies if they met the following inclusion and exclusion criteria:
-
1.
Study design: we included randomized, controlled trials, but also nonrandomized, controlled trials.
-
2.
Population: adult participants (18 years and older).
-
3.
Context: participants training in any setting, that is, hospital, university, community facility or home.
-
4.
Intervention: receiving an isometric resistance exercise intervention with longitudinal follow-up before and after the intervention lasting a minimum of 3 weeks. In order to distinguish between acute and chronic exercise training exposure, a line had to be drawn somewhere. During our IPD protocol development, consistent with previous work, it is unlikely an IRT antihypertensive effect could be observed prior to 2 weeks [28] or even 3 weeks [19].
-
5.
We excluded interventions without an IRT component or head-to-head comparisons of two or more exercise interventions, with no control group. We also excluded studies where only a sub-set of the total number of participants were analysed.
-
6.
Comparator: a no-exercise group defined as no exercise or exercise at 5% or less maximal voluntary contractions (MVC) who carried out their usual daily activities or an attention placebo.
-
7.
Sample size: we placed no restriction on sample size.
Data management
The principal investigators of included trials were invited by E-mail to participate in this IPD meta-analysis and share their anonymized trial data. Included datasets had ethical approval and consent from their sponsors. Each dataset was saved in its original format and then converted and combined into one overall master dataset with standardized variables. All files are stored on a secure password-protected computer server managed in accordance with the data management standard operating procedures of the University of New England. Data from each trial were checked on range, extreme values, internal consistency, missing values, and consistency with published reports. Data discrepancies or missing information were discussed with trial investigators. Where errors or discrepancies occurred, the relevant study author was contacted and asked to check their data and ensure the IPD dataset tallied with the article. There were no unresolved discrepancies. Access to data at all stages of cleaning and analysis was restricted to core members of the research team (N.A.S., D.W. and B.B.).
Main outcomes
In accordance with the study research, objectives we sought IPD for the following outcomes from eligible trials:
-
1.
Resting SBP, DBP and MAP
-
2.
Exercise program characteristics
We also sought individual key baseline participant demographic and clinical data (including age, medication and sex). Details of exercise training prescription (i.e. session frequency, duration and intensity and overall programme duration) were also collected as part of the review. Wherever available, we sought from investigators details, at an individual participant level, of the IRT session adherence.
Collection of data
Investigator contact
Initially all identified trial investigative teams were emailed via the named contact author, as detailed in publications, to inform them about our IPD meta-analysis and to ask them if they were willing to share their original IPD. As part of our previous review [15], a number of investigators were contacted for the purposes of obtaining further data or clarification. We also attempted to identify current contact details of those who did not reply to initial e-mails. Positive responses were received from 12 of 18 potential corresponding authors (see Supplementary file CONSORT statement).
Data format
The procedure for collection and collation of data was coordinated by the project secretariat based at the University of New England. Participating study authors were asked to provide de-identified primary datasets corresponding to minimum data required to answer the primary research objectives. Wherever possible, electronic versions of datasets were sought, together with written details of the coding of the variables.
Data transfer and storage
Methods of receiving raw data from investigators varied depending on the security concerns of their host institutions. However, our default approach to data transfer was either an encrypted data file sent via E-mail to the project secretariat or via a password-protected drop box facility. Each raw data set was saved in its original format and then converted and combined into one overall dataset with standardized variables. We worked with individual trial authors to ensure standardization of variables and to check that our initial analyses of individual datasets were consistent with the published results from the trial.
Data checking
Data from each study was evaluated and compared with the data provided in the available publication. Each dataset was checked for the range of included variables to make sure none of the values were outliers. If any data appeared to be an outlier, the original author was asked to verify. Datasets were also assessed for missing observations, in relation to each variable. These were checked against the original publication. Thereafter, an attempt was made, to replicate the results that were reported in the original publication, including baseline characteristics and outcome data at each available follow-up period, by following the statistical methods as reported by the study authors. Any discrepancy or missing information, between our results and those presented in each original publication, was discussed and clarified with the original study authors. Study authors of the six eligible missing, datasets were e-mailed, and if this failed, they were called (telephone) directly. Our understanding is that one author, Wiley [29], is no longer active in academia. Also because of the time elapsed since the studies were conducted, four datasets were destroyed (Gill et al.[30], Devereux et al.[31], Millar et al.[32] and Taylor et al.[33]). The other author Pagonas [34] refused to participate. Once data checks were complete and satisfactory, individual study datasets were combined to form a new master dataset with a variable added to indicate the original study. Data from individual datasets remain the property of the collaborators who provided the data.
Statistical analysis
Analyses were conducted in accordance with current recommendations for IPD meta-analyses [22]. A detailed statistical analysis plan was prepared (available from authors). All analyses were carried out according to the principle of intention-to-treat (i.e. patients included according to their group allocation) and included only patients with observed baseline data (where required) and outcome data at follow-up. Where missing data was noted within an individual trial, contact with the author was attempted and data added if available, no missing data were imputed. Given the relatively small levels of missing outcome and covariate data within trials, we did not undertake data imputation. We checked for potential small study bias by assessing funnel plot asymmetry and using the Egger test [35].
Descriptive analysis
The study-level and participant-level characteristics of included studies are presented in Table 1. Data from studies participating in the IPD analysis were compared with the data from those that opted not to participate to determine if the collective IPD cohort was a representative (unbiased) sample of the full set of existing studies. Independent t-tests were conducted for baseline characteristics in intervention versus control groups. A P value less than 0.01 was considered significant for all statistical analyses.
TABLE 1.
Studies included in this analysis examining the effects of isometric exercise training on blood pressure
| Reference (year) | Study design | Participants (n) | Exercise mode and intensity | Major findings |
| Badrov et al. [39], 2013 | RCT Medicated Hypertensive Office BP | Ex: 12 Con: 12 13 males, 11 females Age 51–74 years | Alternating bilateral IHG 4 × 2 min, 1 min rest periods 30% MVC; three times a week for for 10 weeks | ↓SBP 80 mmHg, D↓BP 5 mmHg ↓MAP 6 mmHg, D↓BP 4 mmHg |
| Baross et al. [41], 2012 | RCT Hypertensive and prehypertensive Office BP | Ex: 10 (14%) Ex: 10 (8%) Con: 10 (20M) 20 Males Age 45-60 | Bilateral leg extension; ∼14 and ∼8% MVC 4 × 2 min, 2-min rest periods 8 weeks | ↓SBP 11 mmHg, M↓AP 5.0 mmHg ↓HR 4.8, ↓ (14% MVC) Resting BP no change (8% MVC) |
| Baross et al. [40], 2013 | RCT Office BP | Ex: 10 Con: 10 20 Males Age 45–60 yrs | Bilateral leg extensions at 18% MVC; 4 × 2 min, 2-min rest periods thrice weekly for 8 weeks | ↓SBP 10.8 mmHg, ↓MAP 4.7 mmHg ↓HR 4.8 beats/min |
| Carlson et al. [13], 2016 | RCT Prehypertensive and hypertensive Office BP | Ex: 20 Con: 20 15 men, 25 women Age 36–65 years | Unilateral IHG, 4 × 2 min, 3 min Rest periods at 5% (n = 20) or 30% (n = 20) MVC, three times a week for 8 weeks | 5% ↓SBP 2 mmHg, ↓DBP 3 mmHg ↓MAP 3 mmHg, ↓HR 1 mmHg 30% ↓SBP 7 mmHg, ↓DBP 2 mmHg ↓MAP 4 mmHg, ↑HR 2 mmHg |
| Farah et al. [42], 2018 | RCT Hypertensive Ambulatory BP | Ex: 30 Con: 16 14 men, 32 women Age 38–79 years | Alternate bilateral, IHG, 4 × 2 min 30% MVC; 1-min rest; thrice weekly; for 12 weeks | 30% ↓SBP 11 mmHg, ↓DBP 6 mmHg |
| Goessler et al. [43], 2018 | RCT Healthy Ambulatory BP | Ex: 19 Con: 14 30–36 years 15 men, 18 women Age 21–59 years | Daily 4 × 2 mins, 1 min rest Bilateral handgrips 30% MVC for 8 weeks | 30% ↓SBP 4.4 mmHg, DBP ↓3.3 mmHg |
| Gordon et al. [44], 2017 | RCT Outpatient Cardiopulmonary Medicated Hypertensive Office BP | Ex. 6 Con 5 10 men, 1 woman Age 50–80 years | Unilateral IHG, 4 × 2-min at 30% MVC 1-min rest for 6 weeks | 30% no change SBP, DBP |
| Gordon et al. [45], 2017 | Controlled trial Hypertensive Office BP 6 men, 15 women Age 24–60 years | Home (n = 9) Lab (n = 7) Con (n = 5) for 12 weeks | Unilateral; IHG 30% MVC MVC 30%; 4 × 2 min; 1 min rest | 30% Lab SBP ↓9.0 mmHg Home ↓30% ↓8.6 mmHg SBP |
| Hess et al. [46], 2016 | RCT Healthy Office BP | Ex:10 Con:10 13 men, 7 women Age 26–50 years | Unilateral IHG, 4 × 2 min, 3 min, 10% MVC and 5% MVC (control) 1-min rest; 8 weeks | 10% ↓SBP 5.6 mmHg, ↑DBP1.8 mmHg |
| Stiller-Moldovan et al. [12], 2012 | RCT Medicated Hypertensive Office and ambulatory BP | Ex: 11 Con: 9 10 men, 10 women Age 42–76 years | Alternating bilateral IHG 4 × 2 min, 1 min rest periods 8 weeks, 30% MVC. three times a week for bilateral leg extension | No change resting or 24 h ambulatory BP |
| Wiles et al. [48], 2010 | RCT Normotensive office BP | Ex: 22 Con: 11 33 men Age 18–34 | 4 × 2 min, 2 min rest periods 3 days a week for 8 weeks 10 and 21% MVC | ↓SBP 3.7 mmHg in LI ↓SBP 5.2 mmHg in HI ↓DBP 2.6 mmHg in both ↓MAP 2.5 LI & 2.6 HI |
| Wiles et al. [47], 2017 | Randomized Crossover Normotensive Office | Ex: 15 Con: 13 28 men Age 30 ± 7 years | Wall Squat @95% Max HR ∼21% MVC, 4 × 2 min, 2 min rest periods 3 days/week for 4 weeks | 21% ↓SBP 4.2 mmHg, ↓DBP 2.8 mmHg ↓MAP 3.0 mmHg |
All blood pressure readings are reported as means. Ambulatory BP, ambulatory methods were used to measure blood pressure; BA, brachial Artery; Con, control; Ex, exercise; FMD, flow-mediated dilation; HI, high intensity; HR, heart rate; IHG, isometric hand grip; LI, low intensity; MAP, mean arterial pressure; MVC, maximum voluntary contraction; n, number of participants; office BP, office blood pressure measurement was undertaken; PP, pulse pressure; RCT, randomized control trial. ↓, indicates reduction; ←→, indicates no change; ↑, indicates increase.
Individual participant data meta-analysis
In this project, we used both one-step and two-step IPD meta-analysis.
One-step analysis
For the one-step IPD analysis, we fitted mixed effects models to the change in blood pressure (systolic, diastolic and mean arterial) with treatment as a fixed effect, treating each study as a random effect. This is the most logistically demanding, it accounts for the clusters generated by the different studies and allows for analysis of covariates and has the best performance in terms of power [25]. We repeated the analysis with the following moderating variables as factors: medication versus no medication, sex, age (under 45 years and 45 years and over), presence/absence of heart disease, BMI category [underweight 19 or under; normal >19 to 24.9; overweight (25.0–29.9); and obese 30 or over], bilateral versus unilateral IRT and arm versus leg IRT. That is, we assessed treatment by subgroup interaction effects (P values) to determine whether the moderating variables influenced the treatment effect. All analyses followed the principle of intention-to-treat as closely as possible. Specifically, we included all studies that provided relevant outcome data. The one-step analyses were undertaken using R core team software (R Foundation, Vienna, Austria) [36].
Subgroup and medication analysis
We fitted mixed effects models to the change in blood pressure (systolic, diastolic and mean arterial) with study as random effect and treatment as well as the following moderating variables as factors; medication versus no medication, sex, age (under 45 years and 45 years and over), presence/absence of heart disease, BMI category [underweight 19 or under; normal >19 to 24.9; overweight (25.0–29.9); and obese 30 or over], bi-lateral versus unilateral IRT and arm versus leg IRT. We then conducted ANOVA tests to determine whether the moderating variables had a significant effect on the response to treatment.
Regression analyses
Backward stepwise regression was employed to identify, which clinical and study variables may best predict participants’ response to IRT.
Two-step analysis
The two-step analyses were conducted with each trial analysed using a random effects model for each outcome. A random effects model was preferred because of the high degree of clinical heterogeneity across the individual trials, which included different patient populations and comparators [37]. Analyses were completed for continuous data using the mean baseline-follow-up change and change in SD and the number of participants in each group, within or between group P values or 95% CI. Additionally, the I2 and τ2 statistics were reported alongside the associated P value for the results of the main analyses. The two-step analyses were conducted using RevMan 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
Data verification between included and excluded individual participant data
A comparison between the included studies and the studies that were excluded, as IPD data was not provided, was conducted for SBP and DBP analyses.
Study quality
Study quality assessment of included studies was undertaken using the validated TESTEX scale [38].
RESULTS
Search results
An initial search yielded 1386 potential articles. After duplicates were removed, 952 articles remained, 909 were excluded based on title and abstract reviews, leaving 44 full text articles for evaluation. Acute studies accounted for 15 articles, nine were not controlled trials and three did not utilize continuous isometric contractions. Six of the 18 eligible study authors were unable to provide the individual patient data as it had been destroyed or the relevant author could not be contacted/declined to participate (see Table 5 and Consort Statement in Supplementary Files). Twelve studies met the inclusion criteria and provided IPD data [13,39–48] (Table 1) representing 326 participants, 191 assigned to IRT and 135 controls. A summary of the baseline participant characteristics shows both the IRT and control groups to be similarly matched (Table 2). The MVC intensity in the IRT participants was 8% in nine people, 10% in 11 people, 14% in 11 people, 18% in 10 people, 21% in 26 people and 30% in 124 people. None of the data were imputed, but Farah et al. [42] (n = 46) provided no BMI data and Gordon et al.[45] (n = 21) provided no medication data.
TABLE 5.
Randomized, controlled trials not included in this meta-analysis
| Eligible studies unable to provide individual patient data |
| Devereux, 2011 – Data destroyed because of time elapsed |
| Gill, 2015 – Data lost |
| Millar, 2008 – Data destroyed because of time elapsed |
| Pagonas, 2016 – Refused to participate |
| Taylor, 2003 – Data destroyed because of time elapsed |
| Wiley, 1992 – Unable to contact author |
| Noneligible RCTS | Reason for exclusion |
| Ash, 2017 | Sub-set analysis of larger trial |
RCTs, randomized controlled trials.
TABLE 2.
Summary of baseline characteristics of the study participants included in the individual participant data meta-analysis
| Demographic | All (n = 326) | Exercise (n = 191) | Control (n = 135) |
| Agea | 48.58, 16.33, 18/80 | 48.2, 16.4, 18/78 | 49.1, 16.3, 18/80 |
| Maleb | 205 | 120 (59%) | 85 (41%) |
| BMI meana | 28.31, 5.73,18.23/58.06 | 28.4, 5.25, 18.9/51 | 28.1, 6.37, 18.2/58.1 |
| Hypertensionb | 109 | 63 (58%) | 46 (42%) |
| Blood pressure | |||
| Systolic (mmHg)a | 129.54, 15.18, 90.5/188 | 130., 14.7, 95/188 | 128., 15.8, 90.5/167 |
| Diastolic (mmHg)a | 76.19, 10.12, 45/105 | 77.0, 9.52, 54.3/105 | 75.1, 10.9, 45/99 |
| Mean arterial (mmHg)a | 94.31, 10.50, 62.22/131 | 95.1, 9.77, 71.7/131 | 93.2, 11.4, 62.2/119 |
| Medicationsc | |||
| Angiotensin-converting enzyme inhibitor | 58 | 33 (57%) | 25 (43%) |
| Beta blocker | 33 | 21 (64%) | 12 (36%) |
| Calcium channel blocker | 37 | 22 (59%) | 15 (41%) |
| Diuretic | 61 | 39 (64%) | 22 (36%) |
| arb ii antagonist | 67 | 38 (57%) | 29 (43%) |
aReporting mean, SD, min/max.
bReporting totals and percentages for the exercise and control groups.
cReporting totals and percentages for the exercise and control groups without Gordon et al. [45], as the data do not show type of medication for this study.
Primary analysis
One-step analysis of variance showed that IRT treatment had a significant SBP-lowering effect −6.22 mmHg mean difference (MD) (95% CI −7.75 to −4.68; P < 0.00001). Two-step meta-analysis showed the mean difference in SBP to be MD −7.35 mmHg (−8.95 to −5.75; P < 0.00001), I2 = 22% (Fig. 1).
FIGURE 1.
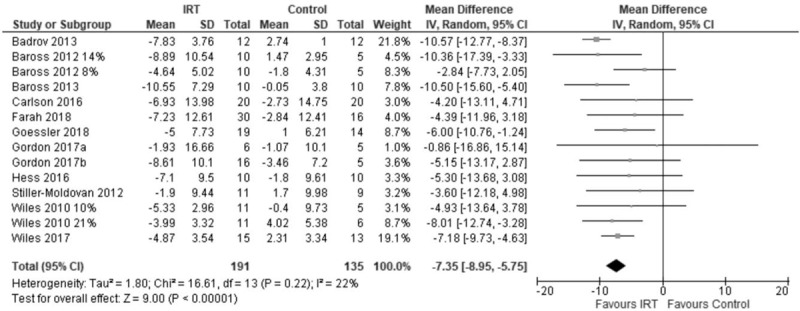
Forest plot of change in SBP using two-step analysis model.
Analysis of variance showed that IRT treatment had a significant mean DBP −2.78 mmHg (95% CI −3.92 to −1.65; P = 0.002). The two-step approach yielded similar results for change in DBP mean difference −3.29 mmHg (95% CI −5.12 to −1.46; P = 0.0005), I2 = 63% (Fig. 2).
FIGURE 2.
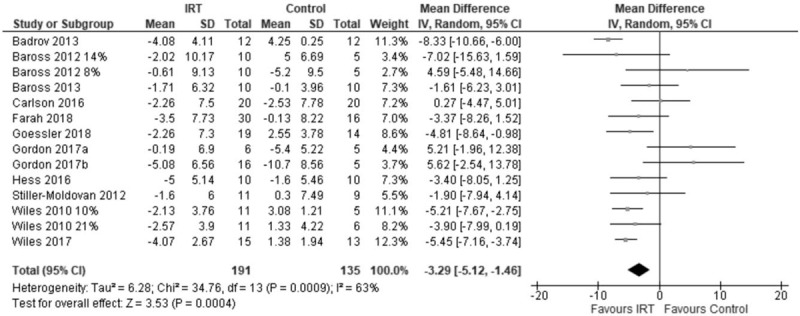
Forest plot of change in DBP using two-step analysis model.
Analysis of variance showed that IRT treatment had a significant MAP IRT −4.12 mmHg (95% CI −5.39, −2.85; P < 0.0001). The two-step approach yielded similar results for change in MAP mean difference −4.63 mmHg (95% CI −6.18 to −3.09: P < 0.00001); I2 = 47% (Fig. 3).
FIGURE 3.
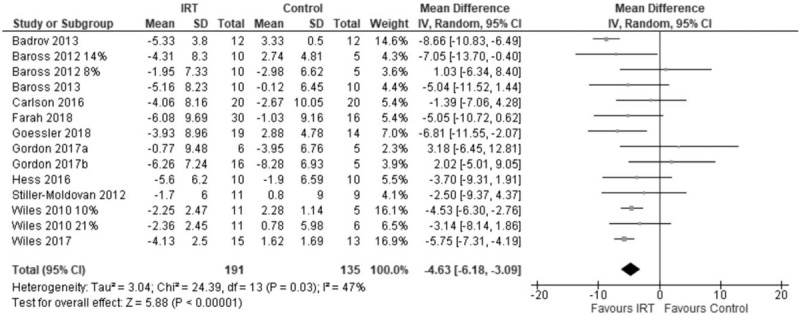
Forest plot of change in mean arterial blood pressure using two-step analysis model.
These results are summarized in Table 3.
TABLE 3.
Baseline versus postintervention changes in blood pressure for isometric resistance training versus control participants included in the individual participant data meta-analysis
| One-step model | Exercise mean difference (mmHg) (95% CI) | Control mean difference (mmHg) (95% CI) | P, treatment effect |
| Δ SBP | −6.22 (−7.75 to −4.68) | −0.14 (−1.93 to 1.65) | <0.00001 |
| Δ DBP | −2.78 (−3.92 to −1.65) | −0.45 (−1.76 to 0.85) | 0.002 |
| Δ MAP | −4.12 (−5.39 to −2.85) | −0.32 (−1.76 to 1.13) | <0.00001 |
| Two-step model, n = 12, included studies exercise versus control mean difference mmHg (95% CI) | Exercise versus control mean difference (mmHg) (95% CI) | P, treatment effect |
| ΔSBP | −7.32 (−8.93 to −5.71); I2 = 22% | <0.00001 |
| ΔDBP | −3.29 (−5.12 to −1.46); I2 = 63% | 0.0004 |
| ΔMAP | −4.63 (−6.18 to −3.09); I2 = 47% | <0.00001 |
| Two-step model, n = 6, excluded studies | Exercise versus control mean difference (mmHg) (95% CI) | P, treatment effect |
| ΔSBP | −5.61 (−9.04 to −2.19); I2 = 82% | 0.001 |
| ΔDBP | −4.27 (−7.92 to −0.62); I2 = 91% | 0.02 |
| ΔMAP | −1.92 (−3.00 to −0.84); I2 = 7% | 0.0005 |
One-step and two-step model analyses. CI, confidence interval; MAP, mean arterial pressure.
One-step versus two-step model comparison
A comparison of the change in blood pressures between the one-step and two-step models revealed that both analyses resulted in statistically significant outcomes for SBP, DBP and MAP. Furthermore, the mean difference values varied by only 1.2 mmHg for SBP, 0.51 mmHg for DBP and 0.51 mmHg for MAP, with the two-step model always producing the greater reductions in all three blood pressure measurements, see Table 3.
Included versus missing or excluded trial data
A comparison of the 12 included, and 6 excluded, randomized, controlled trials was made using a two-step (group-level) meta-analysis. This analysis showed that, for the six excluded studies, the statistical significance of SBP was mean difference −5.61 mmHg (−9.04 to −2.19), P = 0.001; DBP mean difference −4.27 mmHg (−7.92 to −0.62) P = 0.02; MAP mean difference −1.92 (−3.00 to −0.84), P = 0.0005. These values were within 0.61 mmHg of the corresponding value for the 12 included studies for the one-step model equivalents for SBP, and within 1.49 mmHg for DBP, whereas the MAP showed a 1.37 mmHg difference. The summary statistics of these comparisons are in Table 3, whereas the forest plots are in supplementary files.
Secondary analyses
We then conducted sub-analyses based upon the significant findings in SBP, DBP and MAP. We did not detect any significant effects (even if P < 0.05 rather than our stipulated P < 0.01 was used) of sex, medication status, BMI category (underweight, normal, overweight and obese), age (45 years and over versus under 45 years), hypertensive status (normotensive versus hypertensive), bilateral versus unilateral IRT and arm versus leg IRT on the treatment effect. Furthermore, there were insufficient studies with coronary heart disease participants to justify an analysis on the IRT treatment effect.
Backward stepwise regression was employed to identify which participants may best respond to IRT. These regression analyses failed to identify a model formed from any clinical or program variables that significantly predicted blood pressure response to isometric resistance training.
Sex
Eight studies had male and female participants with one study having one female participant only. There was no evidence of an effect of sex on the treatment response for SBP, DBP and MAP.
Antihypertensive medication status
Five studies had both medicated and unmedicated patients. There was no evidence of a medication effect for change in SBP, DBP and MAP. Neither adherence to medication nor changes were recorded in any study, although unmedicated participants who undertook IRT showed a trend towards a greater reduction in SBP than their medicated counterparts; mean difference −10.18 mmHg (−15.50 to −4.86) medicated versus −5.86 mmHg (−9.19 to −2.54) unmedicated.
BMI
All studies reported BMI class, there was little evidence of an effect of BMI class on size of change in SBP, DBP and MAP.
Age
Seven studies had patients both under and over 45 years of age groups, there was no evidence of an effect of age on size of change in SBP, DBP and MAP.
Hypertensive status
Four studies had both normotensive and hypertensive patients, there was no evidence that blood pressure status had an effect on the size of change in SBP, DBP and MAP.
Unilateral versus bilateral isometric exercise
There was no evidence (see Table 1) that unilateral (n = 52 exercise participants from four studies) or bilateral IRT (n = 139 exercise participants from eight studies) had an effect on the size of change in SBP, DBP and MAP.
Arm versus lower limb isometric exercise
There was no evidence (see Table 1) that arm (n = 197 exercise participants from six studies) or leg IRT (n = 129 exercise participants from six studies) had an effect on the size of change in SBP, DBP and MAP.
Study design variables
There were no significant differences observed for comparisons of any of the study design variables; unilateral versus bilateral exercise, arm versus leg, outpatient versus home, office versus ambulatory blood pressure measurement. We did not conduct an analysis of exercise specialists versus nonexercise specialist supervision as so few studies/patients were supervised by an exercise specialist.
Disease status
There were insufficient people with coronary heart disease to draw meaningful conclusions from this analysis.
Risk of bias
Funnel plots revealed some risk of publication bias, which is not surprising as we are aware of six published datasets from which individual patient data were not available.
Study quality
A summary of study quality assessment of included studies, utilizing the TESTEX Scale [38] can be seen in Table 4. Four studies scored 9, seven studies scored 10 and one study scored 11 out of 15, median score was 10.
TABLE 4.
Study quality assessment of included studies (using TESTEX Scale)
| Study name | Eligibility criteria specified | Randomly allocated participants | Allocation concealed | Groups similar at baseline | Assessors blinded | Outcome measures assessed more than 85% of participants# | Intention-to-treat analysis | Reporting of between-group statistical comparisons | Point measures and measures of variability reporteda | Activity monitoring in control group | Relative exercise intensity review | Exercise volume and energy expended | Overall TESTEX |
| Badrov et al. [39], 2013 | YES | YES | Unclear | YES | NO | YES (2) | NO | YES | YES (2) | NO | YES | NO | 9 |
| Baross et al. [40], 2013 | YES | YES | Unclear | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 10 |
| Baross et al. [41], 2012 | YES | YES | Unclear | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 10 |
| Carlson (2017) | YES | YES | YES | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 11 |
| Farah et al. [42], 2018 | YES | YES | NO | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 10 |
| Goessler et al. [43], 2018 | YES | YES | NO | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 10 |
| Gordon et al. [44], 2017 | YES | YES | NO | YES | NO | YES (2) | Unclear | YES | YES (2) | NO | YES | NO | 9 |
| Gordon et al. [45], 2017b | YES | YES | NO | YES | NO | YES (2) | Unclear | YES | YES (2) | NO | YES | NO | 9 |
| Hess (2016) | YES | YES | NO | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 10 |
| Stiller-Moldovan et al. [12] | YES | YES | Unclear | YES | NO | YES (2) | NO | YES | YES (2) | NO | YES | BO | 9 |
| Wiles et al. [48], 2010 | YES | YES | NO | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 10 |
| Wiles et al. [47], 2017 | YES | YES | NO | YES | NO | YES (2) | YES | YES | YES (2) | NO | YES | NO | 10 |
Total out of 15 points. NR, not reported.
Legend: #, three points possible – 1 point if adherence greater 85%, 1 point if adverse events reported, 1 point if exercise attendance is reported.
aTwo points possible – 1 point if primary outcome is reported, 1 point if all other outcomes reported
Post hoc sample size estimate
On the basis of the results of our one-step model SBP analysis, which were −6.22 mmHg 95% CI (−7.75 to −4.68 mmHg), we calculated post hoc sample size (based on converting the standard error from our meta-analysis into a standard deviation). We calculate that the minimum sample size required to detect this magnitude of SBP change, with 90% power would be approximately 85 participants in each group, if one were to factor in 10% attrition then this number would rise to a total sample size of 187.
DISCUSSION
The findings of the present IPD meta-analysis support the capacity for IRT to reduce resting blood pressure, specifically SBP, DBP and MAP. The one-step and two-step analyses were very similar for SBP and DBP whilst there was only a difference of about 2.5 mmHg for the MAP analysis. These values are also in agreement with those reported in previous ‘aggregated data’ meta-analyses [8,15,17]. These results offer new insights into the scope of participants that will reap the benefit following IRT. Taken together, this work supports the use of IRT as a novel nonpharmacologic therapy to manage high blood pressure in selected patients, in line with current recommendations [6] and support future trials to identify the patients most likely to respond to IRT [11,49].
We found no evidence that sex had an effect on the treatment response for SBP, DBP and MAP. Conflicting information from previous work has suggested that both men [15] and women [50] may have greatest potential to reduce blood pressure. Yet other work has suggested that men and women have the potential to reduce blood pressure using IRT by a similar magnitude [51], this is consistent with our analysis.
Our analyses showed unmedicated participants who undertook IRT had a trend towards a greater reduction in SBP than their medicated counterparts. Previous work has suggested that medication use may blunt the antihypertensive effects of IRT [14], possibly because of a shared mechanism.
Our analysis did not suggest a trend towards a greater change in SBP with changing BMI classification. There was also no evidence of an effect of age on size of change in SBP, DBP and MAP. The age cut-off for this analysis, of 45 years, is somewhat arbitrary but does reflect similarity with previous analyses that have examined if the size of blood pressure reductions vary between older and younger participants [15]. All of these sub-analyses were likely to be underpowered to detect change in sex, medication status, BMI classification or age.
Our IPD meta-analysis represents the first time that it has been possible to investigate the potential interaction of specific antihypertensive pharmacological classes on the blood pressure response to IRT, as insufficient data existed previously to conduct meaningful analyses. It has been hypothesized that medicated hypertensive participants might not respond to IRT at the same rate as unmedicated participants because of the potential mechanistic overlap of antihypertensive medications [14]. The results from this analysis suggest that individuals who use blood pressure-lowering medications may exhibit similar antihypertensive response to IRT-based interventions. Moreover, the size of blood pressure reduction observed in this IRT IPD are superior to those observed from aerobic exercise, in a previous meta-analysis [52].
Isometric resistance training antihypertensive effects
The mechanisms responsible for the reduction in resting blood pressure after IRT are unclear [14]. Research has supported a role of IRT to increase endothelial-dependent vasodilation, a marker of nitric oxide bioavailability [27,50,51]. This would suggest that IRT may lower blood pressure by reducing total peripheral resistance, similar to dynamic aerobic exercise [8]. The absence of statistically significant evidence of a relationship with specific classes of antihypertensive medications is an important finding for helping to identify participants most likely to respond to IRT. This finding also supports studies exploring the use of this type of exercise as an adjunct to existing antihypertensive treatment [40]. Previous work on the antihypertensive effects of aerobic exercise training has postulated that 12 weeks’ exposure may lead to enhanced vasodilation and reduced vascular resistance [53,54], however, only two IRT studies have been of 12 weeks’ duration.
The convenience and practicality of employing IRT as a treatment tool are advantageous. IRT exercise can be implemented while seated, at any time of the day, and is easily accessible for most patients with mobility issues. A drawback of IRT is that the benefits to date have been confined to blood pressure, unlike dynamic aerobic exercise, which impacts a number of other cardiovascular risk factors (e.g. body weight, insulin resistance, HDL cholesterol) [55]. Nevertheless, IRT may offer a novel nonpharmacologic lifestyle option for treating hypertension. Our results question previous work that identified key variables, such as sex and age, as barriers to acquiring antihypertensive effects from IRT-based interventions. It is also important to consider that blood pressure reductions can reduce the risk of myocardial infarction, transient ischemic attack, CAD, and mortality [56]. The ever-increasing evidence for IRT warrants larger scale RCT studies to further support its efficacy.
Limitations
As with most IRT research, the available sample size remains small compared with work in aerobic exercise training [8,55]. We recognize that the results are based on efficacy studies, conducted under optimal conditions compared with ‘real world’ settings. As such the generalizability of our results are limited as they are based on studies in restricted samples of volunteers.
A number of participants were using antihypertensive medications during the course of this study. However, there were only a small number of participants that were using multiple antihypertensive medications. There were insufficient numbers of participants taking the various classes of antihypertensive medications to allow analyses between the different agents. No adherence to medication or changes were recorded in any study and small numbers of participants taking some medications precluded an analysis of any specific drug and IRT interaction effects.
Insufficient numbers of people in each of the various BMI (underweight, normal, overweight and obese) categories may have meant these sub-analyses were underpowered to show significant improvements in SBP, DBP and MAP. There were some missing BMI data from Farah et al.[42] (n = 46) and Gordon et al.[45] (n = 21) provided no medication data. Considering that these sub-analyses were quite some way from reaching statistical significance, the missing data could not have altered the findings.
The studies by Stiller-Moldovan et al. [12], Goessler et al.[43], Farah et al.[42] and the recent work by Taylor et al.[57] are the only randomized trials to date to report both clinic and ambulatory BP measurements. Ambulatory measurements, especially nighttime measurements, provide a better approach to the risk estimation compared with office measurements and until ambulatory findings have been confirmed in a larger study population, the true value of IRT as a therapy for hypertension will remain unclear.
Enrolment in trials of behavioural modification in high blood pressure is known to raise subject awareness of interventions, such as medication, weight loss, dietary sodium reduction, etc. That said, these data were not reported, so we did not record any change in prestudy and poststudy body weights, or medication dosage or adherence, so we cannot rule out concomitant weight loss and changes in medication use as confounding factors.
These findings have implications for future research designs as contrary to preexisting arguments, there is no evidence that specific participant characteristics are more or less likely to derive optimal benefit from IRT, and therefore, most people could expect to benefit from an antihypertensive effect of this treatment.
In conclusion, inadequate blood pressure control puts millions of people around the world at risk of the potentially fatal consequences of hypertension. This IPD analysis confirms a clinically meaningful and statistically significant effect of IRT on resting blood pressure. Future prospective clinical trials are needed to confirm the effectiveness of IRT as a nonpharmacologic therapy to treat hypertension.
By demonstrating for the first time, using our robust IPD design, the effectiveness of IRT to lower blood pressure in high-risk hypertensive populations, we provide much needed support for its potential adoption as part of hypertension standard-of-care treatment.
ACKNOWLEDGEMENTS
We would like to acknowledge the statistical advice provided by Dr Theresa Neeman of the Australian National University, Canberra, Australia.
All listed authors were involved in data collection and analysis for one or more databases providing IPD data.
This work has not been previously presented either in whole or part.
There were no sources of any support, for any of the authors, for the work in the form of grants, equipment, drugs, or any combination of these.
No funding was received from National Institutes of Health (NIH); Wellcome Trust; or Howard Hughes Medical Institute (HHMI).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Abbreviations: IRT, isometric resistance training; MAP, mean arterial blood pressure
REFERENCES
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ open 2013; 3:e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 4.Vidal-Petiot E, Elbez Y, Luscher TF, Fox KM, Steg PG. The 2018 ESC-ESH guidelines for the management of arterial hypertension leave clinicians facing a dilemma in half of the patients. Eur Heart J 2018; 39:4040–4041. [DOI] [PubMed] [Google Scholar]
- 5.Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, et al. Hypertension Canada. Hypertension Canada's 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can J Cardiol 2018; 34:506–525. [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 7.Gabb GM, Mangoni AA, Anderson CS, Cowley D, Dowden JS, Golledge J, et al. Guideline for the diagnosis and management of hypertension in adults - 2016. The Medical journal of Australia 2016; 205:85–89. [DOI] [PubMed] [Google Scholar]
- 8.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013; 2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendis S, Davis S, Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015; 46:e121–e122. [DOI] [PubMed] [Google Scholar]
- 11.Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, et al. American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension 2013; 61:1360–1383. [DOI] [PubMed] [Google Scholar]
- 12.Stiller-Moldovan C, Kenno K, McGowan CL. Effects of isometric handgrip training on blood pressure (resting and 24 h ambulatory) and heart rate variability in medicated hypertensive patients. Blood Press Monit 2012; 17:55–61. [DOI] [PubMed] [Google Scholar]
- 13.Carlson DJ, Inder J, Palanisamy SK, McFarlane JR, Dieberg G, Smart NA. The efficacy of isometric resistance training utilizing handgrip exercise for blood pressure management: a randomized trial. Medicine 2016; 95:e5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millar PJ, McGowan CL, Cornelissen VA, Araujo CG, Swaine IL. Evidence for the role of isometric exercise training in reducing blood pressure: potential mechanisms and future directions. Sports Med 2014; 44:345–356. [DOI] [PubMed] [Google Scholar]
- 15.Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res 2016; 39:88–94. [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens 2013; 31:639–648. [DOI] [PubMed] [Google Scholar]
- 17.Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc 2014; 89:327–334. [DOI] [PubMed] [Google Scholar]
- 18.Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 2011; 58:950–958. [DOI] [PubMed] [Google Scholar]
- 19.Millar PJ, Bray SR, McGowan CL, MacDonald MJ, McCartney N. Effects of isometric handgrip training among people medicated for hypertension: a multilevel analysis. Blood Press Monit 2007; 12:307–314. [DOI] [PubMed] [Google Scholar]
- 20.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993; 341:418–422. [DOI] [PubMed] [Google Scholar]
- 21.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340:c221. [DOI] [PubMed] [Google Scholar]
- 22.Tierney JF, Vale C, Riley R, Smith CT, Stewart L, Clarke M, Rovers M. Individual participant data (IPD) meta-analyses of randomised controlled trials: guidance on their use. PLoS Med 2015; 12:e1001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986-1995. Arch Intern Med 2000; 160:2847–2853. [DOI] [PubMed] [Google Scholar]
- 24.Pinto E. Blood pressure and ageing. Postgrad Med J 2007; 83:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 2001; 37:1199–1208. [DOI] [PubMed] [Google Scholar]
- 26.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF. PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015; 313:1657–1665. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor DAHS. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001; 322:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortimer J, McKune AJ. Effect of short-term isometric handgrip training on blood pressure in middle-aged females. Cardiovasc J Africa 2011; 22:257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiley RL, Dunn CL, Cox RH, Hueppchen NA, Scott MS. Isometric exercise training lowers resting blood pressure. Med Sci Sports Exerc 1992; 24:749–754. [PubMed] [Google Scholar]
- 30.Gill KF, Arthur ST, Swaine I, Devereux GR, Huet YM, Wikstrom E, et al. Intensity-dependent reductions in resting blood pressure following short-term isometric exercise training. J Sports Sci 2015; 33:616–621. [DOI] [PubMed] [Google Scholar]
- 31.Devereux GR, Wiles JD, Swaine I. Markers of isometric training intensity and reductions in resting blood pressure. J Sports Sci 2011; 29:715–724. [DOI] [PubMed] [Google Scholar]
- 32.Millar PJ, Bray SR, MacDonald MJ, McCartney N. The hypotensive effects of isometric handgrip training using an inexpensive spring handgrip training device. J Cardiopulm Rehabil Prev 2008; 28:203–207. [DOI] [PubMed] [Google Scholar]
- 33.Taylor AC, McCartney N, Kamath MV, Wiley RL. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc 2003; 35:251–256. [DOI] [PubMed] [Google Scholar]
- 34.Pagonas N, Vlatsas S, Bauer F, Seibert FS, Zidek W, Babel N, et al. Aerobic versus isometric handgrip exercise in hypertension: a randomized controlled trial. J Hypertens 2017; 35:2199–2206. [DOI] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Team RC. R:A language and environment for statistical computing. R Foundation for Statistical Computing. 2018. Available at: https://www.R-project.org/. [Google Scholar]
- 37.Higgins JPTTS, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smart NA, Waldron M, Ismail H, Giallauria F, Vigorito C, Cornelissen V, Dieberg G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 2015; 13:9–18. [DOI] [PubMed] [Google Scholar]
- 39.Badrov MB, Horton S, Millar PJ, McGowan CL. Cardiovascular stress reactivity tasks successfully predict the hypotensive response of isometric handgrip training in hypertensives. Psychophysiology 2013; 50:407–414. [DOI] [PubMed] [Google Scholar]
- 40.Baross A, Wiles JD, Swaine IL. Double-leg isometric exercise in older men. Open Access J Sports Med 2013; 4:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baross AW, Wiles JD, Swaine IL. Effects of the intensity of leg isometric training on the vasculature of trained and untrained limbs and resting blood pressure in middle-aged men. Int J Vasc Med 2012; 2012:964697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farah BQ, Rodrigues SLC, Silva GO, Pedrosa RP, Correia MA, Barros MVG, et al. Supervised, but not home-based, isometric training improves brachial and central blood pressure in medicated hypertensive patients: a randomized controlled trial. Front Physiol 2018; 9:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goessler KF, Buys R, VanderTrappen D, Vanhumbeeck L, Cornelissen VA. A randomized controlled trial comparing home-based isometric handgrip exercise versus endurance training for blood pressure management. J Am Soc Hypertens 2018; 12:285–293. [DOI] [PubMed] [Google Scholar]
- 44.Gordon BDH, S W, Lavis A, King I, Zacherle E, Doyle S, et al. Isometric handgrip exercise training in rehabilitation patients. Paper presented at American College of Sports Medicine Southeast Regional Chapter, 16–18 February 2017. Greenville, South Carolina. [Google Scholar]
- 45.Gordon BDH, V E, Jones C, Warren-Findlow J, Howden R. Blood pressure reductions following a 12-week isometric training program: Lab-based vs. homebased approach. ASCM Annual Meeting, 31 May 2017, Denver, CO, USA. [Google Scholar]
- 46.Hess NC, Carlson DJ, Inder JD, Jesulola E, McFarlane JR, Smart NA. Clinically meaningful blood pressure reductions with low intensity isometric handgrip exercise. A randomized trial. Physiol Res 2016; 65:461–468. [DOI] [PubMed] [Google Scholar]
- 47.Wiles JD, Goldring N, Coleman D. Home-based isometric exercise training induced reductions resting blood pressure. Eur J Appl Physiol 2017; 117:83–93. [DOI] [PubMed] [Google Scholar]
- 48.Wiles JD, Coleman DA, Swaine IL. The effects of performing isometric training at two exercise intensities in healthy young males. Eur J Appl Physiol 2010; 108:419–428. [DOI] [PubMed] [Google Scholar]
- 49.Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, et al. Hypertension Canada's 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol 2016; 32:569–588. [DOI] [PubMed] [Google Scholar]
- 50.Badrov MB, Bartol CL, Dibartolomeo MA, Millar PJ, McNevin NH, McGowan CL. Effects of isometric handgrip training dose on resting blood pressure and resistance vessel endothelial function in normotensive women. Eur J Appl Physiol 2013; 113:2091–2100. [DOI] [PubMed] [Google Scholar]
- 51.Badrov MB, Freeman SR, Zokvic MA, Millar PJ, McGowan CL. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur J Appl Physiol 2016; 116:1289–1296. [DOI] [PubMed] [Google Scholar]
- 52.Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res 2015; 39:88–94. [DOI] [PubMed] [Google Scholar]
- 53.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 2003; 108:530–535. [DOI] [PubMed] [Google Scholar]
- 54.Goto C, Nishioka K, Umemura T, Jitsuiki D, Sakagutchi A, Kawamura M, et al. Acute moderate-intensity exercise induces vasodilation through an increase in nitric oxide bioavailiability in humans. Am J Hypertens 2007; 20:825–830. [DOI] [PubMed] [Google Scholar]
- 55.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005; 46:667–675. [DOI] [PubMed] [Google Scholar]
- 56.Turnbull F. Blood Pressure Lowering Treatment Trialists Colloboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 57.Taylor KA, Wiles JD, Coleman DA, Leeson P, Sharma R, O’Driscoll JM. Neurohumoral and ambulatory haemodynamic adaptations following isometric exercise training in unmedicated hypertensive patients. J Hypertens 2018; (doi: 10.1097/HJH.0000000000001922). [DOI] [PubMed] [Google Scholar]


