Abstract
The brainstem is known to be an important brain area for nociception and pain processing, and both relaying and coordinating signaling between the cerebrum, cerebellum, and spinal cord. Although preclinical models of pain have characterized the many roles that brainstem nuclei play in nociceptive processing, the degree to which these circuitries extend to humans is not as well known. Unfortunately, the brainstem is also a very challenging region to evaluate in humans with neuroimaging. The challenges for human brainstem imaging arise from the location of this elongated brain structure, proximity to cardiorespiratory noise sources, and the size of its constituent nuclei. These challenges can require dedicated approaches to brainstem imaging, which should be adopted when study hypotheses are focused on brainstem processing of nociception or modulation of pain perception. In fact, our review will highlight many pain neuroimaging studies that have reported some brainstem involvement in nociceptive processing and chronic pain pathology. However, we note that with recent advances in neuroimaging leading to improved spatial and temporal resolution, more studies are needed that take advantage of data collection and analysis methods focused on the challenges of brainstem neuroimaging.
Keywords: Brainstem; Imaging; fMRI; Ultrahigh field; Periaqueductal gray, Rostroventromedial medulla, Diffuse noxious inhibitory control, Descending inhibition, Medulla, Pons, Midbrain
1. Introduction
The brainstem is a critical area for nociception and pain processing, as well as relaying and coordinating signaling between the cerebrum, cerebellum, and spinal cord. It is composed of 3 distinct subregions—the medulla (most caudal), pons, and midbrain (most cranial). Human functional magnetic resonance imaging (fMRI) studies have evaluated acute and chronic pain processing in the brainstem at both conventional (eg, 3 T) and ultrahigh-field (7 T and above) magnet strength, whereas structural MRI studies have assessed how gray matter volume and white matter integrity in the brainstem are associated with acute pain processing and are altered by chronic pain. Our review will highlight important challenges in brainstem neuroimaging (reviewed more in-depth elsewhere108), particularly for nuclei purported to be linked with pain processing. We will also provide promising examples of research demonstrating progress in human brainstem imaging to better understand the encoding of nociception and pain.
2. Brainstem nuclei involved in nociception and pain processing
Nociception is defined as the neural processes of encoding harmful stimuli,72 and the brainstem plays a cardinal role in both nociception and acute pain processing. In fact, most animal studies of pain processing have been focused on nociceptive encoding and have contributed heavily to our mechanistic understanding of the brainstem's role in pain processing. Multiple brainstem nuclei are known to be involved in pain processing, and include the periaqueductal gray (PAG) and nucleus cuneiformis (NCF) in the midbrain; dorsal raphe nuclei and median raphe nuclei, parabrachial nucleus, and locus coeruleus (LC) in the pons; and rostral ventromedial medulla (RVM, composed mainly of nucleus reticularis gigantocellularis (NGc) and nucleus raphe magnus), ventrolateral medulla (VLM), and dorsal reticular nucleus (DRt) in the medulla (Fig. 1). In addition, nociceptive input from the face and viscera enters the medulla and terminates in the spinal trigeminal nucleus (SpV) and nucleus tractus solitarii (NTS).29,103 These sensory nuclei also play an important role in both nociception and acute/chronic pain processing.
Figure 1.
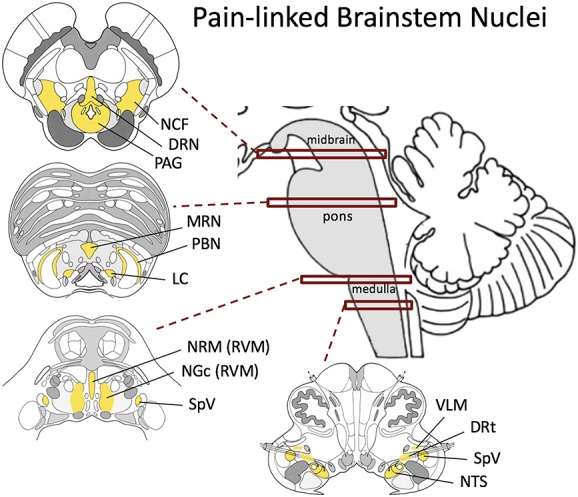
Schematic of brainstem nuclei linked with pain processing. DRN, dorsal raphe nucleus; DRt, dorsal reticular nucleus; LC, locus coeruleus; MRN, median raphe nucleus; NCF, nucleus cuneiformis; NGc, nucleus gigantocellularis; NRM, nucleus raphe magnus; NTS, nucleus tractus solitarii; PAG, periaqueductal gray; PBN, parabrachial nucleus; RVM, rostral ventromedial medulla; SpV, spinal trigeminal nucleus; VLM, ventrolateral medulla.
The PAG is a key region for pain processing. Although simple withdrawal responses to acute noxious stimuli are organized at the level of the spinal cord (or analogous brainstem nuclei for craniofacial reflexes), more complex and arguably more critical behavioral responses to noxious stimuli are integrated in higher brainstem regions such as in the PAG. The PAG surrounds the mesencephalic aqueduct and is composed of several columns with neurons associated with different neurotransmitter systems. The PAG has no clear cytoarchitectonic boundaries. However, distinct behavioral, cardiovascular, and afferent and efferent connection patterns have resulted in the PAG being divided into 4 longitudinal columns along the aqueduct, the dorsomedial PAG (dmPAG), dorsolateral PAG (dlPAG), lateral PAG (lPAG), and ventrolateral PAG (vlPAG) subdivisions.60 Active coping behavioral responses to pain that are critical for survival such as fight and flight can be evoked by direct stimulation of the lPAG, whereas passive coping behaviors such as quiescence are mediated by the vlPAG.6 In addition to the motor responses characterizing active and passive behaviors, direct PAG stimulation alters arterial pressure, heart rate, and blood flow patterns to support these behaviors. Stimulation also modulates vigilance and reactivity, and all of these behavioral/physiological responses are coupled to a powerful analgesia. While the expression of these behaviors can be modulated by afferent inputs to the PAG from higher brain regions such as the dorsomedial/orbital prefrontal cortex, cingulate cortex, and central nucleus of the amygdala,5 the PAG itself contains all the neural hardware required to produce these integrated defensive behaviours.60 Furthermore, nociceptive inputs to the lPAG are arranged somatotopically with SpV projections terminating in the rostral lPAG, and cervical and lumbar spinal projections at progressively more caudal levels.10,126 In addition, somatic and visceral nociceptive inputs are column specific, with afference from superficial nociceptors conveyed to lPAG and dlPAG columns (mainly through A-delta fibers), whereas the vlPAG receives afference from muscle/fascia and cutaneous C-fiber nociceptors, as well as visceral afference through the NTS.10,98 Given the fundamental nature of these animal model–derived behavioral responses, it is assumed that the circuitry responsible for them is preserved in humans, although this is yet to be definitively established.
As described above, PAG stimulation can produce a powerful analgesia, inhibiting incoming noxious information at the dorsal horn and SpV through PAG projections to the RVM.25,44,51 The PAG is heavily interconnected with the RVM, which is composed of so-called ON, OFF, and NEUTRAL classes of neurons within the NGc and nucleus raphe magnus, which can both facilitate and inhibit incoming noxious information. OFF-cells are silent during nociceptive input43 and were found to inhibit incoming nociceptive inputs.56 Alternatively, pain facilitation and behavioral hyperalgesia have been associated with increased activity of RVM ON-cells.45 Although the analgesia that occurs in concert with active and passive behaviors likely aids the individual in avoiding threatening situations, PAG–RVM pathways supporting descending pain inhibition also appear to be critical in mediating “higher-order” analgesic responses such as stress-induced analgesia. Periaqueductal gray–RVM pathways supporting pain facilitation132 through RVM ON-cells have been proposed to underlie some chronic pain conditions, manifested by increased PAG–RVM response during evoked noxious input.97 However, with respect to human neuroimaging, preclinical studies have clearly shown that the various RVM cell types are anatomically intermingled, and thus, activity of individual populations cannot be separated with typical fMRI approaches in humans.
In addition to the well-described PAG–RVM pain modulatory system, other brainstem nuclei have been implicated in nociceptive input regulation. These include 2 regions in the medulla, the DRt and caudal VLM.46,48,70 The DRt is critical for conditioned pain modulation, an analgesic response whereby central nervous system response to one noxious stimulus is inhibited by the application of a second noxious stimulus, and acts by inhibiting nociceptive inputs within the spinal and medullary (ie, SpV) dorsal horns. By contrast, the presence of documented ON- and OFF-cells in the VLM points to a dual inhibitory/facilitatory role, similar to the RVM.99 In the pons, the LC is a source for noradrenergic input to the brain, regulating attention and arousal104 but also highly involved in pain processing, such as with distraction analgesia. Optogenetic activation of different subpopulations of LC neurons has been shown to exert both pronociceptive and antinociceptive effects in rats, suggesting a bidirectional influence of this nucleus.57 In the midbrain, the NCF is also involved in both ascending transmission and modulation of nociceptive afference. Importantly, the NCF also contains ON-/OFF-cells55 and, like the PAG, projects to the RVM. In fact, its proximity to the PAG (the NCF is ventral and lateral to the PAG) has led some authors to mistakenly attribute NCF fMRI response to the PAG.
Although these brainstem nuclei can clearly alter incoming nociceptive information, more research is needed to delineate the roles they play in the initiation and/or maintenance of various chronic pain conditions in humans.
3. Challenges of brainstem neuroimaging
Preclinical studies have been successful at defining extremely small groups of neurons within the brainstem responsible for discrete functions, and it is assumed that the relatively primitive functions of these brainstem nuclei are preserved across species. Noninvasive neuroimaging is a powerful tool to evaluate brain activity in humans. However, the challenges for human brainstem imaging are many and arise from the location of this elongated brain structure, its proximity to cardiorespiratory noise sources, and the size of its constituent nuclei. These challenges can require dedicated approaches to brainstem imaging, which should be adopted when study hypotheses are focused on brainstem processing of nociception or modulation of pain perception.
First, many brainstem nuclei are elongated, with an average cross-sectional diameter of only a few millimeters or less in humans, and are considerably smaller than our current delineations of regional functional specializations in higher cortical and subcortical structures.1 The typical in-plane fMRI spatial resolution is 2 to 4 mm at 3 T, whereas ultrahigh-field (7 T and above) fMRI spatial resolution can be on the order of 1 mm isotropic (Fig. 2).12,14,26,32,42,100,105,109,123 Multiband acceleration can significantly improve spatiotemporal resolution for echo planar imaging fMRI, both at 3 and 7 T. However, caution should be used with very high acceleration factors because of potentially reduced signal-to-noise ratio at high acceleration factors. This has been noted for resting-state connectivity101 and task-evoked fMRI metrics, which early reports suggest may not benefit from the higher temporal resolution afforded by multiband fMRI.33 How multiband imaging specifically impacts the brainstem fMRI signal is not well understood, and parameters such as slice orientation and slice thickness can significantly impact the upper limits on acceleration factors. Regardless of which acceleration factor is chosen, the relatively limited spatial resolution for most fMRI methods poses a major challenge for imaging brainstem function in humans, and some of the standard analysis procedures used when exploring activity changes in higher-order brain regions are less appropriate when investigating brainstem function. From an analysis perspective, owing to the small cross-sectional area of most brainstem nuclei, correction for multiple comparisons should use voxel-based correction approaches, as opposed to cluster-based approaches that are skewed to larger activation clusters. In fact, cluster correction approaches typically used for whole-brain imaging will, many times, identify brainstem clusters only when such clusters cover high-noise cerebrospinal fluid or blood vessel voxels surrounding the brainstem parenchyma. Furthermore, nonparametric approaches (eg, permutation testing) or Bayesian statistics may be preferred over parametric general linear models, as smoothness assumptions associated with the latter are often not met in the brainstem.8
Figure 2.
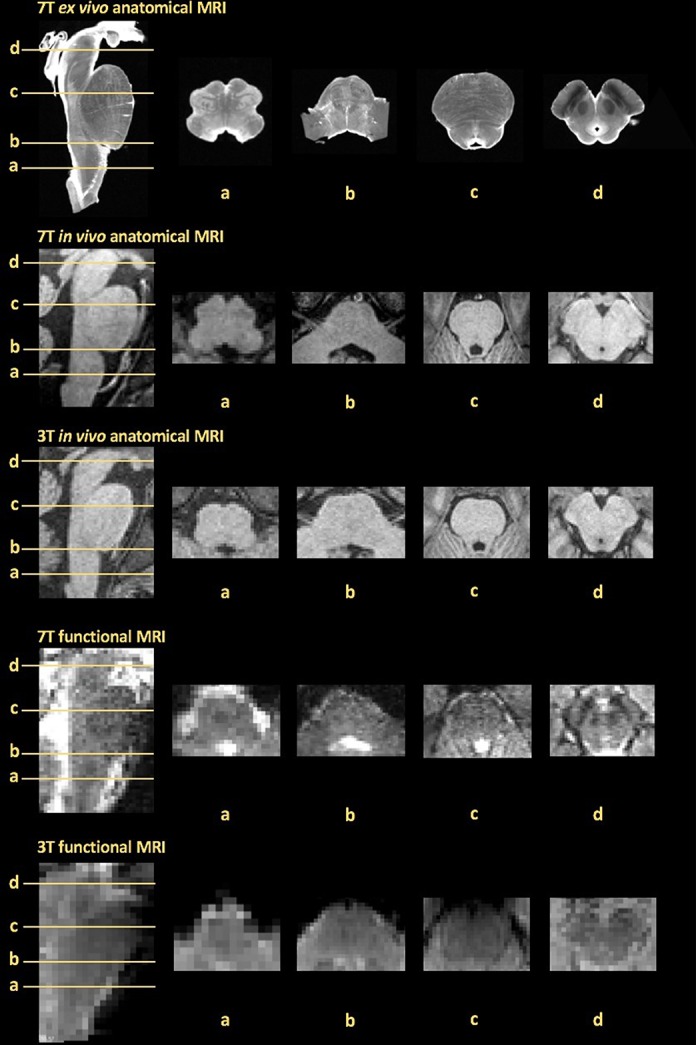
Representative examples of anatomical and functional brainstem MRI data quality obtained at different magnetic field strengths (7 vs 3 T). Axial slices (a–d) include pain-associated brainstem nuclei of interest from Figure 1. From the top: ex vivo anatomical obtained at 7 T (0.2-mm isotropic voxels, B0 image from DTI acquisition) generously provided by the laboratory of Dr. Alan Johnson23; in vivo anatomical obtained at 7 T (0.75-mm isotropic voxels, Multi-Echo MPRAGE); in vivo anatomical obtained at 3 T (1-mm isotropic voxels, Multi-Echo MPRAGE); functional MRI data obtained at 7 T (1.2-mm isotropic voxels, TR = 0.99 seconds, TE = 23 ms, phase-encoding R-L, Simultaneous Multi Slice, SMS factor = 2); and functional MRI data obtained at 3 T (2-mm isotropic voxels, TR = 1.25 seconds, TE = 33 ms, phase-encoding A-P, SMS factor = 5). DTI, diffusion tensor imaging; MRI, magnetic resonance imaging; TR, repetition time; TE, echo time.
Physiological (ie, cardiorespiratory) noise sources can significantly degrade the MRI signal. Such noise can stem from magnetic field changes due to chest motion (off-resonance B0 effects present in both functional and structural MRI), as well as from the propagation of cardiac and respiratory pulse pressure waves in arteries, cerebrospinal fluid spaces, and parenchyma. Moreover, compared with higher brain regions, caudal brainstem locations are closer in proximity to noise-generating sources such as the heart and lungs,19 potentially increasing the level of cardiorespiratory noise. Although fMRI physiological noise increases with field strength,66,117 its contribution is mitigated by decreasing voxel size,15 a strategy commonly used in ultrahigh-field fMRI. Many pain-processing brainstem regions lie close to areas of high susceptibility as evident in unmasked brainstem fMRI data using independent component analyses.9 Hence, some authors have advocated a number of techniques to limit these potential artefacts such as restricting fMRI brainstem analyses to an anatomically defined tight brainstem mask,7,82,109 applied before any spatial smoothing, thus limiting the extension of cardiorespiratory physiological noise near the surface from corrupting the fMRI signal from deeper brainstem nuclei. In addition, chest motion effects can be partially compensated by retrospective physiological noise correction strategies, which use physiological recordings for cardiac and respiratory frequency band estimates19,50 or respiratory-related information extracted from the image phase.13
In addition to potential physiological noise, brainstem MRI is plagued by magnetic susceptibility–induced distortions because of its proximity to air-filled cavities and the steep magnetic susceptibility gradient produced by the air–tissue boundary.49 Although similar susceptibilities occur in higher brain regions such as the prefrontal cortex (due to the frontal air sinus) and the temporal lobe (due to the mastoid air cells)—for the brainstem, such distortions can hamper coregistration and transformation to a standard space template. Furthermore, the brainstem is located in a narrow bony canal, which narrows as it extends caudally, leading to further susceptibility-induced distortions in caudal regions due to this bone–tissue proximity. Solutions have included the use of an anatomical reference data set with identical distortion to the BOLD fMRI data, applied both at 3 and 7 T,52,102,116 enabling improved masking of brainstem structures by transforming a brainstem mask defined in standard space to individual functional space109 and the use of a brainstem isolation and an anatomical specific template.38
Another challenge for brainstem imaging stems from a lack of dedicated, comprehensive probabilistic brainstem atlas that includes the large number of nuclei across the midbrain, pons, and medulla. Although existing atlases34,37,62,118 released with the common neuroimaging software (eg, FSL, FreeSurfer, and SPM) include several cortical and subcortical regions, most brainstem nuclei and their subdivisions are not available. Atlases including a limited number of brainstem nuclei, such as the substantia nigra, red nucleus, and subthalamic nucleus,28,62,67,78,84 are available, although their subdivisions are not. Recently, Mori et al. showed the feasibility of ex vivo diffusion tensor imaging (DTI) of several nuclei important for motor and cranial nerve functional systems in a single postmortem brainstem specimen.2 However, further attempts are ongoing, particularly at 7 T,11,12 and in the future, a dedicated probabilistic atlas for a larger number of brainstem nuclei in a standard space will greatly enhance localization. Given the difficulty in localization for many brainstem nuclei, it is recommended that imaging results be shown with axial slices from a tilted (ie, pitched along a medial–lateral axis in the sagittal plane) brainstem underlay, to match existing published atlases.22,91
Difficulty in brainstem atlas creation also extends to the copious white matter and densely crossing fiber tracts that pass through this brain region. Fiber crossings and decussations for major white matter tracts create challenges for accurate 3D determination of the pathways that interconnect different brainstem nuclei, and connect the spinal cord and brainstem to higher cortical structures. Spatial resolution is an important consideration for white matter tracking as well, and Mori et al. developed and publicly released an ex vivo DTI atlas of brainstem white matter tracts.84,85 Further efforts are ongoing to better account for microstructure and crossing fibers in the brainstem, and a recently published brainstem white matter atlas, based on DTI data from the publicly released Human Connectome Project database, delineated 23 main brainstem white matter bundles covering motor and sensory tracts, as well as the cerebellar peduncles.114
Ultimately, improved spatial resolution provided by high-field MRI will provide the basis for improved exploration of regional brainstem function. For instance, Satpute and coauthors used ultrahigh-field (7 T) fMRI to image the PAG with a 0.75-mm isotropic spatial resolution,105 while exposing participants to emotionally aversive images. Activation was localized to the lPAG and dmPAG rostrally, and to the vlPAG caudally, consistent with observations from animal studies,87,88 and further supporting the feasibility of exploring the functional architecture of small, difficult to image brainstem nuclei with ultrahigh-field fMRI.
4. Brainstem neuroimaging for nociception and acute pain processing
Neuroimaging studies have attempted to extend preclinical animal research, which has mostly focused on neural networks responsible for acute pain processing. Early brainstem-focused fMRI studies were able to demonstrate activation in brainstem nuclei such as PAG, NCF, ventral tegmental area, substantia nigra, and dorsolateral pons (ie, parabrachial nucleus and LC) in response to both somatic cutaneous and visceral (eg, rectal) nociceptive stimuli.40 In addition, the orofacial system provides a unique opportunity to explore pain processing at the primary afferent synapse in the SpV, located in the medulla and caudal pons. A number of studies have shown activation of the SpV during acute cutaneous and muscle noxious stimuli applied to the orofacial region,16,31,68,94,131 supporting the well-characterized pathways described by animal research.25 An important lesson from such studies is that adequate spatial resolution and functional/structural coregistration are critical for robustly determining fMRI response in elongated, small cross-sectional area nuclei such as the SpV and NTS in the medulla and pons. Thus, data collection and analysis methods for brainstem imaging may need to deviate from conventional cortical imaging approaches.
In addition, acute pain fMRI studies were recently extended to assess brainstem circuitry responsible for the nociceptive processing phenomena of temporal summation and conditioned pain modulation. A recent brain imaging study used the application of a noxious muscle stimulus to the leg to inhibit orofacial acute pain, ie, conditioned pain modulation. They revealed that conditioned pain modulation is associated with reduced fMRI signal response in the DRt and dorsolateral pons, as well as the brainstem region receiving noxious orofacial afferents, ie, SpV.129 Reduced conditioned pain modulation responsiveness has been linked to chronic pain, underscoring the importance of further neuroimaging research on this phenomenon. Furthermore, a recent brain imaging study has linked activity in the PAG–RVM axis with temporal summation of pain,17 a phenomenon related to nociception wind-up in animal models of chronic pain and in individuals suffering from chronic pain.96,120
Although we have described the spatial resolution limitations of brainstem imaging, temporal resolution is also a limitation. Most functional brain imaging protocols collect a volume every 2 or more seconds, which effectively limits the temporal range of pain processing that can be explored. For example, temporal summation of pain requires a stimulus frequency of approximately 1 Hz, which is typically above the temporal resolution of fMRI, impacting event separability due to ambiguity in the delayed hemodynamic response function. Higher field strength scanners can allow for subsecond acquisition time frames. However, cruder forms of time-resolved brainstem responses during painful stimuli have been previously assessed with lower field fMRI. For example, instead of simple averaging over multiple repetitions of an evoked pain stimulus, independent modeling of each serial block (or event) from typical fMRI study designs allows for the assessment of temporal variations in brainstem activation.92 This approach was used to resolve nonlinear (eg, U-shaped) midbrain activation to cuff pain stimuli across the pain intensity spectrum73 and dishabituation phenomena for patients with chronic pain (see section 5.2 below). Brainstem processing may in fact be a major determinant for temporally variable pain perception with repeated nociceptive stimuli, and future studies should apply advanced neuroimaging analysis approaches to better assess time-resolved brainstem response to evoked pain stimuli.
It is important to note that although the brainstem pain-modulating circuits described above can modulate incoming nociceptive input at the primary afferent synapse, it is thought that higher brain areas can also modulate pain by influencing these brainstem circuits. For instance, a number of human neuroimaging studies have begun to evaluate how higher cognitive functions can modulate such circuitry. For example, Keltner et al.61 linked cognitive expectancy modulation of pain intensity with NCF responses during nociceptive stimuli, supporting the importance of brainstem mechanisms for cognitive modulation of pain. Similarly, Brooks et al.18 found that when high cognitive load reduced thermal pain ratings, there was a concomitant temperature × task interaction in LC fMRI response. In addition, Tinnermann et al.115 recently noted that value information (eg, “expensive” vs “cheap” pain cream) can upregulate nocebo hyperalgesia, an effect mediated by pregenual anterior cingulate cortex responses to heat pain stimuli and its connectivity to the vlPAG, which was also more activated during the high value nocebo condition. In fact, the measure of functional connectivity, ie, the strength of signal covariation between different brain regions, has also been commonly used to assess communication between brainstem and higher telencephalic regions in both resting and pain-processing studies. For instance, a recent 3 T fMRI study using resting-state fMRI revealed connections between the vlPAG and brain regions associated with descending pain modulation (anterior cingulate cortex, dorsal pons, and medulla), whereas the lPAG and dlPAG were connected with brain regions implicated in cognitive/executive functions (eg, middle frontal gyrus).30
In fact, given the critical role for the PAG in pain behaviors revealed in experimental animal investigations,25 PAG fMRI activity has been a common focus for many acute pain neuroimaging studies, although thus far mainly at conventional field strengths such as 3 T.71 As we noted the well-described fine parcellation of the PAG, it is important that future research is aimed at exploring this structure in greater detail using higher field strength scanners. Indeed, Hahn et al. compared fMRI responses to painful vs innocuous electrical stimulation at 3 and 7 T,53 adopting similar in-plane resolutions for both field strengths (1.48 × 1.48 mm2 at 3 T and 1.5 × 1.5 mm2 at 7 T), and found that PAG activation for painful vs innocuous stimulation was found only with 7 T fMRI, likely due to increased BOLD signal-to-noise ratio at higher field strengths. These results support an expanded role for ultrahigh-field fMRI in evaluating brainstem nociceptive circuitries, where spatial and temporal resolution can better target discrete brainstem nuclei such as the subdivisions of PAG, NCF, and the medullary components (RVM and VLM) of the descending pain modulatory system.
Of course, in addition to the sensory perceptual aspect of acute pain processing, noxious stimuli are often coupled to autonomic changes, and it is well known from extensive experimental animal investigations that autonomic nervous system activity is closely tied to pain perception. In addition to known nociception processing nuclei, the brainstem also contains sympathetic and parasympathetic premotor nuclei, some of which overlap with the pain modulation circuitry noted above. In fact, a recent 7 T fMRI study found that sustained (6 minutes) experimental pain reduced cardiovagal modulation (high-frequency heart rate variability [HF-HRV]), and brainstem nuclei associated with this pain-evoked HF-HRV reduction included RVM, ventral nucleus reticularis (Rt)/nucleus ambiguus, dorsal motor nucleus of the vagus/NTS, and LC.109 Such studies, combining high spatial resolution fMRI and high temporal resolution HF-HRV data, hold promise for multimodal imaging of brainstem circuitries supporting pain-associated autonomic responses, which have been shown to contribute to biomarker development for clinical pain perception.69
5. Brainstem neuroimaging for assessment of chronic pain mechanisms
The significant involvement of the brainstem in nociceptive processing also makes this brain region a likely key contributor to the pathophysiology of many chronic pain conditions. Chronic pain can develop after injury to the central nervous system above the level of the brainstem, such as that following thalamic stroke.64 However, it has been shown that even chronic pain conditions that involve injury to peripheral structures are characterized by changes in pain modulatory regions located within the brainstem.20,80,122 Indeed, brainstem imaging for chronic pain has been mainly applied on clinical pain disorders associated with cranial sensory nerves that enter the pons and medulla, such as trigeminal neuralgia, trigeminal neuropathy, and migraine. Notably, investigations in other chronic pain disorders have also begun to explore aberrant brainstem processing, particularly related to the PAG–RVM descending pain modulatory system.
5.1. Trigeminal neuralgia
Trigeminal neuralgia is a neuropathic pain disorder with high morbidity and is thought to arise from neurovascular compression of the trigeminal nerve at the root entry zone, within the pontine cistern.74 Although gross neurovascular compression is not always evident with standard clinical MRI, in a recent brainstem-focused study, DTI acquired with 1-mm in-plane resolution was used to assess white matter microstructure in the trigeminal nerve rootlets.36 Trigeminal neuralgia patients demonstrated lower fractional anisotropy in the affected (ipsilateral to the pain) trigeminal nerve. Fractional anisotropy is a DTI marker linked with white matter integrity, and a lower value in peripheral nerves, in conjunction with increased radial and mean diffusivity, suggests the existence of neuroinflammation and/or edema. This same group later found that effective surgical therapy reversed these DTI abnormalities, and change scores were correlated with pain relief.35 Such studies represent a nice example of a brainstem-linked chronic pain pathology, imaged with appropriate spatial resolution to assess hypothesis-driven brainstem-related morphological alterations, which are then linked to clinical outcomes after therapy. Interestingly, another trigeminal neuralgia DTI study suggested that fractional anisotropy may instead be elevated within brainstem areas consistent with the SpV.124 Future studies will be needed to corroborate and reconcile the few structural imaging findings that have been reported for this chronic pain population, and to extend these observations to better understand the functional plasticity associated with structural changes in trigeminal neuralgia. Although only few fMRI studies have evaluated functional alterations in brainstem circuitry associated with trigeminal neuralgia, a previous study did find that allodynia at the cutaneous trigger zone was associated with greater SpV activation.83
5.2. Migraine
Migraine is a neurovascular disorder characterized by altered neural processing in the central nervous system.3,107,111 Importantly, hyperalgesia, allodynia, and impaired habituation have been commonly reported in patients with migraine, even during the interictal phase (between attacks),21 suggesting impaired brainstem pain modulation circuitry. In fact, imaging studies have demonstrated that patients with migraine show interictal abnormalities in subcortical and brainstem regions including PAG, dorsal pons, and SpV, as well as activation of the dorsal pons and PAG during the migraine attack itself.3,54,75,89,90 These studies support the hypothesis that the PAG–RVM axis, which mediates descending inhibition and facilitation, is likely altered during migraine. Indeed, a recent fMRI study found that interictal migraineurs demonstrate reduced PAG activation in response to orofacial heat pain stimulation but enhanced resting PAG/RVM connectivity during evoked pain using a psychophysiological interaction analysis.77 In this same study, migraineurs also displayed greater pain-evoked activation in the SpV and reduced resting SpV/RVM connectivity. Another recent fMRI study found that although SpV response to innocuous trigeminosensory stimuli in interictal migraine patients was not greater than healthy adults, the transfer of information from the SpV was actually amplified in higher cortical regions such as hypothalamus and posterior insula (ie, elevated ratio of fMRI response in hypothalamus/insula vs SpV fMRI response), an effect modulated by patients' relative interictal phase.68 In fact, several recent studies have highlighted differential brain processing during the period immediately before a migraine attack relative to the interictal phase. Immediately before a migraine, there is increased SpV responses to innocuous trigeminosensory stimulation,68 increased Sp5 connectivity to the hypothalamus during noxious trigeminal stimulation,106 and increased amplitude of low-frequency oscillations in the resting fMRI signal for several brainstem regions including PAG and SpV.79
In addition to such functional brainstem neuroimaging studies, other forms of imaging have implicated important brain structural and functional changes in migraineurs. For instance, a recent positron emission tomography study with [11C]PBR28, a radioligand that binds to the 18 kDa translocator protein (TSPO) which is a marker of glial activation, noted elevated SpV uptake in migraineurs with aura.4 Structural T1-weighted MRI data have been used to evaluate deformable mesh models of different brainstem regions allowing for shape analyses in migraineurs. Using this technique, it was found that outward deformations in the lateral medulla and dorsolateral pons occur in migraineurs,27 implicating regions containing nuclei such as SpV in migraine pathophysiology. Furthermore, brainstem DTI studies in migraine have shown altered diffusivity in the PAG, RVM, and SpV in migraineurs,76 again implicating altered descending pain modulatory circuits in the pathophysiology of migraine. Future studies are warranted, particularly with improved spatial resolution and focusing on trigeminal nerve rootlets entering the pontomedullary junction, given the trigeminal entry zone DTI studies noted above for trigeminal neuralgia.
5.3. Temporomandibular disorder
Temporomandibular disorder (TMD) is a relatively common craniofacial pain disorder characterized by (mainly) myofascial pain within the temporomandibular joint and/or masticatory muscles adjacent to this joint. In TMD, nociceptive afference is directed to the brainstem along the trigeminal nerve, and DTI studies have found reduced fractional anisotropy and increased mean diffusivity and radial diffusivity in the trigeminal nerve roots entering the pons.81 These DTI changes were subsequently corroborated by another group,125 strongly suggesting that although gross abnormalities in peripheral nerve anatomy are not present for this idiopathic chronic pain disorder, microstructural changes along the trigeminal nerve can indeed be found. Structural MRI studies using voxel-based morphometry have also found altered SpV gray matter volume in patients with TMD, both increased127 and decreased,125 relative to healthy controls—a discrepancy that needs further research. Although BOLD fMRI assessments for brainstem processing in TMD have been scarce, an arterial spin labeling study assessed regional cerebral blood flow for patients with TMD and found that patients show increased blood flow in SpV compared with healthy controls, suggesting that painful TMD may be maintained by sustained activation of peripheral nociceptors in the temporomandibular joint and/or adjacent masticatory muscles.128 In summary, structural MRI studies point to altered neuroanatomy in brainstem structures primary to trigeminal pain processing. Functional MRI assessment of altered brainstem neurophysiology in TMD needs more research attention, although promising evidence has linked SpV physiology with TMD. Future research using dedicated brainstem fMRI methods is needed to further probe altered brainstem neurophysiology for this disorder.
5.4. Mechanisms of therapeutic interventions for chronic pain
Finally, it should be noted that brainstem imaging has also been used to assess therapeutic mechanisms. For instance, deep brain stimulation (DBS) of the PAG and periventricular gray has been applied for chronic pain, although the mechanisms of action are not completely understood. A recent positron emission tomography (PET) study used [11C]diprenorphine (DPN, an opioid radioligand) in a small cohort of patients with implanted PAG/periventricular gray DBS systems demonstrated decreased [11C]DPN binding in the caudal and dorsal PAG following DBS in the rostral dlPAG, suggesting a focal release of opioid peptides.110 While PET spatial resolution is more limited, we should note that ultrahigh-field MRI may indeed play an important role in presurgical planning and MR-guided surgery for precise lead placement,24 although clinical benefits of 7 T MRI have yet to be seen for DBS of the brainstem.119
Noninvasive neuromodulatory approaches for pain have also targeted brainstem nuclei by electrically stimulating cranial nerve innervated territories. One promising approach is the targeting of the NTS with transcutaneous vagus nerve stimulation (tVNS). Vagus nerve stimulation, which involves surgical placement of electrodes coiled around the cervical vagus nerve within the carotid sheath, has demonstrated efficacy for multiple disorders (eg, epilepsy and depression) and, recently, migraine.58,86,130 Despite the therapeutic potential of VNS, adverse events and complications associated with surgery and chronic stimulation limit broad applicability.41 Importantly, the NTS and SpV also receive somatosensory afference through the auricular branch of the vagus nerve (ABVN).63,95 Noninvasive (transcutaneous) methods of ABVN stimulation (tVNS) have been proposed,121 and preliminary 3 T neuroimaging studies have found that tVNS modulates brainstem and cortical areas similar to classical VNS,39,65 although a clinical trial suggested that tVNS may also reduce the frequency of migraine episodes.112 Interestingly, the dorsal medullary vagal system operates in tune with respiration and ABVN stimulation gated to exhalation may enhance tVNS outcomes for pain.93 Other studies have demonstrated that respiratory-gated tVNS can also enhance targeting of specific brainstem nuclei such as NTS, as recently shown for patients suffering from migraine.47 In fact, NTS response to auricular tVNS may also benefit from a more focal identification, by applying ultrahigh-field fMRI. Furthermore, future brainstem neuroimaging studies could use cranial nerve stimulation techniques such as tVNS (known to target distinct medullary nuclei) to optimize stimulation parameters for enhanced therapeutic response and, from a methodological point of view, to improve fMRI pulse sequences and analysis approaches for brainstem neuroimaging applications.
6. Conclusions and future directions
The brainstem is a critical structure for nociception and pain processing, both for acute experimental pain and chronic pain pathology. Unfortunately, the brainstem is also a very challenging region to evaluate in humans with neuroimaging. Many previous pain neuroimaging studies have reported some brainstem involvement in nociceptive processing and chronic pain pathology. However, most of these studies have been designed to assess cortical and/or supra-brainstem morphology and physiology, with only serendipitous brainstem findings reported when evident.
With recent advances in multiband accelerated neuroimaging leading to improved spatial and temporal resolution, more brainstem-focused studies are needed with modified data collection and analysis methods, taking into account the unique location of this brain region and relatively small size of many brainstem nuclei, compared with typical telencephalic structures. Such dedicated brainstem imaging approaches will surely improve the sensitivity and replicability of brainstem neuroimaging studies for pain.
In fact, different neuroimaging techniques are needed to assess brainstem physiology beyond BOLD fMRI. For instance, it has been known for some time that glial cells, which contribute greatly to pain processing,59 also influence neurovascular coupling and hence the BOLD fMRI signal.113 Thus, future brainstem imaging might also extend beyond “neuroimaging,” using PET ligands sensitive to microglia and astrocyte activity. These techniques hold great promise in exploring glial mechanisms for human pain disorders, and future studies should make greater use of emerging PET techniques with novel, more specific ligands.
Ultimately, although the challenges of brainstem imaging are daunting, recent advances in image acquisition and analysis methods have helped improve the feasibility and robustness of dedicated brainstem imaging research. Furthermore, given the important role that nuclei within this brain region play in the processing of nociception and pain, the coming years should see a notable increase in published neuroimaging research focused on the brainstem.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgements
The authors thank the following organizations for funding support: US National Institutes for Health (NIH), Office of the Director (OT2-OD023867 to V.N.); National Center for Complementary and Integrative Health (NCCIH), NIH (P01-AT009965, R61-AT009306, R33-AT009306, and R01-AT007550 to V.N.); and National Institute for Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NIH (R01-AR064367 to V.N.).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Afshar F, Watkins F, Yap J. Stereotaxic atlas of the human brainstem and cerebellar nuclei: a variability study. New York, NY: Raven Press, 1978. [Google Scholar]
- [2].Aggarwal M, Zhang J, Pletnikova O, Crain B, Troncoso J, Mori S. Feasibility of creating a high-resolution 3D diffusion tensor imaging based atlas of the human brainstem: a case study at 11.7 T. Neuroimage 2013;74:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 2011;12:570–84. [DOI] [PubMed] [Google Scholar]
- [4].Albrecht D, Mainero C, Ichijo E, Ward N, Granziera C, Zürcher N, Akeju O, Bonnier G, Price J, Hooker J, Napadow V, Loggia M, Hadjikhani N. Imaging of neuroinflammation in migraine with aura—a [11C]PBR28 PET/MR study. Neurology 2019;92:e2038–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol 1998;401:455–79. [PubMed] [Google Scholar]
- [6].Bandler R, Keay KA. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res 1996;107:285–300. [DOI] [PubMed] [Google Scholar]
- [7].Beissner F, Baudrexel S. Investigating the human brainstem with structural and functional MRI. Front Hum Neurosci 2014;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beissner F, Deichmann R, Baudrexel S. fMRI of the brainstem using dual-echo EPI. Neuroimage 2011;55:1593–9. [DOI] [PubMed] [Google Scholar]
- [9].Beissner F, Schumann A, Brunn F, Eisenträger D, Bär KJ. Advances in functional magnetic resonance imaging of the human brainstem. Neuroimage 2014;86:91–8. [DOI] [PubMed] [Google Scholar]
- [10].Benarroch EE. Periaqueductal gray: an interface for behavioral control. Neurology 2012;78:210–7. [DOI] [PubMed] [Google Scholar]
- [11].Bianciardi M, Strong C, Toschi N, Edlow BL, Fischl B, Brown EN, Rosen BR, Wald LL. A probabilistic template of human mesopontine tegmental nuclei from in vivo 7 T MRI. Neuroimage 2018;170:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bianciardi M, Toschi N, Edlow BL, Eichner C, Setsompop K, Polimeni JR, Brown EN, Kinney HC, Rosen BR, Wald LL. Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems. Brain Connect 2015;5:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bianciardi M, van Gelderen P, Duyn JH. Investigation of BOLD fMRI resonance frequency shifts and quantitative susceptibility changes at 7 T. Hum Brain Mapp 2014;35:2191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blazejewska AI, Schwarz ST, Pitiot A, Stephenson MC, Lowe J, Bajaj N, Bowtell RW, Auer DP, Gowland PA. Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology 2013;81:534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bodurka J, Ye F, Petridou N, Murphy K, Bandettini PA. Mapping the MRI voxel volume in which thermal noise matches physiological noise—implications for fMRI. Neuroimage 2007;34:542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Borsook D, Burstein R, Moulton E, Becerra L. Functional imaging of the trigeminal system: applications to migraine pathophysiology. Headache 2006;46(suppl 1):S32–38. [DOI] [PubMed] [Google Scholar]
- [17].Bosma RL, Ameli Mojarad E, Leung L, Pukall C, Staud R, Stroman PW. Neural correlates of temporal summation of second pain in the human brainstem and spinal cord. Hum Brain Mapp 2015;36:5038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brooks JC, Davies WE, Pickering AE. Resolving the brainstem contributions to attentional analgesia. J Neurosci 2017;37:2279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brooks JC, Faull OK, Pattinson KT, Jenkinson M. Physiological noise in brainstem FMRI. Front Hum Neurosci 2013;7:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci 2002;22:5129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci 2015;35:6619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Büttner-Ennever JA, Horn AK. Olszewski and Baxter's cytoarchitecture of the human brainstem. Eur Surg Res 2013;51:101–92.24217574 [Google Scholar]
- [23].Calabrese E, Hickey P, Hulette C, Zhang J, Parente B, Lad SP, Johnson GA. Postmortem diffusion MRI of the human brainstem and thalamus for deep brain stimulator electrode localization. Hum Brain Mapp 2015;36:3167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chansakul T, Chen PN, Jr, Lee TC, Tierney T. Interventional MR imaging for deep-brain stimulation electrode placement. Radiology 2016;281:940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia 2017;37:613–26. [DOI] [PubMed] [Google Scholar]
- [26].Cho ZH, Kang CK, Son YD, Choi SH, Lee YB, Paek SH, Park CW, Chi JG, Calamante F, Law M, Kim YB. Pictorial review of in vivo human brain: from anatomy to molecular imaging. World Neurosurg 2014;82:72–95. [DOI] [PubMed] [Google Scholar]
- [27].Chong CD, Plasencia JD, Frakes DH, Schwedt TJ. Structural alterations of the brainstem in migraine. NeuroImage Clin 2017;13:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chowdhury R, Lambert C, Dolan RJ, Düzel E. Parcellation of the human substantia nigra based on anatomical connectivity to the striatum. Neuroimage 2013;81:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst 1982;6:303–22. [DOI] [PubMed] [Google Scholar]
- [30].Coulombe MA, Erpelding N, Kucyi A, Davis KD. Intrinsic functional connectivity of periaqueductal gray subregions in humans. Hum Brain Mapp 2016;37:1514–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci 2002;22:8183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Deistung A, Schäfer A, Schweser F, Biedermann U, Güllmar D, Trampel R, Turner R, Reichenbach JR. High-resolution MR imaging of the human brainstem in vivo at 7 tesla. Front Hum Neurosci 2013;7:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Demetriou L, Kowalczyk OS, Tyson G, Bello T, Newbould RD, Wall MB. A comprehensive evaluation of increasing temporal resolution with multiband-accelerated protocols and effects on statistical outcome measures in fMRI. Neuroimage 2018;176:404–16. [DOI] [PubMed] [Google Scholar]
- [34].Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80. [DOI] [PubMed] [Google Scholar]
- [35].DeSouza DD, Davis KD, Hodaie M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. PAIN 2015;156:1112–23. [DOI] [PubMed] [Google Scholar]
- [36].DeSouza DD, Hodaie M, Davis KD. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. PAIN 2014;155:37–44. [DOI] [PubMed] [Google Scholar]
- [37].Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010;53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 2006;33:127–38. [DOI] [PubMed] [Google Scholar]
- [39].Dietrich S, Smith J, Scherzinger C, Hofmann-Preiss K, Freitag T, Eisenkolb A, Ringler R. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed Tech (Berl) 2008;53:104–11. [DOI] [PubMed] [Google Scholar]
- [40].Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci 2005;25:7333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fahy BG. Intraoperative and perioperative complications with a vagus nerve stimulation device. J Clin Anesth 2010;22:213–22. [DOI] [PubMed] [Google Scholar]
- [42].Faull OK, Jenkinson M, Clare S, Pattinson KT. Functional subdivision of the human periaqueductal grey in respiratory control using 7 tesla fMRI. Neuroimage 2015;113:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci 1983;3:2545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 1991;14:219–45. [DOI] [PubMed] [Google Scholar]
- [45].Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature 1983;306:684–6. [DOI] [PubMed] [Google Scholar]
- [46].Foong FW, Duggan AW. Brain-stem areas tonically inhibiting dorsal horn neurones: studies with microinjection of the GABA analogue piperidine-4-sulphonic acid. PAIN 1986;27:361–71. [DOI] [PubMed] [Google Scholar]
- [47].Garcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R, Wasan AD, Edwards RR, Rosen BR, Hadjikhani N, Napadow V. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. PAIN 2017;158:1461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gebhart GF, Ossipov MH. Characterization of inhibition of the spinal nociceptive tail-flick reflex in the rat from the medullary lateral reticular nucleus. J Neurosci 1986;6:701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gizewski ER, Maderwald S, Linn J, Dassinger B, Bochmann K, Forsting M, Ladd ME. High-resolution anatomy of the human brain stem using 7-T MRI: improved detection of inner structures and nerves? Neuroradiology 2014;56:177–86. [DOI] [PubMed] [Google Scholar]
- [50].Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 2000;44:162–7. [DOI] [PubMed] [Google Scholar]
- [51].Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Grabner G, Poser BA, Fujimoto K, Polimeni JR, Wald LL, Trattnig S, Toni I, Barth M. A study-specific fMRI normalization approach that operates directly on high resolution functional EPI data at 7 Tesla. Neuroimage 2014;100:710–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hahn A, Kranz GS, Seidel EM, Sladky R, Kraus C, Kublbock M, Pfabigan DM, Hummer A, Grahl A, Ganger S, Windischberger C, Lamm C, Lanzenberger R. Comparing neural response to painful electrical stimulation with functional MRI at 3 and 7 T. Neuroimage 2013;82:336–43. [DOI] [PubMed] [Google Scholar]
- [54].Harriott AM, Schwedt TJ. Migraine is associated with altered processing of sensory stimuli. Curr Pain Headache Rep 2014;18:458. [DOI] [PubMed] [Google Scholar]
- [55].Haws CM, Williamson AM, Fields HL. Putative nociceptive modulatory neurons in the dorsolateral pontomesencephalic reticular formation. Brain Res 1989;483:272–82. [DOI] [PubMed] [Google Scholar]
- [56].Heinricher MM, Ingram SL. 5.41—the brainstem and nociceptive modulation A2—Masland, Richard H. In: Albright TD, Albright TD, Masland RH, Dallos P, Oertel D, Firestein S, Beauchamp GK, Bushnell MC, AI Basbaum, Kaas JH, Gardner EP, editors. The senses: a comprehensive reference. New York: Academic Press, 2008. pp. 593–626. [Google Scholar]
- [57].Hickey L, Li Y, Fyson SJ, Watson TC, Perrins R, Hewinson J, Teschemacher AG, Furue H, Lumb BM, Pickering AE. Optoactivation of locus ceruleus neurons evokes bidirectional changes in thermal nociception in rats. J Neurosci 2014;34:4148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain 2003;4:530–4. [DOI] [PubMed] [Google Scholar]
- [59].Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Keay K, Bandler R. Emotional and behavioral significance of the pain signal and the role of the midbrain periaqueductal gray (PAG). In: Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, Albright TD, Dallos P, editors. The senses: a comprehensive reference. Boston, MA: Elsevier, 2008. pp. 627–34. [Google Scholar]
- [61].Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci 2006;26:4437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Keuken MC, Bazin PL, Crown L, Hootsmans J, Laufer A, Muller-Axt C, Sier R, van der Putten EJ, Schafer A, Turner R, Forstmann BU. Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. Neuroimage 2014;94:40–6. [DOI] [PubMed] [Google Scholar]
- [63].Kiyokawa J, Yamaguchi K, Okada R, Maehara T, Akita K. Origin, course and distribution of the nerves to the posterosuperior wall of the external acoustic meatus. Anat Sci Int 2014;89:238–45. [DOI] [PubMed] [Google Scholar]
- [64].Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 2009;8:857–68. [DOI] [PubMed] [Google Scholar]
- [65].Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal—a pilot study. Brain Stimul 2013;6:798–804. [DOI] [PubMed] [Google Scholar]
- [66].Krüger G, Glover GH. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn Reson Med 2001;46:631–7. [DOI] [PubMed] [Google Scholar]
- [67].Kwon DH, Kim JM, Oh SH, Jeong HJ, Park SY, Oh ES, Chi JG, Kim YB, Jeon BS, Cho ZH. Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol 2012;71:267–77. [DOI] [PubMed] [Google Scholar]
- [68].Lee J, Lin RL, Garcia RG, Kim J, Kim H, Loggia ML, Mawla I, Wasan AD, Edwards RR, Rosen BR, Hadjikhani N, Napadow V. Reduced insula habituation associated with amplification of trigeminal brainstem input in migraine Cephalalgia 2016;37:1026–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lee J, Mawla I, Kim J, Loggia M, Ortiz A, Jung C, Chan S, Gerber J, Edwards R, Wasan A, Schmithorst V, Berna C, Kong J, Kaptchuk T, Gollub R, Rosen B, Napadow V. Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. PAIN 2019;160:550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lima D, Almeida A. The medullary dorsal reticular nucleus as a pronociceptive centre of the pain control system. Prog Neurobiol 2002;66:81–108. [DOI] [PubMed] [Google Scholar]
- [71].Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 2012;60:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. PAIN 2008;137:473–7. [DOI] [PubMed] [Google Scholar]
- [73].Loggia ML, Edwards RR, Kim J, Vangel MG, Wasan AD, Gollub RL, Harris RE, Park K, Napadow V. Disentangling linear and nonlinear brain responses to evoked deep tissue pain. PAIN 2012;153:2140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia—diagnosis and treatment. Cephalalgia 2017;37:648–57. [DOI] [PubMed] [Google Scholar]
- [75].Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011;70:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marciszewski KK, Meylakh N, Di Pietro F, Macefield VG, Macey PM, Henderson LA. Altered brainstem anatomy in migraine. Cephalalgia 2018;38:476–86. [DOI] [PubMed] [Google Scholar]
- [77].Marciszewski KK, Meylakh N, Di Pietro F, Mills EP, Macefield VG, Macey PM, Henderson LA. Changes in brainstem pain modulation circuitry function over the migraine cycle. J Neurosci 2018;38:10479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Menke RA, Jbabdi S, Miller KL, Matthews PM, Zarei M. Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson's disease. Neuroimage 2010;52:1175–80. [DOI] [PubMed] [Google Scholar]
- [79].Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA. Deep in the brain: changes in subcortical function immediately preceding a migraine attack. Hum Brain Mapp 2018;39:2651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mills EP, Di Pietro F, Alshelh Z, Peck CC, Murray GM, Vickers ER, Henderson LA. Brainstem pain-control circuitry connectivity in chronic neuropathic pain. J Neurosci 2018;38:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Moayedi M, Weissman-Fogel I, Salomons TV, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. PAIN 2012;153:1467–77. [DOI] [PubMed] [Google Scholar]
- [82].Moher Alsady T, Blessing EM, Beissner F. MICA-A toolbox for masked independent component analysis of fMRI data. Hum Brain Mapp 2016;37:3544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Moisset X, Villain N, Ducreux D, Serrie A, Cunin G, Valade D, Calvino B, Bouhassira D. Functional brain imaging of trigeminal neuralgia. Eur J Pain 2011;15:124–31. [DOI] [PubMed] [Google Scholar]
- [84].Mori S, Oishi K, Faria AV. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol 2009;22:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008;40:570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mosqueira AJ, Lopez-Manzanares L, Canneti B, Barroso A, Garcia-Navarrete E, Valdivia A, Vivancos J. Vagus nerve stimulation in patients with migraine. Revista de neurologia 2013;57:57–63. [PubMed] [Google Scholar]
- [87].Moss MS, Basbaum AI. The peptidergic organization of the cat periaqueductal gray. II. The distribution of immunoreactive substance P and vasoactive intestinal polypeptide. J Neurosci 1983;3:1437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Moss MS, Glazer EJ, Basbaum AI. The peptidergic organization of the cat periaqueductal gray. I. The distribution of immunoreactive enkephalin-containing neurons and terminals. J Neurosci 1983;3:603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moulton EA, Becerra L, Johnson A, Burstein R, Borsook D. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS One 2014;9:e95508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One 2008;3:e3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Naidich T, Duvernoy H, Delman B, Sorensen A, Kollias S, Haacke E. Duvernoy's atlas of the human brain stem and cerebellum. Austria: Springer-Verlag Wien, 2009. [Google Scholar]
- [92].Napadow V, Dhond R, Park K, Kim J, Makris N, Kwong KK, Harris RE, Purdon PL, Kettner N, Hui KK. Time-variant fMRI activity in the brainstem and higher structures in response to acupuncture. Neuroimage 2009;47:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Napadow V, Edwards RR, Cahalan CM, Mensing G, Greenbaum S, Valovska A, Li A, Kim J, Maeda Y, Park K, Wasan AD. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med 2012;13:777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nash PG, Macefield VG, Klineberg IJ, Murray GM, Henderson LA. Differential activation of the human trigeminal nuclear complex by noxious and non-noxious orofacial stimulation. Hum Brain Mapp 2009;30:3772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold's nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res 1984;292:199–205. [DOI] [PubMed] [Google Scholar]
- [96].O'Brien AT, Deitos A, Triñanes Pego Y, Fregni F, Carrillo-de-la-Peña MT. Defective endogenous pain modulation in fibromyalgia: a meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain 2018;19:819–36. [DOI] [PubMed] [Google Scholar]
- [97].Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010;120:3779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Parry DM, Macmillan FM, Koutsikou S, McMullan S, Lumb BM. Separation of A- versus C-nociceptive inputs into spinal-brainstem circuits. Neuroscience 2008;152:1076–85. [DOI] [PubMed] [Google Scholar]
- [99].Pertovaara A, Almeida A. Chapter 13 Descending inhibitory systems. Handb Clin Neurol 2006;81:179–92. [DOI] [PubMed] [Google Scholar]
- [100].Prats-Galino A, Soria G, de Notaris M, Puig J, Pedraza S. Functional anatomy of subcortical circuits issuing from or integrating at the human brainstem. Clin Neurophysiol 2012;123:4–12. [DOI] [PubMed] [Google Scholar]
- [101].Preibisch C, Castrillón G JG, Bührer M, Riedl V. Evaluation of multiband EPI acquisitions for resting state fMRI. PLoS One 2015;10:e0136961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Renvall V, Witzel T, Wald LL, Polimeni JR. Automatic cortical surface reconstruction of high-resolution T1 echo planar imaging data. Neuroimage 2016;134:338–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rhoton AL, Jr, O'Leary JL, Ferguson JP. The trigeminal, facial, vagal, and glossopharyngeal nerves in the monkey. Afferent connections. Arch Neurol 1966;14:530–40. [DOI] [PubMed] [Google Scholar]
- [104].Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 2009;10:211–23. [DOI] [PubMed] [Google Scholar]
- [105].Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle JT, Wald LL, Barrett LF. Identification of discrete functional subregions of the human periaqueductal gray. Proc Natl Acad Sci USA 2013;110:17101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93. [DOI] [PubMed] [Google Scholar]
- [107].Schwedt TJ, Chiang CC, Chong CD, Dodick DW. Functional MRI of migraine. Lancet Neurol 2015;14:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sclocco R, Beissner F, Bianciardi M, Polimeni JR, Napadow V. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage 2018;168:412–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sclocco R, Beissner F, Desbordes G, Polimeni JR, Wald LL, Kettner NW, Kim J, Garcia RG, Renvall V, Bianchi AM, Cerutti S, Napadow V, Barbieri R. Neuroimaging brainstem circuitry supporting cardiovagal response to pain: a combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Philos Trans A Math Phys Eng Sci 2016;374:20150189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sims-Williams H, Matthews JC, Talbot PS, Love-Jones S, Brooks JC, Patel NK, Pickering AE. Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans. Neuroimage 2017;146:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sprenger T, Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr Opin Neurol 2012;25:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain 2015;16:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Takata N, Sugiura Y, Yoshida K, Koizumi M, Hiroshi N, Honda K, Yano R, Komaki Y, Matsui K, Suematsu M, Mimura M, Okano H, Tanaka KF. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia 2018;66:2013–23. [DOI] [PubMed] [Google Scholar]
- [114].Tang Y, Sun W, Toga AW, Ringman JM, Shi Y. A probabilistic atlas of human brainstem pathways based on connectome imaging data. Neuroimage 2018;169:227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Büchel C. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science 2017;358:105–8. [DOI] [PubMed] [Google Scholar]
- [116].Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Functional analysis of V3A and related areas in human visual cortex. J Neurosci 1997;17:7060–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage 2005;26:243–50. [DOI] [PubMed] [Google Scholar]
- [118].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89. [DOI] [PubMed] [Google Scholar]
- [119].van Laar PJ, Marinus Oterdoom DL, Ter Horst GJ, van Hulzen AL, de Graaf EK, Hoogduin H, Meiners LC, van Dijk JM. Surgical accuracy of 3-tesla versus 7-tesla MRI in deep brain stimulation for Parkinson's disease. World Neurosurg 2016;93:410–2. [DOI] [PubMed] [Google Scholar]
- [120].Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain 2013;17:299–312. [DOI] [PubMed] [Google Scholar]
- [121].Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst 2000;16:101–2. [DOI] [PubMed] [Google Scholar]
- [122].Wang R, King T, De Felice M, Guo W, Ossipov MH, Porreca F. Descending facilitation maintains long-term spontaneous neuropathic pain. J Pain 2013;14:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wargo CJ, Gore JC. Localized high-resolution DTI of the human midbrain using single-shot EPI, parallel imaging, and outer-volume suppression at 7T. Magn Reson Imaging 2013;31:810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA. Anatomical changes at the level of the primary synapse in neuropathic pain: evidence from the spinal trigeminal nucleus. J Neurosci 2015;35:2508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA. Anatomical changes within the medullary dorsal horn in chronic temporomandibular disorder pain. Neuroimage 2015;117:258–66. [DOI] [PubMed] [Google Scholar]
- [126].Yezierski RP. Spinomesencephalic tract: projections from the lumbosacral spinal cord of the rat, cat, and monkey. J Comp Neurol 1988;267:131–46. [DOI] [PubMed] [Google Scholar]
- [127].Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. PAIN 2010;149:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Youssef AM, Gustin SM, Nash PG, Reeves JM, Petersen ET, Peck CC, Murray GM, Henderson LA. Differential brain activity in subjects with painful trigeminal neuropathy and painful temporomandibular disorder. PAIN 2014;155:467–75. [DOI] [PubMed] [Google Scholar]
- [129].Youssef AM, Macefield VG, Henderson LA. Pain inhibits pain; human brainstem mechanisms. Neuroimage 2016;124:54–62. [DOI] [PubMed] [Google Scholar]
- [130].Yuan H, Silberstein SD. Vagus nerve stimulation and headache. Headache 2017;57(Suppl 1):29–33. [DOI] [PubMed] [Google Scholar]
- [131].Zhang WT, Mainero C, Kumar A, Wiggins CJ, Benner T, Purdon PL, Bolar DS, Kwong KK, Sorensen AG. Strategies for improving the detection of fMRI activation in trigeminal pathways with cardiac gating. Neuroimage 2006;31:1506–12. [DOI] [PubMed] [Google Scholar]
- [132].Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol 1997;78:746–58. [DOI] [PubMed] [Google Scholar]


