Abstract
Thyroid hormones (THs) are essential for brain development, but few rodent models exist that link TH inefficiency to apical neurodevelopmental endpoints. We have previously described a structural anomaly, a heterotopia, in the brains of rats treated in utero with propylthiouracil (PTU). However, how the timing of an exposure relates to this birth defect is unknown. This study seeks to understand how various temporal treatments of the mother relates to TH insufficiency and adverse neurodevelopment of the offspring. Pregnant rats were exposed to PTU (0 or 3 ppm) through the drinking water from gestational day 6 until postnatal day (PN) 14. On PN2 a subset of pups was cross-fostered to a dam of the opposite treatment, to create 4 conditions: pups exposed to PTU prenatally, postnatally, during both periods, or not at all (control). Both PTU and TH concentrations were characterized in the mother and offspring over time, to capture the dynamics of a developmental xenobiotic exposure. Brains of offspring were examined for heterotopia presence and severity, and adult littermates were assessed for memory impairments. Heterotopia were observed under conditions of prenatal exposure, and its severity increased in animals in the most prolonged exposure group. This malformation was also permanent, but not sex biased. In contrast, behavioral impairments were limited to males, and only in animals exposed to PTU during both the gestational and postnatal periods. This suggests a distinct TH-dependent etiology for both phenotypes, and illustrates how timing of hypothyroxinemia can induce abnormal brain structure and function.
Keywords: hypothyroidism, brain development, learning and memory, cortical malformation, developmental neurotoxicity, thyroid hormone
Thyroid hormone (TH) homeostasis during pregnancy is crucial for proper brain development and organization to occur in the fetus (Williams, 2008; Zoeller and Rovet, 2004). This is highlighted in cases of severe maternal and fetal hypothyroidism, caused by conditions like iodine deficiency, Hashimoto’s disease, or premature birth. This endocrine dysregulation during pregnancy and the postpartum period has been directly linked to an array of neurodevelopmental phenotypes including cretinism, mental retardation, and attention deficit hyperactivity disorder (ADHD) (see review by Berbel et al., 2014). Although the association between TH and neurodevelopment is well supported, the impact of slightly reduced or “subclinical” levels of hypothyroidism and hypothyroxinemia is much less understood. As such, there is increasing concern for the potential human health consequences of environmental endocrine disrupting chemicals (EDCs). Many EDCs are known to alter circulating levels of THs; specifically, the levels of thyroxine (T4) and the active counterpart triiodothyronine (T3) (Brucker-Davis, 1998; Duntas, 2015; Gilbert and Zoeller, 2010; Gore et al., 2015; US EPA, 2013). To understand the potential risk of thyroid disrupting chemicals on brain development, it is critical to understand (1) a chemical’s dosimetry in the mother and offspring, (2) the consequent endocrine response to that chemical, and (3) its relationship to downstream, quantifiable, neurodevelopmental endpoints.
We have previously reported the presence of an ectopic body of cells, referred to as a cortical heterotopia, referred to as a cortical heterotopia, in rat pups born to hypothyroid dams (Gilbert et al., 2014; Goodman and Gilbert, 2007; Powell et al., 2012; Spring et al., 2016). This heterotopia comprises glutamatergic and GABAergic neurons, and is positioned bilaterally in the corpus callosum; (Gilbert et al., 2014) additionally, cells in this abnormality are structurally and functionally connected to cortical neurons (Goodman and Gilbert, 2007). Animals with this birth defect also exhibit hippocampal synaptic transmission and plasticity impairments, white matter abnormalities, increased seizure sensitivity, and learning and memory deficits (Gilbert, 2004, 2011; Gilbert et al., 2014, 2016, 2017; Gilbert and Sui, 2008, 2006; Sharlin et al., 2008). Interestingly, the severity of the phenotype is dependent on the degree of hypothyroidism, and is observable under low or moderate levels of maternal TH disruption induced by propylthiouracil (PTU) and methimazole (Gilbert et al., 2014; Goodman and Gilbert, 2007; Powell et al., 2012; Shibutani et al., 2009; Spring et al., 2016). Given these properties, the heterotopia is a useful downstream indicator of TH insufficiency and provides a means to investigate the mechanistic basis of TH role in brain development. In humans cortical heterotopias are often associated with mental retardation, neurodevelopmental disorders, and epilepsy in afflicted patients (Davies, 2014; Watrin et al., 2015; Werling and Geschwind, 2013). Although certain genetic mutations are directly associated with formation of heterotopias in humans (Guerrini and Carrozzo, 2002; Moro et al., 2002; Watrin et al., 2015), it is unknown what, if any, environmental influences are also linked to this malformation.
Given the potential utility of the described rat heterotopia for evaluating the effects of thyroid disrupting chemicals, understanding its developmental underpinnings is crucial. For example, elucidating how the degree, duration, and timing of developmental TH insufficiency impacts observed morphological and behavioral impairments. To begin to address these questions, a cross-fostering study design was employed using a low dose of the hormone synthesis inhibitor PTU. Serum concentrations of PTU and THs in both dams and pups were examined to characterize the status of the thyroid axis across different exposure scenarios. Changes in heterotopia incidence and volume were also monitored over the lifespan of the animal to determine any exposure-specific effects. Finally, because neurodevelopmental disorders in humans are often male biased (Davies, 2014; Werling and Geschwind, 2013), heterotopia incidence and cognitive impairments were examined in both male and female offspring. This work represents a significant extension of our characterization of the conditions requisite for heterotopia formation, and provides a systematic evaluation of its properties.
MATERIALS AND METHODS
Animals and treatment.
All animal treatments were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Timed pregnant Long Evans rats (n=32) were obtained from Charles River (Raleigh, NC) on gestational day (GD) 2 (vaginal sperm detection=GD0), and housed individually in standard plastic hanging cages in an AAALAC-approved animal facility. Animal rooms were maintained on a 12:12 light:dark schedule, and rats were permitted free access to food (Purina 5008 chow) and deionized water. The experimental design is depicted in Figure 1A. Beginning on GD6, pregnant dams were exposed either to 0 ppm (control, N=16) or 3 ppm (0.0003%, N=16) 6-propyl-2-thiouracil (Sigma, St. Louis MO) dissolved in the drinking water until postnatal day (PN) 14. All control dams produced viable litters (n=16 litters), and one PTU treated dam did not give birth (n=15 litters). On PN2, pups were standardized to five males and five females per litter. Blood from extra pups was pooled within a litter for hormone and chemical analysis. All remaining female pups from half the litters of each treatment group were fostered to a dam of the opposite dose group. The other half of dams from each treatment group had all male pups fostered to a dam of the opposite dose group (Figure 1B). This scheme created four treatment conditions in the offspring and allowed sex of the pup to serve as treatment group identifier. As depicted in Figure 1B, the four treatment groups were comprised of pups that received no treatment gestationally or postnatally (CON-CON), pups that received PTU via the milk beginning on PN2 (CON-PTU), pups that received PTU during gestation until PN2 (PTU-CON), and finally a group of pups that received PTU from early gestation until mid-lactation (PTU-PTU). PTU treatment ceased on PN14 at which time all dams received deionized water until the pups were weaned, and the dams were sacrificed on PN21. At weaning, pups were transferred to plastic hanging cages (2–3/cage with same sex littermates), and permitted free access to chow (Purina 5001) and filtered tap water.
Figure 1.
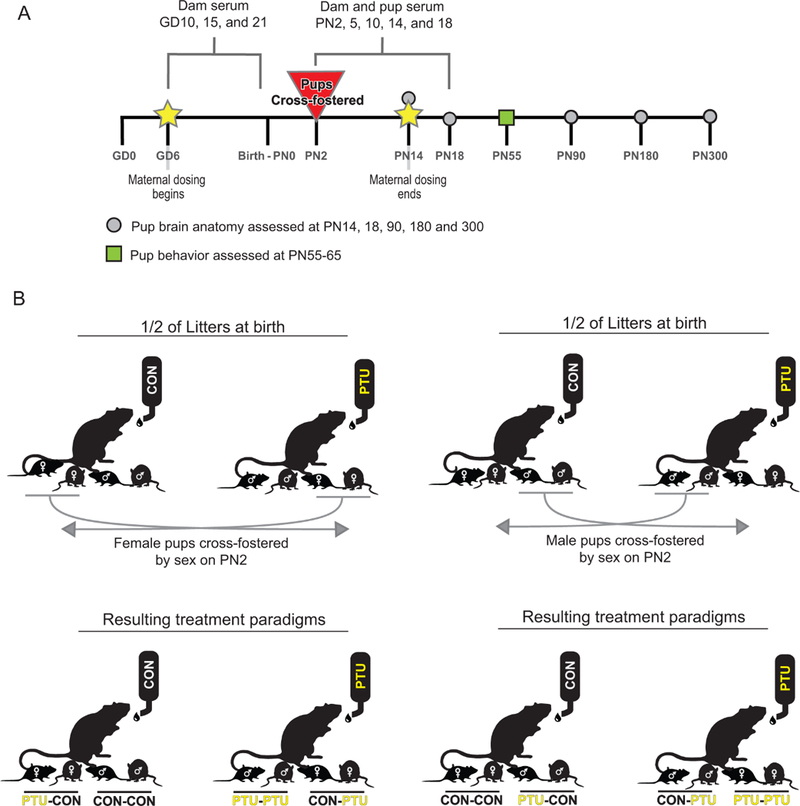
(A) Experimental design of the presented study. Dams were treated with either 0 or 3ppm propylthiouracil (PTU) from gestational day (GD)6 to postnatal day (PN)14. Blood was sampled the dams at several time points throughout the study. Litters were culled to five males and five females on PN2 before cross-fostering. Pups were euthanized on PN5, 10 14 18, 90, 300, blood collected, and brains fixed for histological analysis. Male and female offspring were assessed on fear conditioning between PN55–PN65. (B) Cross-fostering schematic. After standardization of litters on PN2 to equal number of pups, female offspring from half of the control and dosed dams were cross-fostered to dams of the opposite dose group. In the remaining half of the litters, the males were cross-fostered. This created four pup exposure conditions (CON-CON, CON-PTU, PTU-CON, and PTU-PTU) and permitted tracking of exposure history by sex.
In the cross-fostering study male and female pups were not siblings, as cross-fostering occurred by sex on PN2. To more directly assess potential sex-dependent effects, a second experiment (Experiment 2) was conducted in which dams were exposed to 0 (n=8) or 3ppm (n=6) PTU from GD6 through PN14 (equivalent to PTU-PTU). Male and female littermates were then assayed for heterotopia on PN15 (see histology methods).
General toxicity assessment.
Dam body weight was monitored throughout gestation and lactation (GD2–PN21), and pups were weighed by sex and litter on PN2, PN5, PN10, PN14, and PN18. Eye opening was examined once daily from PN13 to PN17, and the number of pups in each treatment condition in each litter with both eyes open was recorded. To evaluate for indicators of thyroid toxicity, pup thyroid glands were dissected and weighed at the time sacrifice on PN10, 14, and 18. Dam thyroid glands were removed and weighed on PN21.
Serum collection in dams and pups.
Blood was sampled in a subset of dams by intravenous tail puncture on GD10, GD15, GD20, PN5, PN10, PN14, and PN18 to provide a profile of serum PTU and serum THs over the course of pregnancy and lactation. The postnatal sampling time in dams corresponded to dates of pup sacrifice. Tail blood was collected using a 21-gauge heparinized butterfly needle inserted into the tail vein, where 1ml of whole blood was collected; all animals were briefly restrained in a Tailveiner (Braintree Scientific Inc, Braintree, MA) for this procedure. On PN21, dams were euthanized by decapitation and whole trunk blood collected.
Pups culled on PN2 were euthanized by decapitation and trunk blood collected, pooled across littermates, and assessed for PTU and TH levels. One male pup from each litter (representing the 4 distinct treatment conditions) was sacrificed by decapitation on PN5, PN10, and PN14. On PN18, four days after termination of PTU treatment, female pups were similarly sampled. For all dam and pup samples, whole blood was collected and allowed to clot on ice for thirty minutes. Serum was then isolated by centrifugation (3000 x g), and stored at –80°C for hormone and chemical analyses.
Serum PTU analysis: extraction and quantification.
PTU was extracted from serum using a liquid-liquid extraction method described previously by Hassan et al. (2017). An aliquot (50 ml) of serum was transferred into a 2.0-ml polypropylene centrifuge tube, followed by 10 ml of PTU-d5 as the internal standard (IS). Dithiothreitol (250 ml of 1% in phosphate-buffered saline [PBS]) was added to each sample, and allowed to denature for 30 min in a 65°C water bath. Equilibration to room temperature and additionof 1-ml of ethyl acetate was followed by PTU extraction into the organic layer by pulse vortex-mixing and centrifugation. The upper organic layer was transferred to an autosampling vial and evaporated under N2 at 50°C. For analysis by liquid chromatography/tandem mass spectrometry (LC/MS/MS), the extracts were reconstituted in 50 ml of methanol/water (5:95, vol/vol) with 0.1% formic acid. The LC/LC/MS system consisted of a Thermo Scientific Surveyor LC system and TSQ Quantum Ultra AM Mass Spectrometer. Chromatographic separation was performed on a Phenomenex Kinetex EVO-C18 column (2.6 mm, 100mm x 2.1mm) at 50°C. A linear gradient using 0.1% of formic acid in water as mobile phase A and 0.1% formic acid in methanol as mobile phase B were used at a flow rate of 0.3 ml/min; the starting mobile phase composition was 95% A and 5% B. After 2 min, the gradient was ramped to 100% B within 5 min, and held at 100% B for an additional 2 min. The column was then re-equilibrated to starting conditions for 5 min. Analytes were detected by positive-ion heated electrospray ionization (ESI), and quantification of PTU and PTU-d5 was achieved by selected reaction monitoring.
Matrix-matched calibration standards were used for quantitation, and calibration standards were prepared and analyzed in the same manner as project samples. The calibration curve used a minimum of 5 points over a range of 1–1000 ng/ml, and the coefficient of determination for the calibration curve was ≥0.995. Each sample batch consisted of a method blank, matrix matched laboratory control sample, and a continuing calibration verification sample. Quality control parameters were experimentally determined by a method validation study. The study consisted of a recovery, method detection limit, matrix effect, and inter- and intra-day precision study. The calculated concentrations for PTU in the laboratory control sample and continuing calibration verification sample were within 80%–132% of actual spike amount as determined by the method validation study. PTU levels in all the control samples were below the limit of detection but set to the limit of detection of 0.3 ng/ml for statistical purposes.
Serum TH extraction and quantification.
Total thyroxine (T4) and triiodothyronine (T3) were measured in dam and pup serum using LC/MS/MS methods previously described (Hornung et al., 2015) with the following exceptions. In this study, serum samples were processed using solid phase extraction (SPE) 96-well plates. Briefly, 20 ml of dam serum or 40 ml of pup serum was placed into individual wells of a 96-well collection plate and spiked with stable isotopes, 13C6-T3 and 13C6-T4. To each 20 ml dam serum sample, the following reagents were added: 20 ml 1N HCl, 100 ml H2O, and 60 ml of 50% H2O:50% acetonitrile (vol/vol) with 0.1% formic acid. The reagent volumes were doubled for pup serum. The samples were vortexed, incubated at 37°C for 2h, cooled to ambient temperature, diluted with an aqueous solution of 0.1% acetic acid, and vortexed. The hydrolyzed samples were processed through SPE well plates (Evolute CX, 96-well SPE plate, 10 mg, 1ml, Biotage, Charlotte, NC) using a positive pressure manifold (PPM-96, Biotage). The SPE wells were conditioned with methanol followed by an aqueous solution of 0.1% acetic acid. The hydrolyzed samples were then transferred to the SPE wells and low pressure was applied. The SPE wells were washed with 0.1% acetic acid and then methanol. A collection plate was placed below the SPE plate and TH were eluted using 2.5% NH4OH in methanol. The SPE extracts were evaporated to dryness with heated N2 using a 96-well plate SPE drying unit (SPE Dry 96, Biotage). The SPE dried extracts were reconstituted with 100 ml of 25% acetronitrile:75% H2O (vol/vol) with 0.1% formic acid and transferred to amber microsampling vials prior to quantification (Agilent Technologies, Santa Clara, CA).
The SPE reconstituted extracts from serum were analyzed for TH by stable isotope dilution-LC/MS/MS using an Agilent 1290 ultra-high pressure LC with a 6490 triple quadrupole mass spectrometer. Chromatographic separation was performed by injecting 20 ml of sample into an Agilent Zorbax SB-C18 RRHT column (1.8 mm, 50mm x 2.1mm) which was maintained at 30_C. THs were eluted under gradient conditions with 5% acetonitrile:95% H2O (vol/vol) with 0.1% formic acid (mobile phase A) and 90% acetonitrile: 10% H2O (vol/vol) with 0.1% formic acid (mobile phase B) at a flow rate of 0.4 ml/min. The LC column effluent was introduced into the mass spectrometer equipped with an ESI source and heated jet spray interface. The ESI source was operated in positive ion mode with multiple reaction monitoring. Analyte concentrations were determined using the IS method of quantification with stable isotopes 13C6-T3 and 13C6-T4 as ISs for T3 and T4, respectively. Calibration curves for T3 and T4 were linear with correlation coefficients ≥0.996. Stable isotopes 13C6-T3 and 13C6-T4, spiked into each sample at sample processing initiation, were used to correct TH concentrations in each sample for procedural recovery and for MS ion suppression. Quality control samples consisted of procedural blanks, matrix spiked stripped serum, and duplicate samples. Mean precision (n=16), reported as relative percent difference between duplicate samples, was 5.4% and 6.5% for T3 and T4, respectively. Mean recovery (n=3) of T3 and T4 in matrix spiked samples was 101.4% and 111.3%, respectively. Procedural blanks were below the lower limit of quantification of 0.025 ng/ml for T3 and T4.
Brain collection for histology.
The brains of male and female offspring were collected on PN14, PN15 (experiment 2), and PN18 to analyze for the presence and severity of the cortical heterotopia. The brain was removed from the skull and immersion fixed in 4% paraformaldehyde (Fisher Scientific, Hampton NH) in 0.1M PBS pH 7.4–7.6 at 4°C for 48 h. The fixative was then removed and replaced with DeOlmos solution (30% sucrose, 30% ethylene glycol, and 1% polyvinylpyrrolidone in 0.1M PBS pH 7.4) before storage at 4°C as previously described (Gilbert et al., 2014; Goodman and Gilbert, 2007). To examine characteristics of the heterotopia in adult animals, female offspring on PN90, and male and female offspring on PN280–300 were euthanized by an overdose of Euthasol administered intraperitoneally, and the brain fixed by cardiac perfusion according to the standard methods. Briefly, the cardiovascular system was flushed with 0.1M PBS pH 7.4, followed by fixation with 4% paraformaldehyde. The brain was kept in the skull in situ, and postfixed in 4% paraformaldehyde overnight. The brain was then removed from the skull, postfixed again in fresh 4% paraformaldehyde overnight, and transferred to DeOlmos solution for long term storage at 4°C.
Immunohistochemistry and heterotopia analysis.
As the cortical heterotopia is characterized by the presence of ectopic mature neurons, we routinely assay for this defect by analyzing NeuN immunoreactivity according to previously published methods (Gilbert et al., 2014; Goodman and Gilbert, 2007; Spring et al., 2016). Brains were sectioned coronally at 60 mm in Tris-buffered saline at pH 7.4, and were further processed using free-floating immunohistochemistry. Sections were incubated overnight at 4°C in NeuN primary antibody (MAB377, Millipore, Temecula, CA), diluted 1:2500, followed by a biotinylated secondary antibody (1:400) in conjunction with avidin-biotin amplification (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Signal was detected by diaminobenzidine tetrahydrochloride as the chromogen. Following immunohistochemistry, sections were mounted on gelatin-coated glass slides, dried, and cover slipped. Slides were imaged using an Aperio AT2 slide scanner, scanned at x 20 magnification (Leica Biosystems, Buffalo Grove, IL).
Each tissue section was inspected for the presence and size of the heterotopia using Aperio ImageScope software, and all analyses were done blinded to the exposure group. If a heterotopia was detected, the area of this region was calculated by tracing the perimeter of the NeuN-positive cell cluster. From these area measures, heterotopia volume was estimated by summing the total calculated heterotopia areas across sections, and then multiplying by both section thickness (60 mm) and interval (1) according to procedures of Gunderson and Jensen (1987). To control for discrepancies in brain size across individuals, treatments, and developmental stages, whole brain area was calculated on every third section to estimate a mean brain area. As previously reported (Gilbert et al., 2014; Spring et al., 2016), occasionally small cluster(s) of NeuN-positive cells within a similar anatomical region as the heterotopia was detected in control animals. Therefore, to correct for this normal variation in neuron localization, a heterotopia was defined as a cluster of neurons with a volume in excess of 0.003 mm3. This calculation is based on previous work across multiple studies, and this minimum threshold volume was used here to calculate the incidence of this cortical defect (Gilbert et al., 2014; Spring et al., 2016).
Behavioral assessment.
Trace fear conditioning, cue learning, and context learning were assayed in both male and female offspring between PN55 and PN65. Testing was performed in 14–18 rats/sex/treatment, with no more than two animals from any given litter represented in any treatment condition. Procedures were similar to those previously used in our laboratory and described elsewhere (Gilbert et al., 2016; Oshiro et al., 2014). Briefly, two training trials were given. After a 2-minute (min) baseline period, a compound 15-second (s) light/tone stimulus was presented, terminating 30 s before (the trace interval) administration of a brief, mild footshock (0.5 s, 1 mA). An intermittent noncontingent “distractor” stimulus (low luminescence flashing light) was randomly presented throughout the training period. Activity was monitored via a motion detector. The following day, animals were returned to the same training box and activity monitored for five min as an index of contextual learning. Approximately 1–2 hours later, animals were placed in a different testing box with visual, tactile, and olfactory cues distinct from the training/context testing box and housed in a different test room. Activity was monitored for two minutes before and after presentation of the compound light/tone stimulus previously paired with footshock to evaluate conditioning to cue. Statistical analyses. All statistics were performed using SAS (version 9.2, SAS Institute, Cary, NC), and with the exception of fear conditioning and heterotopia incidence (see below), litter was considered the unit of analysis. Repeated measures analysis of variance (ANOVA) was performed to analyze for statistical differences in serum PTU and hormones, body weight, pup eye opening, and the number of cortical hemispheres in which a heterotopia was present. Main effects of treatment, age, and sex were included in the model where appropriate. Significant main effects in the overall repeated measures ANOVA of pup serum hormones were followed by planned comparisons at each age using step-down ANOVAs and Dunnett’s mean contrast test where appropriate. Independent repeated measures ANOVA were conducted for prenatal and postnatal dam serum hormones. These were followed by planned comparison step-down ANOVAs at each age in the event of a significant Dose x Day interaction. Heterotopia volume was assessed using Friedman nonparametric ANOVA. Overall heterotopia incidence was evaluated using Kruskal-Wallis nonparametric test and mean contrast tests were conducted using the Dwas, Steel, Critchlow-Fligner method. A criterion for inclusion for incidence was nominally set at >0.003mm3 as described above. Overall incidence of the heterotopia was calculated by expressing frequency of heterotopia in all animals examined, collapsing across age and sex. Fear conditioning training and cue learning data were analyzed using repeated measures ANOVA. Context learning was assessed using a one-way ANOVA. Up to 2 same sex littermates were assessed and no fewer than 7 litters represented for each sex in each treatment condition.
RESULTS
Indicators of Developmental Toxicity Neither body weight in dams (Figure 2A) nor litter size (data not shown) were affected by PTU treatment. Female pup body weight was not different across treatment groups in the early postnatal period. However, on PN14 and PN18, slight reductions in PTU-CON body weights relative to CON-CON females were detected (Figure 2B) [Treatment x Age interaction F(12, 108)=2.06, p=.02]. No significant body weight changes were evident by PN35 in female offspring (data not shown). Male pups from the PTU-PTU treatment condition had slightly lower body weights with significant differences detected on PN5, PN14, PN18, and PN21 (Figure 2D). These findings are supported by an observed significant main effect of Treatment [F(3, 27)=6.65, p=.0017], and of a Treatment x Age interaction [F(12, 108)=2.79, p=.0023]. Body weight decrements persisted to PN35 in males, but resolved when assessed on PN80 (data not shown). A delay in eye opening was evident in pups receiving PTU during the postnatal period (both CON-PTU and PTU-PTU groups), but not in pups that were only exposed during gestation (PTUCON) (Figure 2C). The CON-PTU group had a lower percentage of animals with both eyes open on PN14, but were not significantly different than CON-CON pups by the following day. PTU-PTU animals exhibited delayed eye opening by an additional day (Figure 2C). These findings are supported by a significant main effects of Treatment [F(3, 58)=17.35, p<.001], and of Day [F(5, 290)=547, p=.001], as well as a significant Treatment_Day interaction [F(15, 290)=9.48, p<.0001].
Figure 2.
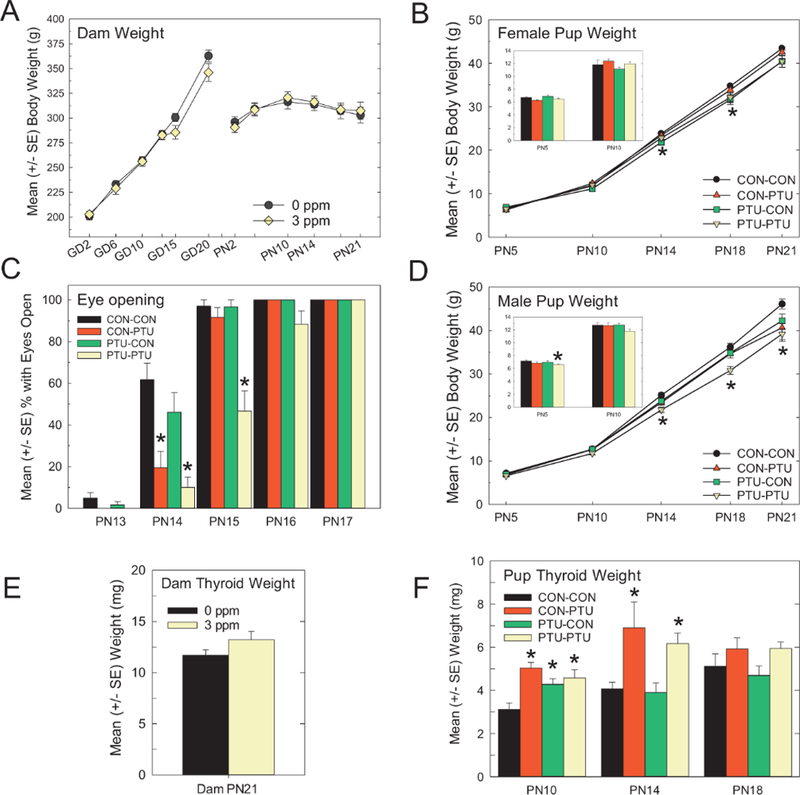
(A) Mean (±SE) body weight in dams over gestation and the postnatal period. No significant alterations were detected at any time point. (B,D) Mean (±SE) body weight of pups from PN5 to PN21. The bar graph insert represents weights at PN5 and PN10 for clarity. Slight reductions were seen and appeared greater in male than in female offspring. (C) Pup eye opening was slightly delayed in pups from CON-PTU and PTU-PTU conditions. No effect was seen in pups with exposure confined primarily to the gestational period (PTU-CON). Significant main effects in ANOVA were followed by mean contrast tests, Dunnett’s t-test, *p<.05. (E) Mean (±SE) thyroid gland weight did not differ in dams euthanized on PN21, 1 week after termination of exposure. (F) Mean (±SE) thyroid gland weights were significantly higher at PN10 in all animals receiving PTU treatment. By PN14 only animals concurrently receiving PTU had larger thyroid glands, which had returned to control levels by PN18 on termination of exposure 4 days earlier. Significant main effects in ANOVA were followed by mean contrast tests, Dunnett’s t-test, *p<.05.
Thyroid Gland Weights
Thyroid gland weights in dams on PN21, seven days after termination of the exposure, were similar in control and PTU-treated animals (Figure 2E) [F(1, 29)=2.44, p=.12]. Pup thyroid glands were larger in treated animals, and the differences among exposure groups varied over developmental time (Figure 2F). Results of an overall ANOVA revealed significant main effects of Treatment, Age, and of a Treatment x Age interaction (all p-values<.001). Step down ANOVAs were conducted by stage and confirmed that thyroid gland weights were increased in all PN10 pups exposed to PTU [F(3, 23)=7.06, p=.0016]. By PN14, thyroid gland weights in PTU-CON pups were similar to CONCON, but thyroid gland weights were increased in both groups of animals nursed by PTU dams (gland weight increased by 75% in CON-PTU and 55% in PTU-PTU, Figure 2F) [F(3, 24)=4.73, p=0.01]. By PN18, four days following the termination of dam PTU exposure, no significant differences were observed in pup thyroid gland weights under treatment conditions [F(3, 25)=1.99, p=.14].
PTU Serum Concentrations
Analysis of PTU levels in the serum of treated animals confirmed the internal dose of PTU received via the drinking water in the dam (Figure 3A), and via the milk in the nursing pup (Figure 3D). Expectedly, all control dams and CON-CON pups were below the limit of detection of the assay. PTU-treated dams exhibited higher serum chemical concentrations during the gestational period, which dropped by approximately 50% at parturition; however, serum PTU levels remained elevated until after termination of exposure on PN14 (Figure 3A). By PN18, no PTU was detected in the dam serum. These findings are supported by ANOVA which revealed significant effects of Treatment, Time, and of a Treatment_Time interaction (all p-values<.0001). Prior to cross-fostering on PN2, pup serum PTU concentrations were similar to what was observed in dams during late gestation (~350 ng/ml in both dams and pups, Figs. 3A and 3D). By PN5, control pups cross-fostered to PTU dams (CON-PTU) had serum chemical levels comparable with pups who had remained with treated dams (PTU-PTU) (Figure 3D). In contrast, serum PTU dropped precipitously to CON-CON levels by PN5 in PTU pups fostered to control dams 3 days earlier (PTU-CON). PTU concentrations were comparable throughout lactation in PTU-PTU and CON-PTU offspring by PN5, and dropped to nondetectable levels by PN18, similar to what was observed in dams (Figure 3D vs Figure 3A). These findings were confirmed by significant main effects of Treatment, Age, and of a Treatment x Age interaction (all p-values<.0001).
Figure 3.
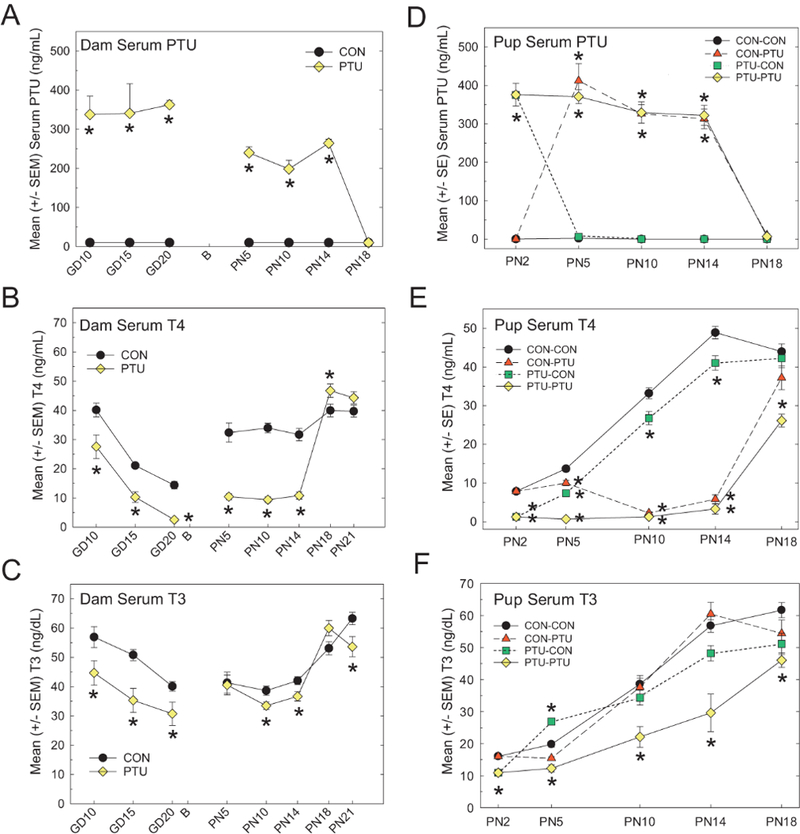
(A) Dam serum concentrations of PTU were highest during gestation but remained elevated until termination of exposure on PN14. DamserumT4 (B) previously reported in Hassan et al., 2017) and T3 (C) dropped over the course of pregnancy and were reduced in PTU exposed dams. Serum T4 recovered by PN14 (B), whereas serum T3 was only slightly reduced when compared with controls on PN10 and PN14. (D) Pup PTU serum concentrations were higher in pups than in dams, indicating significant lactational transfer of the chemical. PTU concentrations dropped in PTU-CON pups by PN5 and increased in the CON-PTU animals as well on PN5 (cross-fostered on PN2). Similar to what was observed in dams, serum PTU fell to control levels by PN18 (exposure termination on PN14). (E) Pup serum T4 increased as a function of age in CONCON animals. T4 reduction in CON-PTU pups was first detected on PN10. (F) Continuously exposed pups (PTU-PTU) had lower T3 throughout the postnatal period. PTUCON pups showed recovery of T4 by PN5, but remained slightly lower than controls until PN18. Control pups fostered to PTU dams (CON-PTU) showed slight reductions in T3 on PN5 but were similar to controls thereafter despite exposure until PN14. PTU-CON pups exhibited slightly elevated serum T3 on PN5, which normalized to control concentrations on PN10. A significant decrease in serum T3 was again observed on PN14. Dunnett’s t-test after significant ANOVA, *p<.05.
Dam Serum Thyroid Hormones
Dam serum TH concentrations are summarized in Figures 3B and 3C. An overall ANOVA confirmed main effects of Dose, Day, and of a Dose x Day interaction for both T4 (Figure 3B) and T3 (Figure 3C) (all p-values<.0001). Independent analyses were then conducted for the prenatal and the postnatal time points. Under control conditions, serum T4 declined over the course of gestation, and PTU treatment produced a significant decrease in serum T4 at all gestational time points (Figure 3B). These observations are supported by an overall effect of Dose [F(1, 40)=49.32, p<.0001], Day [F(2, 40)=78.54, p<.0001], but not of a Dose x Day interaction [F(2, 40)=0.09, p=.91]. After parturition, dam serum T4 concentrations were higher relative to what was observed in late gestation, but treated animals remained significantly suppressed compared with controls (Figure 3B). Analysis of serum T4 in the postnatal period also revealed significant main effects of Dose [F(1, 114)=37.17, p<.0001], Day [F(4,14)=60.29, p<.0001], and of a Day x Dose interaction [F(4,114)=26.88, p<.0001]. Step down ANOVAs at each age confirmed significant decrements in dam serum T4 ranging between 65% and 71% on PN5, PN10, and PN14 (all p-values<.0001). Hormone recovery was swift when exposure ended on PN14, with a marginally significant increase in serum T4 observed on PN18 [F(1, 29)=4.58, p=.04]. No significant differences were detected in dam T4 on PN21 [F(1, 29)=2.51, p=.12].
PTU also significantly reduced dam serum T3 concentrations at all gestational time points tested (Figure 3C), which is supported by observed significant main effects of Dose [F(1,40)=20.93, p<.0001], Day [F(2, 40)=10.59, p=.0002], but not of a Dose x Day interaction [F(2, 40)=0.43, p=.65]. Distinct from serum T4, however, reductions in T3 were more modest and inconsistent over time. Step down ANOVAs confirmed no significant differences between treated and control dams on PN5 [F(1, 14)=0.03, p=.87], with slight reductions (12%–15%) observed on PN10 [F(1, 13)=.32, p=.025] and PN14 [F(1, 29)=6.60, p=.015]. Following termination of exposure on PN14, a slight but insignificant increase in serum T3 (12%) was detected in treated dams relative to control [F(1, 29)=4.01, p=.055]. On PN21, a significant 16% decrease in serum T4 was detected in PTU-exposed dams [F(1, 29)=5.76, p=.023].
Pup Serum Thyroid Hormones
Pup serum TH concentrations are summarized in Figures 3E and 3F. An overall ANOVA confirmed main effects of Dose, Day and of a Dose x Day interaction for both T4 and T3 (all p-values <.0001). Under control conditions, serum T4 increased as a function of age, from the lowest concentrations observed on PN2 to peaking on PN14 (Figure 3E). Serum T4 levels were significantly reduced in pups from PTU-treated dams before cross-fostering on PN2 [F(1, 17)=104.56, p<.0001]. On PN5, T4 remained suppressed in PTU-PTU animals whereas PTU-CON pups exhibited some recovery; however, PTU-CON serum levels were still significantly decreased as compared to CON-CON pups [F(3, 26)=35.54, p<.0001]. Also, on PN5, significant decreases in serum T4 emerged in control pups fostered to PTU dams (CON-PTU, Figure 3E). By PN10 and PN14, serum T4 was equally suppressed in CON-PTU and PTU-PTU pups, while T4 concentrations in the PTU-CON animals continued to rise, though remaining significantly lowered when compared with CON-CON pups [PN10: F(3, 27)=220.94, p<.0001, PN14: F(3, 27)=244.8, p<.0001]. By PN18, recovery to control levels was achieved in all exposure conditions except for the PTU-PTU group [F(3, 26)=12.39, p<.0001].
As with serum T4, T3 concentrations increased in CON-CON animals as a function of age. Gestational exposure to PTU induced a significant reduction in serum T3 in pups sampled on PN2, prior to cross-fostering (Figure 3F) [F(1, 17)=114.81, p=.0036]. Relative to T4, serum T3 showed a much less dramatic pattern of change as a function of treatment condition (Figure 3F vs Figure 3E). On PN5, T3 was elevated above control values in PTU-CON pups, while significantly suppressed in PTUPTU animals [F(3, 26)=40.84, p<.0001]. On PN10 and 14, only pups exposed to PTU during gestation and the postnatal period exhibited significantly reduced T3 (PTU-PTU) [F(3, 27)=7.74, p=.0007 and F(3, 27)=13.34, p<.0001 respectively]. Similarly on PN18, serum T3 was comparable with controls in all treatment groups except for the PTU-PTU animals, which remained significantly suppressed relative to CON-CON pups despite exposure termination on PN14 [F(3, 26)=4.47, p=.0117].
Heterotopia Formation Requires Gestational Hormone Insufficiency
A cluster of NeuN-positive cells comprising the heterotopia was located in the posterior forebrain, within the corpus callosum proximal to both the cingulum and subiculum of the hippocampus. No such formation is evident in a representative section from a control brain taken at an equivalent anterior-posterior position (Fig. 4A and A0). Analysis of sections from PN300 brains demonstrates the presence of a heterotopia in animals exposed prenatally to PTU (PTU-CON and PTU-PTU), but not in animals receiving PTU beginning on PN2 (CON-PTU) (Figure 4B). Using stereological techniques to measure heterotopia volume, this observation was confirmed in brains of male offspring on PN14 (Figure 4C) [F(3, 26)=11.27, p<.0001], and female offspring on PN18 (Figure 4D) [F(3, 23)=6.74, p=.002]. Mean contrast tests revealed heterotopia in brains of animals exposed throughout gestation to mid-lactation (PTU-PTU), and in animals where exposure only occurred during the gestational and early postnatal period (PTU-CON). Heterotopias were not observed in controls (CON-CON), or in animals with postnatal exposure only (CON-PTU). In the PN18 female brain, an extended exposure period (PTU-PTU) increased the size of the heterotopia relative to animals experiencing a prenatal exposure alone (Figure 4D). However, this treatment effect was not observed in male pups on PN14 (Figure 4C). A similar pattern was reflected in heterotopia incidence, defined as the frequency in which this defect was identified across serial sections in a given animal. PTU-PTU and PTU-CON male pups on PN14 (Figure 4E), and female pups on PN18 (Figure 4F), exhibited a higher heterotopia incidence (ie, hemispheres counts) than in animals from either the CONCON or CON-PTU exposure conditions [F(3, 26)=10.28, p<.0001 and F(3, 23)=13.67, p<.0001, respectively].
Figure 4.
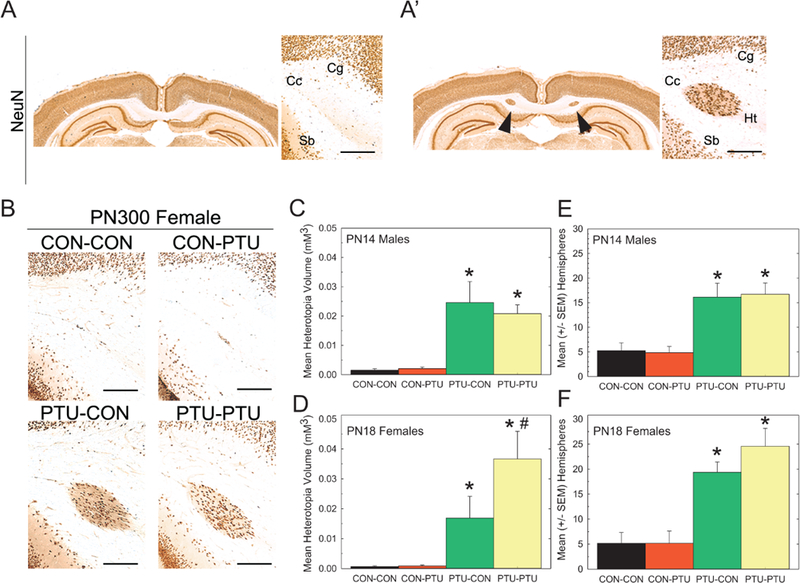
(A) Coronal section of a CON-CON and (A’) PTU-PTU exposed pup showing presence and anatomical position of a heterotopia in NeuN-immunostained section. (B) Heterotopia were present in pups from PTU-CON and PTU-PTU treatment conditions, but not in controls or pups with exposure beginning on PN2 (CON-PTU). (C,D) Mean (±SE) heterotopia volume determined stereologically and the number of hemispheres a heterotopia was observed in male offspring on PN14 was higher in treatments with prenatal exposure (PTU-CON and PTU-PTU) than in control. (E,F) Similarly, heterotopia volume and hemisphere incidence was greater in PN18 females with prenatal exposure, suggesting that gestational exposure is necessary for heterotopia formation. Scale bar=50 mm. Cg, cingulum; Cc, corpus callosum; Sb, subiculum; Ht, heterotopia. Duncan’s t-test following significant ANOVA. *Significantly different from CON-CON; #significantly different from PTU-CON, p<.05.
Heterotopias Are a Permanent Cortical Malformation
The findings above confirm that gestational/early postnatal TH insufficiency is necessary for the formation of a heterotopia, and that the heterotopia can be observed by the second postnatal week. To determine if the heterotopia represents a permanent structural defect, or if animals experienced an exacerbation or possible recovery in its severity over time, the brains of female offspring on PN18, PN90, and PN300 were examined for the presence of a heterotopia(s) (Figs. 5A–C). This cortical abnormality was again detected in the PTU-CON and PTU-PTU treatment groups at all ages tested, and notably persisted into late adulthood (PN300, Figure 5C). This observation is confirmed in analysis of heterotopia volume, where significant main effects of Treatment [F(3, 69)=75.50, p<.0001] and Age [F(2, 69)=9.09, p=.0003] were detected, but not of a Treatment_Age interaction [F(6, 69)=1.07, p=.38]. Similar to observations on the PN18, heterotopia volume in PTU-PTU and PTU-CON animals were again significantly different from both CON-CON and CON-PTU treatments (Figs. 5A–C). Therefore, while the PTU-CON exposure was sufficient to induce this birth defect, a more prolonged TH deficit resulted in a more severe phenotype.
Figure 5.
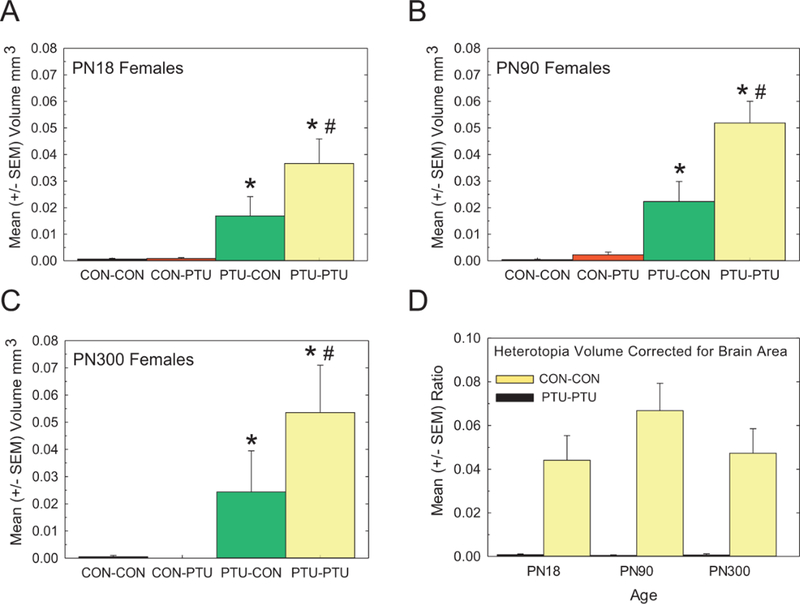
Effect of Age. Quantification of heterotopia volume was performed in female offspring on PN18 (A) PN90 females (B), and PN300 (C). Data in (A) is from Figure 4D but is included for comparison. Heterotopia were evident throughout the life of the animal and did not increase in size with age. Restriction to animals exposed during the gestational period reinforces findings at earlier ages. The overall volume was increased in animals with more prolonged exposure (PTU-PTU vs PTU-CON). (D) Mean (±SE) ratio of heterotopia volume to brain section area showed a similar pattern indicating that results are not confounded by any changes in overall brain size. Duncan’s t-test following significant ANOVA. *Signifies significantly different from CON-CON; #signifies significantly different from PTU-CON, p<.05.
Hypothyroidism and tissue processing artifacts can influence brain weight, size, and area measures, thereby introducing potential confounders to estimates of heterotopia volume changes across ages (Gilbert et al., 2017; Oatridge et al., 2002; Shibutani et al., 2009; Spring et al., 2016). Therefore, whole brain area was measured in every 3rd section to account for potential PTU-induced changes of the brain (see methods). Although slight increases in brain area were evident as a function of age and PTU treatment, none of these changes were statistically significant (data not shown). An analysis of heterotopia volume expressed as a ratio to mean section area eliminated the main effect of Age in the statistical analysis reported above, confirming the heterotopia remained approximately the same size throughout the life stages examined (Figure 5D).
Heterotopia Formation or Severity Is Not Sex Dependent
As neurodevelopmental disorders in human are often sex biased, potential sex differences in the observed heterotopia were analyzed. Heterotopia volume of male and female offspring were compared on PN300 (Experiment 1) and PN15 (Experiment 2). In both studies, heterotopias of equivalent volumes were seen in both sexes (Figs. 6A and 6B). Statistical analyses of the cross-fostering cohort on PN300 revealed a significant main effect of Treatment [F(3, 35)=25.45, p<.0001], but neither of a Sex [F(1, 35)=1.70, p=.20], nor of a Treatment x Sex interaction [F(3, 35)=1.01), p=.39] (Figure 6A). To more directly assess potential sex-dependent effects on heterotopia formation, Experiment 2 was conducted in which dams were exposed from GD6 through PN14 (equivalent to PTU-PTU), and male and female littermates were directly compared for heterotopia volume (Figure 6B). The results of this experiment confirmed the findings in the PN300 cross-fostered pups, whereby a significant main effect of Treatment was observed, without a main effect of Sex, or of a Treatment x Sex interaction.
Figure 6.
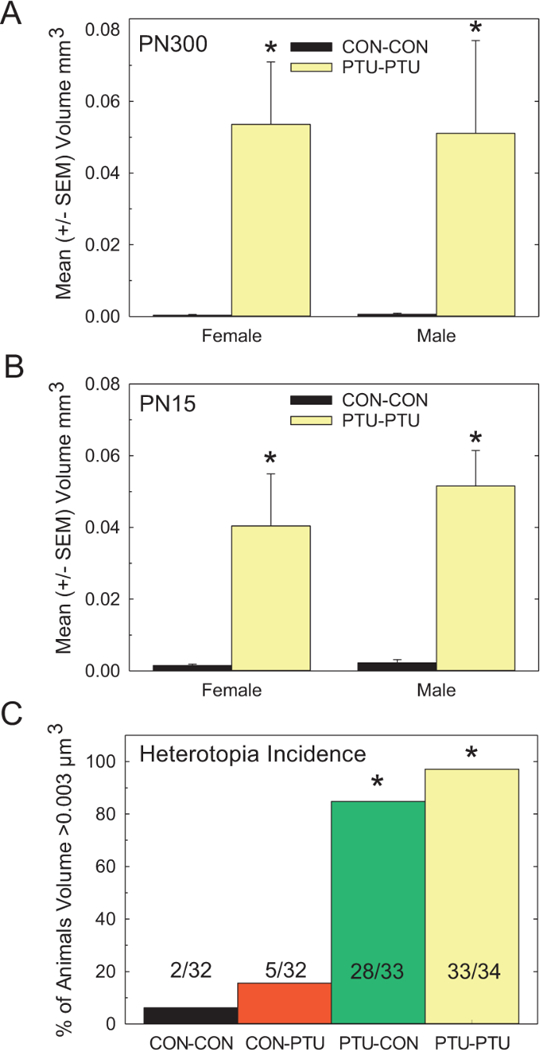
Effect of Sex. (A) Mean (±SE) heterotopia volume in adult female and male brains. Heterotopia were present in the brains of male and female PTUPTU offspring and did not differ in size as a function of sex. (B) As males and females were derived from different dams as a function of the cross-fostering protocol, Experiment 2 was conducted and confirmed the lack of effect of sex on heterotopia formation in male and female littermates sacrificed on PN15. (C) The overall incidence of heterotopia (criterion>0.003mm3) was calculated across all animals examined in the cross-fostering study (32–34 animals/ treatment condition). This assessment confirms the confinement of heterotopia to animals exposed to PTU during the gestational period. *Signifies significantly different from CON-CON.
The Overall Incidence of Heterotopia
Finally, the overall penetrance of the cortical heterotopia was calculated for all animals analyzed in this cross-fostering study (Figure 6C). Each animal was scored for the presence (nominal volume>0.003mm3) or absence (volume<0.003mm3) of this defect, and this measurement was collapsed across all ages and sexes. This permitted assessment of 32–34 animals for each treatment condition. PTU exposure confined primarily to the prenatal period (PTU-CON) induced a heterotopia with a similar penetrance as animals with a prolonged exposure (PTU-PTU), consistent with the conclusion that prenatal TH insufficiency is required to induce this structural malformation (Kruskal-Wallis (3)=85.0, p<.0001). However, despite a comparable incidence of heterotopia, TH insufficiency that extended well into the postnatal period increased the size of this defect (Figure 6C). In contrast, animals whose exposure began in the early postnatal period and extended to PN14 (CON-PTU) were not significantly different from nonexposed animals (CON-CON).
Males Offspring Exhibit Alterations in Fear Conditioning
As adults, male and female offspring were assessed for neurobehavioral dysfunction using a trace fear conditioning task; trace fear condition has been previously demonstrated as a metric sensitive to PTU-induced TH insufficiency (Gilbert, 2011; Gilbert et al., 2016). For fear training, an overall analysis of activity counts was conducted for main effects of Treatment and Sex across the first twenty-four 15-s time bins (corresponds to baseline, conditioned stimulus [CS, tone/light], unconditioned stimulus [US, shock], and the 2-min postshock period). A complex Treatment x Sex x Time interaction was observed [F(57, 2166)=2.32, p<.0001]. Thus, independent ANOVAs were conducted for fear conditioning in both male and female offspring. No effects of PTU exposure were seen in baseline levels of activity during training in males (Figure 7A). Males of the PTU-PTU exposure condition did not suppress their activity levels to the same extent as animals from the other conditions. A repeated measures ANOVA for the baseline and response to the first shock revealed a significant main effect of Treatment [F(3, 56)=3.30, p=.0267], Time [F(19, 1064)=122.45, p<.0001], and of a Dose X Time interaction [F(3, 064)=2.84, p<.0001]. No treatment effect on baseline activity was observed, but a significant difference in the degree of suppression of activity in response to shock application was limited to the PTU-PTU condition [F(3, 56)=4.33, p=.0082]. In female offspring, baseline activity levels did not differ among conditions, and all groups exhibited a comparable degree of activity suppression in response to shock (Figure 7B). The overall ANOVA showed a significant main effect of Time [F(19, 1102)=120.3, p<.0001], but neither an effect of Treatment [F(3, 58)=1.60, p=.19], nor a Treatment x Time interaction [F(57, 1102)=1.05, p=.38].
Figure 7.
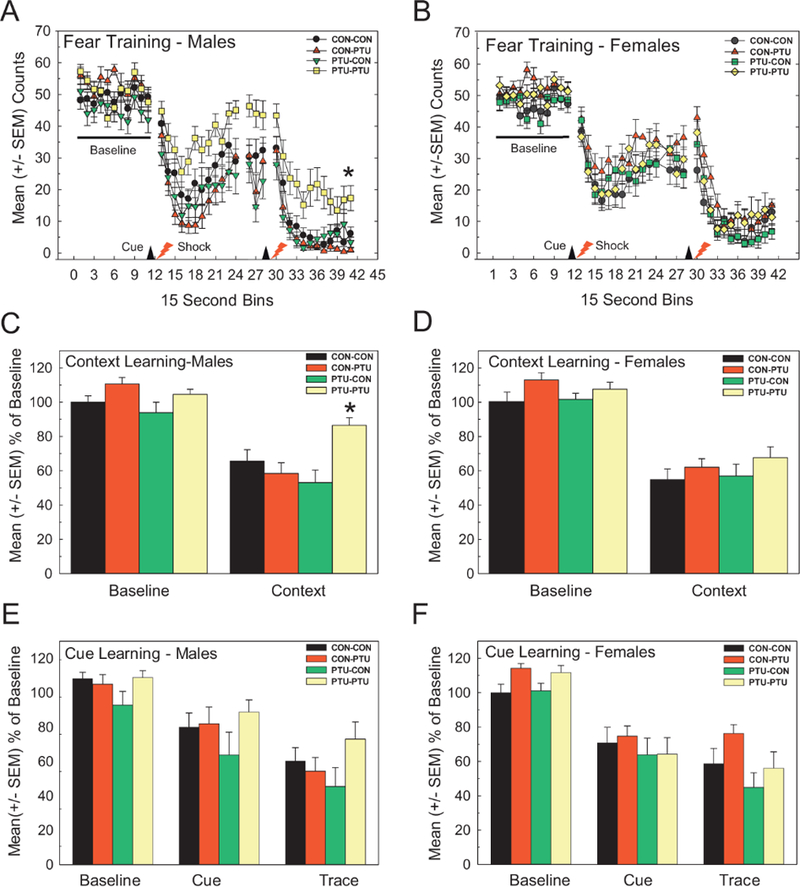
Distract Trace Fear Conditioning. (A,B) Activity was monitored in 15 s bins for 2 min before (Baseline, BL) and after presentation of a compound light/tone CS following 15-s later by a brief low intensity footshock. (A) Male offspring from PTU-PTU condition exhibited a less suppression of activity in response to footshock than other groups during fear acquisition training. No effects on baseline activity measures were observed. (B) No treatment related effects on activity were observed in females. (C,D) Conditioning to Context: A suppression of activity relative to baseline counts during training on Day 1 on return to the training box the following day indicates significant conditioning to context. (C) Males of PTU-PTU condition were impaired in context learning exhibiting higher activity counts relative to CON-CON. No effects on context conditioning were evident in female offspring (D). (E,F) Conditioning to Cue: On introduction to a novel testing box, suppression of ongoing activity with presentation of the CS and the immediate trace period that follows is evidence of cue learning. No effect on cue learning was seen in male (E) or female offspring (F). *Dunnett’s t-test p<.05 following significant effects identified by ANOVA.
Context Learning
Male offspring of the PTU-PTU treatment demonstrated impairments in context conditioning (Figure 7C). Counts expressed as a percent of training confirm a lack of effect in baseline activity levels in the training phase. A significant suppression of activity upon reintroduction to the training box is evidence of conditioning to context. The degree of suppression was curtailed in the PTU-PTU treatment condition relative to control. These conclusions are supported by a repeated measures ANOVA where significant effects of Treatment [F(3, 56)=4.54, p<.0064], Time [F(1, 56)=114.13, p<.0001], and of a Treatment x Time interaction [F(3, 56)=4.16, p<.0100] were observed. No effects in context learning of female offspring were detected (Figure 7D).
Cue Learning
A similar pattern was observed in response to conditioning to cue. Data are expressed as a percent of activity in the cue and trace interval as a function of the activity counts in the 2-min baseline period preceding the CS presentation. All animals demonstrated a moderate reduction in activity during the presentation of the CS, with a much stronger suppression of activity in the trace interval period. There was no treatment related differences in cue learning of male (Figure 7E) or female offspring (Figure 7F) (all p-values>.22).
DISCUSSION
A systematic evaluation of a TH-dependent cortical defect is presented. Confirming and extending previous work, this study quantified both anatomical and behavioral defects resulting from developmental TH insufficiency in the rat. Additionally, chemical disposition and hormone kinetics were assessed over time in the serum, permitting a comprehensive understanding of the dynamic responses of the thyroid axis in both mother and offspring. The present findings demonstrate that maternal exposure to PTU during gestation is required to induce a cortical heterotopia in the pups, and that this malformation is permanent. The overall penetrance of this phenotype is comparable across exposure scenarios that induced gestational TH insufficiency (PTU-CON and PTU-PTU); however, a prolonged exposure duration was associated with an increased severity of the heterotopia. While no sex differences related to this cortical malformation were observed, learning and memory was impaired in male but not female rats. This behavioral deficit was only detected in males subjected to the prolonged PTU exposure (PTU-PTU). This dissonance between the morphological and functional assessments suggests that an independent TH mediated mechanism may underlie these two observed phenotypes.
Internal Dosimeters—PTU and Serum Hormone Concentrations
Consistent with previous reports, maternal serum T4 dropped over the course of pregnancy under control conditions, rebounding shortly after parturition (Fukuda et al., 1980; Hapon et al., 2003). Serum TH reductions were induced by PTU exposure in both the mother and offspring, but reductions were more severe in pups than in the dams. PTU-exposed pups fostered to control dams on PN2 showed decreases in serum PTU by PN5, and corollary increases in T4. Pups removed from exposure by crossfostering to control dams on PN2 showed recovery of serum T4 by PN5. As with dams, T3 was not as severely impacted and recovery was rapid in all groups on termination of exposure. Our previous work demonstrated that in the late term fetus, the parent compound of PTU successfully enters fetal circulation, but at concentrations about half of those seen in the dam (Hassan et al., 2017). The higher PTU levels in the mother when compared with the fetus suggests that PTU disposition is in part regulated by the placenta, and/or there exists differential metabolism of PTU in the fetus versus the dam. As the fetal thyroid gland begins to function on approximately GD17 (Morreale de Escobar et al., 2004), these data suggest an interesting exposure scenario of the PTU exposed pups during the perinatal period. With respect to the data presented here, it is likely that pups in the PTU-CON and PTU-PTU conditions receive a “double hit” of TH dysfunction during late gestation. This double hit stems from (1) a reduced contribution of maternally derived T4 during late gestation due to TH insufficiency of the dam and (2) reduced TH production at the pup’s own thyroid gland due to direct exposure to PTU.
Following parturition, the serum PTU profiles in the neonate indicate significant transfer of the parent compound to the neonate through the milk. Serum PTU concentrations in pups nursed by PTU-exposed dams (PTU-PTU and CON-PTU) exhibit a 50% higher internal dose of PTU during the first 2 postnatal weeks when compared with the mothers. These data are also consistent with the observed differences in serum TH, with pups exhibiting a greater TH deficit than the dams during the first postnatal week. As environmental chemicals are often identified as thyroid disruptors by their effects on circulating levels of THs (Brucker-Davis, 1998; Gilbert and Zoeller, 2010; Gore et al., 2015; US EPA 2013), cognizance of differing placental and lactational transfer of compounds is of paramount importance when evaluating chemical risk. As pharmacokinetics can vary widely across chemicals and life stage, a comprehensive evaluation of serum hormone profiles in rodent dams, fetuses, newborns, and neonates is necessary if THs are to be used to predict the potential of chemicals to induce developmental neurotoxicity.
Heterotopia and Developmental Timing—Prenatal Exposure Is Essential
The primary finding of this study is clear demonstration that a primarily gestational exposure window was sufficient for heterotopia formation (GD6–PN2). Others have demonstrated phenotypic consequences due to gestational hypothyroidism in rodents, notably disorganization of hippocampal and cortical cytoarchitecture. In these studies, hypothyroidism and/or TH insufficiency was induced by methimazole (Auso et al., 2004; Cuevas et al., 2005), iodine deficiency (Lavado-Autric et al., 2003), or in models of premature birth (Berbel et al., 2010). These works, in conjunction with the data presented here, suggest that heterotopia may result from a similar cellular etiology that is dependent on prenatal TH insufficiency. Interestingly, our exposure that began in early gestation and extended into neonatal period (GD6–PN14) did not alter the incidence of heterotopia, but exacerbated its size, whereas the exposure that began on PN2 (CON-PTU) did not result in this malformation. These CONPTU animals did not exhibit a significant suppression of serum T4 on PN5, but did by PN10. As such, the effective postnatal exposure in the CON-PTU condition was limited, raising the possibility that the timing, degree, and duration of exposure may all contribute to our observed prenatal window of susceptibility. However, the lack of effect of an isolated postnatal exposure on heterotopia formation is consistent with our previous report using a higher concentration of PTU (10 ppm) beginning on the day of birth and extending to PN21 (Goodman and Gilbert, 2007). Together, these data suggest that in the absence of prenatal hormone suppression, an isolated postnatal exposure that maintained TH suppression beyond PN14 would still not be sufficient to induce a heterotopia.
Fear Conditioning Is Impaired in Hypothyroid Offspring
Two previous reports from our laboratory have demonstrated differences in acquisition and context learning phases in PTU treated animals, consistent with the findings presented here (Gilbert, 2011; Gilbert et al., 2017). In this fear training paradigm, a suppression in activity is expected in response to shock delivery. As in previous studies, higher activity levels were observed in the 3 min postshock period in males of PTU treated dams during fear training. We have argued that this activity does not appear to be associated with alterations in sensitivity to shock, but rather reflects impairment(s) in fear acquisition, habituation failure, and/or the emotional responsivity to the aversive stimulus (Gilbert, 2011). Deficits in context, but not cue learning, reported here are also consistent with previous findings. The new information contained herein reveals that PTU exposure to PN21 was not necessary to induce these deficits, as they were observed in animals exposed only until PN14. Additionally, these impairments were only detected in male rats, and in the treatment group with the most extended exposure (PTU-PTU). We have previously reported dose-dependent deficits in hippocampal-based learning tasks, adult hippocampal neurogenesis, hippocampal neurotrophin expression, and hippocampal synaptic transmission following exposures equivalent to PTU-PTU group of the present study (Gilbert, 2011; Gilbert et al., 2016, 2017). Alterations in hippocampal excitatory synaptic transmission are evident following treatments that reduce serum T4 in the dam (ie, perchlorate and iodine deficiency), with minimal TH alterations in the pup at weaning (Gilbert et al., 2013; Gilbert and Sui, 2008). Under these conditions, no effect on long-term potentiation (LTP) or on hippocampal tests of spatial learning or fear conditioning were observed, despite significant reductions in excitatory synaptic transmission observed in both. In contrast, synaptic transmission, LTP, and learning are all impaired following treatment with PTU that suppresses THs during both the fetal and neonatal periods (Gilbert, 2011; Gilbert and Sui, 2006). The hippocampus, amygdala, and prefrontal cortex are key structures upon which fear circuitry is based (Alonso et al., 2002; Czerniawski et al., 2011; Guadano-Ferraz et al., 2003; Saxe et al., 2006; Sui et al., 2006). The necessity of a combined prenatal and postnatal TH deficit to induce such learning impairments may result from the extensive postnatal development of these structures in rodents (Altman and Bayer, 1990a, 1990b; Rakic et al., 2009; Rodier, 1994), and is consistent with the emergence of LTP in the postnatal period (Bekenstein and Lothman, 1991; Michelson and Lothman, 1989). Collectively, these findings suggest that impairments in both synaptic transmission and plasticity in the hippocampus may be required before this behavioral manifestation of TH insufficiency becomes apparent.
Effects of Sex
Sex differences in TH-dependent alterations in neuroanatomy and hippocampal function have not been routinely examined. Although independent studies have reported heterotopia in male (Goodman and Gilbert, 2007) and female offspring (Gilbert et al., 2014; Spring et al., 2016), no direct comparison of the sexes had been conducted to date. Investigations of cognitive function, synaptic transmission, and hippocampal plasticity have largely been confined to reports in males (Dong et al., 2005; Gilbert, 2011; Gilbert and Sui, 2006; Sui et al., 2005; Wang et al., 2013). The present findings revealed a significant impairment in fear conditioning that was restricted to male offspring. Although the underlying mechanism is unknown, sex differences in the expression of brain derived neurotrophic factor (BDNF) protein have been reported in response to developmental TH insufficiency (Lasley and Gilbert, 2011). BDNF plays a role in hippocampal-based learning and the structural and functional plasticity upon which learning is based (Bramham and Messaoudi, 2005; Bruel-Jungerman et al., 2007; Messaoudi et al., 2002; Tyler et al., 2002). We recently reported that activity dependent transcription of BDNF is severely suppressed in adult offspring following developmental TH insufficiency (Gilbert et al., 2016). Furthermore, it has been shown that developmental PTU exposures produce greater decrements in BDNF protein in the hippocampus of adult males than in adult female offspring (Lasley and Gilbert, 2011), possibly conferring a greater susceptibility to fear conditioning impairments in this sex. The restriction of behavioral deficits in only PTU-PTU male offspring, despite the presence of heterotopia in both sexes of the PTUPTU and PTU-CON conditions, suggests that differing developmental mechanisms underlie these two indices of TH disruption, and that the heterotopia is likely not causal to the learning deficits.
Considerations for Extrapolation From Animals to Humans
Important considerations when extrapolating neurotoxicological findings across species include differences in life stage, chemical pharmacokinetics, and developmental timing between rodent and human brain development. Brain morphogenesis in the newborn rat is approximately equivalent to a human fetus at the end of the second trimester (Barez-Lopez et al., 2017; Bernal, 2015; Loosen, 1992), and thus, the resulting chemical exposure scenarios are dramatically different between these two species. Differential partitioning of xenobiotics in the maternal, fetal, and neonatal compartments, and how that may impact TH concentrations at different life stages, is critical to understanding the potential effects of thyroid disrupting chemicals on the developing brain. The results presented here underscore the importance of adequate supplies of THs in both the pre- and postnatal periods for normal brain development in the rodent. Additionally, our data are consistent with human reports, in which maternal indices of TH disruption are associated with structural and functional abnormalities of the brain, including deficits in learning and memory (Haddow et al., 1999; Korevaar et al., 2016; Wheeler et al., 2015; Willoughby et al., 2014).
In humans, cortical heterotopias are associated with a host of neurodevelopmental disorders including epilepsy, IQ deficits, ADHD, and autism (Davies, 2014; Werling and Geschwind, 2013); a number of these disorders are also associated with developmental hypothyroidism (Berbel et al., 2014; Roman et al., 2013). Although we do not claim that TH insufficiency induces these neurodevelopmental disorders in humans, the parallels are nonetheless intriguing. In the present report, there is an apparent dissociation between the cortical heterotopia and the observed learning deficits, suggesting that the TH-dependent pathways underlying each phenotype are independent. Alternatively, the behavioral consequences of the heterotopia may not be fully captured by a conditioned fear response, and thus has eluded full detection by our current metrics. Incorporation of more sophisticated behavioral assessments that test different cognitive, attentional, and social behaviors may prove more successful in further characterization of this hypothyroid-induced heterotopia model.
In summary, this study has identified different developmental window that are sufficient to induce both structural and neurobehavioral impairments in the TH insufficient rat. These findings have constructed a basis for the design of future studies that could elucidate the TH-dependent mechanisms underlying heterotopia formation. Identification of such will improve our ability to identify when, and how, thyroid disrupting chemicals could potentially impair brain development.
ACKNOWLEDGEMENTS
The authors are indebted to the excellent technical assistance provided by Stephanie Spring, Michelle Hotchkiss, Susan Thomas, Richard (Luke) Ford, Alan Tennant, and Dr. Michael Narotsky. Comments of Drs. William Boyes and Stephen Lasley on an earlier version of this manuscript are also gratefully acknowledged.
FUNDING
This work is supported by US Environmental Protection Agency.
Footnotes
Disclaimer: This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
The authors have no conflicts of interest to disclose.
REFERENCES
- Alonso M, Vianna MR, Izquierdo I, and Medina JH (2002). Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell. Mol. Neurobiol. 22(5–6), 663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, and Bayer SA (1990a). Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. The Journal of comparative neurology 301(3), 365–81. [DOI] [PubMed] [Google Scholar]
- Altman J, and Bayer SA (1990b). Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J. Comp. Neurol. 301(3), 343–64. [DOI] [PubMed] [Google Scholar]
- Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, and Berbel P (2004). A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145(9), 4037–47. [DOI] [PubMed] [Google Scholar]
- Barez-Lopez S, Obregon MJ, Bernal J, and Guadano-Ferraz A (2017). Thyroid Hormone Economy in the Perinatal Mouse Brain: Implications for Cerebral Cortex Development. Cerebral cortex doi: 10.1093/cercor/bhx088, 1–11. [DOI] [PubMed] [Google Scholar]
- Bekenstein JW, and Lothman EW (1991). An in vivo study of the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Brain Res. Dev. Brain Res. 63(1–2), 245–51. [DOI] [PubMed] [Google Scholar]
- Berbel P, Navarro D, Auso E, Varea E, Rodriguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC, and de Escobar GM (2010). Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb. Cortex 20(6), 1462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbel P, Navarro D, and Roman GC (2014). An evo-devo approach to thyroid hormones in cerebral and cerebellar cortical development: etiological implications for autism. Frontiers in endocrinology 5, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J (2015). Thyroid Hormones in Brain Development and Function. . In Endotext (De Groot LJ, Beck-Peccoz GCP, Dungan K, Grossman A, Hershman JM, C. Koch R, and McLachlan MN, Rebar R, Singer F, Vinik A, and Weickert MO, , Eds.). MDText.com, Inc., South Dartmouth (MA). [Google Scholar]
- Bramham CR, and Messaoudi E (2005). BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 76(2), 99–125. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F (1998). Effects of environmental synthetic chemicals on thyroid function. Thyroid : official journal of the American Thyroid Association 8(9), 827–56. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, and Laroche S (2007). Brain plasticity mechanisms and memory: a party of four. Neuroscientist 13(5), 492–505. [DOI] [PubMed] [Google Scholar]
- Cuevas E, Auso E, Telefont M, Morreale de Escobar G, Sotelo C, and Berbel P (2005). Transient maternal hypothyroxinemia at onset of corticogenesis alters tangential migration of medial ganglionic eminence-derived neurons. Eur. J. Neurosci. 22(3), 541–51. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Ramamoorthi K, Kumata Y, and Otto TA (2011). The importance of having Arc: expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism. J. Neurosci. 31(31), 11200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W (2014). Sex differences in attention Deficit Hyperactivity Disorder: candidate genetic and endocrine mechanisms. Frontiers in neuroendocrinology 35(3), 331–46. [DOI] [PubMed] [Google Scholar]
- Dong J, Yin H, Liu W, Wang P, Jiang Y, and Chen J (2005). Congenital iodine deficiency and hypothyroidism impair LTP and decrease C-fos and C-jun expression in rat hippocampus. Neurotoxicology 26(3), 417–26. [DOI] [PubMed] [Google Scholar]
- Duntas LH (2015). Chemical contamination and the thyroid. Endocrine 48(1), 53–64. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Ohshima K, Mori M, Kobayashi I, and Greer MA (1980). Sequential changes in the pituitary-thyroid axis during pregnancy and lactation in the rat. Endocrinology 107(6), 1711–6. [DOI] [PubMed] [Google Scholar]
- Gilbert M, and Zoeller R (2010). Thyroid hormone - impact on the developing brain: possible mechanisms of neurotoxicity In Neurotoxicology, 3rd edition (Harry G, and Tilson H, Eds.), Vol. 3, pp. 79–111. Informa Healthcare USA, Inc, New York. [Google Scholar]
- Gilbert ME (2004). Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Brain research. Developmental brain research 148(1), 11–8. [DOI] [PubMed] [Google Scholar]
- Gilbert ME (2011). Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol. Sci. 124(2), 432–45. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Goodman JH, Gomez J, Johnstone AF, and Ramos RL (2016a). Adult hippocampal neurogenesis is impaired by transient and moderate developmental thyroid hormone disruption. Neurotoxicology 59, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Goodman JH, Gomez J, Johnstone AF, and Ramos RL (2017). Adult hippocampal neurogenesis is impaired by transient and moderate developmental thyroid hormone disruption. Neurotoxicology 59, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Hedge JM, Valentin-Blasini L, Blount BC, Kannan K, Tietge J, Zoeller RT, Crofton KM, Jarrett JM, and Fisher JW (2013). An animal model of marginal iodine deficiency during development: the thyroid axis and neurodevelopmental outcome. Toxicol. Sci. 132(1), 177–95. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Ramos RL, McCloskey DP, and Goodman JH (2014). Subcortical band heterotopia in rat offspring following maternal hypothyroxinaemia: structural and functional characteristics. J. Neuroendocrinol. 26(8), 528–41. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sanchez-Huerta K, and Wood C (2016b). Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology 157(2), 774–87. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, and Sui L (2008). Developmental exposure to perchlorate alters synaptic transmission in hippocampus of the adult rat. Environ. Health Perspect. 116(6), 752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, and Sui L (2006). Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res. 1069(1), 10–22. [DOI] [PubMed] [Google Scholar]
- Goodman JH, and Gilbert ME (2007). Modest thyroid hormone insufficiency during development induces a cellular malformation in the corpus callosum: a model of cortical dysplasia. Endocrinology 148(6), 2593–7. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, and Zoeller RT (2015). EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine reviews 36(6), E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Benavides-Piccione R, Venero C, Lancha C, Vennstrom B, Sandi C, DeFelipe J, and Bernal J (2003). Lack of thyroid hormone receptor alpha1 is associated with selective alterations in behavior and hippocampal circuits. Mol. Psychiatry 8(1), 30–8. [DOI] [PubMed] [Google Scholar]
- Guerrini R, and Carrozzo R (2002). Epileptogenic brain malformations: clinical presentation, malformative patterns and indications for genetic testing. Seizure 11 Suppl A, 532–43; quiz 544–7. [PubMed] [Google Scholar]
- Gundersen HJ, and Jensen EB (1987). The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 147(Pt 3), 229–63. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, et al. (1999). Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. The New England journal of medicine 341(8), 549–55. [DOI] [PubMed] [Google Scholar]
- Hapon MB, Simoncini M, Via G, and Jahn GA (2003). Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction 126(3), 371–82. [DOI] [PubMed] [Google Scholar]
- Hassan I, El-Masri H, Kosian P, Ford J, Degitz S, and Gilbert M (2017). Neurodevelopment and Thyroid Hormone Synthesis Inhibition in The Rat: Quantitative Understanding Within The Adverse Outcome Pathway Framework. Toxicological Science 160(1), 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung MW, Kosian PA, Haselman JT, Korte JJ, Challis K, Macherla C, Nevalainen E, and Degitz SJ (2015). In Vitro, Ex Vivo, and In Vivo Determination of Thyroid Hormone Modulating Activity of Benzothiazoles. Toxicological sciences : an official journal of the Society of Toxicology 146(2), 254–64. [DOI] [PubMed] [Google Scholar]
- Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, Steegers EA, Visser TJ, White T, Tiemeier H, et al. (2016). Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. The lancet. Diabetes & endocrinology 4(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Lasley SM, and Gilbert ME (2011). Developmental thyroid hormone insufficiency reduces expression of brain-derived neurotrophic factor (BDNF) in adults but not in neonates. Neurotoxicology and teratology 33(4), 464–72. [DOI] [PubMed] [Google Scholar]
- Lavado-Autric R, Auso E, Garcia-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, and Morreale de Escobar G (2003). Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J. Clin. Invest. 111(7), 1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosen PT (1992). Effects of thyroid hormones on central nervous system in aging. Psychoneuroendocrinology 17(4), 355–74. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, and Bramham CR (2002). Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J. Neurosci. 22(17), 7453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson HB, and Lothman EW (1989). An in vivo electrophysiological study of the ontogeny of excitatory and inhibitory processes in the rat hippocampus. Brain Res. Dev. Brain Res. 47(1), 113–22. [DOI] [PubMed] [Google Scholar]
- Moro F, Carrozzo R, Veggiotti P, Tortorella G, Toniolo D, Volzone A, and Guerrini R (2002). Familial periventricular heterotopia: missense and distal truncating mutations of the FLN1 gene. Neurology 58(6), 916–21. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar GM, Obregon MJ, and del Rey FE (2004). Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract. Res. Clin. Endocrinol. Metab. 18(2), 225–48. [DOI] [PubMed] [Google Scholar]
- Oatridge A, Barnard ML, Puri BK, Taylor-Robinson SD, Hajnal JV, Saeed N, and Bydder GM (2002). Changes in brain size with treatment in patients with hyper- or hypothyroidism. AJNR. American journal of neuroradiology 23(9), 1539–44. [PMC free article] [PubMed] [Google Scholar]
- Oshiro WM, Beasley TE, McDaniel KL, Taylor MM, Evansky P, Moser VC, Gilbert ME, and Bushnell PJ (2014). Selective cognitive deficits in adult rats after prenatal exposure to inhaled ethanol. Neurotoxicology and teratology 45, 44–58. [DOI] [PubMed] [Google Scholar]
- Powell MH, Nguyen HV, Gilbert M, Parekh M, Colon-Perez LM, Mareci TH, and Montie E (2012). Magnetic resonance imaging and volumetric analysis: novel tools to study the effects of thyroid hormone disruption on white matter development. Neurotoxicology 33(5), 1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, and Dominguez MH (2009). Decision by division: making cortical maps. Trends in neurosciences 32(5), 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM (1994). Vulnerable periods and processes during central nervous system development. Environ. Health Perspect. 102 Suppl 2, 121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB, Verhulst FC, and Tiemeier H (2013). Association of gestational maternal hypothyroxinemia and increased autism risk. Annals of neurology 74(5), 733–42. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 103(46), 17501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharlin DS, Tighe D, Gilbert ME, and Zoeller RT (2008). The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology 149(5), 2527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M, Woo GH, Fujimoto H, Saegusa Y, Takahashi M, Inoue K, Hirose M, and Nishikawa A (2009). Assessment of developmental effects of hypothyroidism in rats from in utero and lactation exposure to anti-thyroid agents. Reprod. Toxicol. 28(3), 297–307. [DOI] [PubMed] [Google Scholar]
- Spring SR, Bastian TW, Wang Y, Kosian P, Anderson GW, and Gilbert ME (2016). Thyroid hormone-dependent formation of a subcortical band heterotopia (SBH) in the neonatal brain is not exacerbated under conditions of low dietary iron (FeD). Neurotoxicol. Teratol. 56, 41–46. [DOI] [PubMed] [Google Scholar]
- Sui L, Anderson WL, and Gilbert ME (2005). Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicological sciences : an official journal of the Society of Toxicology 85(1), 647–56. [DOI] [PubMed] [Google Scholar]
- Sui L, Wang F, Liu F, Wang J, and Li BM (2006). Dorsal hippocampal administration of triiodothyronine enhances long-term memory for trace cued and delay contextual fear conditioning in rats. J. Neuroendocrinol. 18(11), 811–9. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, and Pozzo-Miller LD (2002). From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 9(5), 224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (2013). State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures. . [Google Scholar]
- Wang Y, Wei W, Wang Y, Dong J, Song B, Min H, Teng W, and Chen J (2013). Neurotoxicity of developmental hypothyroxinemia and hypothyroidism in rats: Impairments of long-term potentiation are mediated by phosphatidylinositol 3-kinase signaling pathway. Toxicol. Appl. Pharmacol. 271(2), 257–65. [DOI] [PubMed] [Google Scholar]
- Watrin F, Manent JB, Cardoso C, and Represa A (2015). Causes and consequences of gray matter heterotopia. CNS Neurosci. Ther. 21(2), 112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, and Geschwind DH (2013). Sex differences in autism spectrum disorders. Current opinion in neurology 26(2), 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SM, McLelland VC, Sheard E, McAndrews MP, and Rovet JF (2015). Hippocampal Functioning and Verbal Associative Memory in Adolescents with Congenital Hypothyroidism. Front. Endocrinol. (Lausanne) 6, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR (2008). Neurodevelopmental and neurophysiological actions of thyroid hormone. Journal of neuroendocrinology 20(6), 784–94. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, McAndrews MP, and Rovet JF (2014). Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid 24(3), 576–84. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, and Rovet J (2004). Timing of thyroid hormone action in the developing brain - clinical observations and experimental findings. J. Neuroendocrinol. 16(10), 809–818. [DOI] [PubMed] [Google Scholar]


