Supplemental Digital Content is available in the text.
Keywords: CTLA-4, immune checkpoint inhibitors, immune response, melanoma, PD-1, PD-L1, predictive biomarkers
Abstract
Immune checkpoint inhibitors (ICIs), which target CTLA-4 or PD-(L)1 molecules, have shown impressive therapeutic results. Durable responses, however, are only observed in a segment of the patient population and must be offset against severe off-target immune toxicity and high costs. This calls for biomarkers that predict response during ICI treatment. Although many candidate biomarkers exist, as yet, there has been no systematic overview of biomarkers predictive during. Here, we provide a systematic review of the current literature of ICI treatment to establish an overview of candidate predictive biomarkers during ICI treatment in melanoma patients. We performed a systematic Medline search (2000–2018, 1 January) on biomarkers for survival or response to ICI treatment in melanoma patients. We retrieved 735 publications, of which 79 were finally included in this systematic review. Blood markers were largely studied for CTLA-4 ICI, whereas tumor tissue markers were analyzed for PD-(L)1 ICI. Blood cytology and soluble factors were more frequently correlated to overall survival (OS) than response, indicating their prognostic rather than predictive nature. An increase in tumor-infiltrating CD8 + T-cells and a decrease in regulatory T-cells were correlated to response, in addition to mutational load, neoantigen load, and immune-related gene expression. Immune-related adverse events were also associated frequently with a favorable response and OS. This review shows the great variety of potential biomarkers published to date, in an attempt to better understand response to ICI therapy; it also highlights the candidate markers for future research. The most promising biomarkers for response to ICI treatment are the occurrence of immune-related adverse events (especially vitiligo), lowering of lactate dehydrogenase, and increase in activated CD8 + and decrease in regulatory T-cells.
Introduction
Immune checkpoint inhibitors (ICIs) represent a major breakthrough in the treatment of metastatic melanoma. They target CTLA-4 or PD-(L)1 molecules on T-cells, which results in prolonged T-cell activation responses. Impressive long-term survival has been observed in advanced melanoma patients upon treatment with ICIs [1]. Unfortunately, the response rates remain low to moderate. Fewer than 20% of advanced melanoma patients experience a long-term response in ipilimumab. The response to PD-(L)1 ICIs is higher (30–40%) and improves to 60% with combination therapy [2]. Still, this means that no durable responses are observed in 40–60% of patients.
ICIs are high-cost therapies; ipilimumab, for example, costs 120 000 euros per patient/year, and yet yield a 40–60% response rate. ICI treatment can also bring with it severe and potentially life-threatening side-effects, such as diarrhea, enterocolitis, hepatitis, hypophysitis, skin rash, and pruritus. These immune-related adverse events (irAEs) were observed in up to 80% of patients in clinical trials of ipilimumab [3].
The reasons underscoring the clinical need for biomarkers that predict the treatment response of ICIs in metastatic melanoma are three-fold: (a) such biomarkers enable personalized treatment with ICI, by selecting patients who are likely to benefit from ICI, and eliminating delay in treatment for others by immediately starting secondary therapies. (b) Their identification and application decreases potentially severe adverse effects without clinical benefit. (c) Their use saves costs by providing therapy only to patients who are likely to respond.
Recently, we performed a systematic review of predictive baseline biomarkers for response to immunotherapy in melanoma patients [4], here we found a large number of different biomarkers measured or measured differently. No single biomarker measured before or at the start of therapy, however, is capable of accurately predicting response. Biomarkers measured during therapy have also been the subject of publication. These do provide the possibility to predict therapy response at an early phase during treatment, and may facilitate decisions on treatment continuation or prolongation.
The aim of this paper is to systematically review the current literature dealing with the clinical data of ICI treatment to provide an overview of candidate predictive biomarkers – measured during therapy – for ICI response in melanoma patients. Our study used the PICO tool, as recommended by the Cochrane Collaboration: (P) advanced melanoma patients treated with ICIs, (I) that measured biomarkers during treatment, (C) and identified differences in biomarker levels, (O) comparing therapy responsiveness: comparing response to therapy (measured in different outcomes).
Methods
Study design and search strategy
In this systematic review, work was carried out in accordance with the PRISMA statement: an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses [5,6].
We performed a systematic OVID Medline search of publications in English appearing in the period 1 January 2000 to 1 January 2018, to identify those dealing with biomarkers predicting clinical response to ICI treatment of human melanoma. We checked the availability of systematic reviews on this topic, but only included original articles in our own review. Original articles were found by safely excluding reviews and editorials by means of double negation (i.e. excluding ‘reviews’ as publication type, except when terms indicating observational studies or trials were present; the same approach was used to safely exclude animal studies). To find both known and unknown biomarkers, the concepts of melanoma and ICI were combined with either (I) general terms for biomarkers or predictive factors or (II) known specific factors combined with terms for prognosis, correlation, predictors and terms for survival, mortality, and clinical response (see Supplementary Additional File 1, Supplemental digital content 1, http://links.lww.com/MR/A127, for the entire Medline search strategy). We cross-checked the reference lists and studies cited through Web of Science for relevant papers, and review studies and adapted the search in case of additional relevant studies. The bibliographic records retrieved were imported and de-duplicated in ENDNOTE.
Participants, interventions, and comparators
Multiple study designs were considered for this review – including clinical trials (phase I, II, and III), prospective or retrospective cohort studies and case series, including more than five patients – that reported on clinical response and/or survival outcomes to ICI therapy. Case reports and other types of publications, including reviews, viewpoints, or conference reports, were excluded. All original research publications in English were included. The patient population of this review comprised AJCC stage 3 or 4 melanoma patients who are eligible to receive ICI therapy. Inclusion eligibility required publications to report on melanoma patients treated with either anti-CTLA-4 antibodies (ipilimumab, tremelimumab, and ticilimumab) or PD-(L)1 inhibitors (nivolumab, pembrolizumab, atezolizumab, avelumab, and durvalumab). Further eligibility required biomarker analysis during treatment. No restrictions were set on the type of outcome parameter for response or survival. No restrictions in age, sex, or ethnicity were applied. Publications were excluded when reporting on (a) nonmelanoma patients, (b) exclusively biomarkers at baseline, (c) animal studies, (d) combination therapy of ICI with other melanoma drugs (except peptide vaccinations), and (e) if they reported results from fewer than five patients.
Systematic review protocol and data extraction
The selection of publications and data extraction was performed in an unblinded standardized manner by two independent reviewers. Any disagreement between reviewers was resolved by consensus. The following information was extracted from the publications selected: study type, type of ICI therapy, number of patients included in the publication, number of patients included in the biomarker analysis, type of predictive biomarker, time of follow-up, clinical outcome measure, and statistical significance. The outcome parameters of the studies were divided into three groups: (a) clinical response, (b) progression-free survival (PFS), and (c) overall survival (OS). PFS also included disease-free interval, response duration, relapse-free survival, and recurrence-free survival. Clinical response also comprised tumor response, the best overall response rate, immune-related response criteria, and clinical benefit.
Data analysis
This review was carried out to explore and summarize published candidate biomarkers. Unfortunately, statistical meta-analysis was not feasible because of variability in the outcome measures, the timespan between biomarker measurements, and the limited number of studies per biomarker. To provide preliminary insights, therefore, the predictive capacity of biomarkers was extracted and summarized by P value significance. We defined P value significance as less than 0.05, including correction for multiple testing when applicable.
Quality assessment
A risk-of-bias analysis was carried out on all publications. This analysis was based on the Cochrane Collaboration quality checklist for prognostic studies [7], consisting of the following five questions: (a) Are the patients adequately described and are the reasons for any restrictions appropriate? (b) Are assessments of the studied biomarkers properly specified and are they valid and reliable? (c) Are the follow-up data clearly described? (d) Are there sufficient data present on biomarkers in the study population? (e) Are the study parameters (outcome, phase of study) properly addressed and explained? Answers to these questions were in the form of ‘yes’, ‘no’, or ‘questionable’.
Those publications with at least four times ‘yes’ answers in questions 1–5 were considered to have a low risk of bias. Publications scoring 1 ‘questionable’ on either question 4 or question 5, or 2 ‘questionables’ in questions 1–5, were considered to have an intermediate risk of bias. High risk of bias was assigned to publications scoring 2 ‘questionables’ in questions 4 and 5 or any ‘no’. Publications describing the analyses of multiple biomarker studies were assessed separately for the quality of the analysis of each biomarker.
Results
Study selection and characteristics
The systematic Medline search retrieved 735 unique publications (Fig. 1). Reference checking did not yield additional publications. On the basis of the eligibility criteria of title and abstract screening, 571 publications were excluded and 164 publications were screened full text, of which 79 publications fulfilled our selection criteria and were included in this review (Supplementary Additional File 2, Supplemental digital content 2, http://links.lww.com/MR/A128) [8–85]. The publication selection process and the reasons for exclusion are presented in Fig. 1. In total, the 79 publications thus included reported on 57 different types of biomarkers. Of these 79 publications, 43 reported on more than one biomarker, resulting in 218 biomarker studies. Most biomarker studies were based on CTLA-4 ICI therapy (n = 148), whereas 65 studies were carried out for PD-(L)1 ICI therapy biomarkers. A total of five studies were carried out in patients who were treated with either CTLA-4 or PD-(L)1 ICI. Biomarkers were organized into four groups: (a) blood markers, (b) tumor tissue, (c) irAEsk and (d) other (Fig. 2). The blood-based biomarker group included studies on general cytology markers, general soluble factors, immune-related soluble factors, cellular markers of T-cell activation and regulation, and systemic tumor-specific immune responses. These biomarkers were reported in 127 studies relating to CTLA-4 and in 19 studies relating to PD-(L) therapy. The second group, focusing on tumor tissue-based markers such as tumor-infiltrating cells, changes in expression profiles, and genetic alterations, included nine studies for CTLA-4 and 37 for PD-(L). This indicates a predominant interest in these markers for PD-(L)1 ICI. The third group comprised markers based on irAEs included in 13 studies for CTLA-4 ICI and 13 for PD-(L)1 ICI. The final group, consisting of ‘other’ markers, included four studies for CTLA-4 and 1 for PD-(L)1. The median number of patients included in the biomarker studies varied from 101 patients for irAEs to 13 patients in tumor tissue studies (Supplementary Additional File 3, Supplemental digital content 2, http://links.lww.com/MR/A129).
Fig. 1.
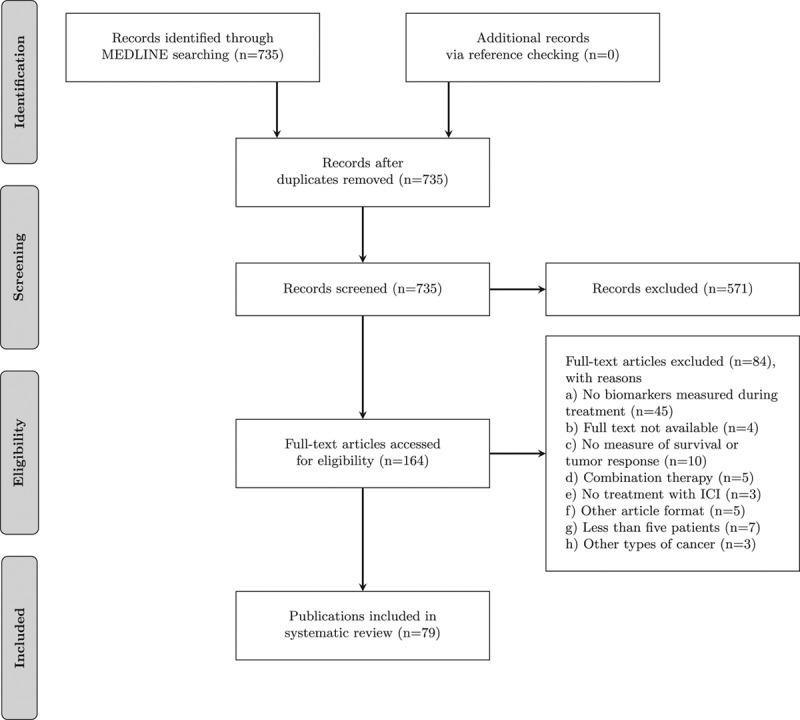
Flowchart of the publications included and reasons for exclusion. ICI, immune checkpoint inhibitor.
Fig. 2.
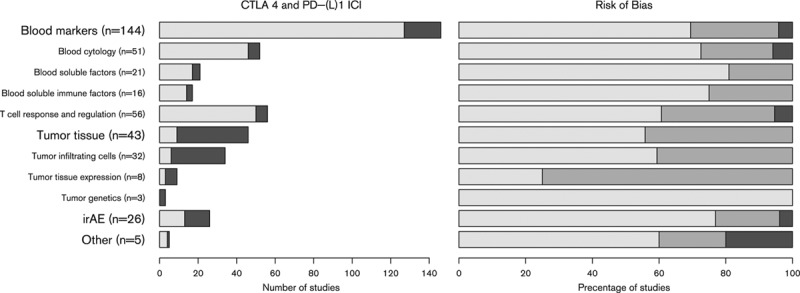
Number of studies per biomarker group for both CTLA-4 (light) and PD-(L)1 (dark) immune checkpoint inhibitor (ICI)-treated patients (left figure) and risk of bias estimation for each biomarker group (right figure). The risk of bias figure shows the percentage of publications per biomarker type for both CTLA-4 and PD-1 immune checkpoint inhibitor with a low (light), intermediate, or high (dark) risk of bias. irAE, immune-related adverse event.
Risk of bias
The risk of bias was assessed for each biomarker type including both CTLA-4 and PD-(L)1 ICI studies (Fig. 2). The assessment indicated a low risk of bias in 67% of all studies, intermediate risk in 29%, and high risk of bias in 4% of publications (Supplementary Additional File 4, Supplemental digital content 4, http://links.lww.com/MR/A130). Studies describing other biomarkers (group 4) contained the highest risk of bias. Within blood biomarker publications, the highest risk of bias was estimated in the T-cell response and regulation subgroup, whereas the lowest risk of bias was found in the blood soluble immune factors subgroup. The studies of tumor tissue biomarker had a higher risk of bias in the tumor tissue-expression subgroup and low risk in tumor genetics (Fig. 2). The risk of bias was largely because of unclear descriptions of patient follow-up data.
Blood biomarkers
The blood cytology biomarkers studies included changes in absolute counts of white blood cells, lymphocytes, granulocytes (eosinophils or neutrophils), monocytes, myeloid-derived suppressor cells, natural killer cells, circulating tumor cells and CD20 + B cells, as well as neutrophils and platelets to lymphocytes ratios (Fig. 3).
Fig. 3.
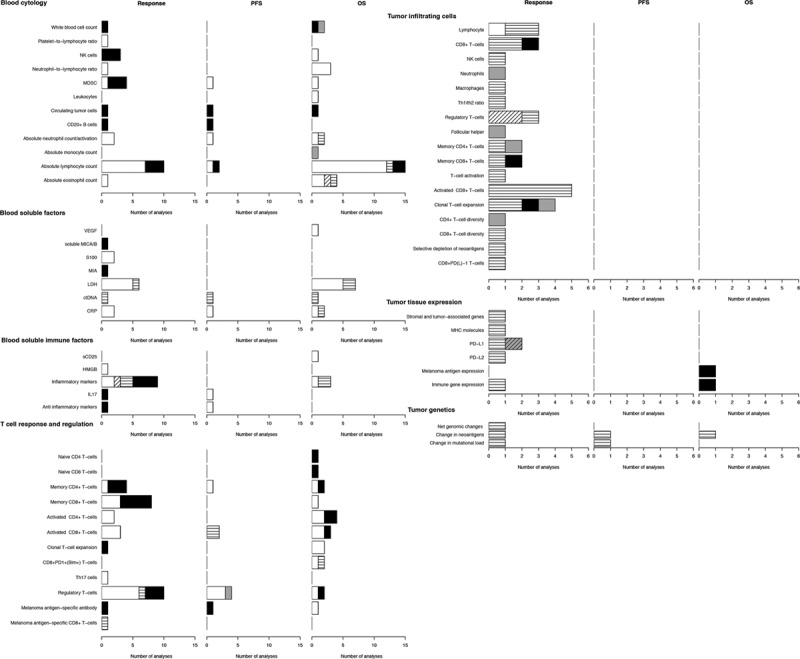
Blood and tumor tissue biomarker analyzed for immune checkpoint inhibitor (ICI) treatment. Graphs show the number of analyses per biomarker type for correlations with clinical response, progression-free survival (PFS), or overall survival (OS) upon ICI therapy. White bars, significant CTLA-4 correlation; black bars, non-significant CTLA-4 correlation; horizontal lines in white bars, significant PD-1 correlation; gray bars, non-significant PD-1 correlation; white bar with diagonal lines, significant both CTLA-4 and PD-1 correlation.
These blood biomarkers were most frequently used to estimate relations with clinical response or OS in the event of CTLA-4 ICI therapy. Significant correlations with response were found in approximately half of the analyses, whereas significant correlations with OS were found in 80% of analyses. The absolute lymphocyte count was most frequently studied and found more frequently to be correlated significantly with OS as opposed to response. Only five analyses were carried out for PD-1, in two publications, including one study of both patient groups treated with CTLA-4 or PD-(L)1 [50,52].
Soluble blood factors studied included vascular endothelial growth factor, major histocompatibility complex class I chain-related protein A and protein B (MICA/B), S100, MIA, lactate dehydrogenase (LDH), circulating tumor DNA, and C-reactive protein (Fig. 3). LDH was studied most frequently, showing not only low levels of LDH at any time during treatment, but also the reduction of LDH levels to be associated with better response and prolonged OS.
Those immune-related soluble factors measured in the blood (Fig. 3) included CD25, HMGB1, multiple inflammatory cytokines [including interleukin (IL)-2, IL-4, IL-6, IL-8, IL-15, INFγ, IP-10, tumor necrosis factor-α], IL-17, and anti-inflammatory cytokines IL-10 and transforming growth factor-β1. Inflammatory cytokines correlated significantly with OS, but their associations with response were only significant in half the analyses. Other markers were measured only once (CD25 and HMBG1 [31,35]) or twice and were significant in half the analyses (IL-17, IL-10, and transforming growth factor-β1) [12,72].
Biomarkers for representing systemic T-cell activation and regulation were studied widely in 56 analyses. These markers consisted of naive-, memory activated-, CD4 + and CD8 + T-cells, as well as T-cell expansion, CD8 + PD-1 + (bim + ) T-cells, Th17 cells, and regulatory T-cells (Fig. 3). Naive T-cells (both CD4 + and CD8 + ) did not correlate to OS and were not analyzed for correlations with response and PFS. In only half the studies did memory T-cells correlate with outcome in any form. Activated T-cells, either CD4 + or CD8 +, correlated with outcome in 2 and 3 analyses, respectively. T-cell regulation was studied quite extensively in up to 10 analyses. The presence of regulatory T-cells was associated negatively with response, PFS, and OS in most studies (1–2 analyses failed to show significant associations). Increased circulating regulatory T-cells showed a beneficiary relation with respect to PFS [71]. CD8 + PD-1 + (bim + ) was the only T-cell-related biomarker associated significantly with OS in more than one study without a nonsignificant association in other studies [26,70]. Four biomarkers (Th17, naive CD4 +, naive CD8 + and melanoma antigen-specific CD8 +, T-cells) were only analyzed once [15,19,66].
The increase in melanoma antigen-specific antibodies (against NY-ESO-1) showed a correlation with OS, but not with PFS or response. In contrast, an increase in MART-1 melanoma antigen-specific CD8 + T-cells was associated with response, but was not analyzed in PFS and OS.
Tumor tissue biomarkers
Predictive biomarkers on the basis of changes in tumor-intrinsic factors or tumor microenvironment were mostly studied for PD-(L)1 ICI and analyzed for response to treatment (Fig. 3). Only seven biomarker studies were carried out for CTLA-4 ICI [34,38,41,59,61], of which three were in a combined setting with PD-(L)1 [41].
Tumor-infiltrating cell analyses reported the number of infiltrating cells, comprising lymphocytes, CD8 + T-cells, natural killer cells, neutrophils, and macrophages, all showing significant correlations to response, except for neutrophils and, in one out of three analyses, CD8 + T-cells. Most analyses on immune regulatory elements infiltrating the tumor environment, including the change in the Th1/Th2 ratio and regulatory T-cells, were associated significantly with response (four out of five), whereas follicular helper cells were not associated with response. Response correlations with CD4 + and CD8 + memory T-cells were significant in one study, but not in another. Activated T-cells were most frequently analyzed (n = 6), of which five analyses focused on activated CD8 + T-cells. All these analyses showed a significant response to treatment. Clonal T-cell expansion and (CD4 + and CD8 + ) diversity showed a significant correlation with response in half of the analyses. Depletion, or loss, of neoantigens during therapy and CD8 + PD(L)-1 T-cells were analyzed only once, but correlated significantly with response.
Changes in tumor tissue-expression during ICI therapy were also investigated as biomarkers for response and survival (Fig. 3). Increased expressions of the major histocompatibility complex molecule HLA class I and PD-L2 were associated significantly with response, but only analyzed once. PD-L1 and immune gene expression were analyzed twice, but were significant in only one analysis. Decrease in melanoma antigen expression was not significant for response.
Changes in genetics (net genomic changes, and changes in neoantigens and mutational load) were investigated six times as biomarkers for response (n = 3), PFS (n = 2), and OS (n = 1) (Fig. 3), and were all associated significantly with these outcomes.
Immune-related adverse event
Most studies did not specify the irAE (16 studies); neither included irAEs of any grade (n = 10) nor grade 3/4 (n = 6) (Fig. 4). Of all these studies, 69% were associated significantly with outcome in response, PFS, and OS. Colitis, the major irAE for ICI, was analyzed twice and was not associated significantly with response and OS. Other irAEs that did not show a significant relation with OS were pneumonitis, thyroid disorders, myalgias, and mucosal toxicity. The two irAEs that did show a significant relation with outcome (response, PFS, and OS) were rash and vitiligo. Of these, vitiligo was the irAE that was analyzed the most (rash = 4 and vitiligo = 10).
Fig. 4.
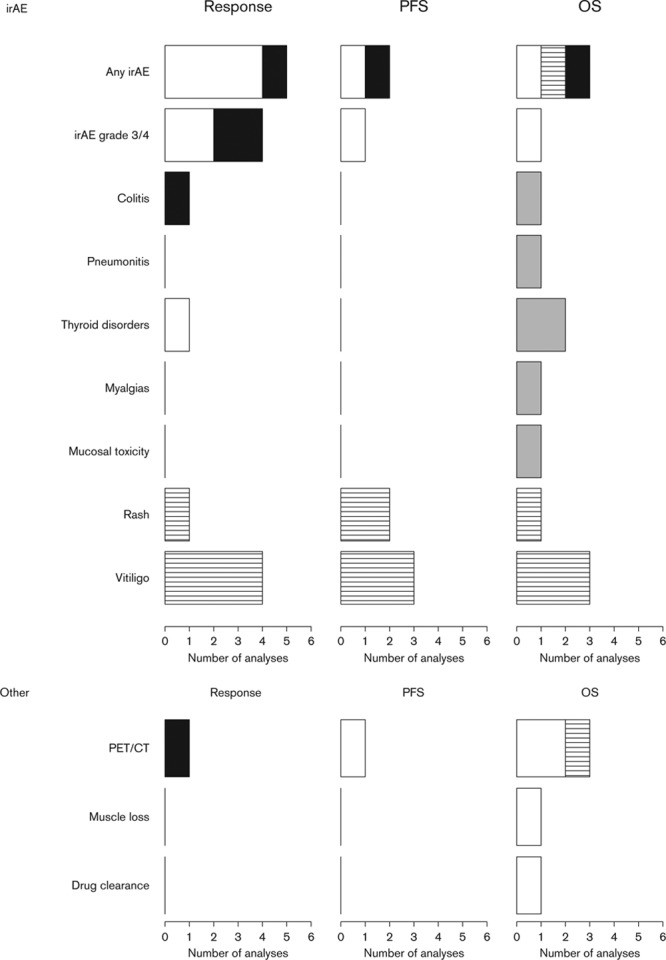
Immune-related adverse events and other biomarkers analyzed for immune checkpoint inhibitor (ICI) treatment. Graphs show the number of analyses per biomarker type for correlations with clinical response, progression-free survival (PFS), or overall survival (OS) upon ICI therapy. White bars, significant CTLA-4 correlation; black bars, non-significant CTLA-4 correlation; horizontal lines in white bars, significant PD-1 correlation; gray bars, non-significant PD-1 correlation; white bar with diagonal lines, significant both CTLA-4 and PD-1 correlation; and gray bars with diagonal lines, non-significant for both CTLA-4 and PD-1 correlation. CT, computed tomography; irAE, immune-related adverse event.
Other
Other predictive biomarkers studied were (fluorine-18-fluorodeoxyglucose) PET/computed tomography (CT), clearance of tremelimumab, and the loss of muscle mass (Fig. 4). All these markers could distinguish patients with good and poor OS at an early stage of treatment. The loss of muscle mass was unfavorable for OS [18]. PET/CT biomarkers were studied most (n = 3), although these studies were limited in the number of patients included. PET-CT did not correlate with response.
Discussion
This review provides a systematic overview of candidate biomarkers, measured during ICI treatment, that might prove useful early in therapy as predictors for response to such treatment.
Biomarkers were divided into four groups (blood markers, tumor tissue, irAEs, and others). Blood markers were studied most frequently, predominantly in patients treated with CTLA-4 ICI, whereas tumor-tissue biomarkers were mostly studied for PD-(L)1 ICI. Outcome analyses focused predominantly on response and OS, whereas PFS correlations were reported less frequently.
Blood biomarkers studies showed considerable variety in the types of cells or soluble factors studied, as well as in the significance of correlations with outcome parameters; this show similarity to our previous findings for baseline biomarkers analyses [4]. CD20 + B cell levels, platelet to lymphocytes counts, were not reported previously as baseline biomarkers. In general, changes in blood cytology biomarkers during ICI therapy show similar correlations with outcome, as baseline values. One exception is the absolute lymphocyte count during ICI therapy, which correlated more frequently with response or OS, than baseline absolute lymphocyte counts [4]. The immune system is able to aid in the eradication of tumors. Yet, inflammation may also be associated with disease progression and adverse outcomes, considered to be because of the inflammatory production of bioactive molecules in the tumor microenvironment [86]. The increase in the absolute lymphocyte count and other immune-related cells may well predict benefit in ICI, but may also fail to account for immune-suppressive versus stimulatory interaction.
Multiple soluble (immune) markers were investigated as potential biomarkers, of which MIA and HMGB were not analyzed previously as baseline biomarkers [4]. These factors did not always show significant correlations to response or OS. The presence of IL-17, an important cytokine in inflammatory bowel disease [87], was shown to be present at higher levels in patients who developed colitis during ICI therapy [72]. However, IL-17 was only related to PFS, but not to response. Conflicting data on the correlation with response were also found for other (anti-)inflammatory markers. This might suggest that the measured soluble (immune) blood factors are more indicative for tumor burden and not directly indicative for response to therapy [4,88].
Biomarker analyses of all stages of T-cell responses from naive, activation, differentiation, to memory formation mediating adaptive-immunity are present in this review. Of these stages, the activation and regulation of (CD8 + ) T-cells were correlated most frequently with outcome. This review shows that these markers individually are not always predictive for response. However, the combination of regulatory T-cell attenuation (e.g. by reducing regulatory T-cell numbers or reducing their suppressive activity in tumor tissues) and the activation of tumor-specific effector T-cells (e.g. by immune checkpoint blockades) may synergize to enhance treatment effects [89,90].
Blood biomarkers that were associated significantly with OS, but not with response to treatment, most likely have a prognostic rather than predictive value, independent of treatment (e.g. most inflammatory markers). Some differences, however, might also be explained by the time to response. Responses, particularly to ipilimumab therapy, may be delayed, so that progression may occur before a benefit is observed [91]. Elevated LDH is known to be prognostic of poor survival in patients with metastatic melanoma [4,42,92]. In our review, however, reduction in LDH during therapy correlated significantly with a favorable response. It seems that even when LDH is elevated at the start of treatment, a reduction in LDH is still favorable for response to therapy. In contrast, baseline LDH only correlated with OS, but not to response [4], suggesting that changes in LDH during therapy are more informative for treatment response than baseline LDH. Both LDH and S100(B) proteins are released by melanoma following tumor cell death [93,94], albeit by different biological mechanisms: S100B interacts with p53 and plays an active role in cell survival and proliferation [95], whereas LDH plays an active role in the mitochondrial metabolism and glycolysis in skin cancer (Warburg effect). LDH converts pyruvate, the final product of glycolysis, into lactate in the absence of oxygen. High concentrations of lactate, in turn, negatively regulate LDH [96,97]. Other blood soluble factors such as CRP reflect disease progression, similar to increasing LDH levels [66].
Most tumor tissue biomarkers were studied for associations with response to treatment rather than PFS or OS. Tumor-infiltrating cells were analyzed in relation to response only. PFS and OS are time-consuming outcome parameters, whereas response to treatment can be measured within a short period of time. Tumor-tissue biomarkers were predominantly analyzed during PD-1 therapy, which may reflect the increasing clinical application of PD-1 compared with CTLA-4 therapy. Moreover, tumor-tissue biomarkers may be more relevant during PD-1 therapy as PD-1 inhibition is considered to act mostly peripherally in the tumor tissue, in contrast to CTLA-4 inhibition acting centrally during T-cell priming. Activated tumor-infiltrating T-cell levels during therapy were associated significantly with response to PD-1 ICI. This corresponds to the frequent associations of activated intratumoral T-cells at baseline with response, as summarized in our previous review [4].
Response correlations of tumor-infiltrating cells during ICI therapy were analyzed for various cellular phenotypes. Ten out of the 17 analyses, however, were carried out in one, single study. Measurement of changes in tumor tissue biomarkers requires a tumor biopsy both before and after treatment, preferably of the same tumor lesion, which limits its practical feasibility as a biomarker. For example, in our previous systematic review, response correlations of baseline PD-L1 expression in the tumor tissue were measured in eight analyses [4], in comparison with only two analyses during therapy in our current systematic review, one of which was performed in both PD-(L)1-treated and CTLA-4 treated patients [39,41].
Just like the blood cytology and T-cell response and regulation markers, tumor-infiltrating cells by themselves are not able to fully predict response to therapy. These markers might, however, have predictive value when analyzed in combination as the balance between tumor-specific effector T-cell activation and tumor-immune suppression, for example, by regulatory T-cells, determines the outcome of antitumor immunity.
Changes in tumor genetics during therapy are of interest as biomarkers on the basis of the observation that a high number of mutations in the tumor are beneficial for response to ICI therapy [98,99]. Yet, these correlations found at the population level might not reach sufficient predictive power for individual patients. Determination of the mutational load and potential neoantigens recognized by tumor-reactive T-cells requires the analysis of a tumor tissue sample before therapy and possibly during therapy as well, which is costly and labor-intensive. Most neoantigens are probably patient-specific and not recurrent, although some mutations may be more frequent than others: frameshift insertions and deletions, for example, were found to be associated with ICI response for melanoma [100].
IrAEs are side effects because of activation of the immune system and may be signs of successful immune checkpoint blockade. In 66% of the analyses, irAEs were a sign that treatment was effective, indicating that having an irAE is no guarantee for treatment benefit. Rash and vitiligo, however, correlated significantly with favorable response to therapy in all studies.
Other markers correlating significantly with OS – change in body composition by the loss of skeletal muscle during treatment and the clearance rate of tremelimumab – may mostly reflect the disease status and physical condition of the patient [18].
PET/CT during ICI therapy was measured only in studies with a small number of patients. These studies also reported response patterns in patients corresponding to the potential mechanism of pseudo-progression, in which apparent tumor growth detected by conventional CT scans may actually reflect an increased density of activated inflammatory cells within the tumor microenvironment [91,101,102].
Limitations
The interpretation and summary of biomarkers across studies remained difficult. This was in part because of the diversity in outcomes parameters used; for example, the definition of response to ICI. ICI response can be measured according to tumor response criteria or as (progression-free) survival prolongation. Variations in values determining response and nonresponse patients can highly affect the results, especially because survival is determined by a great variety of confounding factors, other than a single measured biomarker, all independently influencing survival.
The statistics in the biomarker analyses also varied markedly with respect to biomarkers concentrations, assays, temporal points of measurements, the reporting of univariate/multivariate statistics, numbers of patients, or low statistical power. In future research, meta-analysis of biomarker results will require a well-defined patient population, standardized outcome measures and time points are required to enable meta-analysis of the results of biomarkers studies.
Conclusion
Currently, the research on predictive biomarkers during ICI therapy in melanoma patients is still in a highly exploratory phase. None of the biomarkers during ICI therapy found in this systematic review have been studied extensively. It is therefore difficult to draw conclusions on the feasibility of biomarkers. Nonetheless, a few recommendations for future research can be made. The most promising biomarkers for predicting response to ICI treatment are the occurrence of irAEs (more specifically, the development of vitiligo during treatment) low levels and/or lowering of LDH and an increase in activated (CD8 + ) and decrease in regulatory T-cells. In addition, comprehensive analyses of changes in tumor immune landscape and tumor genetics during ICI therapy hold promise for biomarker discovery. As there is no single marker that can address all paths involved in immunity, a multivariate approach incorporating all facets of the cancer immunity cycle might have merit in predicting ICI therapy outcome.
Acknowledgements
This research was supported by a Postdoc Stipend of the Amsterdam Infection and Immunity Institute.
J.L. constructed the search strategy, implemented by W.O., S.N., and M.B. independently performed the screening procedure, data extraction, and summarized the data under the supervision of W.O., S.N., and M.B. contributed equally. W.O. and R.L. checked the data analysis and validity and composed the figures. W.O. and R.L. wrote the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Mirjam van den Berg and Sanne van der Niet contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.melanomaresearch.com.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma N Engl J Med. 2010; 363:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma N Engl J Med. 2015; 372:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel SP, Woodman SE. Profile of ipilimumab and its role in the treatment of metastatic melanoma Drug Des Devel Ther. 2011; 5:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessurun CAC, Vos JAM, Limpens J, Luiten RM. Biomarkers for response of melanoma patients to immune checkpoint inhibitors: a systematic review Front Oncol. 2017; 7:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement Ann Intern Med. 2009; 151:264–269 [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration J Clin Epidemiol. 2009; 62:e1–e34 [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Green SE; Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available at handbook.cochrane.org
- 8.Alexander M, Mellor JD, McArthur G, Kee D. Ipilimumab in pretreated patients with unresectable or metastatic cutaneous, uveal and mucosal melanoma Med J Aust. 2014; 201:49–53 [DOI] [PubMed] [Google Scholar]
- 9.Arenberger P, Fialova A, Gkalpakiotis S, Pavlikova A, Puzanov I, Arenbergerova M. Melanoma antigens are biomarkers for ipilimumab response J Eur Acad Dermatol Venereol. 2017; 31:252–259 [DOI] [PubMed] [Google Scholar]
- 10.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4 J Clin Oncol. 2005; 23:6043–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4 J Clin Oncol. 2006; 24:2283–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab Oncoimmunology. 2016; 5:e1100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy AJR Am J Roentgenol. 2011; 197:W992–W1000 [DOI] [PubMed] [Google Scholar]
- 14.Cassidy MR, Wolchok RE, Zheng J, Panageas KS, Wolchok JD, Coit D, et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment EBioMedicine. 2017; 18:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients Sci Transl Med. 2014; 6:238ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho SY, Lipson EJ, Im HJ, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma J Nucl Med. 2017; 58:1421–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma J Transl Med. 2008; 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly LE, Power DG, O’Reilly Á, Donnellan P, Cushen SJ, O’Sullivan K, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma Br J Cancer. 2017; 116:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Coaña YP, Wolodarski M, Poschke I, Yoshimoto Y, Yang Y, Nyström M, et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma Oncotarget. 2017; 8:21539–21553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for thetreatment of patients with metastatic melanoma: An early increase in lymphocyte and eosinophil countsis associated with improved survival Ann Oncol. 2013; 24:1697–1703 [DOI] [PubMed] [Google Scholar]
- 21.Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme Cancer Immunol Immunother. 2013; 62:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Giacomo AM, Ascierto PA, Queirolo P, Pilla L, Ridolfi R, Santinami M, et al. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study Ann Oncol. 2015; 26:798–803 [DOI] [PubMed] [Google Scholar]
- 23.Di Giacomo AM, Danielli R, Calabrò L, Bertocci E, Nannicini C, Giannarelli D, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy) Cancer Immunol Immunother. 2011; 60:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma Br J Cancer. 2016; 114:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade Clin Cancer Res. 2007; 13:6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dronca RS, Liu X, Harrington SM, Chen L, Cao S, Kottschade LA, et al. T cell Bim levels reflect responses to anti-PD-1 cancer therapy JCI Insight. 2016; 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farolfi A, Ridolfi L, Guidoboni M, Nicoletti SV, Piciucchi S, Valmorri L, et al. Ipilimumab in advanced melanoma: reports of long-lasting responses Melanoma Res. 2012; 22:263–270 [DOI] [PubMed] [Google Scholar]
- 28.Felix J, Cassinat B, Porcher R, Schlageter MH, Maubec E, Pages C, et al. Relevance of serum biomarkers associated with melanoma during follow-up of anti-CTLA-4 immunotherapy Int Immunopharmacol. 2016; 40:466–473 [DOI] [PubMed] [Google Scholar]
- 29.Felix J, Lambert J, Roelens M, Maubec E, Guermouche H, Pages C, et al. Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response Oncoimmunology. 2016; 5:1136045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes Clin Cancer Res. 2016; 22:886–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab Clin Cancer Res. 2015; 21:5453–5459 [DOI] [PubMed] [Google Scholar]
- 32.Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma Clin Cancer Res. 2015; 21:712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guaraldi F, La Selva R, Samà MT, D’Angelo V, Gori D, Fava P, et al. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution J Endocrinol Invest. 2018; 41:549–556 [DOI] [PubMed] [Google Scholar]
- 34.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma J Transl Med. 2011; 9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannani D, Vétizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25 Cell Res. 2015; 25:208–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab JAMA Dermatol. 2016; 152:45–51 [DOI] [PubMed] [Google Scholar]
- 37.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response Nature. 2017; 545:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang RR, Jalil J, Economou JS, Chmielowski B, Koya RC, Mok S, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans Clin Cancer Res. 2011; 17:4101–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue H, Park JH, Kiyotani K, Zewde M, Miyashita A, Jinnin M, et al. Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma Oncoimmunology. 2016; 5:e1204507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab Cancer Immunol Immunother. 2012; 61:1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, et al. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients Mod Pathol. 2017; 30:1666–1676 [DOI] [PubMed] [Google Scholar]
- 42.Kelderman S, Heemskerk B, van Tinteren H, van Den Brom RRH, Hospers GAP, Van Den Eertwegh AJM, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma Cancer Immunol Immunother. 2014; 63:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoja L, Atenafu EG, Templeton A, Qye Y, Chappell MA, Saibil S, et al. The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma Cancer Med. 2016; 5:2792–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ku GY, Yuan J, Page DB, Schroeder SEA, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival Cancer. 2010; 116:1767–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JH, Long GV, Boyd S, Lo S, Menzies AM, Tembe V, et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma Ann Oncol. 2017; 28:1130–1136 [DOI] [PubMed] [Google Scholar]
- 46.Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma J Immunother. 2006; 29:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martens A, Wistuba-Hamprecht K, Yuan J, Postow MA, Wong P, Capone M, et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab Clin Cancer Res. 2016; 22:4848–4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ménard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, et al. CTLA-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008; 14:5242–5249 [DOI] [PubMed] [Google Scholar]
- 49.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab Cancer Immunol Immunother. 2014; 63:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreira A, Leisgang W, Schuler G, Heinzerling L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy Immunotherapy. 2017; 9:115–121 [DOI] [PubMed] [Google Scholar]
- 51.Najjar YG, Ding F, Lin Y, VanderWeele R, Butterfield LH, Tarhini AA. Melanoma antigen-specific effector T cell cytokine secretion patterns in patients treated with ipilimumab J Transl Med. 2017; 15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy Oncotarget. 2016; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study J Dermatol. 2017; 44:117–122 [DOI] [PubMed] [Google Scholar]
- 54.Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, et al. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy Cancer Immunol Res. 2013; 1:229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Retseck J, VanderWeele R, Lin HM, Lin Y, Butterfield LH, Tarhini AA. Phenotypic and functional testing of circulating regulatory T cells in advanced melanoma patients treated with neoadjuvant ipilimumab J Immunother Cancer. 2016; 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reuben JM, Lee BN, Li C, Gomez-Navarro J, Bozon VA, Parker CA, et al. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma Cancer. 2006; 106:2437–2444 [DOI] [PubMed] [Google Scholar]
- 57.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab Cell. 2017; 171:934–949.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade Clin Cancer Res. 2009; 15:390–399 [DOI] [PubMed] [Google Scholar]
- 59.Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, et al. PD-1 blockade expands intratumoral memory T cells Cancer Immunol Res. 2016; 4:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire Clin Cancer Res. 2014; 20:2424–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance Sci Transl Med. 2017; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients Proc Natl Acad Sci USA. 2015; 112:6140–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study Eur J Nucl Med Mol Imaging. 2015; 42:386–396 [DOI] [PubMed] [Google Scholar]
- 64.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab cutaneous adverse events and their association with disease progression JAMA Dermatol. 2015; 151:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients Ann Oncol. 2017; 28:1988–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma Clin Cancer Res. 2011; 17:896–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seith F, Forschner A, Schmidt H, Pfannenberg C, Guckel B, Nikolaou K, et al. 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start Eur J Nucl Med Mol Imaging. 2017; 22:22. [DOI] [PubMed] [Google Scholar]
- 68.Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, Hu B, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events J Transl Med. 2013; 11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caraco C, Curvietto M, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma Cancer Immunol Immunother. 2014; 63:675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tallerico R, Cristiani CM, Staaf E, Garofalo C, Sottile R, Capone M, et al. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients Oncoimmunology. 2017; 6:e1261242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab PLoS One. 2014; 9:e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma J Immunother Cancer. 2015; 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tietze JK, Angelova D, Heppt MV, Reinholz M, Murphy WJ, Spannagl M, et al. The proportion of circulating CD45RO+CD8+ memory T cells is correlated with clinical response in melanoma patients treated with ipilimumab Eur J Cancer. 2017; 75:268–279 [DOI] [PubMed] [Google Scholar]
- 74.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance Nature. 2014; 515:568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang E, Kang D, Bae KS, Marshall MA, Pavlov D, Parivar K. Population pharmacokinetic and pharmacodynamic analysis of tremelimumab in patients with metastatic melanoma J Clin Pharmacol. 2014; 54:1108–1116 [DOI] [PubMed] [Google Scholar]
- 76.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, et al. Biomarkers on melanoma patient T cells associated with ipilimumab treatment J Transl Med. 2012; 10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma J Clin Oncol. 2013; 31:4311–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilgenhof S, Du Four S, Vandenbroucke F, Everaert H, Salmon I, Lienard D, et al. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma J Immunother. 2013; 36:215–222 [DOI] [PubMed] [Google Scholar]
- 79.Wistuba-Hamprecht K, Martens A, Haehnel K, Geukes Foppen M, Yuan J, Postow MA, et al. Proportions of blood-borne Vδ1+ and Vδ2+ T-cells are associated with overall survival of melanoma patients treated with ipilimumab Eur J Cancer. 2016; 64:116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wistuba-Hamprecht K, Martens A, Heubach F, Romano E, Geukes Foppen M, Yuan J, et al. Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients Eur J Cancer. 2017; 73:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab Proc Natl Acad Sci USA. 2011; 108:16723–16728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit Proc Natl Acad Sci USA. 2008; 105:20410–20415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan J, Zhou J, Dong Z, Tandon S, Kuk D, Panageas KS, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab Cancer Immunol Res. 2014; 2:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma Br J Dermatol. 2016; 174:146–151 [DOI] [PubMed] [Google Scholar]
- 85.Zimmer L, Eigentler TK, Kiecker F, Simon J, Utikal J, Mohr P, et al. Open-label, multicenter, single-arm phase II DeCOG-study of ipilimumab in pretreated patients with different subtypes of metastatic melanoma J Transl Med. 2015; 13:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation Cell. 2011; 144:646–674 [DOI] [PubMed] [Google Scholar]
- 87.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease Inflamm Bowel Dis. 2009; 15:1090–1100 [DOI] [PubMed] [Google Scholar]
- 88.Sanmamed MF, Carranza-Rua O, Alfaro C, Oñate C, Martín-Algarra S, Perez G, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins Clin Cancer Res. 2014; 20:5697–5707 [DOI] [PubMed] [Google Scholar]
- 89.Ouyang Z, Wu H, Li L, Luo Y, Li X, Huang G. Regulatory T cells in the immunotherapy of melanoma Tumor Biol. 2016; 37:77–85 [DOI] [PubMed] [Google Scholar]
- 90.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy Cell Res. 2017; 27:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria Clin Cancer Res. 2009; 15:7412–7420 [DOI] [PubMed] [Google Scholar]
- 92.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification J Clin Oncol. 2009; 27:6199–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brochez L, Naeyaert JM. Serological markers for melanoma Br J Dermatol. 2000; 143:256–268 [DOI] [PubMed] [Google Scholar]
- 94.Jury CS, McAllister EJ, MacKie RM. Rising levels of serum S100 protein precede other evidence of disease progression in patients with malignant melanoma Br J Dermatol. 2000; 143:269–274 [DOI] [PubMed] [Google Scholar]
- 95.Lin J, Yang Q, Wilder PT, Carrier F, Weber DJ. The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma J Biol Chem. 2010; 285:27487–27498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jurisic V, Radenkovic S, Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects Adv Exp Med Biol. 2015; 867:115–124 [DOI] [PubMed] [Google Scholar]
- 97.Li W, Zhao Y. Warburg effect and mitochondrial metabolism in skin cancer J Carcinog Mutagen. 2014; S4:002 [Google Scholar]
- 98.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer Nature. 2013; 500:415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma Science. 2015; 350:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis Lancet Oncol. 2017; 18:1009–1021 [DOI] [PubMed] [Google Scholar]
- 101.Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases Cancer Immunol Immunother. 2009; 58:1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilgenhof S, Du Four S, Everaert H, Neyns B. Patterns of response in patients with pretreated metastatic melanoma who received ipilimumab 3 mg/kg in a European expanded access program: five illustrative case reports Cancer Invest. 2012; 30:712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]


