Abstract
Psychological therapies, such as cognitive behavioral therapy, are widely used multifaceted approaches that have been shown to improve pain-related functioning. A small but growing number of studies have used brain imaging to support the use of psychological therapies for pain. Although these studies have led to an increased understanding of how therapies may engage neural systems, there are multiple technical and conceptual challenges to consider. Based on the current literature, several components of effective psychological therapies for pain may be supported by changes in neural circuitry, which are most consistently represented by diminished activation and/or reduced hyperconnectivity in brain regions related to pain processing, emotion, and cognitive control. Findings may vary based on methodological approaches used and may also differ depending on targets of treatment. To provide a nuanced understanding of the current literature, specific targets and components of effective treatments for which a neural basis has been investigated are reviewed. These treatment components include catastrophic thinking about pain, increasing self-efficacy, mindfulness, anxiety symptom reduction, and exposure-based approaches. In general, such strategies have the potential to normalize regional hyperactivations and reduce hyperconnectivity in brain regions associated with nociceptive processing, cognition, and emotion, although additional research is needed. By determining if there are indeed distinct brain mechanisms engaged by different components of psychological therapy and evidence for specific changes in neural function after these interventions, future therapies may be more optimally tailored for individuals afflicted with chronic pain.
Keywords: Pain, Psychotherapy, Cognitive behavioral therapy, Brain, Neuroimaging, Neural
1. Introduction
Psychological therapies are an important component in the multidisciplinary treatment of chronic pain. Cognitive behavioral therapies (CBTs) are widely used approaches that involve cognitive (eg, reducing catastrophic thinking about pain) and behavioral (eg, relaxation and behavioral activation) strategies to improve coping with pain and pain-related functioning, with some variation in the specific components of CBT across protocols. Cognitive behavioral therapy has been shown to improve functional disability and pain symptoms,46,54 self-efficacy,33,34 pain catastrophizing,33,34 and psychological functioning46,54 in individuals with chronic pain. Although the current review focuses primarily on traditional cognitive and behavioral interventions, other approaches such as mindfulness-based approaches or acceptance-commitment therapy (a variant of CBT that emphasizes acceptance and engagement in valued activities despite pain) may also be beneficial for improving pain outcomes48,69; however, it is unknown how such psychological therapies work. Psychotherapies are multifaceted, and there is even some overlap between seemingly distinct modalities such as CBT and mindfulness. In addition, psychological interventions may work through both specific (eg, reframing negative thoughts) and nonspecific (eg, reduced distress from therapist support and increased psychological flexibility) processes.11–13,20,47 A clearer mechanistic understanding of these interventions can serve as a valuable platform for refining and optimizing treatment delivery.
A small but growing number of studies in the pain field have used brain imaging to explore the effect of psychological therapies for pain, leading to new understanding of how these therapies may influence neural systems.31,62 Emerging findings have been somewhat equivocal, potentially reflecting methodologic differences between studies as well as the possibility that different psychotherapeutic treatment components may engage both distinct and overlapping brain networks. When the current literature specific to pain is examined alongside the broader literature examining the neural basis of the effect of psychotherapy for related conditions such as anxiety and depressive disorders,7,25 there is mounting evidence that successful psychotherapeutic intervention approaches may normalize aberrant brain functioning (ie, diminished regional brain hyperactivations, reduced hyperconnectivity between regions associated with symptoms, affect, and cognition).4 In this review, we also address the methodological challenges of imaging neural changes after psychological therapies for pain, and then focus on extant findings by psychotherapeutic treatment components shown to positively impact pain outcomes (Fig. 1).
Figure 1.
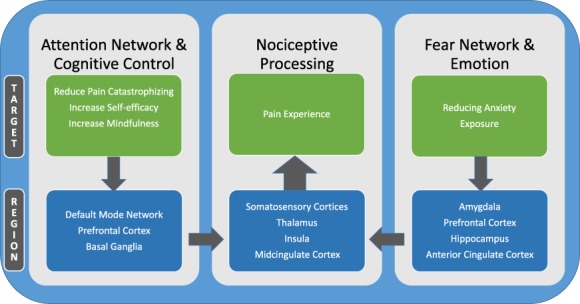
Brain mechanisms influenced by psychological therapies for pain. Psychological therapies for chronic pain may involve multiple components to directly target pain symptoms, such as cognitive restructuring, mindfulness, and exposure to painful stimuli, in addition to integrating treatment components to directly decrease anxiety symptoms. These differing pain treatment components may engage overlapping and partially distinct brain mechanisms, which in turn may independently regulate nociceptive processing and the experience of pain. Thus, combined therapeutic approaches hold the potential to engage multiple modulatory systems to optimize the treatment effect on pain symptoms.
2. How the brain is impacted by chronic pain
To understand pain-related brain changes associated with psychotherapy, we first describe how the brain is impacted by chronic pain. The brain is composed of multiple primary sensory and associated networks that activate and deactivate as distinct assemblies. A whole-brain wide network, conceptualized as the pain connectome, is thought to integrate cognitive, affective, and sensorimotor processes of pain.39 The effect of chronic pain on the brain is broad-ranging,14,55 affecting regions directly related to nociceptive processing (eg, thalamus, and the primary and secondary sensory cortices, insula, and midcingulate cortex), mood (eg, the pregenual anterior cingulate cortex and amygdala), and self-referential processing (eg, medial prefrontal cortex). The insula may link sensory and affective regions and amplify the pain experience.65 Chronic pain may also influence the default mode network (DMN).2,3,56 The DMN is a network of interacting brain regions, including the medial parietal (precuneus and posterior cingulate), bilateral inferior–lateral–parietal, and ventromedial frontal cortices,64 which is active when the mind is “wandering” (ie, not engaged in a specific task)56 or when engaged in self-referential thoughts about pain. The salience network (eg, the anterior insula, midcingulate cortex, temporoparietal junction, and dorsolateral prefrontal cortex [dlPFC]) is also key in its relation to the pain experience, given that this network tracks the degree to which external stimuli (such as a painful stimulus) intrinsically capture attention, and may be represented by sustained activation during attention to pain.40,59 Indeed, chronic pain has a broad-ranging impact on the brain.
Enhanced understanding of the effect of CBT and its components on brain regions/networks could help deepen the understanding of how such therapies work. However, imaging them remains challenging due to several technical considerations.
3. Technical challenges of imaging brain mechanisms underlying psychological therapies for pain
Historically, much of brain imaging has been driven by neuroscientists who use stimulus- or task-based paradigms to evoke neural activity. Accordingly, methodologies have been optimized to image shorter and shorter events. Positron emission tomography of cerebral blood flow was used first for much human imaging to make inferences about brain activity, but the temporal resolution of ∼1 minutes was cumbersome for most tasks.29 The discovery of the BOLD effect and the continued development of functional magnetic resonance imaging have enabled vastly improved temporal resolution.53 Currently, many scanners have the capability to capture whole-brain images in periods of less than 0.5 seconds. Thus, images can be acquired with sufficient speed to accurately capture the hemodynamic response curve elicited by a very brief sensory event. However, in the effort to develop faster and faster image acquisition, the technology for imaging relatively slow or steady-state phenomena (such as chronic pain) has lagged behind.
One of the major limitations of BOLD in this context of imaging psychological therapies for pain is that it fails to accurately image slow phenomena. BOLD is not a fully quantitative technique due to the influence of additional dynamic physiological parameters such as blood volume that are challenging to measure simultaneously.22 Accordingly, the BOLD signal is susceptible to drift, which is often exacerbated by nonphysiological factors such as minute subject movements and scanner heating. Finally, significant signal nonlinearities occur during stimulus/task periods exceeding ∼24 seconds.44 Many phenomena including both chronic pain itself and psychological tasks for pain control last far longer than 30 seconds, and cannot be readily turned on and off. Thus, different approaches to imaging chronic pain and pain improvement, which may be better conceptualized as steady-state phenomena, are critically needed.
The first approach to examining relatively slow phenomena such as pain is to adapt the widely available BOLD technology to examine brain activity in a fashion that still sheds light on mechanisms without the utilization of stimulus-/task-based designs. Examination of functional connectivity provides the means to accomplish this.6 In brief, functional connectivity examines correlations among multiple brain regions during a state that remains relatively stable across the course of imaging series lasting ∼5 to 12 minutes. Individual brain regions exhibit substantial fluctuations in BOLD signal over time, and correlations of these resting or spontaneous fluctuations among multiple brain regions suggest that information is being transferred. Comparisons of these correlation maps before and after treatment, or between patients and controls, thus provide insight into the underlying brain mechanisms. Unfortunately, physiological factors (such as changes in breathing, cardiac activity, and global cerebral blood flow), movement, and scanner artifacts can elicit striking correlations among regions.68 As such, the potential exists for such confounds to dramatically complicate data interpretation. For example, patients experiencing low back pain may be far more prone to move in the scanner than patients whose pain had been relieved by therapy. Accordingly, differences in patterns of correlations may emerge as artifacts of movement rather than engagement of different brain mechanisms. Multiple noise reduction techniques have been developed to address these correlations and such techniques have become widely used.5,30,58 As denoted below, functional connectivity has been the dominant tool for investigating psychological therapies for pain management.
Despite the utility of functional connectivity, imaging either resting or task-related activation offers many advantages for interpretation of mechanisms. For example, comparing resting-state data before and then after intervention will allow for a more specific determination of whether the intervention changed the engagement of brain regions/networks during relative rest. Similarly, engaging in a specific task during imaging (such as practicing a particular pain management strategy) would allow for more definitive conclusions about the specific components of the intervention that may underlie such changes.
In real life (as opposed to being in an MRI scanner), patients can engage in various tasks such as cognitive restructuring, relaxation, and mindfulness to cope with pain for extended periods of time. Brain activation underlying these sustained tasks can be imaged using arterial spin label (ASL) imaging instead of BOLD imaging. Arterial spin label emerged shortly after BOLD imaging,21 but was developed more slowly due to hardware and software limitations. However, as delineated in other studies in this issue (eg, Loggia, Zeidan), ASL provides a highly useful tool for imaging activation during relatively slow phenomena.22 It is fully quantitative and images cerebral blood flow by using magnetically tagged blood as a tracer. Accordingly, ASL can directly assess global changes in cerebral blood flow. This ability becomes particularly useful when the psychological therapy in question is associated with physiological changes that may alter global cerebral blood flow (CBF) (ie, changes in arterial pCO2 that may arise during changes in breathing). Despite these strengths, ASL suffers from a relatively poor signal-to-noise ratio. The perfusion-subtracted images that ASL generates before processing into fully quantified images of CBF are quite variable from one subtraction pair to the next. Accordingly, for reliable quantification, ASL typically requires a minimum of ∼4 minutes of data acquisition to generate one fully quantified CBF volume. Thus, the limitation of a relatively poor temporal resolution is counterbalanced by the strength of obtaining a fully quantified CBF volume when investigating sustained phenomena such as psychological modulation of pain.
4. Neural impact of psychological therapies
Given the limited nature of the psychological intervention literature as it relates to neuroimaging of pain, it is helpful to first understand how psychotherapy generally impacts neural functioning. Several recent reviews have investigated the neural basis of the effect of CBTs and related interventions for the treatment of anxiety and depressive disorders. For example, a 2016 review of 10 neuroimaging studies that examined changes after CBT for depression found that the most common treatment-associated changes involved the anterior cingulate cortex, followed by changes in the posterior cingulate, ventromedial prefrontal cortex/orbitofrontal cortex, and the amygdala/hippocampus regions.25 Furthermore, resting-state activity in the dorsal anterior cingulate cortex may be decreased by CBT for depression, which may result in increased efficiency of a “dorsal cognitive circuit” included in cognitive control and in effortful or top-down emotion regulation.
Similarly,a systematic review of 19 functional magnetic resonance imaging studies (total n = 509) to understand how CBT for anxiety disorders impacts neural functioning suggested that anxiety and related conditions are associated with an exaggerated fear network activation involving regions such as the amygdala, hippocampus, striatum, anterior cingulate cortex, and insula, and CBT may work to increase (ie, normalize) prefrontal control of subcortical structures.7 Furthermore, in studies that specifically involved symptom provocation tasks,52 or exposure-based activities,50 decreased activation in areas associated with these tasks were observed after intervention. In sum, brain regions most associated with emotion processing and emotion regulation (such as the prefrontal cortex and amygdala) may be most sensitive to change after CBT for anxiety and depression. Changes in brain regions associated with emotion regulation may also be characteristic of a positive treatment response to CBT for chronic pain.
Few studies haveexamined neural impact of specific components of CBT including biofeedback, deep breathing, and relaxation. It is plausible that the ventromedial prefrontal cortex (vmPFC) may play a critical role in these treatment strategies, given its association with autonomic regulation. Indeed, the vmPFC is associated with threat-induced changes in heart rate and skin conductance, whereas biofeedback-induced changes in skin conductance are inversely associated with activations of the ventromedial prefrontal cortex and adjacent orbitofrontal cortex.24,51,70 In addition, performance of a biofeedback relaxation task also produces increased activation of the anterior cingulate cortex, basal ganglia, primary somatosensory cortex, inferior parietal cortex, and cerebellar vermis.16,17 Similarly, progressive muscle relaxation may engage many brain regions, activating the superior frontal gyrus and inferior frontal gyrus, and deactivating the posterior cingulate cortex.37 Anatomical studies in monkeys indicate that neurons within the vmPFC, as well as all frontal motor regions, the primary somatosensory cortex, and anterior cingulate cortex, provide afferent input to the adrenal medulla.23,37 Thus, relaxation strategies may engage these brain areas to directly regulate autonomic arousal. However, given the near-total absence of neuroimaging studies in patients with pain, evidence linking brain mechanisms associated with relaxation with pain reduction is lacking—although the vmPFC represents an appealing target for investigation.
Brain mechanismssupporting cognitive reappraisal (CR; often an element of CBT programs) have been extensively investigated, and critically involve the dlPFC. In contrast to studies of relaxation, considerable research has examined brain mechanisms of CR of emotions. A meta-analysis of 48 brain imaging studies reveals that reappraisal activated brain regions involved in cognitive control such as the dlPFC and posterior parietal cortex.9 Assessments of deactivations during reappraisal (ie, identification of brain areas that were greater during baseline emotional tasks) revealed that bilateral portions of the amygdala exhibited decreased activity during reappraisal. Thus, the amygdala may represent a major target of reappraisal therapy. By contrast, the ventromedial prefrontal cortex was not altered—either positively or negatively—during reappraisal. In the case of pain, components of placebo analgesia may engage CR processes.67 Overlapping regions of the dlPFC are activated by both CR as well as placebo.67 Moreover, CBT for chronic pain is associated with increases in dlPFC gray matter volume.60
5. Conceptual challenges to consider when imaging brain mechanisms underlying psychological therapies for pain
In addition to substantial technical challenges, there are a myriad of conceptual challenges when attempting to delineate the impact of psychological therapies for pain on neural functioning. First, and foremost, CBT and related interventions represent a battery of operationally and mechanistically distinct methods for controlling pain. Does the investigator attempt to image resting brain function, functioning during a pain episode (whether real or induced), or during use of a cognitive behavioral strategy to cope with pain? If a pain coping strategy is used, should the participant be able to use the strategy or strategies he/she perceives to be the most effective or should the participant be instructed to use a single strategy? If a single strategy is chosen, is synergy among the different strategies lost, resulting in a concomitant reduction in efficacy and detectability of brain activation? If multiple strategies are chosen, can the resulting patterns of brain activation be reliably interpreted? Second, dissociating the mechanism supporting the psychological therapy from the mechanisms supporting pain may represent a further challenge. There is a considerable overlap between brain regions engaged in nociceptive processing with those engaged in cognitive modulation of pain.38,71 At a simplistic level, during and after therapy, one could anticipate reduced activation in pain-related regions while those associated with various psychological therapies which inhibit pain could exhibit increases. However, what if therapy results in reduced activity of brain regions amplifying pain? Thus, regions involved in cognitive modulation of pain would be predicted to exhibit the same posttherapeutic trend as regions associated with pain. Finally, with any cognitive task, how does the investigator know that the participant was performing the appropriate psychological therapy technique during the imaging window? Despite these many challenges, delineating the multiple brain mechanisms impacted by psychological therapy is a topic of critical importance, given the efficacy of these techniques and the absence of effective pharmacological tools for pain management.
The following sections attempt to distill brain mechanisms associated with several targets and processes of commonly used psychological approaches for the treatment of pain. It is important to note that these findings and the current conclusions drawn are a reflection of an emerging area of research. There have been very few but growing number of studies examining neural mechanisms associated with response to psychological therapies for pain. Although exploring the interventions by subcomponents is useful for distilling potentially distinct mechanisms of treatment, it should be noted that conceptually distinct strategies (ie, reframing catastrophic thinking about pain or mindfulness) may also work in similar ways to reduce pain (eg, through reducing pain catastrophizing), and as a result, there may be overlap in the neural networks/regions impacted. As previously stated, a single treatment program for pain is often multimethod in approach (ie, cognitive and behavioral strategies with mindfulness strategies in a pain-focused CBT protocol, eg) and therefore may impact multiple mechanisms.
6. Psychotherapeutic components
6.1. Reducing catastrophic thinking about pain
Identifying and changing negative appraisals and catastrophic thoughts about pain is a standard component of pain-focused CBT,66 and there is a wealth of literature to suggest that pain-related catastrophizing reduces after CBT for pain.10,34 Catastrophic thoughts are generally self-referential, negative, and may occur automatically (particularly for those with chronic pain who are prone to clinical levels of anxiety),1 and research suggests that they may be reflected by changes within the DMN.35,36 Similarly, mind wandering has been linked to DMN activity.49 Lazaridou et al.43 found that reductions in resting-state functional connectivity between primary somatosensory cortex and several DMN regions (eg, precuneus) were associated with treatment-related reductions in catastrophizing, suggesting that changes in DMN activity may be related to changes in pain-related cognition. However, a similar pattern emerged in both CBT and an educational control, potentially due to shared nonspecific therapeutic effects.12 Another study of healthy individuals undergoing pain-focused CBT showed that no significant associations between DMN activity changes and changes in pain catastrophizing occurred during either CBT or an active control.41 However, it is important to note that the latter study was conducted with healthy persons. This is important because individuals with chronic pain may exhibit altered DMN functioning from the outset. However, healthy individuals may not express the same disrupted DMN activity because such individuals are unlikely to exhibit the automatic negative thoughts that may categorize a chronic pain experience. Hence, psychological therapies in those with chronic pain reduce the tendency to ruminate on maladaptive thoughts about pain, which may in turn be reflected by the neural changes as described above.
In individuals with chronic pain, catastrophic thinking can occur constantly (including in between pain episodes for those with chronic pain conditions who experience a remittance of symptoms between flares) and also during attention-demanding activities, such as during an active pain episode. The latter example may be reflected by changes in the prefrontal and subcortical regions in the brain. However, pain catastrophizing is associated with increased activity in brain regions associated with anticipation of pain, attention, emotion, and motor control.27 Decreased pain catastrophizing after CBT was associated with increased gray matter in the left dorsolateral prefrontal and ventrolateral prefrontal cortices (associated with catastrophizing and cognitive control), right posterior parietal cortex, somatosensory cortices, and pregenual anterior cingulate cortex (regions also associated with attention and nociceptive processing) in a mixed sample of chronic pain patients compared to those who never received CBT.60 Similarly, increased activations were found in the ventrolateral prefrontal/lateral orbitofrontal cortex (regions associated with cognitive control) during a pain-induction task in patients with fibromyalgia who underwent CBT32; however, these studies all used different methodologies (only the latter used pain induction). Despite the various methodological approaches, when considered together, these findings suggest that changes in the brain after improvements in pain-related catastrophizing may involve increased activity of prefrontal and subcortical brain regions or decreased DMN activity (Fig. 1).
6.2. Increasing self-efficacy
Psychotherapies for chronic pain aim to improve self-efficacy in managing pain symptoms by training in adaptive and goal-directed coping strategies. As self-efficacy in coping with pain improves, other maladaptive thoughts and behaviors related to pain, such as pain-related catastrophizing, diminishes. Neuroimaging literature suggests that patients with chronic musculoskeletal pain who exhibited the greatest improvements in self-efficacy after CBT compared to an active control were also those with the greatest reductions between the DMN and the amygdala intrinsic functional connectivity.61 This finding is congruent with literature suggesting that increased self-efficacy, decreased catastrophic thinking, and improved mood may be concurrent outcomes associated with response to pain-focused CBT.34 Furthermore, decreased passive coping and increased self-efficacy after CBT corresponded to increased basal ganglia connectivity with the right secondary somatosensory cortex.61 It is noteworthy that the explicit goal of CBT for pain is to diminish maladaptive coping strategies (such as negative thoughts surrounding pain) and establish new habits (ie, adaptive coping behaviors) to manage pain. Interestingly, the basal ganglia have also been implicated in goal-directed behaviors and in habitual control.57 Thus, it is plausible that these connectivity changes may be related to increases in intentional (goal-directed) or automatic (habitual) use of coping strategies for pain (Fig. 1).
6.3. Targeting global anxiety
When psychotherapy for pain also targets global anxiety (beyond anxiety experienced directly as a result of pain), this may correspond to neural patterns that emerge after successful treatment for anxiety, which are characterized increased prefrontal control of subcortical structures.7 In the pain literature, improvements in symptoms of general anxiety and worry corresponded to reductions in the amygdala and the anterior cingulate cortex regions after cognitive therapy for irritable bowel syndrome.42 Furthermore, decreases in anxiety and in ventrolateral prefrontal cortex activation were found after CBT for pain, but not during an active control,61 suggesting that a reduction in anxiety may be a marker of increased cognitive control. It is possible that reducing anxiety in individuals with chronic pain may bolster the effects of psychotherapy on pain-related outcomes.
Although anxiety and chronic pain commonly co-occur and psychological therapies for pain may serve to decrease anxiety, it is noteworthy that presence of comorbid anxiety may adversely impact response to psychological therapies for pain.18,45 This may be because anxiety management is not consistently integrated into pain-focused treatments,18 although such efforts are underway.19 Again, the neural basis of psychological therapies for anxiety found that treatments may work to increase prefrontal control of structures7 such as the fear network (ie, amygdala and hippocampus), which is associated with improved cognitive appraisal of emotional response. Although additional research is needed in pain, it is possibility that neural circuitry underlying emotional processing and emotional regulation (prefrontal cortex and amygdala) may be most sensitive to change after psychological treatment for anxiety (Fig. 1).
6.4. Exposure
Exposure is traditionally considered a component of successful CBT treatment for anxiety. In the context of pain, exposure has been also used to reduce pain-related fear by promoting gradual increases in activities that may otherwise be avoided due to a real or perceived fear of experiencing pain flares. In a treatment study using elements of exposure (eg, increasing functional behavior in youth with complex regional pain syndrome through an intensive multidisciplinary rehabilitation program), decreased pain-related fear after treatment was associated with decreased hyperconnectivity between the amygdala and the motor and somatosensory cortex, cingulate, and frontal areas.63 Although the treatment approach studied was multifaceted, decreased pain-related avoidance may attenuate the connectivity between regions associated with emotion, sensory processing, and cognition (Fig. 1).
6.5. Mindfulness
Mindfulness approaches (addressed in more detail elsewhere in this issue) generally include attending to experiences in the present moment and acquiring a nonjudgmental attitude towards pain. Some of these strategies have been integrated into CBT and other psychological therapy approaches. Thus, the literature is briefly referenced here. Although not a direct goal of mindfulness, this approach may also reduce catastrophic thinking about pain.15,26 Mindfulness has been shown to be related to increased pain reduction particularly in long-term practitioners,28 and in regions associated with cognitive and emotional control.8 Zeidan et al. (in this issue) summarize multiple studies demonstrating that extensive mindfulness training (>1000 hours) for pain relief is associated with deactivation of the prefrontal cortex and greater activation of somatosensory cortices, which may suggest an ability to diminish pain-related appraisals. Extensive mediation may be considered as conceptually distinct from brief psychotherapies for pain; so, these results are not summarized further in this review.
In a study of healthy individuals undergoing brief mindfulness meditation, reduction in pain intensity during an experimental pain induction task after meditation was associated with increased activity in the anterior cingulate cortex and anterior insula, areas involved in the cognitive regulation of nociceptive processing, whereas reductions in pain unpleasantness ratings were associated with orbitofrontal cortex activation, an area implicated in reframing the contextual evaluation of sensory events.71 Zeidan et al.72 (in this issue) note that dispositional (trait) mindfulness in meditation-naive individuals is associated with lower pain and neural mechanisms related to self-referential processes, although this was not an intervention study. However, brief mindfulness training (<10 hours) for pain is associated with higher-order regulation of nociceptive neural targets such as the thalamus and primary somatosensory cortex, which may suggest an engagement of reappraisal mechanisms.71 The brief mindfulness training approach71 may be more congruent to other nonpharmacological approaches for pain management such as CBT and thus is reviewed further in Table 1. In sum, mindfulness meditation may serve to alter the pain experience through multiple brain mechanisms associated with both cognitive control and evaluation of the pain experience (Fig. 1), but additional research is needed in chronic pain populations.
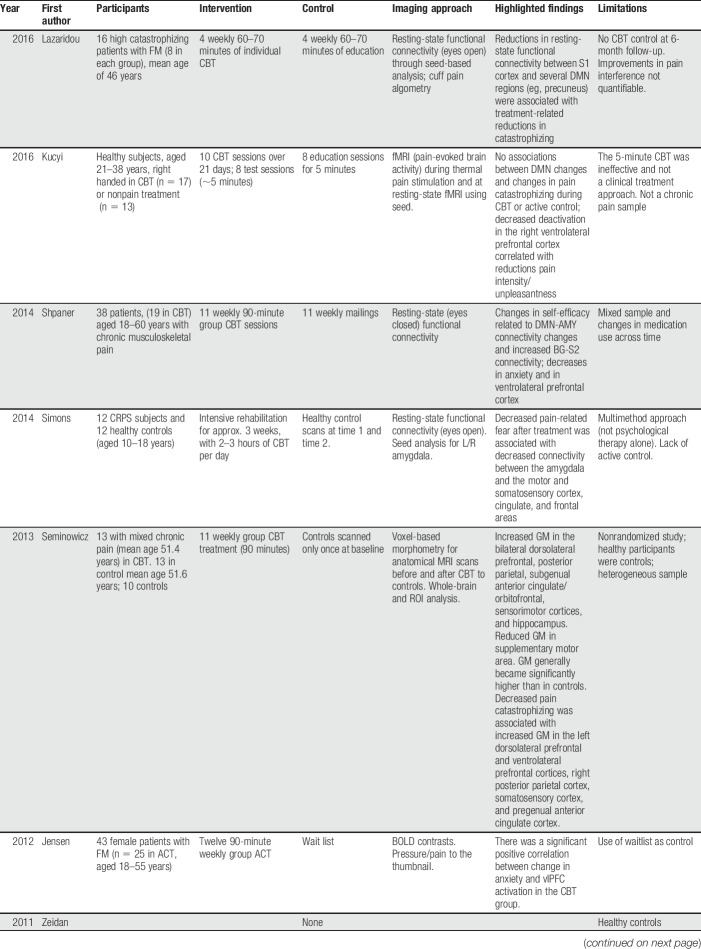
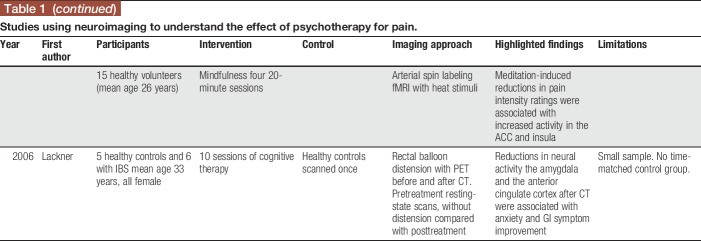
Table 1.
Studies using neuroimaging to understand the effect of psychotherapy for pain.
6.6. The goal: improving pain and functioning
The aim of pain-focused psychotherapy is to improve both pain symptoms and restore daily functioning (ie, reduce pain-related disability); however, the extant literature with regards to neuroimaging has primarily focused on pain and pain-related symptoms as the main outcome of interest. For example, reductions in neural activity (using positron emission tomography) in regions associated with pain perception and self-regulation (ie, amygdala and the anterior cingulate cortex) after cognitive therapy were accompanied by significant improvements in GI symptoms for irritable bowel syndrome patients during a symptom provocation task.42 Furthermore, decreased deactivation in the right ventrolateral prefrontal cortex correlated with reductions in pain intensity and pain unpleasantness after CBT for healthy individuals compared to an active control.41 Although the relationship between neural changes and functional disability after psychological therapy for pain is generally not reported upon in imaging studies, one study that did report changes in function after CBT did not find corresponding changes in brain matter.60 This may be because functional disability is categorized by a constellation of cognitions, feelings, and behaviors, which may be more challenging to map onto specific brain regions. However, this is an area worthy of further investigation.
7. Conclusions
There are multiple technical and conceptual challenges to consider with regards to understanding neural changes after psychological therapies for pain. Different analytic approaches to consider include use of functional connectivity, or use of resting or task-related activation. Psychological therapies that improve clinical outcomes may result in changes in neural circuitry that differ depending on targets and components of treatment (summarized by the conceptual model in Fig. 1). By determining if there are indeed distinct brain mechanisms engaged by different components of psychological therapy, then therapies may be optimally tailored based on mechanistic knowledge. For example, in individuals with chronic pain and high levels of anxiety, use of traditional pain-coping strategies (reducing pain catastrophizing) in conjunction with strategies to specifically manage anxiety may engage distinct brain mechanisms (such as the amygdala and prefrontal cortex) to impact the experience of pain. It would be informative for future studies to directly test how specific elements of a psychological treatment differentially impact neural circuitry of the brain. For example, study designs that dissect the neural mechanisms supporting a positive response to different components of behavioral treatment strategies (eg, relaxation) vs cognitive techniques (eg, reframing catastrophic thoughts) are critically needed to provide mechanistically informed opportunities for tailored treatments capitalizing on synergistic interactions between different mechanisms. Furthermore, psychological therapies may work differently for different pain diagnoses, and may engage distinct mechanisms. Although this emerging area offers promising insights, interpretation is limited, given the variability in treatment designs (ie, differing psychotherapies used and inconsistent use of pain induction) and subjects (age, sex, pain diagnosis vs healthy samples) across studies.
7.1. Future directions
The examination of long-term impact of psychotherapy for pain on brain functioning is important. Using imaging tools to test conceptually driven a priori hypotheses to understand mechanisms of treatment response could be a powerful method for producing replicable findings to advance the field and allowing for a deeper understanding of how treatments for chronic pain work. This emerging research into biobehavioral mechanisms could galvanize the development of more targeted and effective treatments for individuals afflicted with chronic pain.
Disclosures
The authors have no conflict of interest to declare.
N.R. Cunningham is supported by the NIH/NCCIH K23AT009458 grant. S. Kashikar-Zuck is supported by the NIH/NIAMS K24 AR056687 grant. R.C. Coghill is supported by the NIH/NINDS R01 NS085391 and R01 NS101321 grants.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].American Psychological Association. Diagnostic and statistical manual of mental disorders. 5th ed Arlington: American Psychiatric Publishing, 2013. [Google Scholar]
- [2].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006;26:12165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 2008;28:1398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: a systematic and critical review. Prog Neurobiol 2014;114:1–14. [DOI] [PubMed] [Google Scholar]
- [5].Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41. [DOI] [PubMed] [Google Scholar]
- [7].Brooks SJ, Stein DJ. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin Neurosci 2015;17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brown CA, Jones AK. Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. PAIN 2010;150:428–38. [DOI] [PubMed] [Google Scholar]
- [9].Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 2014;24:2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Buhrman M, Nilsson-Ihrfelt E, Jannert M, Ström L, Andersson G. Guided internet-based cognitive behavioural treatment for chronic back pain reduces pain catastrophizing: a randomized controlled trial. J Rehab Med 2011;43:500–5. [DOI] [PubMed] [Google Scholar]
- [11].Burns JW, Day MA, Thorn BE. Is reduction in pain catastrophizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl Behav Med 2012;2:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burns JW, Nielson WR, Jensen MP, Heapy A, Czlapinski R, Kerns RD. Does change occur for the reasons we think it does? A test of specific therapeutic operations during cognitive-behavioral treatment of chronic pain. Clin J Pain 2015;31:603–11. [DOI] [PubMed] [Google Scholar]
- [13].Burns JW, Nielson WR, Jensen MP, Heapy A, Czlapinski R, Kerns RD. Specific and general therapeutic mechanisms in cognitive behavioral treatment of chronic pain. J Consult Clin Psychol 2015;83:1. [DOI] [PubMed] [Google Scholar]
- [14].Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cassidy EL, Atherton RJ, Robertson N, Walsh DA, Gillett R. Mindfulness, functioning and catastrophizing after multidisciplinary pain management for chronic low back pain. PAIN 2012;153:644–50. [DOI] [PubMed] [Google Scholar]
- [16].Critchley H, Melmed R, Featherstone E, Mathias C, Dolan RJ. Brain activity during biofeedback relaxation: a functional neuroimaging investigation. Brain 2001;124:1003–12. [DOI] [PubMed] [Google Scholar]
- [17].Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage 2002;16:909–19. [DOI] [PubMed] [Google Scholar]
- [18].Cunningham NR, Jagpal A, Tran ST, Kashikar-Zuck S, Goldschneider KR, Coghill RC, Lynch-Jordan AM. Anxiety adversely impacts response to cognitive behavioral therapy in children with chronic pain. J Pediatr 2016;171:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cunningham NR, Nelson S, Jagpal A, Moorman E, Pentiuk S, Farrell MK, Kashikar-Zuck S. The development of the Aim to Decrease Anxiety and Pain Treatment (ADAPT) for youth with functional abdominal pain. J Pediatr Gastroenterol Nutr 2018;61:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Day MA, Thorn BE, Burns JW. The continuing evolution of biopsychosocial interventions for chronic pain. J Cog Psychother 2012;26:114–29. [Google Scholar]
- [21].Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med 1992;23:37–45. [DOI] [PubMed] [Google Scholar]
- [22].Detre JA, Rao H, Wang DJ, Chen YF, Wang Z. Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging 2012;35:1026–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci U S A 2016;113:9922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eisenbarth H, Chang LJ, Wager TD. Multivariate brain prediction of heart rate and skin conductance responses to social threat. J Neurosci 2016;36:11987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Franklin G, Carson AJ, Welch KA. Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuropsychiatr 2016;28:61–74. [DOI] [PubMed] [Google Scholar]
- [26].Garland EL, Gaylord SA, Palsson O, Faurot K, Mann JD, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med 2012;35:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gracely R, Geisser M, Giesecke T, Grant M, Petzke F, Williams D, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004;127:835–43. [DOI] [PubMed] [Google Scholar]
- [28].Grant JA, Rainville P. Pain sensitivity and analgesic effects of mindful states in Zen meditators: a cross-sectional study. Psychosom Med 2009;71:106–14. [DOI] [PubMed] [Google Scholar]
- [29].Herscovitch P, Markham J, Raichle M. Brain blood flow measured with intravenous H215O: I. Theory and error analysis. J Nucl Med 1983;24:782. [PubMed] [Google Scholar]
- [30].Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med 1995;34:201–12. [DOI] [PubMed] [Google Scholar]
- [31].Jensen KB, Berna C, Loggia ML, Wasan AD, Edwards RR, Gollub RL. The use of functional neuroimaging to evaluate psychological and other non-pharmacological treatments for clinical pain. Neurosci Lett 2012;520:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgeia. PAIN 2012;153:1495–503. [DOI] [PubMed] [Google Scholar]
- [33].Jensen MP, Turner JA, Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol 2001;69:655. [DOI] [PubMed] [Google Scholar]
- [34].Kashikar-Zuck S, Sil S, Lynch-Jordan AM, Ting TV, Peugh J, Schikler KN, Hashkes PJ, Arnold LM, Passo M, Richards-Mauze MM. Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. J Pain 2013;14:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Knyazev GG. Extraversion and anterior vs. posterior DMN activity during self-referential thoughts. Front Hum Neurosci 2012;6:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Knyazev GG, Savostyanov AN, Volf NV, Liou M, Bocharov AV. EEG correlates of spontaneous self-referential thoughts: a cross-cultural study. Int J Psychophysiol 2012;86:173–81. [DOI] [PubMed] [Google Scholar]
- [37].Kobayashi S, Koitabashi K. Effects of progressive muscle relaxation on cerebral activity: an fMRI investigation. Complement Ther Med 2016;26:33–9. [DOI] [PubMed] [Google Scholar]
- [38].Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci 2005;102:12950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kucyi A, Davis KD. The dynamic pain connectome. Trend Neurosci 2015;38:86–95. [DOI] [PubMed] [Google Scholar]
- [40].Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience-and attention-related brain networks. J Neurophysiol 2012;108:3382–92. [DOI] [PubMed] [Google Scholar]
- [41].Kucyi A, Salomons TV, Davis KD. Cognitive behavioral training reverses the effect of pain exposure on brain-network activity. PAIN 2016;157:1895–904. [DOI] [PubMed] [Google Scholar]
- [42].Lackner JM, Lou Coad M, Mertz HR, Wack DS, Katz LA, Krasner SS, Firth R, Mahl TC, Lockwood AH. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav Res Ther 2006;44:621–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lazaridou A, Kim J, Cahalan CM, Loggia ML, Franceschelli O, Berna C, Schur P, Napadow V, Edwards RR. Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin J Pain 2017;33:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol 2004;66:735–69. [DOI] [PubMed] [Google Scholar]
- [45].Matcham F, Norton S, Scott DL, Steer S, Hotopf M. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology 2016;55:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McCracken LM, Turk DC. Behavioral and cognitive–behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine 2002;27:2564–73. [DOI] [PubMed] [Google Scholar]
- [47].McCracken LM, Velleman SC. Psychological flexibility in adults with chronic pain: a study of acceptance, mindfulness, and values-based action in primary care. PAIN 2010;148:141–7. [DOI] [PubMed] [Google Scholar]
- [48].McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol 2014;69:178. [DOI] [PubMed] [Google Scholar]
- [49].Mittner M, Boekel W, Tucker AM, Turner BM, Heathcote A, Forstmann BU. When the brain takes a break: a model-based analysis of mind wandering. J Neurosci 2014;34:16286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Morgieve M, N'diaye K, Haynes W, Granger B, Clair AH, Pelissolo A, Mallet L. Dynamics of psychotherapy-related cerebral haemodynamic changes in obsessive compulsive disorder using a personalized exposure task in functional magnetic resonance imaging. Psychol Med 2014;44:1461–73. [DOI] [PubMed] [Google Scholar]
- [51].Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage 2004;22:243–51. [DOI] [PubMed] [Google Scholar]
- [52].Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:901–10. [DOI] [PubMed] [Google Scholar]
- [53].Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci 1990;87:9868–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Palermo TM, Eccleston C, Lewandowski AS, Williams ACdC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. PAIN 2010;148:387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 2000;30:263–88. [DOI] [PubMed] [Google Scholar]
- [56].Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci 2010;11:760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 2014;90:449–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 2013;14:1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shpaner M, Kelly C, Lieberman G, Perelman H, Davis M, Keefe FJ, Naylor MR. Unlearning chronic pain: a randomized controlled trial to investigate changes in intrinsic brain connectivity following cognitive behavioral therapy. Neuroimage Clin 2014;5:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev 2014;39:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel A, Serrano P, Sethna N, Berde C, Becerra L, Borsook D. The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. PAIN 2014;155:1727–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 2009;106:13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci 2009;29:2684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Thorn BE, Boothby JL, Sullivan MJ. Targeted treatment of catastrophizing for the management of chronic pain. Cog Behav Pract 2002;9:127–38. [Google Scholar]
- [67].Van Der Meulen M, Kamping S, Anton F. The role of cognitive reappraisal in placebo analgesia: an fMRI study. Soc Cog Affect Neurosci 2017;12:1128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012;59:431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. PAIN 2011;152:533–42. [DOI] [PubMed] [Google Scholar]
- [70].Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 2009;47:821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 2011;31:5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zeidan F, Salomons T, Farris SR, Emerson NM, Adler-Neal A, Jung Y, Coghill RC. Neural mechanisms supporting the relationship between dispositional mindfulness and pain. PAIN 2018;159:2477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]