Supplemental digital content is available in the text.
Key Words: rheumatoid arthritis, rituximab, observational study
Background/Objective
There is evidence supporting that there are no relevant clinical differences between dosing rituximab 1000 mg or 2000 mg per cycle in rheumatoid arthritis (RA) patients in clinical trials, and low-dose cycles seem to have a better safety profile. Our objective was to describe the pattern of use of rituximab in real-life practice conditions.
Methods
Rituximab for RA in clinical practice (RITAR) study is a retrospective cohort study from 2005 to 2015. Eligibility criteria were RA adults treated with rituximab for active articular disease. Response duration was the main outcome defined as months elapsed from the date of rituximab first infusion to the date of flare. A multivariable analysis was performed to determine the variables associated with response duration.
Results
A total of 114 patients and 409 cycles were described, 93.0% seropositive and 80.7% women. Rituximab was mainly used as second-line biological therapy. On demand retreatment was used in 94.6% of cases versus fixed 6 months retreatment in 5.4%. Median response duration to on demand rituximab cycles was 10 months (interquartile range, 7–13). Multivariable analysis showed that age older than 65 years, number of rituximab cycles, seropositivity, and first- or second-line therapy were associated with longer response duration. The dose administered at each cycle was not significantly associated with response duration.
Conclusions
Our experience suggests that 1000 mg rituximab single infusion on demand is a reasonable schedule for long-term treatment of those patients with good response after the first cycles, especially in seropositive patients and when it is applied as a first- or second-line biological therapy.
Rheumatoid arthritis (RA) is a chronic autoimmune disease with a heterogeneous genetic background whose pathogenesis involves different cells of the immune system. Rituximab is a B-lymphocyte depleting monoclonal antibody that was approved for RA treatment in Europe in 2006. The approved indication in this disorder is as a second-line biological therapy, which is for patients who have had an inadequate response or intolerance to 1 or more tumor necrosis factor (TNF) inhibitor therapies, if possible in combination with methotrexate (MTX). Nevertheless, EULAR recommendations for RA management, as well as other consensus documents, suggest that rituximab can be used as a first-line biological therapy under certain circumstances.1,2
The schedule of rituximab retreatment administration remains controversial. The summary of product characteristics suggests that for RA 2 intravenous infusions of 1000 mg, on days 1 and 15, should be administered and then treatment can be repeated after 24 weeks. This ambiguous statement is the consequence of early pivotal studies exploring both fixed retreatment and on demand schedules.3
Current evidence from clinical trials and registries challenges the existence of relevant efficacy differences between dosing rituximab 1000 mg or 2000 mg per cycle, and the low-dose schedule seems to have a better safety profile in terms of immunoglobulin G count.4–6 In addition, rituximab is more effective in RA patients seropositive for rheumatoid factor (RF) or anticitrullinated protein antibodies (ACPAs).7,8 Considering these findings, the Spanish Rheumatology Society and Hospital Pharmacy Society Consensus for biologics optimization recommended the use of 1000 mg rituximab cycles on demand retreatment and preferentially in RF or/and ACPA-seropositive patients.9
The rituximab for RA in clinical practice (RITAR) study objective was to describe the pattern of use of rituximab in real-life practice conditions over 11 years in our hospital. In addition, we collected information about clinical response duration in those cycles prescribed on demand to identify factors associated with this outcome.
METHODS
Patients
RITAR is a retrospective, open-label, observational cohort study that describes the patterns of use of rituximab in a tertiary university hospital in Madrid, Spain, from 2005 to 2014 and a follow-up period that covered until December 2015.
Eligibility criteria were RA adults treated with at least 1 rituximab infusion for articular active disease. Patients were identified from the Hospital Pharmacy Department database, and clinical charts were reviewed to confirm the RA diagnosis according to the American College of Rheumatology 1987 criteria.10 Patients that did not fulfill the criteria, those having a history of another autoimmune disease or RA patients in which rituximab was prescribed because of systemic complications, were excluded from the study. Those rituximab cycles that were lost from follow-up and those cycles administered under a clinical trial protocol were also excluded.
Data were retrospectively collected from our electronic medical record software. During the study period and follow-up, treatment decisions were taken under daily clinical practice criteria. Patients treated with on demand RTX cycles have scheduled visits at 3, 6, 8, 10, 12, and 14 months to early detect relapses. Some of these visits are conducted by our specialized nurse. Patients have also the possibility to contact the nurse by phone if they feel they are relapsing, and then the nurse can provide them with an extra appointment with the responsible physician.
RA Activity Measurements
DAS28 (disease activity score based on a 28-joint count) was systematically registered at the time of rituximab infusion (baseline visit of each cycle). However, because this is a retrospective noninterventional study, some data required to assess disease activity with objective measures (ie, DAS28) were missing in subsequent follow-up visits. Response duration was defined as the time (in months) elapsed from the date of the first rituximab infusion to the date of flaring (DAS28 > 3.2) recorded in the clinical chart. In those cases in which there was no information on the date of flare, the date of the next rituximab administration was used as the flare date. Ineffectiveness was considered if the physician in charge of the patient described absence of improvement of disease activity or improvement lasting for less than 4 months. Information about effectiveness was obtained from the data and commentaries in the clinical chart.
Study Variables
For each rituximab cycle, we collected number of cycle, rituximab dose per cycle (1000 mg or 2000 mg), retreatment schedule (fixed retreatment every 6 months or on demand), concomitant disease-modifying antirheumatic drugs (DMARDs), and number of previous biological therapies. In case of rituximab discontinuation, the reason for it was also collected (lack of effectiveness, adverse reactions, and others). In addition, we recorded patient RF or/and ACPA status at baseline.
Additional Variables
Baseline characteristics of the patients were recorded, including demographic characteristics (sex, age at disease onset, age at rituximab start) and presence of comorbidities (chronic obstructive pulmonary disease, congestive heart disease, depression, diabetes mellitus, gastric ulcer, hypercholesterolemia, hypertension, ischemic heart disease, osteoporosis, stroke, solid, or hematological neoplasms), shown in Supplemental Digital Content Table 1, http://links.lww.com/RHU/A126, with patient’s comorbidities, and RA systemic and extra-articular manifestations (amyloidosis, atlantoaxial luxation, episcleritis, Felty syndrome, lung disease, rheumatoid vasculitis, serositis, Sjögren syndrome) shown in Supplemental Digital Content Table 2, http://links.lww.com/RHU/A127, with complications associated to RA.
Concomitant DMARDs, previous nonbiological and biological DMARDs, were also recorded including their biological line number, dates of onset and withdrawal, as well as the main reason for discontinuation (lack or loss of effectiveness, adverse reactions, others). Glucocorticoids and nonsteroid anti-inflammatory drugs, intra-articular and periarticular glucocorticoid infiltrations, and other concomitant treatments are frequently used but were not registered.
Ethical Statements
RITAR study was conducted according to the principles expressed in the Helsinki Declaration of 1983. RITAR was considered by the Spanish Agency of Medicines and Medical Devices as an observational study, and it was approved by the Research Ethics Committee of Hospital Universitario La Princesa (code number 2903A), which did not consider necessary to ask for the written consent of patients. Nevertheless, patients that continued their follow-up at the Rheumatology Department were informed about the study, and verbal consent was obtained.
Statistical Analysis
For the description of the population means ± standard deviation (SD), medians and interquartile range (IQR) and absolute and relative frequencies were used depending on the distribution of variables. Student t test was used for continuous variables with normal distribution. Discrete variables or those continuous not normally distributed were analyzed with the Mann-Whitney U test or Kruskal-Wallis test, if more than 2 categories existed. Categorical variables were analyzed with the χ2 test or Fisher test when any expected frequency was lower than 1, or when 20% of the expected frequencies were 5 or less.
To determine which variables were associated with response duration, and considering that we studied repeated measurements (different rituximab cycles) for each patient, a multivariable analysis using population-averaged panel data models through the command xtgee of Stata was used. This command allows fitting multivariable analysis using generalized estimation equations nested by patient and cycle to adjust for repeated measurements. Adding all variables with a p value less than 0.15 in the bivariate analysis performed the first model. The final model was constructed through backward stepwise method removing all variables with p greater than 0.15 and also considering quasi-likelihood estimation based on the independence model information criterion and Wald tests.11 In addition, to estimate the average response duration of 1000 mg and 2000 mg rituximab cycles, we forced this variable in the final model, and then the adjusted mean ± standard error were estimated with the xtgee postestimation command margins. Statistical analyses were performed using the statistical package Stata v. 12.
RESULTS
Patients
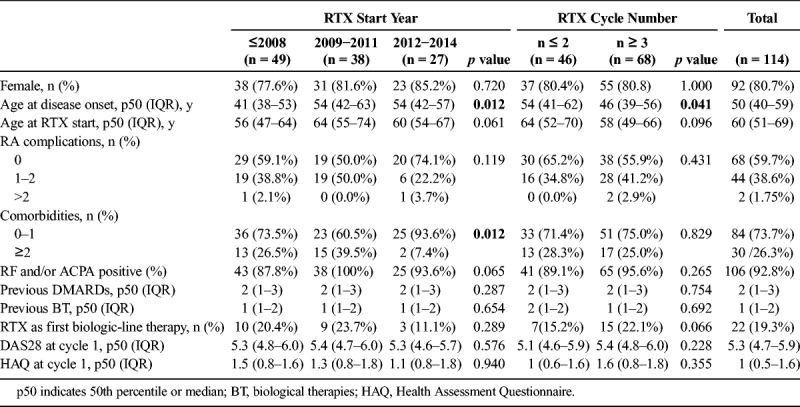
One hundred sixty-one patients were treated with rituximab. Eighteen patients treated under other diagnoses different from RA, 8 patients in whom rituximab was prescribed because of RA systemic manifestations, and 21 patients who were not followed up in our hospital after the first rituximab infusion were excluded. In addition, those rituximab cycles that belonged to clinical trials or were lost during follow-up were excluded from the analysis. Finally, 114 patients were included and 409 rituximab cycles were described. Median follow-up was 2.95 years (IQR, 1.24–5.54).
Age at rituximab start was 60 years (IQR, 51–69) and disease duration was 11 years (IQR, 6–16). Most patients experienced seropositive disease (93%) and 80.7% of them were women. Because, after approval of rituximab, new biological therapies became available for RA treatment, we decided to cluster patients in 3 groups considering the year of rituximab start (before 2009, 2009–2011, and 2012–2014). As it is shown in Table 1, patients treated with rituximab before 2009 were younger and seronegative cases were more frequent. In addition, those treated after 2012 tended to suffer less comorbidities (p = 0.018) and systemic complications (p = 0.119). No differences were observed in terms of previous DMARDs, biological therapies, or disease activity at the beginning of treatment.
TABLE 1.
Baseline Characteristics of Patients
Patterns of Rituximab Use
As it is shown in Table 1, rituximab was mainly used as second-line biological therapy. Nevertheless, rituximab was the first biological treatment in 19.3% of overall cases, with tendency to be less frequent since 2012 and more common among those patients who received more rituximab cycles (Table 1). Previous therapies to rituximab treatment start are shown in Supplemental Digital Content 3, http://links.lww.com/RHU/A112. Median number of rituximab cycles per patient was 3 (IQR, 2–6), ranging from 1 to 11.
On demand retreatment schedule was used in 94.6% of total rituximab cycles versus fixed retreatment every 6 months in 5.4%. Rituximab fixed retreatment was more frequent during the first years of rituximab use in our center (until 2008 12% of cycles), but this pattern of use gradually decreased (7.9% of cycles 2009–2011) and disappeared after 2012. In addition, fixed retreatment schedule was more common in the initial courses of treatment reaching 12.5% at first cycle, 6.9% at second, 3.3% at third, and 2.0% at fourth or later. For those patients receiving 5 or more cycles, the on demand schedule was always used.
Rituximab dose was 1 × 1000 mg in 31.8% of total cycles. There were no relevant differences in the percentage of 1000 mg and 2000 mg cycles over time (p = 0.151). However, we observed that the use of 1 × 1000 mg cycles was significantly more frequent in cycle 3 or successive (45%) compared with the 2 first cycles (17%; p < 0.001). Specifically, in 91.5% of first rituximab cycles dose was 2000 mg. Then, the use of rituximab 1000 mg per cycle gradually increased up to 80% to 100% in those patients with more than 6 cycles.
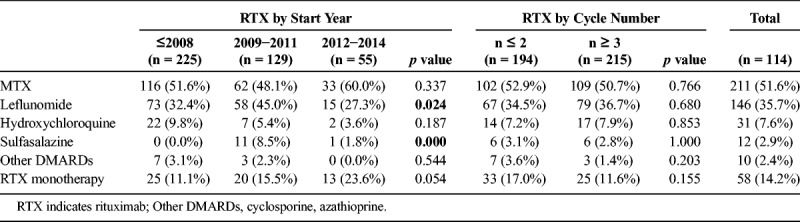
Rituximab was used without concomitant DMARDs in 12.2% of patients, and the use as monotherapy gradually increased over time (Table 2). Regarding concomitant DMARD treatment, the most frequent was MTX (53.8%), followed by leflunomide (36.7%), hydroxychloroquine (7.6%), sulfasalazine (2.9%), and others (azathioprine and cyclosporine, 7.6%). In 16.2% of the cycles, concomitant DMARDs were used in combination, being MTX plus leflunomide the most frequent.
TABLE 2.
Concomitant DMARDs in Rituximab Cycles
Response Duration
As it is shown in Figure 1A, there was an average disease activity improvement after rituximab administration at each cycle, although the degree of response was clearly heterogeneous. In addition, Figure 1B shows that baseline disease activity was the worst at the first rituximab cycle. Then, although most of the successive rituximab cycles were prescribed on demand when the disease flared, on average, retreatment was administered with lower levels of disease activity than the first cycle.
FIGURE 1.
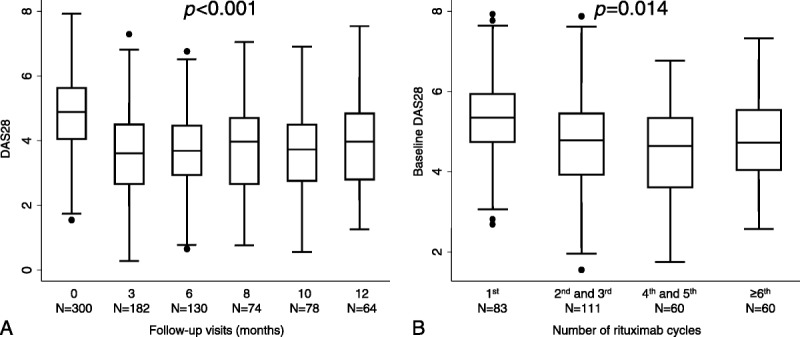
Evolution of disease activity with rituximab treatment in RITAR. A, Average disease activity assessed with DAS28 by follow-up visit. B, Baseline disease activity at different rituximab cycles. Data are presented as interquartile range (p75 upper edge, p25 lower edge, p50 midline), p95 (line above the box), and p5 (line below the box). Dots represent the outliers. Statistical significance was estimated with the nptrend Stata command, which is an extension of the Wilcoxon rank-sum test that performs a nonparametric test for trend across ordered groups.
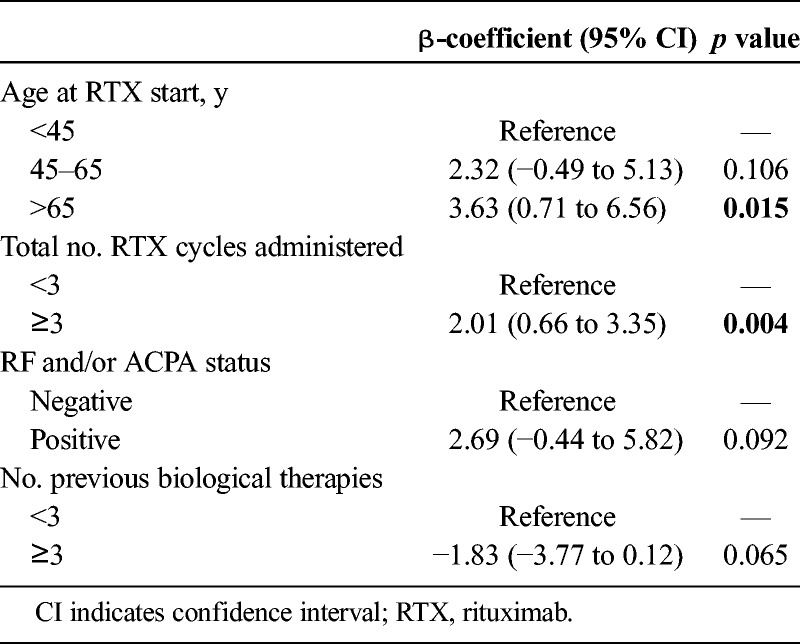
Response duration to rituximab could only be determined in 314 cycles administered on demand. Median response duration was 10 months (IQR, 7–13). Because there were some differences in the use of rituximab along the 3 periods, we decided to perform a multivariable analysis to determine which variables better explained response duration in our study. The best-fitted model (Table 3) showed that older age and higher number of rituximab cycles were significantly associated with longer response duration. Of interest, response duration was almost 3 months shorter in seronegative than in seropositive patients, although the differences did not reach statistical significance (Table 3). Finally, when rituximab was used as the third or even later line of biological therapy, the response duration was almost 2 months shorter than in those cycles in which rituximab was used as first or second line, although statistical significance was not reached (Table 3).
TABLE 3.
Variables Associated With Response Duration (Months) to Rituximab
Interestingly, the variable dose per cycle was not significantly associated with response duration. Furthermore, we estimated the mean response duration for 1000 mg and 2000 mg rituximab cycles adjusted for the variables included in the model shown in Table 3. As Figure 2 shows, the differences in response duration were negligible. Other variables such as sex, date of rituximab start, or use in monotherapy were not associated with response duration either. Nevertheless, female sex and use of rituximab in monotherapy showed a trend to lower response duration (Supplemental Digital Content 4, http://links.lww.com/RHU/A128). When rituximab was used in combination with DMARDs, there were no relevant differences between the different drugs.
FIGURE 2.

Response duration by dose of rituximab. Data are shown as the prediction of the mean response duration in months (top of the histograms) and its 95% confidence intervals (bars) adjusted by the relevant variables included in the multivariable analysis provided in Table 3 (for further information, see Statistical Analysis section).
Reasons for Rituximab Withdrawal
Of 114 patients that started rituximab because of active RA, 60 (52.6%) were still under this treatment at December 2015, with a median follow-up of 4.80 years (IQR, 2.15–7.17). Withdrawal from rituximab was due to ineffectiveness in 18.4% of cases (n = 21), adverse events in 7.0% (n = 8), and other reasons such as lost follow-up or non–rituximab-related deaths in 22.0% (n = 25).
Focusing on discontinuation caused by ineffectiveness, 71.4% patients stopped after first or second rituximab cycles (n = 15), 23.8% after 3 to 5 cycles (n = 5) and 1.8% afterward (n = 1) (p = 0.002). Biological line in which rituximab was used did not seem to be related with ineffectiveness as the reason for discontinuation (p = 0.805).
DISCUSSION
RITAR, a real-life observational study that described different rituximab strategies used during 11 years, provides significant information about how the use of this drug has evolved in our center since its approval for RA treatment. We observed a tendency to abandon the fixed retreatment every 6 months schedule in favor of the on demand use. In addition, the recommended rituximab dose of 2000 mg per cycle was frequently replaced by 1000 mg per cycle administered as a single infusion, especially beyond the third cycle. The most frequent pattern consisted on starting rituximab 2000 mg in the first and second cycles and then reducing to rituximab 1000 mg per cycle in successive retreatments. The main reasons for this schedule are cost-saving and the attempt to reduce adverse effects such as hypogammaglobulinemia and infections.12 This strategy was similar to that used in the SMART trial, a French study that demonstrated noninferiority in different rituximab schedules: 2000 mg per cycle versus a first course rituximab 2000 mg followed by rituximab 500 mg × 2 in the consecutive ones.5 In addition, a meta-analysis supported similar efficacy of low- versus high-dose rituximab in different subsets of patients.4 Comparing to SMART strategy, RITAR treatment schedule has advantages in terms of better cost-effectiveness13 and patient comfort, because low-dose cycles were administered as a single infusion instead of 2 infusions of 500 mg.5
Rituximab was mainly used as second-line biological therapy. Nevertheless, rituximab was prescribed as first-line biological therapy in 1 of 5 patients, which is a remarkable proportion. We could not determine the reasons for this; probably cost-saving, anti-TNF contraindication, and preference due to patient lifestyle are some of the possibilities. In any case, recently, the ORBIT trial, a head-to-head adalimumab versus rituximab randomized controlled study, showed that rituximab is noninferior to TNF inhibitors in patients naive to biological treatment.14 In addition, in a long-term Finnish study, almost 28% of patients had never used biologics before rituximab.15
In our study, rituximab was widely administered with 1 or more concomitant DMARDs being the most frequently used MTX and/or leflunomide. Previous evidence supports the use of leflunomide in this context.2,16 Twelve percent of our rituximab cycles were prescribed without concomitant DMARD. This is lower than another Spanish cohort in which rituximab monotherapy was used in 29% of patients.17 Interestingly, rituximab monotherapy has increased in our center from 11.1% to 23.6% in recent years. The reasons for this trend are unclear and should be analyzed, because lower response magnitude and duration have been described when rituximab is used as monotherapy.18
Average rituximab response duration in our patients was 10 months, which is in agreement with the data reported in the literature, ranging from 8.5 months in SMART5 and RESET19 trials to 11 to 13 months observed in a long-term real-life Finnish study.15 However, the longer time to retreatment in Finnish patients led to disease flare in a significant proportion of patients. The SMART study showed a time to retreatment 1 month shorter than ours, but a similar time to retreatment in the rituximab 1000 mg course (8.6 months) than in 2000 mg per cycle strategy (8.4 months) in the 2-year follow-up. This later finding has also been observed in our study.
In our multivariable model, the variables significantly associated with longer response duration were cycles later than second and age higher than 65 years. These are likely indication biased because those patients with the worst response to rituximab were withdrawn before the third cycle. In addition, we probably delayed rituximab retreatment in elderly patients because of safety reasons.
Additional variables associated to a trend to longer response duration in our study were seropositivity (RF and/or ACPA) and previous treatment with 2 or less biological therapies. On average, seropositive patients showed 3 months longer response duration than seronegative ones. This is in accordance with previous studies.7,8,17 It is feasible that the pathogenesis of RA in those patients with autoantibodies is more dependent on B-cells, thus explaining their better response to rituximab.3 Furthermore, those patients that received 3 or more previous biological lines experienced almost 2 months shorter response duration than those that were treated with rituximab in an earlier biological line. This is again in agreement with previous reports and the well-known lower response to RA treatments in more refractory patients.17
Our study has some limitations commonly present in retrospective observational studies: missing data, high percentage of unexplained lost cycles from follow-up, indication bias, and so on. However, this kind of study offers complementary information to that obtained in clinical trials, because observational studies are able to provide data from real-life patients that are excluded from clinical trials and schedules of treatment that are different from the conventional approved ones.
In summary, our data suggest that, after a successful first cycle of 2000 mg, retreatments with rituximab can be scheduled as cycles of 1000 mg single infusion, because a lower number of B-lymphocytes are detected at early phases of relapse when this drug is used on demand (Lopez et al, unpublished data). In our experience, this retreatment schedule provides median response duration of 10 months, resulting in a comfortable schedule of treatment with acceptable control of disease activity in most patients. Rituximab use in seropositive patients, especially if it is applied as first or second biological line, seems to provide longer response duration.
KEY POINTS
Rituximab 1 × 1000 mg cycles in on demand retreatment schedule is useful for maintaining response in responder RA patients, providing median response duration of 10 months.
Patients older than 65 years, seropositive disease, and rituximab use in first or second biological line seem to result in longer response duration.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Teresa Velasco, BSN, for her help in collecting data; Tomás Gallego, PharmD, and Alberto Morell, PharmD, for the pharmacy compounding data; and Alicia Humbría, MD, PhD, Rosario García-Vicuña, MD, PhD, Ana M. Ortiz, MD, PhD, Esther Patiño, MD, PhD, Eva G. Tomero, MD, and Esther F. Vicente, MD, PhD, for their work at the clinic.
Special thanks to Manuel Gómez-Gutierrez for the writing assistance.
Footnotes
Conflicts of interest and sources of funding: I.C., L.M., J.L., I.L., E.R.H., J.B., and C.M. have nothing to disclose. A.G.V. reports personal nonfinancial support from Lilly and Pfizer. J.M.Á.G. reports personal fees from Abbvie; personal fees and nonfinancial support from Bristol-Myers Squibb; personal fees and nonfinancial support from Merck Sharp & Dohme; grants, personal fees, and nonfinancial support from Roche; grants and personal fees from UCB; personal fees and nonfinancial support from Pfizer; personal fees and nonfinancial support from Lilly; personal fees from Sanofi; personal fees from Janssen-Cilag; and personal fees from Novartis, outside the submitted work. S.C. reports grants from Ministerio de Economía y Competitividad (Instituto de Salud Carlos III, PI12/01578) and grants from Pfizer España during the course of the study; personal fees from Abbvie; personal fees from Amgen; personal fees from Bristol-Myers Squibb; personal fees from Gebro Pharma; personal fees from Lilly; personal fees from Merck Sharp & Dohme; personal fees from Pfizer; and personal fees from Roche, outside the submitted work. I.G.Á. reports grants from Instituto de Salud Carlos III, during the course of the study; personal fees from Lilly; grants, personal fees, and nonfinancial support from UCB; personal fees and nonfinancial support from Bristol-Myers Squibb; personal fees and nonfinancial support from Pfizer; grants from Roche; personal fees and nonfinancial support from Abbvie; and nonfinancial support from Merck Sharp & Dohme, outside the submitted work; in addition, he also has a patent PCT/ES2015/070182 issued. Our manuscript was supported by grants RD16/0012/0011 and PI14/00442 from the Ministerio de Economía y Competitividad (Instituto de Salud Carlos III) and cofunded by the European Regional Development Fund.
I.C. is now currently working as pharmacist in Direction of Pharmaceutical Management, Autonomous Community of Madrid, Madrid, Spain. L.M. is now currently affiliated with the Rheumatology Department, Hospital San Pedro, Logroño, Spain. J.L. is now currently affiliated with the Centro de Estudios y Desarrollos Sanitarios CEyDES, Zaragoza, Spain and Centro de Análisis Genéticos Citogen, Zaragoza, Spain.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.jclinrheum.com).
REFERENCES
- 1.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martín Mola E, Hernández B, García-Arias M, et al. Consensus on the use of rituximab in rheumatoid arthritis. A document with evidence-based recommendations. Grupo de Expertos en Rituximab. Reumatol Clin. 2011;7:30–44. [DOI] [PubMed] [Google Scholar]
- 3.Cambridge G, Torre Ide L. Response to rituximab: has the original hypothesis been confirmed? Curr Pharm Des. 2015;21:212–220. [DOI] [PubMed] [Google Scholar]
- 4.Bredemeier M, Campos GG, de Oliveira FK. Updated systematic review and meta-analysis of randomized controlled trials comparing low- versus high-dose rituximab for rheumatoid arthritis. Clin Rheumatol. 2015;34:1801–1805. [DOI] [PubMed] [Google Scholar]
- 5.Mariette X, Rouanet S, Sibilia J, et al. Evaluation of low-dose rituximab for the retreatment of patients with active rheumatoid arthritis: a non-inferiority randomised controlled trial. Ann Rheum Dis. 2014;73:1508–1514. [DOI] [PubMed] [Google Scholar]
- 6.Chatzidionysiou K, Lie E, Nasonov E, et al. Effectiveness of two different doses of rituximab for the treatment of rheumatoid arthritis in an international cohort: data from the CERERRA collaboration. Arthritis Res Ther. 2016;18:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaacs JD, Cohen SB, Emery P, et al. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann Rheum Dis. 2013;72:329–336. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Mola E, Balsa A, Garcia-Vicuna R, et al. Anti-citrullinated peptide antibodies and their value for predicting responses to biologic agents: a review. Rheumatol Int. 2016;36:1043–1063. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Alvaro I, Martinez-Fernandez C, Dorantes-Calderon B, et al. Spanish Rheumatology Society and Hospital Pharmacy Society Consensus on recommendations for biologics optimization in patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis. Rheumatology (Oxford). 2015;54:1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 11.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. [DOI] [PubMed] [Google Scholar]
- 12.Gottenberg JE, Ravaud P, Bardin T, et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum. 2010;62:2625–2632. [DOI] [PubMed] [Google Scholar]
- 13.Seror R, Mariette X. Cost-effectiveness of rituximab strategies in rheumatoid arthritis. Lancet. 2017;389:365–366. [DOI] [PubMed] [Google Scholar]
- 14.Porter D, van Melckebeke J, Dale J, et al. Tumour necrosis factor inhibition versus rituximab for patients with rheumatoid arthritis who require biological treatment (ORBIT): an open-label, randomised controlled, non-inferiority, trial. Lancet. 2016;388:239–247. [DOI] [PubMed] [Google Scholar]
- 15.Valleala H, Korpela M, Hienonen-Kempas T, et al. Long-term real-life experience with rituximab in adult Finnish patients with rheumatoid arthritis refractory or with contraindication to anti-tumor necrosis factor drugs. J Clin Rheumatol. 2015;21:24–30. [DOI] [PubMed] [Google Scholar]
- 16.Narvaez J, Diaz-Torne C, Ruiz JM, et al. Comparative effectiveness of rituximab in combination with either methotrexate or leflunomide in the treatment of rheumatoid arthritis. Semin Arthritis Rheum. 2011;41:401–405. [DOI] [PubMed] [Google Scholar]
- 17.Narvaez J, Diaz-Torne C, Ruiz JM, et al. Predictors of response to rituximab in patients with active rheumatoid arthritis and inadequate response to anti-TNF agents or traditional DMARDs. Clin Exp Rheumatol. 2011;29:991–997. [PubMed] [Google Scholar]
- 18.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. [DOI] [PubMed] [Google Scholar]
- 19.Haraoui B, Bokarewa M, Kallmeyer I, et al. Safety and effectiveness of rituximab in patients with rheumatoid arthritis following an inadequate response to 1 prior tumor necrosis factor inhibitor: the RESET Trial. J Rheumatol. 2011;38:2548–2556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


