Abstract
Differentiating subtypes of chronic pain still remains a challenge—both from a subjective and objective point of view. Personalized medicine is the current goal of modern medical care and is limited by the subjective nature of patient self-reporting of symptoms and behavioral evaluation. Physiology-focused techniques such as genome and epigenetic analyses inform the delineation of pain groups; however, except under rare circumstances, they have diluted effects that again, share a common reliance on behavioral evaluation. The application of structural neuroimaging towards distinguishing pain subtypes is a growing field and may inform pain-group classification through the analysis of brain regions showing hypertrophic and atrophic changes in the presence of pain. Analytical techniques such as machine-learning classifiers have the capacity to process large volumes of data and delineate diagnostically relevant information from neuroimaging analysis. The issue of defining a “brain type” is an emerging field aimed at interpreting observed brain changes and delineating their clinical identity/significance. In this review, 2 chronic pain conditions (migraine and irritable bowel syndrome) with similar clinical phenotypes are compared in terms of their structural neuroimaging findings. Independent investigations are compared with findings from application of machine-learning algorithms. Findings are discussed in terms of differentiating patient subgroups using neuroimaging data in patients with chronic pain and how they may be applied towards defining a personalized pain signature that helps segregate patient subgroups (eg, migraine with and without aura, with or without nausea; irritable bowel syndrome vs other functional gastrointestinal disorders).
Keywords: Chronic pain, Migraine, IBS, Machine learning
1. Introduction
Chronic pain is one of the most frequently encountered medical conditions and still remains an enormous clinical, behavioral, and social challenge.16 Understanding how elements of pain differ between individuals is critical towards optimizing medical care. Attempts at differentiating subtypes of chronic pain, even within a particular disease state, may evaluate several features. For example, a person's phenotype can be indexed to evaluate the behavioral or clinical evaluation of pain symptoms, whereas their biotype can provide information relating to the expression of genetic markers, or by evaluating brain data, how pain information is processed differently between individuals. Phenotypic analyses have dominated clinical and research investigations to date and commonly group patients experiencing chronic pain symptoms under a single identifier to understand the etiology of pain symptoms. Routinely, clinical evaluation has depended on factors such as psychosocial factors, pain variability and quality, sleep and fatigue, quantitative sensory testing and sensory profiling (notably, pain intensity), and conditioned pain modulation to define a pain condition.19 Phenotypic evaluations are inherently subjective and can have a high degree of temporal variability,52 intersubject variability,27 and be susceptible to factors such as observational bias, as noted by Cowen et al.15 who argue for more objective tools when evaluating pain. This variability in symptom reporting can place limitations on developing techniques such as genome-wide association studies30,76 and research aimed at resolving pain mechanisms and outlining therapies. New methods that use objective criteria may provide critical insight to improve how chronic pain is understood.
1.1. Neuroimaging of chronic pain
Chronic pain is a complex process that integrates brain networks implicated in a myriad of behavioral, affective, cognitive, and motor functions. Thus, aside from sensory measures, psychological factors such as depression2 and anxiety3 can significantly influence pain levels and are associated with discrete brain networks.50 Other processes such as cognitive domains implicated in pain include attention and emotional circuitry25 that alone integrate frontal, temporal, and subcortical brain structures.28 Brain imaging of these networks provides a potentially integrative and objective measure that is intended to be independent of factors such as observer bias and provide more stable metrics of pain in patient groups (see Ref. 17 for discussion). In other words, behaviors are defined by brain networks. Techniques such as voxel-based morphometry, as well as resting-state functional neuroimaging use static or non–task-related images of the brain, and therefore are (in theory) removed from certain forms of bias because they rely on statistical methods for quantifying anomalous data in pain cohorts.
Clinical evaluation of pain has proved difficult to segregate subtypes in many disease states. For example, in persons with Parkinson's disease, attempts have been made to create subtypes from clinical features such as the extent of motor symptoms58; however, the extensive variability both in symptom breadth and in their temporal presentation represent formidable barriers because they present as overlapping spectral, rather than categorical, features.46 Similar findings are observed in persons with headache69 and gastrointestinal disorders65 that rely heavily on patient-reported symptoms. The concept of overlapping pain conditions and associated pain symptom reporting adds further complexity to the clinical diagnosis, which may be informed by brain imaging of pain-relevant brain networks that occur on a subliminal level.8 For example, the insula is a region implicated in pain salience in patients with migraine whose structural morphology and functional activity are clinically related.7 As well, subcortical brain structures including the amygdala, brainstem, and cerebellum are involved in the processing of emotional elements of a painful stimulus such as fear of pain condition.4 Notably, because pain is an “experience” and therefore tied to perception,56 it remains to be determined how brain imaging can be applied towards defining pain subtypes and whether insights are best applied either independently or cooperatively to inform patient diagnostics.
1.2. Two clinical conditions
Delineating pain groups using phenotypic criteria is fundamentally limited in its ability to resolve group differences in patients with chronic pain. It remains to be determined if neuroimaging can provide additional data and extend knowledge on a group of patients who share similar pain phenotypes. Two chronic pain conditions that share phenotypic features and are often evaluated in tandem for their shared etiology and clinical presentation are irritable bowel syndrome (IBS) and migraine. Notably, we refer to a patient's phenotype as it relates to pain symptomology and specifically the evolution and characteristics of how pain symptoms present over the course of an individual's life. For example, both conditions begin in childhood, involve intermittent attacks, female predisposition, have autonomic components that include nausea and vomiting,10 and commonly both conditions occur in the same patient.53 Migraine has been associated with functional gastrointestinal disorders and other bowel disease, including colic, gastroparesis, celiac disease, and inflammatory bowel disease.18,29
2. Neuroimaging comparisons between migraine and irritable bowel syndrome
The aim of this review is to evaluate the potential of structural neuroimaging to inform the clinical divisions between 2 chronic pain conditions. We focus on the objectives of (1) comparing patient groups with migraine and IBS by outlining changes in brain structure in the 2 cohorts, (2) providing a meta-analysis of stereotaxic data reported from the 2 conditions, and (3) providing a review of research evaluating machine learning on structural neuroimaging data in these cohorts. Reports of brain regions can be found in Tables 1 and 2, as well as in Figure 1.
Table 1.
Structural brain changes in patients with IBS.
Table 2.
Structural brain changes in patients with migraine.
Figure 1.

Regional structural changes in IBS and migraine cohorts presented using chord diagrams. Chords represent reported changes with the center of each chord being the frontal (F) cortex presented for convenience. The machine-learning diagram presents both increases and decreases together. Line weights are proportional to the number of studies reported. IBS, irritable bowel syndrome.
2.1. Brain regions
Data extracted from neuroimaging studies reflected average thickness and volume from cortical and subcortical regions of the brain. White matter integrity was evaluated using diffusion-weighted imaging1 and measures reflecting water diffusion along the axial (AD = sensitive to axonal damage), radial (RD = sensitive to myelin level), and a mean (MD = measure sensitive to edema) of all directions, as well as a measure anisotropic water diffusivity (fractional anisotropy; FA = nonspecific marker of neuropathology). The patient data reflect individuals with IBS and migraine from approximately 20 to 60 years of age. Patient cohorts ranged in size from 8 to 110 and reflected mainly comparisons between patients and healthy controls.
2.1.1. Frontal cortex
Although structural brain changes are reported in both cohorts, more diffuse abnormalities are presented within persons with migraine than IBS within the frontal cortex. In IBS cohorts, evidence of decreased cortical thickness within the middle frontal gyrus13,62 as well as the DLPFC and DMPFC33 was observed. For persons with a migraine diagnosis, an increase in surface area of the left suborbital gyrus was observed in one study,49 whereas a decrease in brain volume was reported in regions including the right middle frontal lobe, left superior frontal sulcus, and left precentral sulcus.37 In the migraine cohort, an increase in FA was found20 and an increase in gray matter density in the orbitofrontal cortex62 and a decrease in brain volume was reported by 4.13,33,41,62 A review on persons with migraine supports a general decrease in brain volume within the frontal cortex.35
2.1.2. Visual cortex
The visual cortex does seem to be impacted in migraine subjects in a manner that was not observed those with IBS. A decrease in brain volume in the visual cortex has been reported for migraine37 as well as IBS.13,62 In patients with IBS, a small cluster of cortex within the occipital cortex was found to show decreased thickness relative to healthy controls62 and a small region within the cuneus.13 In migraine cohorts, an increase in brain volume is reported within the visual cortex in the lateral occipital–temporal cortex74 and broadly across the visual cortex when associated with visual aura.23 As such, differences in pain conditions within the visual cortex seem to be related to the visual symptoms associated with migraine patients.
2.1.3. Somatosensory cortex
Similar trends are reported across structural brain data within the somatosensory cortex in patients with IBS and migraine. In persons with IBS, an increase in cortical thickness36,41,62,70 was reported with only one study reporting a decrease in cortical thickness.20 Specifically, female patients with IBS have been reported to have greater cortical thickness in the postcentral gyrus than healthy female,36,41 as well as in the secondary somatosensory cortex.62 Healthy controls have been reported to have increased cortical thickness in the somatosensory cortex relative to persons with migraine32 as well as an increased gyrification index (ie, increased folding) in left postcentral gyrus.74 Relative to controls and patients without aura, patients with aura had decreased cortical thickness of the right central sulcus.49 Together, findings suggest that similar trends are reported within the somatosensory cortex, even when considering subtypes of patients with migraine who may experience aura.
2.1.4. Parietal cortex
Structural neuroimaging data from patients with IBS and migraine within the parietal cortex were similar between cohorts. No evidence of increased cortical thickness from the reviewed studies was found for the parietal regions in either the IBS or migraine cohorts. In patients with IBS, there are consistent reports of a decrease in the posterior parietal cortex,13,33,62 and a similar decrease is found in migraine patients in both the posterior parietal cortex39 as well as the inferior parietal lobe.61 As such, similar regions and trends are reported within the parietal cortex.
2.1.5. Temporal cortex
Differences in how brain structure is implicated between patients with IBS and migraine are found within the temporal cortex. In the context of IBS, evidence to support a structural change is limited, with only one study identifying lower volumes in bilateral hippocampi.41 Alternatively, in migraine, there is evidence to support a decrease in gray matter volumes57,61,67 including gray matter density in the parahippocampal area61 as well as increases45,49,74 in regions such as the left inferior temporal cortex.35Therefore, differences in pain groups within the temporal cortex are found where persons with migraine may demonstrate gray matter changes external to the hippocampus and parahippocampus.
2.1.6. Motor cortex
In migraine and IBS cohorts, similar changes in brain structure are observed. Findings in persons with IBS support the presence of an increase in the primary motor cortex in female relative to male IBS patients36 and a decrease in brain volume within the premotor cortex62 as well as lower FA in motor regions.20 In patients with migraine, an increase in cortical thickness around the central gyrus35,45 as well as a decrease in motor and premotor regions35,39,49,67 are reported.
2.1.7. Limbic regions
The limbic regions of the brain experience similar changes in brain structure between IBS and migraine cohorts. Several studies report an increase in brain volume in persons with IBS within the anterior cingulate cortex and posterior cingulate cortex, insula, as well as increased FA in the fornix and external capsule next to the right posterior insula.11,33,54,62 Patients also reported a decrease in the anterior midcingulate cortex and right caudal anterior cingulate cortex, and female patients seem to have higher MD in the cingulate white matter than male patients with IBS do.5,13,20,31,36,42,70 In patients with migraine, a decrease in gray matter volume within the left cingulate gyrus, right anterior cingulate cortex, and bilateral insular cortex is reported.32,37,39,45,60,67,74 No significant increases within limbic regions were reported in the migraine cohort.
2.1.8. Subcortical regions
In terms of brain regions, group trends were largely similar in terms of how subcortical regions were impacted structurally. However, it seems that distinct subcortical structures are impacted in each clinical cohort. In persons with IBS, Ellingson et al.20 reported higher MD in regions in the internal capsule and thalamus and a lower FA and MD in the globus pallidus. An increase in gray matter density in the hypothalamus5 and decreased gray matter density were found in the thalamus62 and left cuneus13 have also been observed, thus focusing attention largely on the thalamus within IBS cohorts. In migraine cohorts, an increase in the gray matter of cerebellar regions74 and the periaqueductal gray (PAG)57 and a decrease in gray matter volume of the cerebellum37 were found. The former (PAG) has previously been implicated in having a role in descending pain modulation44 and is a notable difference between groups. That is, contrary to reports in IBS, the volume expansion12 as well as atypical functional activity have been reported within the PAG in persons with migraine, which has been proposed as generator for migraine attacks.71 As such, subcortical structures may provide novel insight into specific pain conditions.
2.1.9. Sex differences and psychometrics
Biological sex has been shown to influence changes in brain morphology. As shown in Table 1, a significant portion of subjects evaluated were female. In persons with IBS, Jiang et al. found that brain changes were most observant in female subjects. Females with IBS were found to have lower cortical thickness within the bilateral subgenual anterior cingulate cortex and greater cortical thickness within the precentral gyrus and postcentral gyrus relative to female healthy controls.36 Alternatively, in migraineurs, an ALE analysis comparing 191 migraine patients and 199 healthy controls found no effect of sex.35 It has been reported that changes in brain morphology in persons with IBS correlate with patient health variables such as sex, anxiety, depression, IBS bowel subtype, and early-life trauma.70 In persons with migraine, an ALE analysis found that estimated frequency of attack has been reported to correlate with changes in the GM volume of the right claustrum, left cingulated gyrus, right anterior cingulate, amygdala, and left parahippocampal gyrus.35 Thus, the influence of biological sex and clinical features seems to influence IBS and migraine in distinct manners.
2.2. Meta-analysis
To further examine the brain regions implicated in the 2 evaluated cohorts, a meta-analysis of reported stereotaxic coordinates was performed. Of the included studies (limited by author reporting of coordinates), stereotaxic coordinates were extracted from 9 experiments (5 from migraine and 4 from IBS), totaling 473 subjects and 107 coordinates (Fig. 2). Only studies that reported coordinates comparing the sample population relative to healthy controls were included. One cluster of stereotaxic coordinates was found in the combined data set and was located within the inferior frontal gyrus of the right hemisphere (Montreal Neurological Institute [MNI] coordinates: 62, 14, 10; extrema value: 0.0167). This cluster contained only studies from the migraine cohort.39,57,67 Notably, changes within this region are not reported in the IBS literature from our review (see above). However, reported clustering in the right hemisphere aligns with prior literature suggesting a right hemisphere focus for pain processing.66 As outlined in the current review, a large number of brain regions are found to display altered morphology within the frontal cortex in both groups, and altered changes within the inferior frontal gyrus have been specifically implicated in pain processing.40 The right hemisphere inferior frontal gyrus has been implicated in cognitive processes such as attentional switching26 and response inhibition.22 As such, the current meta-analysis findings suggest that group differences may be observed within the frontal lobe between groups in relation to factors that may influence motor tasks.
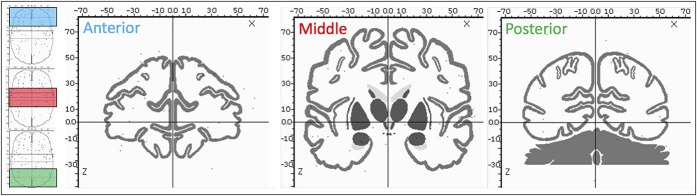
Figure 2.
Coordinate evaluated in meta-analysis. Three sections are presented going from the anterior (blue) through the middle (red) and posterior (green) aspects of the brain. Black dots represent stereotaxic coordinates reported in the included studies within the window presented on the left. Image is presented in MNI space.
2.3. Defining pain signatures and subgroups using higher level classification tools
The availability of large-scale data has instigated the use of more mathematically driven techniques to understand data emerging from brain imaging. One of the most popular forms of this is machine learning where regression-based analyses (for example) can be applied to understand data trends in ways that either reinforce a priori knowledge or provide data-driven knowledge (see Ref. 51 for review of machine learning in neurological disorders). In the former, called supervised learning, knowledge regarding group designations may be supplied (eg, designating if a participant is a healthy control or migraine patient) to determine how well-evaluated metrics (eg, functional Magnetic Resonance Imaging fMRI BOLD data) are able to predict group membership. Unsupervised learning is where data are submitted without a priori knowledge, which enables a more data-driven approach to evaluate trends emerging from that data set. Accordingly, machine learning has the capacity to be applied as a classification tool in persons with pain to aid in the delineation of variables that may be used to differentiate existing group membership, or to supply novel data that extend current pain-group knowledge.
Research evaluating patients with IBS and migraine using structural neuroimaging data and machine learning has been minimal. Regions delineating patients with IBS from healthy controls using morphological criteria can achieve an accuracy of 70%. Although this level of accuracy is low, it provides a list of regions that help distinguish healthy from IBS persons: the horizontal ramus (R), subcallosal area (L), intraparietal sulcus (L) and transverse parietal sulci (L), fusiform gyrus (L), transverse temporal sulcus (R), superior precentral gyrus (L) and primary interoceptive cortex (L), posterior segment of the lateral sulcus (L), and posterior insula/inferior parietal sulci (L).42 Evaluating migraine patients without aura using gray matter data, the top 10 brain regions that classified patients relative to controls (accuracy of 71.43%) included the hippocampus (L/R), parahippocampus (L/R), precentral gyrus (L/R), superior frontal gyrus (L/R), supplementary motor area (L), superior frontal gyrus (R), and inferior frontal gyrus (R) (opercular part). Notably, these patients had elevated depression and anxiety scores that may also have impacted structural brain data.75 Using a support vector machine model, Garcia-Chimeno et al.24 found that they could correctly classify controls vs patients (chronic migraine) at a level of 94% using diffusion tensor imaging from the left uncinate, and left cingulate gyrus that also integrated patient reports of pain and their analgesic use. Accordingly, the value of using brain imaging data is best exemplified when used in conjunction with patient-related data.
In patients with chronic pain, the use of machine-learning classifiers has been yielding results that are at times strong but largely variable between studies. The accuracy of such classifiers ranges from approximately 50% to 90% and only addresses the differentiation between pain and nonpain groups, and not addressing the diversity in underlying pain subtypes. Although research has shown in both IBS and migraine cohorts that distinct brain regions can be identified through structural metrics to classify patients relative to healthy controls, no research has evaluated these 2 conditions in tandem. Despite this, evidence suggests that a relatively high level of accuracy can be obtained in relation to healthy populations (approximately 70%–90%). Comparing results from the above investigations with those shown to contribute towards subliminal pain processing (see Ref. 8), it is interesting to note that only cortical structures are observed. The prefrontal cortex is inherently involved in the conscious awareness of pain, and temporal lobe structures such as the hippocampus are involved with conscious and unconscious memories. Both of these regions were identified through the above machine-learning studies in the IBS and migraine cohorts and align with a neuronal basis of pain chronification. Pursuing future research that directly compares pain cohorts with machine-learning algorithms is likely to yield highly valuable clinical information.
3. Discussion
In this review, 2 highly phenotypically related pain conditions were chosen to evaluate and compare how structural neuroimaging data can distinguish the 2 groups. Findings highlight unique differences between these patient groups that may be exploited in future work attempting to delineate pain subtypes using single study or advanced classification tools.
3.1. Are differences in pain groups observable and relevant?
In the current review, a comparison was performed between 2 chronic pain conditions with similarities in terms of their clinical phenotype. Interestingly, a large number of regions displayed similar trends between groups that align with those reported in prior work on the “pain matrix,”47,48 consistent with a core set of brain regions being implicated in the pain response. However, beyond these regions, the 2 populations diverged. Group differences were observed mainly within the visual, temporal, and subcortical regions, and findings from the meta-analysis highlighted a difference in the inferior frontal gyrus. An increase in cortical thickness within the visual cortex was observed in the migraine and not IBS group, which may align with the visual component of migraine symptoms.68 Although both groups showed decreases in temporal lobe volumes, only the migraine cohort showed evidence of an increase in brain volume. The finding of hippocampal changes in patients with migraine has been attributed towards stress38 but is has also been suggested to play an important role in the pathophysiology of migraine.45 Interestingly, a very pain-relevant area was found in the migraine but not IBS cohort (PAG), which plays a role in descending pain modulation.44 Finally, findings from the inferior frontal gyrus may relate to a loss of motor control for patients with migraine,73 the extent of the pain (right sided), or how the pain influences nearby motor structures such as speech perception (Brodmann Area 44: Broca's area). Together, findings from the review and meta-analysis support an identifiable difference between conditions that share very similar phenotypic presentations.
Findings from the reviewed machine-learning studies highlighted unique features. That is, in the IBS group, dominant regions were reported that included frontal, parietal, motor, and limbic regions. Alternatively, in the migraine group, frontal, temporal, and limbic regions of the cortex were reported. In both cases, only cortical regions were reported as features to the associated algorithms. This is a novel finding and contrasts with findings from single studies (Fig. 1) that report both cortical and subcortical structures as being active, as well as prior findings that implicate subcortical structures in pain processing.8 Reasons for this may include low variability in subcortical volumes, inconsistent labeling of subcortical structures, and a lower relative contribution to pain symptoms in comparison with cortical structures. The precise nature of this will be the subject of future research. Notably, the output from such algorithms produced varying degrees of success, ranging from 50% to 90% in accuracy,6,9,55,64 and suggests that the performance of pain-group classifiers may currently be limited in terms of reproducibility or stability. Changes in brain regions highlighted outside of the pain matrix have been suggested to correspond to comorbidities63 such as depression59 or anxiety21 and may have influenced brain morphology. This would align with higher ratings of depression and anxiety found in the migraine cohort and may account for findings in the limbic (eg, insula) and prefrontal (eg, orbitofrontal cortex) regions. Considering that the currently referenced work is at the level of differentiating pain conditions from healthy control cohorts, comparing pain subtypes is likely to present even greater instability.
3.2. Integrating neuroimaging to improve pain classification
It has been previously argued by our group that pain processing continues, even if it does not reach conscious awareness.8 To this point, analysis of neuroimaging data would provide invaluable knowledge that could not be addressed using existing phenotypic evaluation criteria. However, the delineation of brain regions implicated in specific pain cohorts has received a paucity of attention because the pain-neuroimaging field first validates the ability to distinguish regions that are implicated in the elementary response to pain.34,47,48
To improve the stability and accuracy of machine-learning classifiers, future analyses should integrate multimodal data. As in phenotypic as well as biotype analyses, the isolation of a single or even a set of brain regions is not pathognomonic for a particular pain disorder.43 Because each domain has limitations, the use of metadata from multiple modalities likely has a critical role in defining pain subtypes in future research. The use of phenotypic data definitely has a critical role in the evaluation of patients with chronic pain because it reflects the impact that a disease has on factors such as quality of life; however, understanding subliminal factors such as neuronal processing of pain information and genetic susceptibility will not only help delineate new pain subtypes but also improve the precision of personalized medicine. The use of large pain imaging repositories such as PainRepository.org will contribute to the development of accurate pain-group classifiers by improving their accuracy and reproducibility.42
3.3. Limitations
This study has several limitations that require address. First, the literature search was not exhaustive and therefore we may have missed studies that are published. The impact of missing a publication on findings from this review is likely minimal as our findings integrated prior reviews. Second, the performed meta-analysis is limited in terms of the number of studies that were included. We were constrained in terms of how many studies we could extract reported stereotaxic coordinates from as not all studies reported coordinates from statistical comparisons. Similar to other reviews, we chose to align our reporting with the brain regions reported by study authors. Third, a lack of investigations examining the age effects of chronic pain conditions constrained our ability to comment on the impact of age in the evaluated clinical conditions. Although prior research has shown age-dependent effects in terms of the clinical presentation of migraine,72 as well as the functional organization of the brain,14 this is an area that requires greater attention from researchers. Due to the limited nature of the available literature, investigations evaluated in this review were focused on young adults. Finally, our choice of pain conditions for evaluation was based on prior literature examining both IBS and migraine both independently and in tandem. If different pain conditions were chosen, it is possible that study findings may have been marginally different, although we are confident that conclusions would remain the same.
4. Conclusion
The capacity to identify subgroups of patients and achieve personalized medical practice will require the integration of a person's brain type. Information retrieved from brain imaging is likely to play a significant role in this process because it offers a bridge that connects the variance in a person's innate biology with their presenting phenotype and can provide insight into mechanisms of pain perception and persistence. The specific delineation of pain subgroups is likely to be best fulfilled by integrating metadata from diverse modalities and using higher level computing to discern trends using supervised and semisupervised techniques. Future research will be required to determine the optimal integration of each technique to define pain subtypes.
Disclosures
The authors have no conflict of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2008:26:316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003;163:2433. [DOI] [PubMed] [Google Scholar]
- [3].Bandelow B. Generalized anxiety disorder and pain. Mod Trends Pharmacpsychiatry 2015;30:153–65. [DOI] [PubMed] [Google Scholar]
- [4].Bingel U, Quante M, Knab R, Bromm B, Weiller C, Büchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. PAIN 2002;99:313–21. [DOI] [PubMed] [Google Scholar]
- [5].Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 2010;138:1783–9. [DOI] [PubMed] [Google Scholar]
- [6].Boissoneault J, Sevel L, Letzen J, Robinson M, Staud R. Biomarkers for musculoskeletal pain conditions: use of brain imaging and machine learning. Curr Rheumatol Rep 2017;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Borsook D, Veggeberg R, Erpelding N, Borra R, Linnman C, Burstein R, Becerra L. The insula: a “Hub of activity” in migraine. Neuroscientist 2016;22:632–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Borsook D, Youssef AM, Barakat N, Sieberg CB, Elman I. Subliminal (latent) processing of pain and its evolution to conscious awareness. Neurosci Biobehav Rev 2018;88:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Callan D, Mills L, Nott C, England R, England S. A tool for classifying individuals with chronic back pain: using multivariate pattern analysis with functional magnetic resonance imaging data. PLoS One 2014;9:e98007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chang FY, Lu CL. Irritable bowel syndrome and migraine: bystanders or partners? J Neurogastroenterol Motil 2013;19:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen JYW, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res 2011;1392:121–31. [DOI] [PubMed] [Google Scholar]
- [12].Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S. Volume expansion of periaqueductal gray in episodic migraine: a pilot MRI structural imaging study. J Headache Pain 2017;18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chua CS, Bai CH, Shiao CY, Hsu CY, Cheng CW, Yang KC, Chiu HW, Hsu JL. Negative correlation of cortical thickness with the severity and duration of abdominal pain in Asian women with irritable bowel syndrome. PLoS One 2017;12:e0183960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Colon E, Ludwick A, Wilcox SL, Youssef AM, Danehy A, Fair DA, Lebel AA, Burstein R, Becerra L, Borsook D. Migraine in the young brain: adolescents vs. young adults. Front Hum Neurosci 2019;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cowen R, Stasiowska MK, Laycock H, Bantel C. Assessing pain objectively: the use of physiological markers. Anaesthesia 2015;70:828–47. [DOI] [PubMed] [Google Scholar]
- [16].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults. MMWR Morb Mortal Wkly Rep 2018;67:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Davis KD, Flor H, Greely HT, Iannetti GD, Mackey S, Ploner M, Pustilnik A, Tracey I, Treede RD, Wager TD. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol 2017;13:624–38. [DOI] [PubMed] [Google Scholar]
- [18].Drossman D. The functional gastrointestinal disorders and the Rome III Process. Gastroenterology 2016;130:1377–90. [DOI] [PubMed] [Google Scholar]
- [19].Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, Brell J, Bujanover S, Burke LB, Carr D, Chappell AS, Cowan P, Etropolski M, Fillingim RB, Gewandter JS, Katz NP, Kopecky EA, Markman JD, Nomikos G, Porter L, Rappaport BA, Rice ASC, Scavone JM, Scholz J, Simon LS, Smith SM, Tobias J, Tockarshewsky T, Veasley C, Versavel M, Wasan AD, Wen W, Yarnitsky D. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. PAIN 2016;157:1851–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. PAIN 2013;154:1528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fonzo GA, Etkin A. Affective neuroimaging in generalized anxiety disorder: an integrated review. Transl Res 2017;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WPM, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci 2008;28:9790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gaist D, Hougaard A, Garde E, Reislev NL, Wiwie R, Iversen P, Madsen CG, Blaabjerg M, Nielsen HH, Krøigård T, Østergaard K, Kyvik KO, Hjelmborg J, Madsen K, Siebner HR, Ashina M. Migraine with visual aura associated with thicker visual cortex. Brain 2018;141:776–85. [DOI] [PubMed] [Google Scholar]
- [24].Garcia-Chimeno Y, Garcia-Zapirain B, Gomez-Beldarrain M, Fernandez-Ruanova B, Garcia-Monco JC. Automatic migraine classification via feature selection committee and machine learning techniques over imaging and questionnaire data. BMC Med Inform Decis Mak 2017;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garland EL. Pain processing in the human nervous system. Prim Care 2012;39:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hampshire A, Chamberlain S, Monti M, Duncan J, Owen A. The role of the right inferior frontal gyrus: inhibition and attention control. Neuroimage 2010;50:1313–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum 2005;52:3670–4. [DOI] [PubMed] [Google Scholar]
- [28].Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC Neurosci 2012;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van Hemert S, Breedveld AC, Rovers JMP, Vermeiden JPW, Witteman BJM, Smits MG, de Roos NM. Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front Neurol 2014;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Holliday KL, McBeth J. Recent advances in the understanding of genetic susceptibility to chronic pain and somatic symptoms. Curr Rheumatol Rep 2011;13:521–7. [DOI] [PubMed] [Google Scholar]
- [31].Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, Smith SR, Tillisch K, Naliboff B, Mayer EA. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci 2014;34:14252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hougaard A, Amin FM, Arngrim N, Vlachou M, Larsen VA, Larsson HBW, Ashina M. Sensory migraine aura is not associated with structural grey matter abnormalities. Neuroimage Clin 2016;11:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hubbard CS, Becerra L, Smith JH, DeLange JM, Smith RM, Black DF, Welker KM, Burstein R, Cutrer FM, Borsook D. Brain changes in responders vs. non-responders in chronic migraine: markers of disease reversal. Front Hum Neurosci 2016;10:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back). Exp Brain Res 2010;205:1–12. [DOI] [PubMed] [Google Scholar]
- [35].Jia Z, Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin 2017;14:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, Ashe-McNalley C, Hong JY, Tillisch K, Toga AW, Mayer EA. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS One 2013;8:e73932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J. Structural and functional abnormalities in migraine patients without aura: brain abnormalities in migraine patients without aura. NMR Biomed 2013;26:58–64. [DOI] [PubMed] [Google Scholar]
- [38].Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem 2015;22:411–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim J, Suh SI, Seol H, Oh K, Seo WK, Yu SW, Park KW, Koh SB. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 2008;28:598–604. [DOI] [PubMed] [Google Scholar]
- [40].Kong J, Loggia ML, Zyloney C, Tu P, LaViolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. PAIN 2010;148:257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Tillisch K, Ebrat B, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. PAIN 2014;155:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Labus JS, Van Horn JD, Gupta A, Alaverdyan M, Torgerson C, Ashe-McNalley C, Irimia A, Hong JY, Naliboff B, Tillisch K, Mayer EA. Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. PAIN 2015;156:1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded. Prog Neurobiol 2011;93:111–24. [DOI] [PubMed] [Google Scholar]
- [44].Loyd DR, Murphy AZ. The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural Plast 2009;2009:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct 2013;218:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Marras C, Chaudhuri KR. Nonmotor features of Parkinson's disease subtypes. Mov Disord 2016;31:1095–102. [DOI] [PubMed] [Google Scholar]
- [47].Martucci KT, Mackey SC. Imaging pain. Anesthesiol Clin 2016;34:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015;125:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Messina R, Rocca MA, Colombo B, Valsasina P, Horsfield MA, Copetti M, Falini A, Comi G, Filippi M. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology 2013;268:170–80. [DOI] [PubMed] [Google Scholar]
- [50].Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry 2015;77:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev 2012;36:1140–52. [DOI] [PubMed] [Google Scholar]
- [52].Parry E, Ogollah R, Peat G. Significant pain variability in persons with, or at high risk of, knee osteoarthritis: preliminary investigation based on secondary analysis of cohort data. BMC Musculoskelet Disord 2017;18:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Perveen I. Prevalence of irritable bowel syndrome (IBS), migraine and co-existing IBS- migraine in medical students. J Clin Diagn Res 2016;10:OC09–OC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Piché M, Chen JI, Roy M, Poitras P, Bouin M, Rainville P. Thicker posterior insula is associated with disease duration in women with irritable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain 2013;14:1217–26. [DOI] [PubMed] [Google Scholar]
- [55].Robinson ME, O'Shea AM, Craggs JG, Price DD, Letzen JE, Staud R. Comparison of machine classification algorithms for fibromyalgia: neuroimages versus self-report. J Pain 2015;16:472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Robinson ME, Staud R, Price DD. Pain measurement and brain activity: will neuroimages replace pain ratings? J Pain 2013;14:323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 2006;37:1765–70. [DOI] [PubMed] [Google Scholar]
- [58].van Rooden SM, Colas F, Martínez-Martín P, Visser M, Verbaan D, Marinus J, Chaudhuri RK, Kok JN, van Hilten JJ. Clinical subtypes of Parkinson's disease. Mov Disord 2011;26:51–8. [DOI] [PubMed] [Google Scholar]
- [59].Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TGM, Bos D, Ikram MA, Vernooij MW, Niessen WJ, Tiemeier H, Hofman A, Wittfeld K, Grabe HJ, Janowitz D, Bülow R, Selonke M, Völzke H, Grotegerd D, Dannlowski U, Arolt V, Opel N, Heindel W, Kugel H, Hoehn D, Czisch M, Couvy-Duchesne B, Rentería ME, Strike LT, Wright MJ, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Goya-Maldonado R, Gruber O, Krämer B, Hatton SN, Lagopoulos J, Hickie IB, Frodl T, Carballedo A, Frey EM, van Velzen LS, Penninx BWJH, van Tol MJ, van der Wee NJ, Davey CG, Harrison BJ, Mwangi B, Cao B, Soares JC, Veer IM, Walter H, Schoepf D, Zurowski B, Konrad C, Schramm E, Normann C, Schnell K, Sacchet MD, Gotlib IH, MacQueen GM, Godlewska BR, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Hall J, Sussmann JE, Li M, Walter M, Aftanas L, Brack I, Bokhan NA, Thompson PM, Veltman DJ. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017;22:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schmidt-Wilcke T, Leinisch E, Gänbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. PAIN 2006;125:89–97. [DOI] [PubMed] [Google Scholar]
- [61].Schmitz N, Arkink EB, Mulder M, Rubia K, Admiraal-Behloul F, Schoonmann GG, Kruit MC, Ferrari MD, van Buchem MA. Frontal lobe structure and executive function in migraine patients. Neurosci Lett 2008;440:92–6. [DOI] [PubMed] [Google Scholar]
- [62].Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 2010;139:48–57.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain 2013;14:663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Smith A, López-Solà M, McMahon K, Pedler A, Sterling M. Multivariate pattern analysis utilizing structural or functional MRI—in individuals with musculoskeletal pain and healthy controls: a systematic review. Semin Arthritis Rheum 2017;47:418–31. [DOI] [PubMed] [Google Scholar]
- [65].Soares RL. Irritable bowel syndrome: a clinical review. World J Gastroenterol 2014;20:12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Symonds LL, Gordon NS, Bixby JC, Mande MM. Right-lateralized pain processing in the human cortex: an fMRI study. J Neurophysiol 2006;95:3823–30. [DOI] [PubMed] [Google Scholar]
- [67].Valfrè W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine: January 2008. Headache J Head Face Pain 2007;48:109–17. [DOI] [PubMed] [Google Scholar]
- [68].Vincent MB. Vision and migraine. Headache J Head Face Pain 2015;55:595–9. [DOI] [PubMed] [Google Scholar]
- [69].Weatherall MW. The diagnosis and treatment of chronic migraine. Ther Adv Chronic Dis 2015;6:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Weaver KR, Sherwin LB, Walitt B, Melkus GD, Henderson WA. Neuroimaging the brain-gut axis in patients with irritable bowel syndrome. World J Gastrointest Pharmacol Ther 2016;7:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Welch KMA, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache J Head Face Pain 2001;41:629–37. [DOI] [PubMed] [Google Scholar]
- [72].Wilcox SL, Ludwick AM, Lebel A, Borsook D. Age- and sex-related differences in the presentation of paediatric migraine: a retrospective cohort study. Cephalalgia 2018;38:1107–18. [DOI] [PubMed] [Google Scholar]
- [73].Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry 2007;78:600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhang J, Wu YL, Su J, Yao Q, Wang M, Li GF, Zhao R, Shi YH, Zhao Y, Zhang Q, Lu H, Xu S, Qin Z, Cui GH, Li J, Liu JR, Du X. Assessment of gray and white matter structural alterations in migraineurs without aura. J Headache Pain 2017;18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang Q, Wu Q, Zhang J, He L, Huang J, Zhang J, Huang H, Gong Q. Discriminative analysis of migraine without aura: using functional and structural MRI with a multi-feature classification approach. PLoS One 2016;11:e0163875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zorina-Lichtenwalter K, Meloto CB, Khoury S, Diatchenko L. Genetic predictors of human chronic pain conditions. Neuroscience 2016;338:36–62. [DOI] [PubMed] [Google Scholar]