Supplemental Digital Content is available in the text.
Keywords: 28-day mortality rate, pneumonia severity index, randomized controlled trial, severe community-acquired pneumonia, XueBiJing injection
Abstract
Objectives:
To investigate whether XueBiJing injection improves clinical outcomes in critically ill patients with severe community-acquired pneumonia.
Design:
Prospective, randomized, controlled study.
Setting:
Thirty-three hospitals in China.
Patients:
A total of 710 adults 18–75 years old with severe community-acquired pneumonia.
Interventions:
Participants in the XueBiJing group received XueBiJing, 100 mL, q12 hours, and the control group received a visually indistinguishable placebo.
Measurements and Main Results:
The primary outcome was 8-day improvement in the pneumonia severity index risk rating. Secondary outcomes were 28-day mortality rate, duration of mechanical ventilation and total duration of ICU stay. Improvement in the pneumonia severity index risk rating, from a previously defined endpoint, occurred in 203 (60.78%) participants receiving XueBiJing and in 158 (46.33%) participants receiving placebo (between-group difference [95% CI], 14.4% [6.9–21.8%]; p < 0.001). Fifty-three (15.87%) XueBiJing recipients and 84 (24.63%) placebo recipients (8.8% [2.4–15.2%]; p = 0.006) died within 28 days. XueBiJing administration also decreased the mechanical ventilation time and the total ICU stay duration. The median mechanical ventilation time was 11.0 versus 16.5 days for the XueBiJing and placebo groups, respectively (p = 0.012). The total duration of ICU stay was 12 days for XueBiJing recipients versus 16 days for placebo recipients (p = 0.004). A total of 256 patients experienced adverse events (119 [35.63%] vs 137 [40.18%] in the XueBiJing and placebo groups, respectively [p = 0.235]).
Conclusions:
In critically ill patients with severe community-acquired pneumonia, XueBiJing injection led to a statistically significant improvement in the primary endpoint of the pneumonia severity index as well a significant improvement in the secondary clinical outcomes of mortality, duration of mechanical ventilation and duration of ICU stay.
Community-acquired pneumonia (CAP), an important public health problem, is the leading infectious cause of death worldwide (1). The annual number of hospitalizations for CAP in the United States is expected to increase to up to 1 million in 2020, with similar trends in many other countries (2, 3). Severe CAP (SCAP) remains a major cause of mortality, and despite antibiotic therapy, 12–36% of patients admitted to ICUs die within a short period of time (4). In addition to having a high mortality rate, critically ill patients with SCAP have longer durations of mechanical ventilation and have longer ICU and hospital lengths of stay (5–7).
Empiric antibiotic recommendations for patients with SCAP have not changed substantially from those in previous guidelines (8, 9). However, the prevalence of bacterial resistance for community infections is on the rise, and due to the increasing complexity of patients with SCAP, these patients frequently encounter drug toxicities (10). Therefore, there is a need for nonantibiotic, adjunctive therapeutic options to improve outcomes.
XueBiJing, a Chinese herbal derived therapeutic, has been approved to treat severe infections (sepsis) in critically ill patients (China Food and Drug Administration; Beijing, China, Number Z20040033). XueBiJing has long been hypothesized to improve outcomes for serious lung infections in China (11–13). XueBiJing has potential mechanisms as an anti-inflammatory and an immune function enhancer (14–16) (chemical composition and reaction mechanism of XueBiJing are described in Supplemental Content 1, http://links.lww.com/CCM/E703).
Although pilot, randomized controlled trials (RCTs) reported that XueBiJing use was associated with significantly decreased mortality and a shorter hospital length of stay (17) in patients with severe pneumonia, these trials were too small to influence practice. Placebo-controlled evidence to support the benefit of XueBiJing is also weak, leaving concerns about safety in critically ill patients. To answer these questions, we conducted a large randomized trial to compare XueBiJing with placebo in critically ill patients with SCAP.
MATERIALS AND METHODS
Design and Setting
The study protocol, which includes a detailed description of the intervention, has been published (18). The study was a randomized, double-blind, placebo-controlled trial of XueBiJing, 100 mL, q12 hours, for at least 5 days up to a maximum of 7 days in adult patients with SCAP. The trial was approved by the Medical Ethics Committee of Zhongshan Hospital, Fudan University (2011–2038 [3]). Written informed consent was obtained from each patient or from his or her legal guardian. Participants were enrolled at 33 public tertiary teaching hospitals in China (three settings were missed in published protocol, see Protocol Amendments, Supplemental Content 3, http://links.lww.com/CCM/E705). Recruitment started in September 2013 and ended in December 2015; follow-up was completed in January 2016.
Study Population
Patients were eligible for inclusion if they met the following criteria: adults 18–75 years old, clinical symptoms suggestive of CAP, acquired outside of the hospital or less than 48 hours after hospital admission, met SCAP criteria (defined by the American Thoracic Society [8]), fulfilled three or more of the following criteria: Pao2/Fio2 ratio less than or equal to 250 mm Hg, respiratory rate greater than or equal to 30 breaths/min, blood urea nitrogen greater than 20 mg/dL, leukopenia (WBC count < 4,000 cells/mm3) not due to other causes, thrombocytopenia (platelet count < 100,000 cells/mm3), hypothermia (core temperature < 36°C), new onset mental confusion, receiving treatment with vasopressors at therapeutic doses after adequate fluid resuscitation, or radiographic findings of new pulmonary infiltrate(s) consistent with CAP diagnosis. Patients with pneumonia of sufficient severity requiring ICU management and patients meeting at least one of the severity criteria, such as receiving mechanical ventilatory support or septic shock with the need for vasopressors, were also included in the study.
Patients were excluded from enrollment if life expectancy was 48 hours or less, if pregnant or lactating, if diagnosed with severe primary diseases, if using immunosuppressants and/or cytotoxic drugs, if diagnosed with lung disease induced by obstructive lung tumors, if diagnosed with psychiatric disorders, or if allergic to two or more substances. Further exclusion criteria included participating in other clinical trials 30 days before enrollment, use of prohibited medicine 7 days prior to enrollment, if the patient was unable to complete the investigation, and a diagnosis of severe acute respiratory distress syndrome (ARDS) (if patient had multilobar infiltrates, a low Pao2/Fio2 ratio [< 100 mm Hg] and met the definition of severe ARDS, he/she was excluded).
Randomization and Blinding
Eligible participants were randomly administered with a 1:1 ratio of XueBiJing or placebo for 5–7 days. Randomization was generated centrally by using an interactive web response system. The independent drug administrators received group information based on a random number, and then they assigned the study drug to the nurses. The mixture of drugs was prepared by the nurses in a separate room. Photophobic brown color infusion bags and infusion devices for both groups were visually inspected by the pharmacy to ensure identical appearance. This mixture of drugs is standard procedure for XueBiJing, and all the nurses had experience in performing this task and signed a confidentiality agreement about patient allocation. The participants, as well as all the members of the study and the healthcare team, were blinded to the study drug assignment. Data analysis was performed by a researcher who was blinded to patient allocation.
Intervention
The participants received the solvent only (normal saline, 200 mL, q12 hr) in the placebo group and the solvent plus XueBiJing (normal saline 100 mL + XueBiJing 100 mL, q12 hr) in the XueBiJing group. XueBiJing, specification 10 mL/ampule, packaging 10 ampules/container, concentration 0.1 g/mL, were manufactured by a Good Manufacturing Practice certified company in China (Tianjin Chase Sun Pharmaceutical Co., Tianjin, China; China lot number 1304291, 1401091 and, 1501261). Generally, the treatment duration of the study was at least 5 days. In this SCAP trial, the recommended total duration of treatment was 7 days (maximum 7 d). Both groups received a standard therapy (such as antibiotics) chosen by the attending physician according to the 2007 American Thoracic Society/Infectious Diseases Society of America guideline (8). In cases that were judged as high initial inflammatory response, the participants were given low-dose (1 mg/kg/d) methylprednisolone for 2–3 days. Low molecular weight heparin was indicated in patients with acute respiratory failure when D-dimer level increased (according to physician’s judgment whether patient has potential risk of pulmonary embolism formation based on pulmonary embolism Caprini score) (19). Culture results were used reassess the appropriateness of the initial therapy prescribed, along with the patient’s clinical progress and other investigation results (20–22).No animal products or restricted herbal ingredients were used in this study.
Data Collection and Follow-Up Processes
The participants were followed for 28 days after randomization. The contract research organization periodically monitored the individual sites by assessing medical records and case report forms for accuracy. All adverse events (AEs) were recorded in the case report form by the principal investigators. Serious AEs were reported to the sponsor within 24 hours.
Outcome Measurements
A composite endpoint was defined as: Pneumonia severity index (PSI) improvement rate, n (%) = [(significantly effective+ effective)/the total number] × 100% The PSI is a validated predictor of mortality for pneumonia (23, 24). Previous studies have shown that higher PSI scores indicate increased mortality in SCAP patients; thus, the PSI was used as a surrogate for mortality in those patients (24, 25). Thus, improvement in PSI risk rating was defined as the primary outcome. Whether the primary outcome was achieved was determined 8 days after randomization. Secondary outcomes included 28-day mortality, the time of mechanical ventilation, and total duration of ICU stay. Detailed outcomes were reported and are summarized in the study protocol (18). There are some omissions in the published protocol. Five indicators (clinical scores improvement) were presented as secondary outcomes, and corresponding raw score were not listed. These are outcomes that may be of interest, and they may indirectly reflect important outcomes.
Power Calculation and Statistical Analyses
To estimate the sample size, we assumed the improvement of PSI risk rating to be 70% in the control group and 80% in the treatment group based on the results of previous studies (26). The study needed 710 participants to achieve 80% power with a two-sided α level of 0.05. These calculations included a 15% dropout or withdrawal rate.
The primary outcome was analyzed by fitting a logistic regression model, adjusted for sites and stratification factors. Additionally, adjusted differences and 95% CIs between groups were calculated using bootstrapping. Patients who discontinued without providing a post-baseline improvement in the PSI risk rating were considered ineffective at day 8.
Kaplan-Meier survival curves and the log-rank test were used to compare the two groups. Mortality in the two treatment groups was also compared using Cox regression models, with the hazard ratio (HR) as an effect measure that considered clinical sites as a random effect. The CIs for the HRs were constructed with standard errors derived from the model. For other continuous variables, comparisons between treatment groups were assessed using the general linear model, adjusted for baseline value and sites. Categorical variables were compared using the logistic regression model with bootstrap CIs of 95%, adjusted for sites.
Descriptive statistics were used for demographics, baseline characteristics, and safety variables. All the analyses were performed on the modified intention-to-treat population, which comprised all the patients who were randomized to treatment and received at least one dose of the study drug. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC), with a two-sided p value of less than 0.05 considered significant.
RESULTS
Of the 710 participants enrolled at 33 centers, 334 were assigned to XueBiJing and 341 to placebo. Table 1 shows the baseline characteristics of the participants. The mean PSI score before study entry was 116.14 for XueBiJing recipients and 113.83 for placebo recipients. Figure 1 shows the disposition of the study participants. In Table 2, the proportions of patients with septic shock, ARDS and the baseline settings of mechanical ventilation did not differ between groups. As the study was monitored, 21 XueBiJing and 14 placebo recipients were identified who failed to receive treatments. These 35 patients were excluded from the baseline summaries and primary analyses. Microbial investigations were done in 297 patients (XueBiJing: 144 [43.11%] vs control: 153 [44.87%]). The microbial etiology was determined in 85 of 144 (59%) cases in the XueBiJing group, and 104 of 153 (68%) cases in control group (Table S1, Supplemental Content 2, http://links.lww.com/CCM/E704). And there was no difference in receiving inappropriate treatment in XueBiJing group and control group (65 [19.46%] vs 71 [20.82%]; p = 0.66)
TABLE 1.
Comparison of Demographic and Basal Clinical Characteristics of Patients Between XueBiJing Injection and Placebo Groups
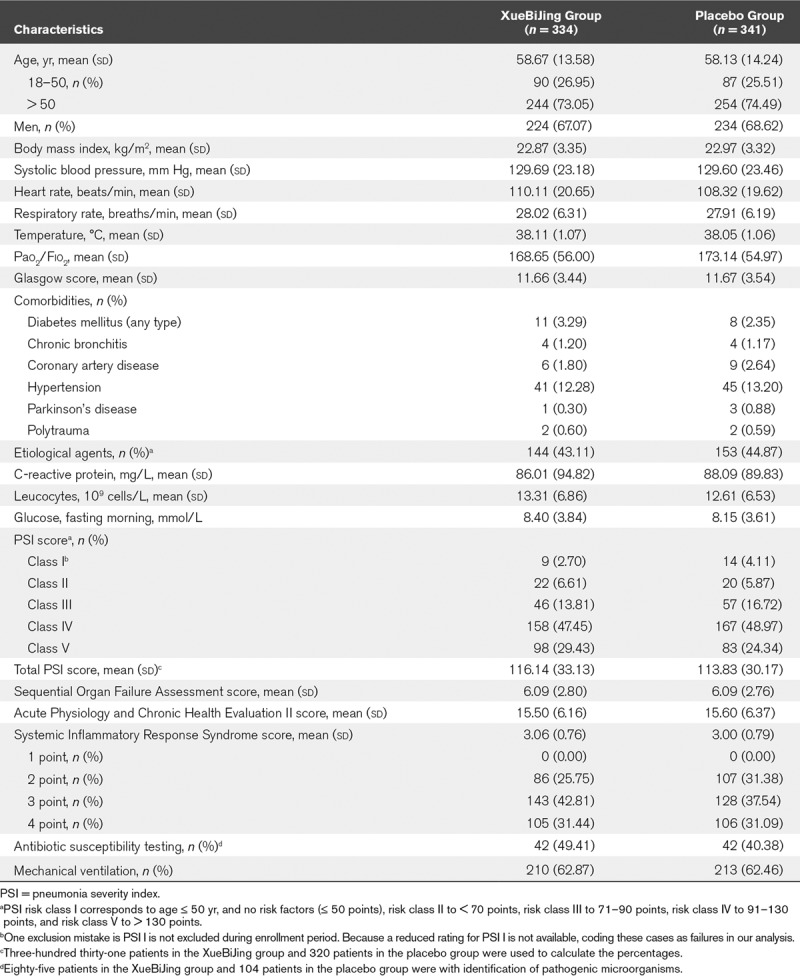
Figure 1.
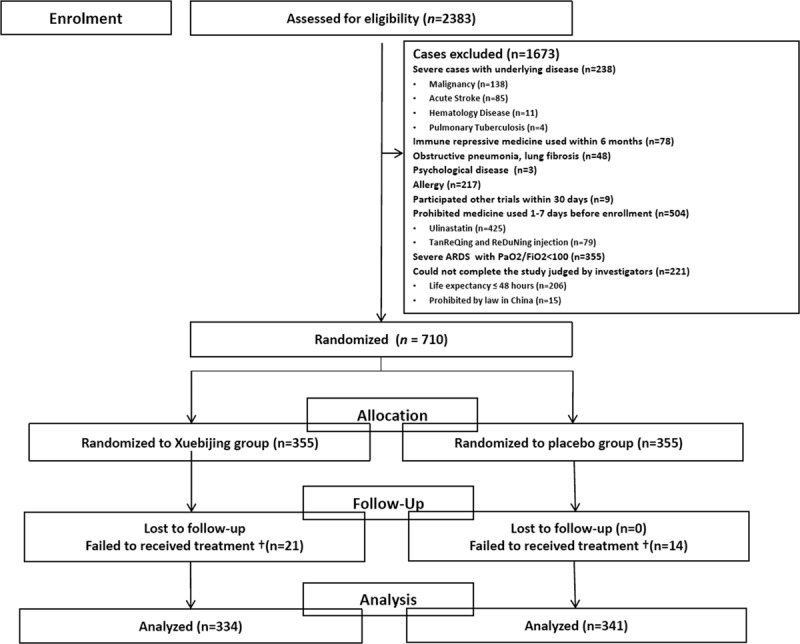
Study flow diagram. †Data were removed (the 35 patients who failed to received treatments). ARDS = acute respiratory distress syndrome.
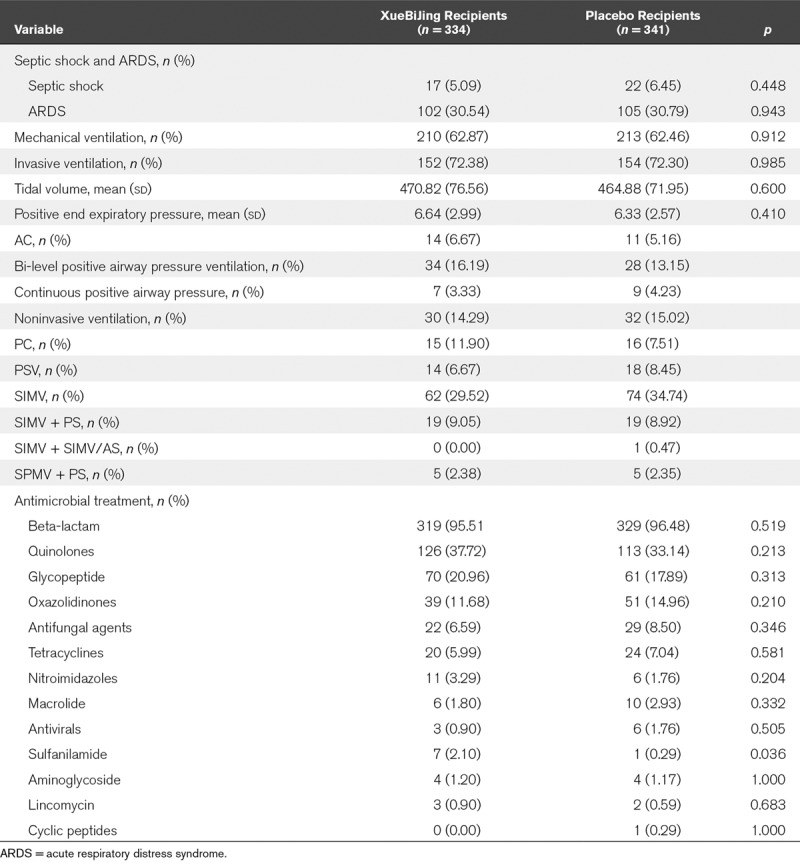
TABLE 2.
Rate of Patients With Acute Respiratory Distress Syndrome and Septic Shock, the Baseline Settings of Mechanical Ventilation, and the Frequency of Antimicrobial Prescriptions for XueBiJing Group Versus Placebo Group Using Descriptive Statistics for the Intention-to-Treat Populations
Improvement in the PSI risk rating, as a previously defined endpoint, occurred in 203 (60.78%) participants receiving XueBiJing and 158 (46.33%) of those receiving placebo. Patients in the XueBiJing group had a significantly greater improvement in the PSI risk rating (between-group difference [95% CI], 14.4% [6.9–21.8%]; p < 0.001) (Table 3). The outcome data were not available for nine participants who received XueBiJing and 14 who received placebo, and these cases were coded as failures. Because a reduced rating for PSI was not available, coding these cases as failures represented a conservative approach.
TABLE 3.
The Primary and Three Secondary Outcomesa
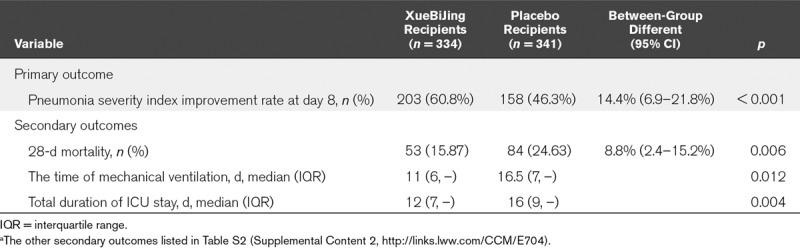
The XueBiJing group had a significantly lower 28-day mortality rate (15.87%) than the control group (24.63%) (8.8% [2.4–15.2%]; p = 0.006) died within 28 days (Fig. 2). Patients in the XueBiJing group had a significantly shorter duration of mechanical ventilation (11 vs 16.5 d; p = 0.012) and ICU stay (12 vs 16 d; p = 0.004). Some secondary outcomes are listed in the Table S2 (Supplemental Content 2, http://links.lww.com/CCM/E704).
Figure 2.
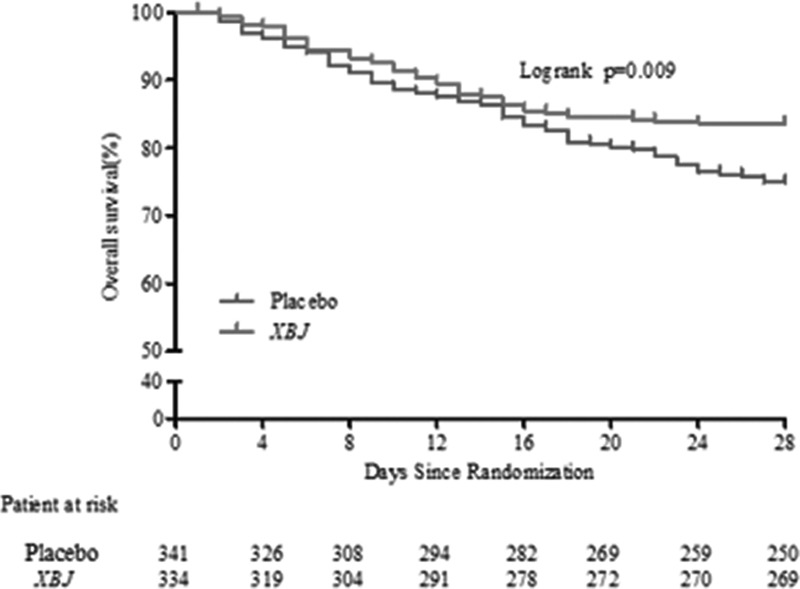
Death from randomization to 28 d. The overall survival during the study period in XueBiJing (XBJ) and control groups.
There were no significant differences in AEs among patients in the XueBiJing and placebo groups (119 [35.63%] vs 137 [40.18%]; p = 0.235]). Table S3 (Supplemental Content 2, http://links.lww.com/CCM/E704) shows an overall list of AEs and clinically significant laboratory abnormalities.
DISCUSSION
To our knowledge, this is the first double-blind placebo-controlled trial using a strict and well-accepted methodological protocol to compare XueBiJing with placebo for patients with SCAP. The results demonstrated that the administration of XueBiJing was associated with more PSI rating improvement and better 28-day survival in a prospectively identified population of patients with SCAP. The 28-day mortality in the placebo group is consistent with a previous study (27). The 28-day mortality was reduced from 24.63% to 15.87% in patients treated with XueBiJing (p < 0.01), there was an 8.76% absolute reduction in mortality in patients who received XueBiJing. And this is the largest multicenter study to date of nonantibiotic therapeutic options in high-risk population, where 94.07% of the participants were from ICUs. A MEDLINE search for studies published in English up to February 2018 revealed dozens of SCAP clinical trials. Most had small sample sizes or were conducted in single institutions or among non-ICU patient samples. In a meta-analysis of hospitalized adults with CAP, it was found that systemic corticosteroid therapy may reduce mortality by approximately 3% (28). Although these results are compelling, despite the small sample size, they are relevant to a select patient sample and are not generalizable to most ICU patients. Despite the multicenter design, therapeutic strategies (i.e., antibiotic therapy, ventilation protocols, and adjunctive support therapies) were standardized according to the 2007 ATS/IDSA guideline.
XueBiJing is one of the most common medical treatments in China, and it has been used in a large number of critically ill patients for over 10 years (13); the route of administration and safety of use for XueBiJing has been clinically well established. The dominant components of XueBiJing that have been monitored include Hydroxysafflor yellow A, Oxypaeoniflorin, Senkyunolide I, and Benzoylpaeoniflorin by ultra-high performance liquid chromatography-quadrupole-orbitrap mass spectrometry (29). There is evidence that XueBiJing may modulate cytokine production, particularly tumor necrosis factor-α and interleukin-6, which are known to be involved in the inflammatory response (30). Liu et al (31) also reported that XueBiJing may markedly improve the survival rate in septic mice model. This result is consistent with the present finding of the effect of XueBiJing on 28-day mortality rates over this short therapy duration.
Our study highlights some important variables influencing efficacy in SCAP trials, especially use of antibiotics, methylprednisolone, and heparin. Our Study, a trial protocol, including indications of concomitant medications, was developed and implemented in all study centers to standardize the procedure. There was no difference in antimicrobial treatment between two groups, except the use of sulfanilamide (Table 2). Furthermore, the use of other antibiotics, the total duration of active antibiotic therapy, methylprednisolone, and heparin were similar between the randomized groups.
This study provides data about the safety of XueBiJing, 100 mL, q12 hours, and shows that the treatment was seldom discontinued because of an AE (judged by the investigator to be related to the study medication). AEs were evenly distributed across the two groups (Table S3, Supplemental Content 2, http://links.lww.com/CCM/E704).
LIMITATIONS
Our study has several limitations. A major limitation was that all the participants were from ICUs and emergency departments in China, which may limit generalizability to other countries. Another limitation was the outcome measurement. Then, what outcomes should be measured in a clinical trial of SCAP? Mortality may be the most robust outcome of a noninferiority trial, but until now, there has been no universal agreement on primary outcome of a superiority study. From the results of a MEDLINE search, there are a variety of primary outcomes in these trials, including the proportion of patients not reaching clinical stability (32), the composite outcome of treatment failure (33), the number of ventilator-free days (34), and the clinical cure and length of hospital stay (35). A literature search of different RCTs in ICUs reported that relatively few trials (10/72) using mortality as a primary outcome showed a beneficial effect of the intervention on the survival of critically ill patients. Although numerous studies and systematic review suggest that XueBiJing reduce mortality of patients (13, 36). In this superiority trial, the PSI has been carefully selected as a primary outcome. It was derived and validated with data on patients with CAP by use of well-accepted methodological standards and is the best prediction aid that has been empirically shown the better discriminatory power than another commonly used predictors of outcome from pneumonia-the CURB-65 (confusion, uremia, respiratory rate, BP, age ≥ 65 years) Score. The PSI predicts mortality using age, gender, concurrent diseases, mental status, vital signs, and laboratory values. Because of its accuracy, methodologic rigor, and effectiveness and safety as a prediction aid, the PSI has become the reference standard for outcome predictor of CAP. We also investigated the reliability of the PSI as an outcome measure by examining clinical outcomes in patients with high-risk PSI scores (PSI IV/V) (Fig. S1, Supplemental Content 2, http://links.lww.com/CCM/E704). To demonstrate some clinical improvements of SCAP with XueBiJing, we incorporated several established outcomes as secondary outcome measures for improvement into our study design. Finally, the trial excluded patients over 75 years old.
CONCLUSIONS
In conclusion, the addition of XueBiJing administration to standard, guideline-adherent treatment for SCAP led to a statistically significant improvement in the primary outcome of improvement in the PSI score. It also led to a statistically significant in the clinically relevant secondary endpoints of mortality, duration of mechanical ventilation and duration of ICU stay. XueBiJing may be a new and beneficial treatment candidate that can widen the therapeutic options for treating SCAP.
ACKNOWLEDGMENTS
We thank Eric Seeley (Department of Pulmonary and Critical Care Medicine, University of California, San Francisco, CA) for his help with article revision. We wish to gratefully acknowledge all of the study centers and study participants who have devoted their time and effort to this trial. We would like to acknowledge the Data and Safety Monitoring Board members. We also thank Tianjin University of Traditional Chinese Medicine, Institute of Clinical Evaluation for data management and quality control, and Peking University Clinical Research Institute for the statistical analysis.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by a Tianjin Science and Technology committee grant (14ZXLJSY00230) and National Natural Science Foundation of China (81630001,81490533).
Drs. X. Yu and Zhi Liu disclosed work for hire. Dr. B. Zhang disclosed government work. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Clinical Trial Registration: http://www.chictr.org.cn/index.aspx. Unique identifier: ChiCTR-TRC-13003534.
REFERENCES
- 1.Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team: Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis 2000; 31:347–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team: Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers JD. Severity assessment tools to guide ICU admission in community-acquired pneumonia: Systematic review and meta-analysis. Intensive Care Med 2011; 37:1409. [DOI] [PubMed] [Google Scholar]
- 5.Ryan D, Connolly R, Fennell J, et al. Aetiology of community-acquired pneumonia in the ICU setting and its effect on mortality, length of mechanical ventilation and length of ICU stay: A 1-year retrospective review. Crit Care 2014; 18:P64 [Google Scholar]
- 6.Horita N, Otsuka T, Haranaga S, et al. Adjunctive systemic corticosteroids for hospitalized community-acquired pneumonia: Systematic review and meta-analysis 2015 update. Sci Rep 2015; 5:14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers JD. Identifying severe community-acquired pneumonia: Moving beyond mortality. Thorax 2015; 70:515–516 [DOI] [PubMed] [Google Scholar]
- 8.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–S72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran GJ, Rothman RE, Volturo GA. Emergency management of community-acquired bacterial pneumonia: What is new since the 2007 Infectious Diseases Society of America/American Thoracic Society guidelines. Am J Emerg Med 2013; 31:602–612 [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Giesler DL, Gellad WF, et al. Antibiotic therapy for adults hospitalized with community-acquired pneumonia: A systematic review. JAMA 2016; 315:593–602 [DOI] [PubMed] [Google Scholar]
- 11.Qi F, Liang ZX, She DY, et al. A clinical study on the effects and mechanism of xuebijing injection in severe pneumonia patients. J Tradit Chin Med 2011; 31:46–49 [DOI] [PubMed] [Google Scholar]
- 12.Fang K, Wang XL. [Treatment of multiple organ dysfunction syndrome by Xuebijing Injection: A clinical research]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013; 33:205–207 [PubMed] [Google Scholar]
- 13.Li C, Wang P, Zhang L, et al. Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: A meta-analysis of randomized controlled trials. J Ethnopharmacol 2018; 224:512–521 [DOI] [PubMed] [Google Scholar]
- 14.Jiang M, Zhou M, Han Y, et al. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol 2013; 147:426–433 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Tian X, Cui M, et al. Safflower yellow inhibits angiotensin II-induced adventitial fibroblast proliferation and migration. J Pharmacol Sci 2014; 126:107. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Wu X, Tong X, et al. Xuebijing ameliorates sepsis-induced lung injury by downregulating HMGB1 and RAGE expressions in mice. Evid Based Complement Alternat Med 2015; 2015:860259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu MJ, Zhang G, Hu MH, et al. Stasis-resolving and detoxifying effect of Xuebijing injection on severe pneumonia: A systematic review. Chin J Evid-Based Med 2014; 14:462–468 [Google Scholar]
- 18.Ping W, Song Y, Zhi L, et al. Xuebijing injection in the treatment of severe pneumonia: Study protocol for a randomized controlled trial. Trials 2016; 17:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Clin Microbiol Infect 2011; 17:1–24 [DOI] [PubMed] [Google Scholar]
- 20.Bassetti M, Montero JG, Paiva JA. When antibiotic treatment fails. Intensive Care Med 2018; 44:73–75 [DOI] [PubMed] [Google Scholar]
- 21.Zilberberg MD, Shorr AF, Micek ST, et al. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit Care 2014; 18:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micek ST, Lang A, Fuller BM, et al. Clinical implications for patients treated inappropriately for community-acquired pneumonia in the emergency department. BMC Infect Dis 2014; 14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–250 [DOI] [PubMed] [Google Scholar]
- 24.Menéndez R, Torres A, Zalacaín R, et al. Risk factors of treatment failure in community acquired pneumonia: Implications for disease outcome. Thorax 2004; 59:960–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections - summary. Clin Microbiol Infect 2011; 17:1–24 [DOI] [PubMed] [Google Scholar]
- 26.Julián A. Improved management of community-acquired pneumonia in the emergency department. Arch Bronconeumol 2013; 49:230–240 [DOI] [PubMed] [Google Scholar]
- 27.Restrepo MI, Mortensen EM, Velez JA, et al. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest 2008; 133:610–617 [DOI] [PubMed] [Google Scholar]
- 28.Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med 2015; 163:519–528 [DOI] [PubMed] [Google Scholar]
- 29.Zuo L, Sun Z, Hu Y, et al. Rapid determination of 30 bioactive constituents in XueBiJing injection using ultra high performance liquid chromatography-high resolution hybrid quadrupole-orbitrap mass spectrometry coupled with principal component analysis. J Pharm Biomed Anal 2017; 137:220–228 [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Feng Y, Shen X, et al. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol 2018; 211:358–365 [DOI] [PubMed] [Google Scholar]
- 31.Liu YC, Yao FH, Chai YF, et al. Xuebijing injection promotes M2 polarization of macrophages and improves survival rate in septic mice. Evid Based Complement Alternat Med 2015; 2015:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garin N, Genné D, Carballo S, et al. β-Lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: A randomized noninferiority trial. JAMA Intern Med 2014; 174:1894. [DOI] [PubMed] [Google Scholar]
- 33.Ceccato A, Cilloniz C, Ranzani OT, et al. Treatment with macrolides and glucocorticosteroids in severe community-acquired pneumonia: A post-hoc exploratory analysis of a randomized controlled trial. PLoS One 2017; 12:e0178022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welte T, Dellinger RP, Ebelt H, et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: A randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med 2018; 44:438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oosterheert JJ, Bonten MJ, Schneider MM, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: Multicentre randomised trial. BMJ 2006; 333:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G, Gao Y, Jiang Y, et al. Efficacy and safety of Xuebijing injection combined with ulinastatin as adjunctive therapy on sepsis: A systematic review and meta-analysis. Front Pharmacol 2018; 9:743. [DOI] [PMC free article] [PubMed] [Google Scholar]


