Abstract
Aims:
Cardiovascular disease (CVD) is one of the leading causes of mortality and morbidity worldwide, including in Sweden. The main aim of this study was to explore the temporal trends and spatial patterns of CVD in Sweden using spatial autocorrelation analyses.
Methods:
The CVD admission rates between 2000 and 2010 throughout Sweden were entered as the input disease data for the analytic processes performed for the Swedish capital, Stockholm, and also for the whole of Sweden. Age-adjusted admission rates were calculated using a direct standardization approach for men and women and temporal trends analysis were performed on the standardized rates. Global Moran’s I was used to explore the structure of patterns and Anselin’s local Moran’s I together with Kulldorff’s scan statistic were applied to explore the geographical patterns of admission rates.
Results:
The rates followed a spatially clustered pattern in Sweden with differences occurring between genders. Accordingly, hotspots were identified in northern Sweden with higher intensity identified for men together with clusters in central Sweden. Cold spots were identified in the adjacency of the three major Swedish cities of Stockholm, Gothenburg and Malmö.
Conclusions:
The findings of this study can serve as a basis for distribution of health care resources, preventive measures and exploration of etiological factors.
Keywords: Cardiovascular disease, spatial analysis, spatial autocorrelation analysis, temporal trends, disease clusters
1. Introduction
Cardiovascular disease (CVD) is one of the major causes of morbidity, disability, and mortality in adults worldwide (1, 2) with more than 16.7 million (>28%) of 57 million annual deaths (3) caused by CVD. In Sweden, the mortality of CVD has decreased during recent decades (4). The reasons for this decline are most likely because: (i) overall life conditions, including financial circumstances, have gradually improved, (ii) the amount and the quality of healthcare measures have increased the chance of surviving a heart attack (5–7) and (3) prevention strategies have improved (7). However, although the mortality as well as incidence rate of CVD has continued to decline during the past decades, CVD remains one of the most fatal diseases in Sweden (8). As a result, this has led to an increased need in Sweden to examine additional CVD etiological factors. Moreover, a systematic and comprehensive examination of spatial patterns in disease occurrence is important for health care planning and priority setting and for the identification of potentially modifiable factors that may contribute to such patterns. Thus, an age-standardized spatial analysis of CVD hospital admission rates across geographical units in Sweden was performed in this study to provide new insights about spatial CVD patterns.
Spatial patterns of diseases have long been considered to be important for a better understanding of disease occurrence, prevention, optimal treatment, and health outcomes (9, 10). Several studies have been conducted to explore spatial patterns of several diseases worldwide (11–14). For example, Xia, Bergquist (15) investigated the clusters of schistosomiasis in China in order to identify the distribution of high-risk areas. Loughnan, Nicholls (16) presented the spatial differences between the seasonal patterns of cardiac disease in Australia. Soljak, Samarasundera (17) investigated the geographic variations in the prevalence of three cardiovascular conditions to study the impact of under-diagnosis on population health outcomes in England. Asaria, Fortunato (18) analyzed CVD death rates using Bayesian spatial techniques in England. Ma, Zhao (19) explored the spatial patterns and geographical variations of congenital heart disease in China and discussed possible associated environmental risk factors. Although national and regional clusters of CVD have been explored sporadically in different parts of the world (17, 19, 20), spatial perspectives of CVD occurrence have previously not been investigated in Sweden and thus little data exist on small-area trends. The latter could serve as a basis for the exploration of potential underlying factors causing severe diseases, such as CVD, which has been ignored in spatial analysis previously, probably due to difficulties in large-scale data collection, preparation and analyses. Hence, the main aim of this study was to examine the admissions of CVD across all of Sweden during an 11 year time period as well as in the highly populated Swedish capital of Stockholm, using popular spatial pattern analysis and cluster detection approaches including global and local indices of Moran (21) and spatial scan statistic (22, 23).
2. Methods
2.1. Data acquisition
2.1.1. CVD Data
This study was based on Swedish hospital records of CVD admissions during an 11-year period between Jan 1st, 2000 and Dec 31st, 2010. According to the World Health Organization’s (WHO)’s International Classification of Diseases (ICD 10), the following cardiovascular disorders were applied in this study: Coronary heart disease (CHD) including codes I20, I21, I22, I23, I24, I25; Ischemic stroke including codes I63 (excluding I63.6, i.e. cerebral venous thrombosis), I65, I66, I67.2, I67.8, G45, and G46 (G46 only in combination with any of the other diagnoses), and atherosclerotic and aortic disease including codes I70, I71, I72, I73 (excluding I73.0, I73.1), I74, and I77.1. Hospital admissions were used in this study rather than individual-level incidence rates. The rationale behind using all hospital admissions was because the overall purpose of this study had been to examine the spatial patterns of CVD in all Sweden over eleven years in order to provide a basis for healthcare planning rather than examining the potential mechanisms behind CVD.
Health care data including date of hospital admission were collected from the Swedish National Board of Health and Welfare. Data on age and sex were collected from national population registers (24) and comprised 1,131,375 hospital admissions within Sweden. Moreover, approximate locations representing each patient’s residential area within a 100m buffer zone were also collected and considered as the basis for all spatial calculations and aggregations in this study. Age, sex and an approximate location were hence linked to CVD admission data at the individual level (without aggregation).
2.2. Scale
Three different spatial scales were considered in this study to explore the Swedish CVD patterns including (i) the entire country (ii) all Swedish municipalities, and (iii) BAS, a local sub-county division unit. BAS is derived from a Swedish word, “Basområden”, meaning “base areas”, which are only defined for Stockholm county for administrative purposes. The boundaries of BAS divisions are defined based on homogenous types of buildings to contain approximately 2000 residents. BAS divisions were used in this study as proxies for neighborhoods in Sweden (25). The individual level admission data were aggregated geographically at the three above mentioned levels in Sweden. These three levels were considered in this study as the main geographical area units for the analyses, and all CVD hospital admissions were linked to them accordingly. There are 290 municipalities in Sweden and approximately 1200 BAS units in Stockholm.
2.3. Data preparation
Age-adjusted CVD admission rates were calculated by direct standardization techniques (26) using 5-year age groups for those aged 40 years and older and for men and women separately, with the entire Swedish population in year 2000 as the standard population. All population data were sourced from StatisticsSweden (24), the Swedish Government-owned statistics bureau.
In this way, the adjusted rates were calculated for each area (i.e. the entire country, municipalities, and BAS) on a yearly basis during the study period. However, applying the adjusted rates directly into the analysis might skew the results because there were areas with very small populations, which could lead to disproportionate admission rates and appear highly variable even with a low number of admissions; this might misdirect the results (27). For example, municipalities and also BAS divisions in Sweden had highly uneven populations and as a result less populated areas in some years received unrealistically high admission rates despite a low number of admissions. The imprecise values of the rates were due to the variance instability, which also violates the basic assumption underlying spatial autocorrelation analysis of a constant variance. To overcome this problem, an Empirical Bayes (EB) method, proposed by Clayton and Kaldor (28), was applied in order to pool information across different areas and smoothen the adjusted rates. This approach standardized the rates by considering them as independent and identically distributed variables using the following Poisson distribution:
| (1) |
Under the Poisson distribution, the estimated admission rate for the ith area ( ) was obtained using the following expression:
| (2) |
where:
Ci = the ratio of prior variance to data variance;
=the weighted sample mean;
CARi = CVD admission rate for each area. The CAR values were adjusted by using the empirical Bayes smoothing function prepared in the GeoDa software.
2.4. Temporal Trend Analysis
A joinpoint regression approach (29, 30) was applied in order to examine the trends in the admission rates, based on statistical significance tests, during the study period. The main aim was to identify so-called joinpoints as significant changes (i.e. increase or decrease) in overall admission rates by fitting regression models for both men and women. This method also allowed us to estimate the annual percent change (APC) for each of the detected time trends. All significance tests were two-sided and the statistical significance was defined as p-value < 0.05.
For the comparison of two sets of trends created by joinpoint regression, Kim, Fay (31) proposed pairwise comparison tests. In this study, these tests were used to compare identified changes in CVD rate trend patterns between men and women to examine (i) whether the joinpoint regression functions for men and women were identical (test of coincidence), or (ii) whether the mean functions for admission rates of men and women were parallel with different intercepts (test of parallelism). In other words, the tests determined whether the intercepts or slopes of trends were significantly equal (p-value < 0.05). A permutation procedure was used in the tests to estimate the p-values.
2.5. Spatial statistical analysis
spatial autocorrelation analysis (SAA) and spatial scan statistic are commonly used to explore patterns and clusters of diseases, and to identify significant occurrence of disease hotspots and cold spots. Clusters are defined as “geographically bounded group of occurrences of sufficient size and concentration to be unlikely to have occurred by chance”(32). Cluster detection methods were applied in this study in order to determine whether the study region had a pre-specified cluster structure (33). These methods explore the structure of disease patterns to examine whether the disease is dispersed, random, or clustered. Global Moran’s I test (21) is one of the most commonly used indices for exploring spatial pattern of diseases. However, Moran’s I test is a global statistic index that only estimates the overall degree of spatial autocorrelation (34). In order to gain a more detailed geographical insight about the local patterns of a certain disease, local indicators of spatial associations (LISA) have been frequently applied for different diseases. The Anselin local Moran’s I is one of the frequently used LISA statistics, which is mostly applied to examine the spatial autocorrelation at a local level across a study area (34). On the other hand, Kulldorff (23)’s spatial scan statistic has been applied to a wide variety of public health problems for detecting disease clusters and has also been shown to be the most powerful approach for identification of local clusters(35). In this study, the spatial patterns of CVD were explored to identify previously unnoticed patterns of CVD, both globally and locally. Accordingly, global Moran’s I was used to explore the patterns of CVD admissions in Sweden between 2000 and 2010 globally, and Anselin local Moran’s I was used to explore the spatial autocorrelation at a local level and spatial scan statistic were applied for the identification of the location of CVD clusters.
2.5.1. Global Moran’s I
The classic global Moran’s I was used to examine the global spatial autocorrelation in the spatial distribution of CVD admissions. Hence, in order to calculate global Moran’s I, the contiguity information of municipalities was adjusted into a so-called spatial weights matrix. A k-nearest neighborhood approach (k = 8) was used to generate the weights matrix in this study. A random permutation procedure was used in order to test the stability and reliability of the global statistic calculated using Moran’s I. This procedure was implemented by recalculating several values for Moran’s I statistic in order to create a reference distribution, and then compare the calculated Moran’s I to this distribution. It led to the calculation of a pseudo significance level, which enabled the examination of a global pattern index. In addition to the value of Moran’s I, its theoretical mean (E[I]), mean, and standard deviation (SD) of the empirical distribution were also calculated. Thus, the index was evaluated by 9,999 permutation tests setting the significance level to 0.01. A Moran scatterplot was then produced with the average value of CVD admission rates within each local neighborhood (i.e. spatial lag) on the vertical axis and a standardized CVD admission rate on the horizontal axis. This global index was performed using GeoDa software according to the following equation:
| (3) |
where:
I = Global Moran Index;
= the admission rate of CVD for the ith municipality or BAS unit*;
= the mean admission rate of CVD for all the municipalities or BAS units* in the study area;
= the admission rate of CVD for the jth municipality or BAS unit*;
= a weight parameter for the pair of municipality or BAS unit* i and j that represents proximity using the queen contiguity; equal to the weight between observations i and j;
= the number of municipalities or BAS units*;
S0 = the sum of all , *: Moran’s I has been applied on two different scales with two different administrative units in this study. For all of Sweden, municipalities were considered as the reference administrative units. For Stockholm County, BAS divisions were considered as the main administrative units.
2.5.2. Local Moran’s I
Anselin local Moran’s I (34) was applied to detect clusters of CVD using age adjusted admission rates and a spatial weight matrix. Permutation tests (i.e. 9,999 permutations) were performed afterwards by setting the significance level to 0.01 in order to examine the stability of the results. Hence, values could also be plotted on a map to display the specific locations of clusters, i.e. locations with high or low values with similar neighbors denoted as high-high, or hotspots, and low-low, or cold spots. Potential outliers could also be specified locally, i.e. locations with high or low values with dissimilar neighbors were denoted as high-low and low-high areas on the maps. The Anselin local Moran’s I (Ii) for the ith area was calculated using the following equation:
where:
= the admission rate of CVD for the ith municipality or BAS unit;
= the mean admission rate of CVD for the CVD in the entire study area;
= the admission rate for the jth municipality or BAS unit;
= a weight parameter for the pair of municipality or BAS unit i and j that indicates proximity;
S = the standard deviation of the admission rate of CVD in the entire study area.
2.5.3. Spatial scan statistic
Kulldorff (23)’s spatial scan statistic has been widely used for spatiotemporal cluster analysis of CVD admissions. The approach has been implemented within the SaTScan software package, and is freely available (http://www.satscan.org). It uses a circular window for detecting clusters, which is centered on several possible points positioned throughout the study region with a varying radius. The maximum cluster size in this study was set to be 50% of the population at risk and the space-time scan statistic was considered for the retrospective spatiotemporal analysis of CVD for the whole study period. A Monte Carlo permutation approach was also applied to test the significance level of possible clusters in this study. Hence, 9999 random replications were simulated under the null hypothesis of the null distribution. Two types of clusters were presented by spatial scan statistic approach in this study(i) one region as most likely cluster (ii) one or more regions as secondary likely clusters(23).
3. Results
3.1. Temporal Trend analysis
The results of the joinpoint analysis showed that age-adjusted CVD admission rates across Sweden have been decreasing significantly for both men and women during the 11-year period between 2000 and 2010 (Figure 1). The rates decreased annually by 3.25% (95% confidence interval (CI): 2.9%−3.6%) in men and by 3.1% (95% CI: 2.7%−3.5%) in women. The age-adjusted CVD admission rates were statistically significantly higher for men as compared to women (p<0.05). Therefore, as a result of the identified difference, the spatial patterns of CVD were explored separately for men and women.
Figure 1-.
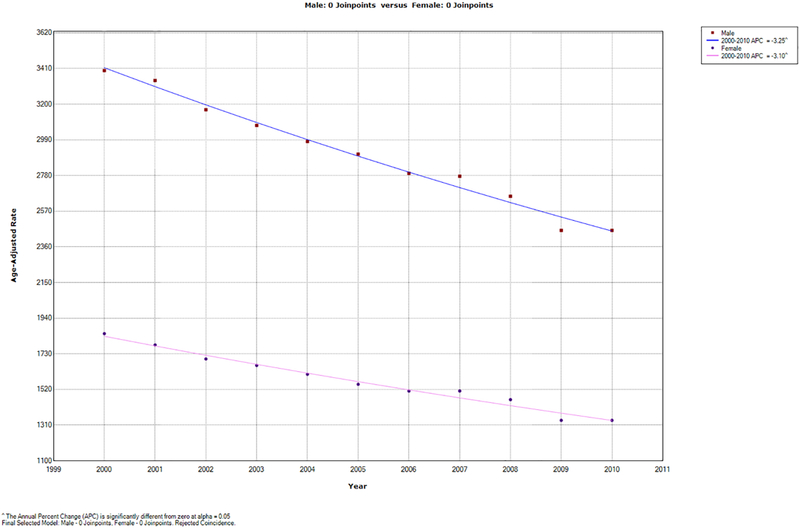
Temporal trends for the age-adjusted CVD admission rates (per 100,000) between 2000–2010
^ The Annual Percent Change (APC) is significantly different from zero at alpha = 0.05
Final Selected Model: Male - 0 Joinpoints. Female- 0 Joinpoints. Rejected Coincidence.
3.2. Spatial pattern analysis
Empirical Bayes-smoothed age-adjusted CVD admission rates were analyzed for men and women separately using quartile maps, highlighting outliers in the data. The results highlighted that the age-adjusted CVD admission rates were highest in the northern municipalities.
The global spatial autocorrelation measures for the smoothed admission rate of CVD were calculated using Global Moran’s I (Table 1). The results indicated that the distribution of CVD admission rates were spatially clustered as most of the global Moran’ I values were significant at the 0.01 significance level during the study period (Table 1). The overall tendencies were higher in some years (e.g. 2004, 2006, and 2010) and the overall degree of clustering for men was higher than for women.
Table 1-.
Global indices of spatial autocorrelation for CVD admission rates in Swedish municipality areas under 0.01 significance level and 9,999 permutations
| Year | Sex | Moran’s I | E[I] | Mean | STD | z-Value | p-Value | Pattern |
|---|---|---|---|---|---|---|---|---|
| 2000 | Females | 0.1002 | −0.0035 | −0.0037 | 0.0275 | 3.7779 | 0.00030 | Clustered |
| 2001 | Females | 0.1068 | −0.0035 | −0.0032 | 0.0269 | 4.0848 | 0.00020 | Clustered |
| 2002 | Females | 0.0533 | −0.0035 | −0.0035 | 0.0272 | 2.0878 | 0.02650 | Random |
| 2003 | Females | 0.0883 | −0.0035 | −0.0033 | 0.0274 | 3.3468 | 0.001500 | Clustered |
| 2004 | Females | 0.1401 | −0.0035 | −0.0026 | 0.0279 | 5.3316 | 0.000100 | Clustered |
| 2005 | Females | 0.0815 | −0.0035 | −0.0033 | 0.0277 | 3.0675 | 0.002300 | Clustered |
| 2006 | Females | 0.1609 | −0.0035 | −0.0026 | 0.0268 | 3.0918 | 0.00100 | Clustered |
| 2007 | Females | 0.1579 | −0.0035 | −0.0034 | 0.0272 | 5.9208 | 0.00010 | Clustered |
| 2008 | Females | 0.1173 | −0.0035 | −0.0036 | 0.0272 | 4.4067 | 0.000200 | Clustered |
| 2009 | Females | 0.1490 | −0.0035 | −0.0035 | 0.0273 | 5.5891 | 0.00010 | Clustered |
| 2010 | Females | 0.1631 | −0.0035 | −0.0034 | 0.0272 | 6.1146 | 0.00010 | Clustered |
| 2000 | Males | 0.0118 | −0.0035 | −0.0034 | 0.0273 | 0.5568 | 0.277900 | Random |
| 2001 | Males | 0.0981 | −0.0035 | −0.0032 | 0.0274 | 3.7107 | 0.00080 | Clustered |
| 2002 | Males | 0.0513 | −0.0035 | −0.0032 | 0.0274 | 1.9870 | 0.030900 | Random |
| 2003 | Males | 0.0993 | −0.0035 | −0.0034 | 0.0274 | 3.7434 | 0.000500 | Clustered |
| 2004 | Males | 0.2112 | −0.0035 | −0.0034 | 0.0272 | 7.8748 | 0.000100 | Clustered |
| 2005 | Males | 0.1629 | −0.0035 | −0.0027 | 0.0279 | 5.9448 | 0.001000 | Clustered |
| 2006 | Males | 0.1609 | −0.0035 | −0.0026 | 0.0268 | 3.0918 | 0.00100 | Clustered |
| 2007 | Males | 0.1304 | −0.0035 | −0.0031 | 0.0272 | 4.8269 | 0.00010 | Clustered |
| 2008 | Males | 0.0990 | −0.0035 | −0.0035 | 0.0272 | 3.7647 | 0.000700 | Clustered |
| 2009 | Males | 0.1753 | −0.0035 | −0.0031 | 0.0271 | 6.5883 | 0.00010 | Clustered |
| 2010 | Males | 0.2643 | −0.0035 | −0.0031 | 0.0273 | 9.8082 | 0.00010 | Clustered |
3.3. Cluster detection
Figure 2 shows clusters of high age-adjusted CVD admission rates for men and women for the time period 2000–2010, with more occurring clusters presented in a deeper color. Figure 2 is an aggregation of yearly clusters, where municipalities had significant local Moran’s I statistics (p-value <0.01). Positive autocorrelations were considered as clusters (high and low admission rates with similar neighbors), while negative autocorrelations were considered as spatial outliers (high and low admission rates with dissimilar neighbors).
Figure 2-.
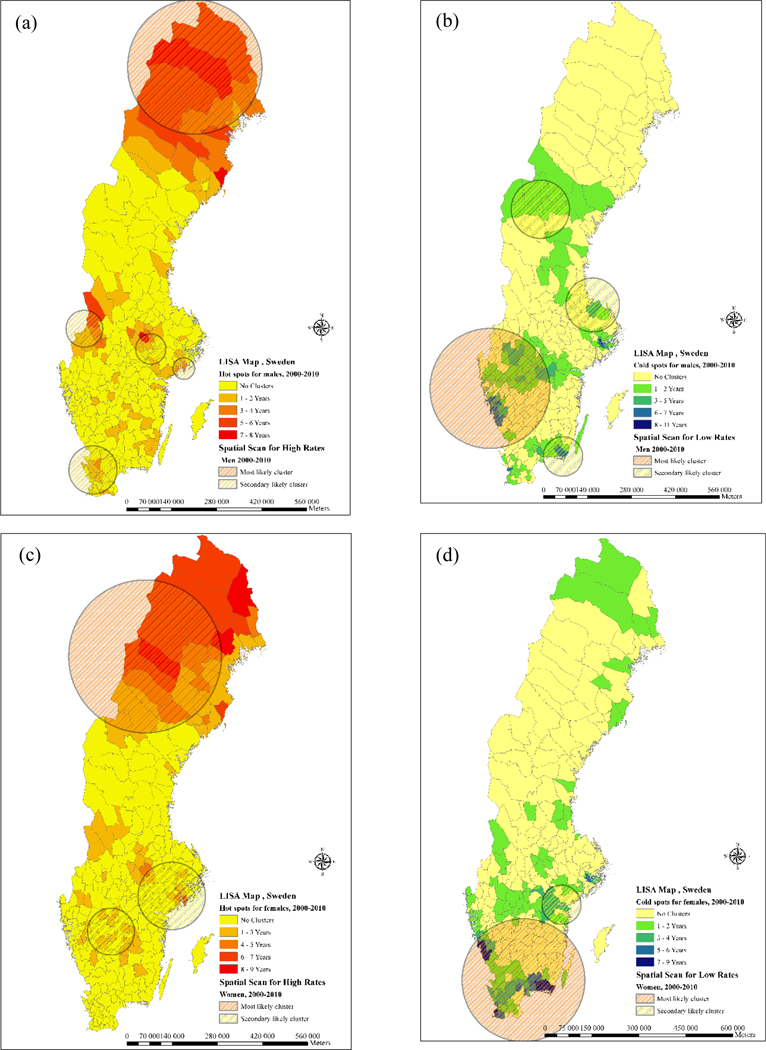
clusters of CVD admission rates during 2000–2010 in Sweden (a) hot spots for males (b) cold spots for males (c) hot spots for females (d) cold spots for males
For both men and women, the clusters of high CVD admission rates, also known as hotspots as depicted in Figure 2, were concentrated in the northern municipalities. Hotspots were also found in some western and central municipalities of Sweden. Furthermore, hotspots were also identified in the vicinity of Stockholm, mostly for women during the study period.
Figure 2 also shows the clusters of low age-adjusted CVD admission rates for men and women for the time period 2000–2010, with more occurring clusters presented in a deeper color. These clusters of low admission rates, also known as cold spots, were seemingly more common for women and predominantly located in the southern parts of the country. The most permanent cold spot during the study period was located in Blekinge. Moreover, cold spots were identified in the proximity of the three largest cities in Sweden; Stockholm, Gothenburg and Malmö. In these cities, the clusters again seemed to be more common in women.
In order to explore the spatial patterns of age-adjusted CVD admission rates on a larger scale, these were also examined with autocorrelation analysis in Stockholm County. Figure 3 was derived in the same way as Figure 2 and shows that CVD hotspots were mostly identified in the more rural neighborhoods of Stockholm County, with a slightly higher frequency for men. On the other side, cold spots were more common in the central part of Stockholm county and mostly pertained to female admissions.
Figure 3-.
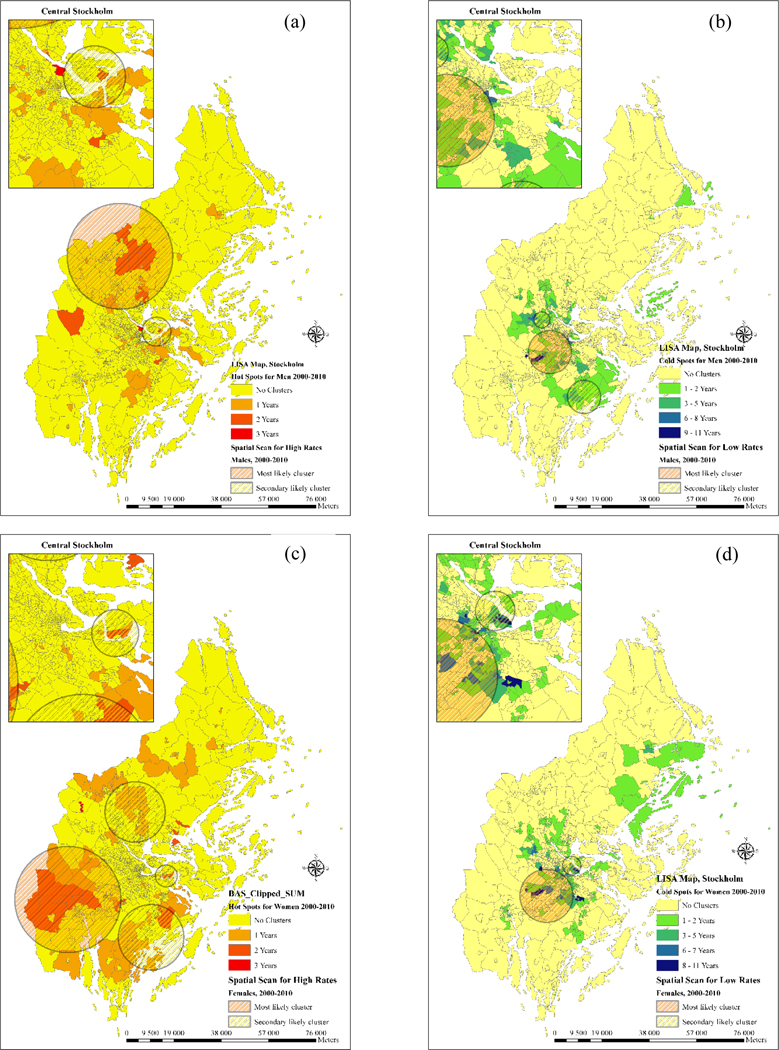
clusters of CVD admission rates during 2000–2010 in Stockholm (a) hot spots for males (b) cold spots for males (c) hot spots for females (d) cold spots for males.
As shown in Figure 2, Figure 3 and Table 2, most likely clusters and secondary clusters of high and low admission rates were detected in Sweden and Stockholm County region from 2000 to 2010. The log likelihood ratio (LLR) indicated a statistical significance both in space and time in the study region which is represented in Table 2. Regarding the geographical distribution of clusters, most of the areas with high frequency of clusters in LISA maps were also detected by spatial scan statistic in both hot spots and cold spots of Sweden and Stockholm county. Secondary likely clusters also matched some of the clusters detected by spatial autocorrelation analysis and highlighted the risk in these areas. The largest cluster was the cold spot detected for females in the southern part of Sweden which covered 100 municipalities and the smallest was the cluster of high admission rates detected in the sparsely populated areas of northern Sweden covering 16 municipalities. Space-time clusters were also detected in the Stockholm county region and indicated a slightly lower relative risk compared to entire Sweden (Table 2).
Table 2-.
Characteristics of the most likely clusters using space-time scan statistics (2000–2010)
| Cluster | Sex | Type | No. Areas | Ratio (observed/ expected) |
RR* | LLR** | P-value |
|---|---|---|---|---|---|---|---|
| Sweden | Male | High | 16 | 1.60 | 1.65 | 4422.412 | < 0.001 |
| Sweden | Female | High | 23 | 1.87 | 1.91 | 4238.011 | < 0.001 |
| Sweden | Male | Low | 93 | 0.89 | 0.91 | 3896.085 | < 0.001 |
| Sweden | Female | Low | 100 | 0.94 | 0.97 | 2299.305 | < 0.001 |
| Stockholm | Male | High | 202 | 1.32 | 1.41 | 3265.511 | < 0.001 |
| Stockholm | Female | High | 183 | 1.26 | 1.34 | 3524.236 | < 0.001 |
| Stockholm | Male | Low | 311 | 0.91 | 0.95 | 4125.984 | < 0.001 |
| Stockholm | Female | Low | 261 | 0.87 | 0.89 | 3956.592 | < 0.001 |
: Relative Risk
: Log likelihood ratio
4. Discussion
4.1. Temporal Trends of CVD
One of the key findings in the present study is that during the study period, 2000–2011, the age-adjusted CVD admission rates on a national level decreased significantly over time, with an approximate 3% decrease per year for both men and women, which might indicate that factors causing the decline in the admissions are quite similar for both genders. During the study period, men consistently had almost twice as high admission rates as women. In absolute numbers, men had a larger decrease than women. This overall declining trend may be partially attributable to changes occurring on a societal level, prevalence of risk factors associated with CVD, changes in the health care system and improved prevention of CVD-related outcomes, as well as changes occurring at the individual level. Moreover, changes in the level of education at the population level and decreased educational inequalities during the past decade could also be considered as one explanatory factor behind decline in CVD admissions(36) which is worth stressing in future studies. Identifying such trends is important from a public health perspective in order to evaluate potential interventions or changes in the health care system. However, it may be difficult to disentangle the separate contributions of changes that may occur during the same time period. This also highlights the need for continuous assessments as it may not be appropriate to assume that the observed declining trends will continue in the future. For instance, in some countries the decline in mortality rates has leveled off during recent years (37, 38), whereas in Sweden a leveling of the mortality rates has not been observed(4).
4.2. Spatial patterns of CVD
Most of the frequently occurred clusters of LISA were also detected by scan statistic’s most likely or secondary likely clusters which highlights the reliability of the resulted maps. However, LISA generates only one value for each location, which could be misleading without any statistical inference. In order to be able to infer LISA results, a random conditional permutation approach (9,999 permutations) was used to obtain a reference distribution for each location. Accordingly, the results were analyzed for possible sensitivity and locations with significant local statistic were detected. However, 0.05 were considered primarily for the level of significance but in order to examine the outliers more precisely the significance was narrowed to 0.001 which resulted in disappearance of all of the outliers and subsequently persisting clusters were considered for the final discussion.
CVD, as the main cause of morbidity and mortality worldwide (3) has only been rarely explored in contrast to other causes of mortality such as cancer, neoplastic diseases, infectious diseases and asthma, whose spatial patterns have been explored more frequently. Moreover, existing studies relevant to CVD were mostly either conducted on a local scale (with a smaller number of occurrences) or have ignored the spatial patterns. To the best of our knowledge, this is the first study to explore the spatial patterns of potential cardiovascular disparities that covers all of Sweden, as well as spatial statistical analysis to explore the geographical variation of hospital admissions of CVD throughout the municipalities of Sweden. Results of the global and local Moran’s I confirmed that CVD admission rates exhibit spatial dependence and several hot and cold spots were identified across Sweden. Furthermore, positive spatial autocorrelations were identified in the patterns of CVD admissions indicating that CVD rates were not spatially homogeneous and were also different for men and women in the study area. In this way, spatial clusters of CVD admissions could be considered as a guide when exploring the potential influential factors causing higher (or lower) admissions of CVD.
Hotspots were generally found in northern municipalities with low population density and high age. There was also a high mobility amongst the younger part of the population. These are also areas with high consumption of welfare resources. The pattern for more rural populations was also observed in parts of southern Sweden such as in the counties of Skåne, Småland and Värmland. The hotspots in the central parts of Sweden include declining industrial areas that are far from commuting ranges. The coastal hotspots in the south were located in scarcely populated remote areas that had an aging population and with fewer opportunities to commute. The spatial patterns reoccurred similarly in women. However, there was a lack of reoccurring clusters in Skåne and Värmland.
Hotspots were found in remote rural areas far from urban centers. These areas are characterized by low population density, aging population and low mobility especially among men. These areas could be old manufacturing areas that are now in decline, which is most likely the case in southern Sweden.
The cold spots for men as well as women clearly showed an axe southeast to southwest in the south of Sweden. The axe was somewhat difficult to explain in the southwest. On commuting distance to Gothenburg, there were four municipalities that showed cold spots over the period. These are rather wealthy municipalities with young populations and a fairly strong industry. Wealthy areas with young populations were also found in suburbs of Skåne and the inner city areas in Stockholm. Some of the patterns might indicate a population with a lifestyle oriented towards outdoor activities and healthy food. The peripheral pattern was quite strong in Stockholm County as shown at the municipality level. In the case of a more centred area it is still in suburbs where cold spots are found. Instead of the more remote areas, as in the hotspots location, there is a more centered pattern but still not in Stockholm municipality, i.e. the urban part of Stockholm County.
The cold spots at every level were concentrated to areas within commuting distance to bigger cities, which perhaps could be an indicator of another lifestyle than in the hotspots. The cold spots also represent areas with a different population structure; a younger, probably healthier and more mobile population. Hotspots were found in different parts of Sweden in different years for both genders, with more consistency in the northern part of the country. On the other side, there were cold spots across large swathes of municipalities in eastern Sweden. The municipalities of Stockholm County frequently experienced hotspots, especially for women, while the other two major cities of Sweden (Malmö and Gothenburg) had several cold spots. This is worth stressing since Stockholm county is a densely populated area and approximately 10% of the total population of Sweden lives there. Thus, spatial patterns of CVD were explored in a more detailed spatial scale in the neighborhoods of Stockholm county in order to highlight neighborhoods with CVD clusters. Accordingly, hotspots located in more rural areas were found mostly for women and some central hotspots were found for men.
Cold spots in central Stockholm were identified in most of the years for women while cold spots pertaining to men were sparse. Moreover, cold spots also occurred in the municipality areas that were located in highly populated areas around the second and third big cities of Sweden (Gothenburg and Malmö), which is also worth some attention. This could perhaps be explained by better socioeconomic status especially a lower proportion of unemployed people; a scenario that is in agreement with other studies investigating the influence of socioeconomic conditions related to CVD, e.g. Wennerholm, Grip (39), Unal, Critchley (5), Kuulasmaa, Tunstall-Pedoe (6).
On several occasions, this study identified municipalities that during the study period were identified as both hot spots and cold spots, mainly located in less densely populated municipalities. An example of such changes occurring over shorter periods of time is the change in Torsby, which went from being a cold spot in 2008 to being a hotspot in 2009 and 2010. We hypothesize that such findings reflect the size of the underlying vulnerable population. From a medical point of view, a year with higher hospital admissions may through mortality or efficient treatments deplete the pool of vulnerable individuals and will likely be followed by subsequent years of less admissions and vice versa. However, the changing distribution of hot and cold spots over time further emphasizes that continuous evaluation is of importance as it may not be appropriate to assume that observed trends will remain in the future. For instance, from a policy and public health perspective such a continuous evaluation would identify the emergence of a cold spot around lake Vänern. These cold spots started to appear during the last two years of the study period, providing tools to retrospectively identify potential changes occurring at the municipality level. Even though such a pattern may be random and difficult to explain from a causal point of view, the identification of trends and spatial patterns are of importance when planning future health care and allocating scarce public health care resources.
There were also some sporadic outliers identified in different parts of the study area. Further investigations indicated that most of the outliers were inconsistent and disappeared in a shorter period of time during the study period. The overall tendencies were higher in some years (e.g. 2004, 2006, and 2010) and the overall degree of clustering for men was higher than for women. One possible explanation for the higher tendencies in some years may be that the size of the vulnerable population changes.
Although both outliers and clusters indicate variations in CVD admission rates, their potential influencing areas are different. The outliers only represent single locations where there were dissimilarities between the calculated statistics in a local neighborhood. On the other hand, the clusters represent the core of the potential influencing area, which likely could be extended to its neighborhood. Therefore, it should be noticed that the spatial extent of the hotspots and cold spots could include much larger areas than represented by their cores alone.
Two cluster detection methods were applied to investigate the spatial pattern of CVD admission rates in Sweden. Unlike spatial scan statistic, LISA can detect any cluster shape(40) but it can result in outliers when the sample has a varying size especially in poorly sampled areas (41). The spatial scan statistic proposed by Kulldorff (23) detects the potential clusters through a circular window and it has difficulties in identifying noncircular clusters. This highlights a potential problem especially with the irregular shapes of BAS areas in the dense region of Stockholm. Flexible spatial scan statistic can be applied to detect clusters of irregular shapes, which does not have that problem when using spatial scan statistic. The latter could be noticed for future studies. However, it only works properly with smaller cluster sizes including maximum 30 irregular shapes and cannot be applied in study areas like Sweden where clusters could include 100 municipalities and 202 BAS divisions(42). It is also worth mentioning that the most likely cluster in SaTScan is usually very large and unrealistically includes neighboring areas that does not have elevated risks (43). Moreover, setting the maximum cluster size at 50% of population also generates sensitivities (44) by delineation of a single large cluster which consists of multiple smaller clusters with lower rates. Hence, a sophisticated method should be applied to find the proper window size and shape in future studies of spatial scan statistic (45), preferably based on large-scale registers to gain statistical power. Finally, from the data reliability perspective, the Swedish nationwide population and health care registry data have a well-documented accuracy and completeness which calls for more attention in spatial analysis approaches for investigating different public health problems.
This study showed a decline in CVD admission rates in Sweden between 2000 and 2010, which has also been found in previous studies. However, the results indicate that there still is a large population at risk and the high-risk areas became more concentrated in some parts of Sweden over time, which calls for a clinical attention and prevention. Although the preventive measures, clinical diagnosis and quality regarding CVD treatment has clearly been successful during the past decades in Sweden, the number of admissions is still high. The complex etiology of CVD, which involves many different endogenous or exogenous factors such as genetic, lifestyle, socio-economic, and environmental factors, makes it difficult to identify the major sources of this public health problem. Based on the results from this study, the identification of hotspots of CVD admission rates should become a vital part in future strategies of disease control and potential clarification of disease etiology. The findings also confirm the link between CVD and space, thereby providing a reference for authorities to mitigate disease burden in hotspots by the allocation of health care resources. Meanwhile, the clusters indicated the center of areas with high admission rates and the preventive efforts should not be confined to just one hot spot, but also to neighboring areas.
Moran’s Index for spatial autocorrelation could also be applied with temporal attributes to assess the spatiotemporal patterns of CVD admission rates across Sweden. This will be presented in future studies of the authors. Moreover, the spatial dependencies of CVD admission rates with socioeconomic or environmental conditions such as social deprivation or air pollution could be examined with spatial regression approaches and constitute another topic for future studies.
5. Conclusion
The temporal trends and spatial patterns of CVD in Sweden were explored in both men and women between 2000 and 2010 in all Swedish municipalities and in all neighborhoods in Stockholm. The results show that the spatial distribution of CVD in Sweden was clustered and that there were spatial variations in CVD admission rates in the country during the 11-year study period. Hotspots were found as well as cold spots and results varied in different parts of Sweden as well as in men and women. Some area units tended to be hotspots or cold spots during long time periods. In future, the spatial distribution of hot spots and cold spots analyzed in all of Sweden can be used to plan healthcare interventions and risk reduction strategies as well as for the exploration of potential environmental or socioeconomic factors that may lead to the development of hotspots.
6.1. Acknowledgments
This study was part of a project funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL116381 to Kristina Sundquist. The first author of this study is financed by the European scholarship program Erasmus Mundus Action 2 project SALAM under scholarship number SALA1203450. The authors greatly appreciate the funding.
6.3. Funding
This study was part of a project funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL116381 to Kristina Sundquist.
Footnotes
Ethical approval
This study was approved by the Ethical Review Board, 2012/795, of the Ministry of Health, Sweden.
Competing interests
None declared.
1. References
- 1.Zöller B, Li X, Sundquist J, Sundquist K. Risk of subsequent coronary heart disease in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. PLoS One. 2012;7(3):e33442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giang KW, Björck L, Novak M, Lappas G, Wilhelmsen L, Torén K, et al. Stroke and coronary heart disease: predictive power of standard risk factors into old age—long-term cumulative risk study among men in Gothenburg, Sweden. European heart journal. 2013;34(14):1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430(6996):242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg J, Björck L, Lappas G, O’Flaherty M, Capewell S, Rosengren A. Continuing decrease in coronary heart disease mortality in Sweden. BMC cardiovascular disorders. 2014;14(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109(9):1101–7. [DOI] [PubMed] [Google Scholar]
- 6.Kuulasmaa K, Tunstall-Pedoe H, Dobson A, Fortmann S, Sans S, Tolonen H, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. The lancet. 2000;355(9205):675–87. [DOI] [PubMed] [Google Scholar]
- 7.Björck L, Rosengren A, Bennett K, Lappas G, Capewell S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. European heart journal. 2009. [DOI] [PubMed] [Google Scholar]
- 8.Rosvall M, Chaix B, Lynch J, Lindström M, Merlo J. Contribution of main causes of death to social inequalities in mortality in the whole population of Scania, Sweden. BMC Public Health. 2006;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinaldi L, Musella V, Biggeri A, Cringoli G. New insights into the application of geographical information systems and remote sensing in veterinary parasitology. Geospatial health. 2006;1(1):33–47. [DOI] [PubMed] [Google Scholar]
- 10.Ostfeld RS, Glass GE, Keesing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends in ecology & evolution. 2005;20(6):328–36. [DOI] [PubMed] [Google Scholar]
- 11.Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. American journal of epidemiology. 1997;146(1):48–63. [DOI] [PubMed] [Google Scholar]
- 12.Chaix B Geographic life environments and coronary heart disease: a literature review, theoretical contributions, methodological updates, and a research agenda. Annual review of public health. 2009;30:81–105. [DOI] [PubMed] [Google Scholar]
- 13.Chow CK, Lock K, Teo K, Subramanian S, McKee M, Yusuf S. Environmental and societal influences acting on cardiovascular risk factors and disease at a population level: a review. International journal of epidemiology. 2009:dyn258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann B, Moebus S, Stang A, Beck E-M, Dragano N, Möhlenkamp S, et al. Residence close to high traffic and prevalence of coronary heart disease. European Heart Journal. 2006;27(22):2696–702. [DOI] [PubMed] [Google Scholar]
- 15.Xia C, Bergquist R, Lynn H, Hu F, Lin D, Hao Y, et al. Village-based spatio-temporal cluster analysis of the schistosomiasis risk in the Poyang Lake Region, China. Parasites & vectors. 2017;10(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughnan ME, Nicholls N, Tapper NJ. Demographic, seasonal, and spatial differences in acute myocardial infarction admissions to hospital in Melbourne Australia. International Journal of Health Geographics. 2008;7(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soljak M, Samarasundera E, Indulkar T, Walford H, Majeed A. Variations in cardiovascular disease under-diagnosis in England: national cross-sectional spatial analysis. BMC cardiovascular disorders. 2011;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asaria P, Fortunato L, Fecht D, Tzoulaki I, Abellan JJ, Hambly P, et al. Trends and inequalities in cardiovascular disease mortality across 7932 English electoral wards, 1982–2006: Bayesian spatial analysis. International journal of Epidemiology. 2012;41(6):1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L-G, Zhao J, Ren Z- P, Wang Y- Y, Peng Z- Q, Wang J- F, et al. Spatial patterns of the congenital heart disease prevalence among 0-to 14-year-old children in Sichuan Basin, P. R China, from 2004 to 2009. BMC public health. 2014;14(1):595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng C-L, Chen Y- C, Liu T- M, Yang Y- HK. Using spatial analysis to demonstrate the heterogeneity of the cardiovascular drug-prescribing pattern in Taiwan. BMC public health. 2011;11(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran PA. The interpretation of statistical maps. Journal of the Royal Statistical Society Series B (Methodological). 1948;10(2):243–51. [Google Scholar]
- 22.Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Statistics in medicine. 1995;14(8):799–810. [DOI] [PubMed] [Google Scholar]
- 23.Kulldorff M A spatial scan statistic. Communications in Statistics-Theory and methods. 1997;26(6):1481–96. [Google Scholar]
- 24.StatisticsSweden. Statistical database. Statistics Sweden; Available at: http://wwwssdscbse (20 March 2017, date last accessed)2000–2010. [Google Scholar]
- 25.Sundquist K, Malmström M, Johansson S. Neighbourhood deprivation and incidence of coronary heart disease: a multilevel study of 2.6 million women and men in Sweden. Journal of epidemiology and community health. 2004;58(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtin LR, Klein RJ. Direct standardization (age-adjusted death rates): US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics; 1995. [Google Scholar]
- 27.Anselin L, Lozano N, Koschinsky J. Rate transformations and smoothing. Urbana. 2006;51:61801. [Google Scholar]
- 28.Clayton D, Kaldor J. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics. 1987:671–81. [PubMed] [Google Scholar]
- 29.Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine. 2000;19(3):335–51. [DOI] [PubMed] [Google Scholar]
- 30.Joinpoint. Joinpoint Regression Program. 4.4.0.0. ed. Calverton, MD: Statistical Research and Applications Branch, National Cancer Institute; 2017. [Google Scholar]
- 31.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60(4):1005–14. [DOI] [PubMed] [Google Scholar]
- 32.Knox EG. Detection of clusters. Methodology of enquiries into disease clustering London: Small Area Health Statistics Unit. 1989;17:20. [Google Scholar]
- 33.Lawson AB. Statistical methods in spatial epidemiology: John Wiley & Sons; 2013. [Google Scholar]
- 34.Anselin L Local indicators of spatial association—LISA. Geographical analysis. 1995;27(2):93–115. [Google Scholar]
- 35.Song C, Kulldorff M. Power evaluation of disease clustering tests. International journal of health geographics. 2003;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulo E, Nygård O, Vollset SE, Igland J, Sulo G, Ebbing M, et al. Coronary angiography and myocardial revascularization following the first acute myocardial infarction in Norway during 2001–2009: Analyzing time trends and educational inequalities using data from the CVDNOR project. International journal of cardiology. 2016;212:122–8. [DOI] [PubMed] [Google Scholar]
- 37.O’Flaherty M, Ford E, Allender S, Scarborough P, Capewell S. Coronary heart disease trends in England and Wales from 1984 to 2004: concealed levelling of mortality rates among young adults. Heart. 2008;94(2):178–81. [DOI] [PubMed] [Google Scholar]
- 38.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the US from 1980 through 2002. Journal of the American College of Cardiology. 2007;50(22):2128–32. [DOI] [PubMed] [Google Scholar]
- 39.Wennerholm C, Grip B, Johansson A, Nilsson H, Honkasalo M-L, Faresjö T. Cardiovascular disease occurrence in two close but different social environments. International journal of health geographics. 2011;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Luo L, Xu W, Ledwith V. Use of local Moran’s I and GIS to identify pollution hotspots of Pb in urban soils of Galway, Ireland. Science of the total environment. 2008;398(1–3):212–21. [DOI] [PubMed] [Google Scholar]
- 41.Gelman A, Price PN. All maps of parameter estimates are misleading. Statistics in medicine. 1999;18(23):3221–34. [DOI] [PubMed] [Google Scholar]
- 42.Tango T, Takahashi K. A flexibly shaped spatial scan statistic for detecting clusters. International journal of health geographics. 2005;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tango T A spatial scan statistic scanning only the regions with elevated risk. Advances in Disease Surveillance. 2007;4:117. [Google Scholar]
- 44.DeChello LM, Sheehan TJ. Spatial analysis of colorectal cancer incidence and proportion of late-stage in Massachusetts residents: 1995–1998. International journal of health geographics. 2007;6(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugumaran R, Larson SR, DeGroote JP. Spatio-temporal cluster analysis of county-based human West Nile virus incidence in the continental United States. International journal of health geographics. 2009;8(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]


