Abstract
Introduction:
The International Association for the Study of Pain has established a global task force to comprehensively investigate the use of cannabinoids and cannabis-based medicines for pain management. This systematic review, the first in this field, will assess the preclinical literature that investigates the antinociceptive effects of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators in animal models of tissue damage, inflammation, or neuropathy.
Methods:
A systematic electronic search of 3 online databases will identify relevant studies in which cannabinoids, cannabis-based medicines, and endocannabinoid system modulators have been tested in animal models of injury-related or pathological persistent pain. Data will be extracted for pain-associated behavioural outcomes, study design, and the reporting of measures to avoid bias. Standardised mean difference meta-analysis will be used to provide summary estimates of efficacy, with the effects of study quality and study design explored using stratified meta-analysis.
Perspective:
The evaluation of the preclinical evidence will quantify the antinociceptive effects of cannabinoids on pain behaviour in animal models of pathological pain in an effort to quantify the presence and prevalence of analgesic efficacy. It will also provide an understanding of the strengths and weaknesses of the preclinical field and inform an agenda for future research.
Keywords: Systematic review, Pain, Animal models, Cannabis, Cannabinoids, Endocannabinoid system modulators
1. Introduction
In 2018, the International Association for Study of Pain (IASP) established a task force on the use of cannabis and cannabinoid-based medicines (CBMs) for pain management. It comprised 4 work packages (WP) focused on (1) basic science, including medicinal chemistry, compound classification, pharmacology, and assessment of efficacy in preclinical studies; (2) evidence synthesis on clinical efficacy and, where possible, effectiveness; (3) evidence synthesis on harms; and (4) the societal impact including changing policy and political practice. This review will inform WP1 and is focused on summarising the preclinical evidence for the efficacy of cannabinoids and CBMs in animal models of pathological or injury-related persistent pain. WP2 will focus on summarising the evidence for both efficacy and harms as measured within randomised controlled trials of cannabinoids and CBMs in addition to providing a review of all previous systematic reviews (PROSPERO IDs CRD42019124714 and CRD420191247). Harms data from randomised controlled trials will be addressed in a third concurrent review (WP3) (PROSPERO IDXXXXXXXXXX [to be inputted when registered]).
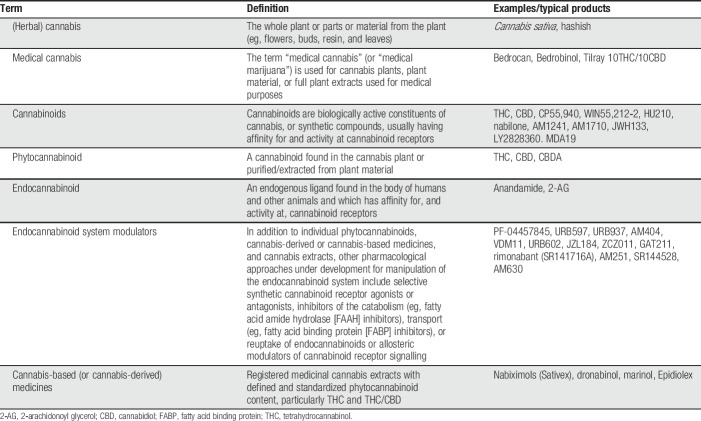
For thousands of years, the plant Cannabis sativa has been used to treat various medical conditions including nausea, convulsions, and pain. The endocannabinoid system—consisting of the cannabinoid 1 receptor (CB1) and cannabinoid 2 receptor (CB2), endogenous cannabinoid ligands (endocannabinoids), and their metabolising enzymes—is implicated in pain signalling pathways. Cannabinoids are a diverse class of biologically active constituents of cannabis or synthetic compounds, which usually have affinity for and activity at cannabinoid receptors. The most studied plant-derived cannabinoids (phytocannabinoids) are Δ9-tetrahydrocannabinol, the main psychoactive constituent in cannabis, attributable to its binding to CB1 receptors, and cannabidiol, which does not bind to cannabinoid receptors (reviewed by Fine and Rosenfeld9); Table 1 provides current terminology, definitions, and examples of typical products. The endocannabinoid system has been studied extensively in animal models, and endocannabinoids, phytocannabinoids, and synthetic cannabinoid receptor agonists have been reported to have antinociceptive effects in animal models of acute, inflammatory, and neuropathic pain (for review see Refs. 11, 15, and 18). Very recently, a mutation in the endocannabinoid signalling pathway has been found to be associated with congenital insensitivity to pain.7
Table 1.
Terminology and definitions (modified from Hauser et al.9).
With increasing scrutiny of the field and mounting global pressure for the legalisation of medicinal cannabis, it is crucial to assess the existing evidence in an unbiased and robust manner including that from preclinical studies. Pain cannot be measured directly in nonhuman animals. Animal models of disease states or toxic challenges known to induce pain in humans have been developed. These models are used to investigate the pathophysiology underlying chronic pain and have enriched our understanding. They have been used to test the efficacy of novel therapeutics and therefore provide justification for clinical trials. The number of preclinical experiments performed each year is rising exponentially but the number of novel interventions reaching the clinic continues to fall. Failure to translate to patients success observed in preclinical studies has led to the questioning of the predictive validity of animal models. There are many reasons for the disparity between animal model and clinical trial results including the complexity of human trials. Lack of scientific rigour and/or transparent reporting across many preclinical fields of biological research may contribute to the lack of translation in addition to methodological differences across studies.1 The extent to which there is conclusive evidence for efficacy of cannabinoids for chronic pain in humans is still somewhat controversial, with different systematic reviews coming to differing conclusions. This aspect is being addressed by the 2 other IASP Cannabinoid Taskforce protocols for systematic reviews of the corresponding clinical literature. Therefore, there is a strong rationale for better understanding the preclinical efficacy data on cannabinoids in animal models of pathological or injury-related persistent pain, to better inform future translational and clinical research. A systematic review conducted in accordance with the guidance as stipulated by Sena et al.13 will provide an unbiased framework to appraise the relevant preclinical literature. It will produce empirical evidence to achieve a dual goal of determining the efficacy of specific cannabinoids and cannabis-based medicines in pain models, and to inform future experimental and clinical trial design.
This review also aims to use a crowd. Preclinical systematic reviews are still novel, but they are vital research tools, important for evidence-based research planning and decision making. In addition, systematic reviews are an effective approach to consolidating the high-volume, rapidly accruing, and often conflicting research on a specific topic. The methods for their conduct are well developed, but they are resource intensive.16 The most labour-intensive stages are screening, annotation, and outcome data extraction, which requires a minimum of 2 reviewers and a third independent reviewer to reconcile any disagreements thereby minimising the risk of bias and ensuring reliability. The time they take to complete weakens their usefulness because they are often out of date once they have been published.14 This problem is being exacerbated by the exponentially increasing number of publications in databases.2 A growing amount of research is conducted in an open collaborative fashion, in projects referred to as “crowd science,” “citizen science,” or “networked science.” Challenging traditional ways of working using a crowd offers a novel opportunity to improve the efficiency and accuracy of a review.9 By breaking down the review process into microtasks, systematic reviews can be conducted more efficiently by a wider range of people and the crowd will be used to conduct tasks that require common scientific skills.
1.1. Aims and objectives
The aims of this systematic review and meta-analysis are to (1) estimate the antinociceptive efficacy of cannabinoids in animal models of pathological or injury-related persistent pain (ie, typically studied over a period of hours, days, weeks, or months, not acute nociception), (2) assess the impact of the studies' internal and external validity on reported behavioural outcome measures, and (3) to identify the presence of publication bias and determine its magnitude.
2. Methods
2.1. Crowd recruitment and training
Ethical approval to use a crowd has been obtained from Imperial College London's Head of Surgery and Cancer and Science, Engineering and Technology Research Ethics Committee.
The recruitment strategy aims to predominantly target bioscientists who are interested in contributing to an important area of research and looking for professional development opportunities. As such, participants will have widely varied experience of the topic and the systematic review process. An advertisement has been developed with IASP, which will be shared widely through the IASP network, collaborators, colleagues, and students using direct communication, e-mail, newsletters, social media, and face–face interactions at conferences and workshops. Participants will be asked to e-mail in response to the advertisement and will then receive a registration form, participant information sheet, and consent form and once these enrolment activities are complete, the participant will be invited to commence the training. There is not a required minimum/maximum number of participants to meet the needs of the review. Reviewers do not need to meet a requisite criteria but will be required to successfully complete the training to participate.
Participants will receive training using an online platform (Learn to SyRF), which will be supported by a training manual with comprehensive instructions. They will need to conduct training for both screening and data extraction. To pass the screening training, participants will need to correctly make the include or exclude decisions for 10 consecutive publications. For data extraction, they will be presented with studies to extract data from, and their data extraction will be compared with a “gold standard” derived following review of a set of studies conducted in house by the team. Once their concordance with that gold standard is greater than 80% for 3 successive studies, they will be considered to have been trained to a sufficient standard. Participants will not be able to contribute to the systematic review if they fail to complete the training or do not meet the required standard.
2.2. Protocol registration
The protocol for this systematic review is registered on PROSPERO (PROSPERO ID CRD4201912804). The protocol uses the template designed by the Systematic Review Centre for Laboratory Animal Experimentation.4
2.3. Study population
Whole in vivo animal models of pathological or injury-related persistent pain (eg, tissue injury, cancer, chemotherapy-induced, toxic neuropathy, inflammation, or nerve damage) that receive any cannabinoid-related therapeutic, including endocannabinoid modulators, will be included. Studies of acute physiological nociception will be excluded.
2.4. Control population
For studies that investigate cannabinoid treatment efficacy in a model of injury-related or pathological persistent pain, the control population is defined as a cohort of animals in which the model has been induced but there has been an appropriate control treatment (eg, injection of vehicle or saline).
2.5. Outcome measures
Pain-associated behavioural measures as declared by the authors will be extracted, ie, pain is declared the reason for the assessment. Behavioural measures not associated with pain or aimed to assess the potential unwanted side effects of cannabinoids will be excluded. Behavioural outcomes are likely to include (but not an exhaustive list)
(1) Evoked limb withdrawal (eg, von Frey withdrawal threshold, Plantar test [Hargreaves'], and thermal footplate)
(2) Mechanical allodynia/hypersensitivity
(3) Cold allodynia/hypersensitivity (eg, acetone test)
(4) Heat hypersensitivity
(5) Spontaneous behavioural outcomes (eg, weight bearing difference, spontaneous foot lifting, burrowing and grimace scale; nocifensive behaviour)
(6) Motor tests (eg, rotarod, CatWalk)
(7) Complex behavioural outcomes (eg, elevated plus maze, open field [thigmotaxis], and conditioned place preference or aversion).
To inform a narrative review, included studies that contain the following will be annotated (systematic identification of relevant studies):
(1) Whether the study investigated the effects of drug on non–pain-related motor activity.
(2) Electrophysiology (eg, wide dynamic range and nociceptive-specific cells)
(3) Markers for neuronal activity (eg, c-Fos, ERK, p38 MAPK)
(4) Whether CB1/CB2 receptor has been confirmed as the target (eg, antagonism, knockout control; inactive enantiomer control, tolerance consistent with receptor mediation)
2.6. Systematic search
To identify all relevant studies PubMed, Embase, and Web of Science databases will be searched. To ensure that the search has the highest probability of identifying all applicable studies, a broad search strategy will be used and optimised for each of the databases. It will include all languages and dates, but publication types will be limited to not include editorial materials, book chapters, biographical items, reviews, notes, letters, or news items. The general search terms are given below; full search strategy can be found on the Open Science Framework Cannabinoid Preclinical SR search strategy:
Cannabinoids OR cannabis OR marijuana OR marihuana OR hemp OR hashish OR cannabinoid OR cannabinoids OR cannabidiol OR tetrahydrocannabinol OR “endocannabinoid modulator” OR FAAH OR MGL OR MAGL OR ABHD6 OR ABHD12 OR “fatty acid binding protein” OR NAAA OR endocannabinoid OR endocannabinoids OR endo-cannabinoid OR FAAH inhibitor OR FAAH inhibition OR MAGL inhibitor OR MAGL inhibition OR MGL inhibitor OR MGL inhibition OR anandamide transport inhibitor OR anandamide transport inhibition OR “ABHD6 inhibitor” OR “ABHD6 inhibition” OR “ABHD12 inhibitor” OR “ABHD12 inhibition” OR NAAA inhibitor OR NAAA inhibition OR “Fatty acid Binding Protein inhibitor” OR “fatty acid binding protein inhibition” OR FABP inhibition OR FABP inhibitor OR allosteric modulator OR “endocannabinoid modulators” OR “endo-cannabinoid modulators” OR “endo-cannabinoid modulator” OR FAAH inhibitors OR MAGL inhibitors OR MGL inhibitors OR anandamide transport inhibitors OR “ABHD6 inhibitors” OR “ABHD12 inhibitors” OR NAAA inhibitors OR “Fatty acid Binding Protein inhibitors” OR FABP inhibitors OR allosteric modulators OR PEA OR palmitoylethanolamide AND Pain OR Hyperalgesia OR pain OR analgesia OR analgesic OR analgesics OR allodynia OR neuralgia OR hypersensitivity OR hyperalgesia OR hyperalgesic OR antinociception OR anti-nociception OR hypoalgesia OR hypoalgesic OR anti-hyperalgesia OR antihyperalgesia OR antihyperalgesic OR anti-hyperalgesic OR anti-allodynic OR antiallodynic OR anti-allodynia OR antiallodynia AND Animal search filters.5,8
2.7. Before screening
The search results will be imported to Endnote and duplicates removed using the Endnote tool. The studies will then be exported from Endnote in XML format into the CAMARDES (Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies, University of Edinburgh) Systematic Review Facility (SyRF; http://syrf.org.uk/), a fully integrated online platform for preclinical systematic reviews.
2.8. Screening and study design criteria
The screening process requires a minimum of 2 independent reviewers, but this review seeks to use a “crowd” as the second reviewer. Any discrepancies will be resolved by a “third” independent reviewer.
Studies that investigate a cannabinoid administered to a whole animal model of pathological or injury-related persistent pain in which pain relevant behavioural outcomes are presented with an appropriate control will be included. Multiple routes of drug administration will be considered (eg, intraperitoneal, intravenous, subcutaneous, intrathecal, intraplantar, intraventricular, and intracranial [eg, periaqueductal gray, rostral ventromedial medulla, and anterior cingulate cortex]). Publications or abstracts without data, retrospective studies, review articles, in vitro studies, case reports, human studies, letters, and comments will be excluded.
Screening will be conducted based on title and abstract (whole text if ambiguous). It is the aim to use machine learning to reduce the number of studies that need to be screened manually. To achieve this, half of the studies will be screened manually to establish a training set. This data set will then be used to train a machine-learning algorithm10 to screen the remainder. However, it may be that the training set does not provide enough instances to train the machine, perhaps due to the low volume of studies and complex categorisations; therefore, it may be a requirement to continue screening manually.
2.9. Animal inclusion criteria
(1) Animal models of pathological or injury-related persistent pain
(2) Transgenic studies for which pain is resultant, eg, diabetes.
(3) Male and female animals
(4) Primary focus will be adolescent and adult animals. Studies in neonatal animals will also be captured if pathological or injury-related persistent pain was induced.
(5) Multiple models presented as a single model, eg, HIV plus antiretroviral drug
2.10. Intervention inclusion criteria
Any monotherapy and/or combination treatment given before/after/in concurrence with the model induction.
2.11. Exclusion criteria
(1) Human studies
(2) Studies of acute physiological nociception
(3) In vitro and ex vivo studies
(4) Not an original research article containing cannabinoid intervention to a pathological persistent pain animal model. Publications or abstracts without data, retrospective studies, review articles, case reports, letters, and comments.
3. Data extraction
Similarly, the data extraction process requires a minimum of 2 independent reviewers but this review seeks to use a “crowd” as the second reviewer. Any discrepancies will be resolved by a “third” independent reviewer.
3.1. Study bibliographic data
The title, first author, corresponding author, year, journal name, and DOI of each study will be extracted.
3.2. Study design characteristics
The number of animals in the experimental and control groups will be extracted. If the number of animals is given as a range, the most conservative estimate will be recorded. The number of different behavioural assessments (therefore, outcome measures) a group of animals was tested on will also be extracted.
3.3. Animal model characteristics
The following model induction information will be extracted:
(1) Animal species
(2) Animal strain
(3) Animal sex
(4) Animal age and/or weight
(5) Animal supplier
(6) The category of model
(7) The method used to induce the model (including dose if pharmacologically induced)
(8) The time between model induction and outcome measurement
(9) Perioperative analgesia used during model induction
3.4. Intervention (treatment) characteristics
(1) Intervention type (and vehicle)
(2) Cannabinoid pharmacological classification
(3) Dosing regimen
(4) Time of first dose
(5) Route of administration
(6) Time between the administration of the treatment and outcome measurement
(7) Time between the model induction and treatment
3.5. Collection of outcome data
Model behavioural outcomes measured must be compared to a suitable control; a separate control group, the same animal cannot be used for both, eg, contralateral is not a suitable control for ipsilateral due to the possibility of contralateral sensory changes that could affect outcome measures. For graphs or if numerical data are described in tables/within the text comparing (pain model + treatment) vs (pain model + vehicle), the data at the time point at which the drug is most effective will be extracted (ie, the time point at which there is the largest difference between treatment and control) to allow for efficacy to be estimated.
The mean and standard error or SD (studies which present the data as medians will be annotated to give reviewers the option to revisit these studies if deemed necessary) will be extracted manually using universal desktop ruler and/or the inbuilt Adobe measuring tool and/or WebPlotDigitizer (reviewers' preference). It may be an option to use bespoke PDF data extraction tools (https://github.com/EPPI-Centre/Graph2Data) that are currently being integrated into the SyRF platform. The tools have been shown to improve accuracy and reduce the time required for data extraction.3
3.6. Risk of bias and study quality
Study quality will be assessed against the reporting of the following items that may represent risks to study validity:
(1) Random allocation of animals to treatment/control groups
(2) Blinded conduct of the experiment
(3) Blinded assessment of outcome
(4) Statement of sample size calculation
(5) Reporting of animal exclusions
In addition:
(1) Statement regarding possible conflicts of interest
(2) Statement of compliance with animal welfare regulations
Reviewers will select whether these criteria are reported. They will then be required to state the method that the experimenters used so that a risk-of-bias assessment can be conducted in terms of high, low, or unclear risk of bias.
4. Data synthesis
The included studies will be organised according to the type of disease/injury model being studied, e.g., inflammation- or chemotherapy-induced neuropathy, followed by outcome measure and then drug (classification, drug name, dose, and route of administration) and whether it was administered before or after model induction (prophylactic or therapeutic intervention) and if in combination (eg, FAAH-COX, with an opioid, gabapentin, or antidepressant).
A meta-analysis will be conducted in accordance with the guidelines described by Vesterinen et al.17 and aims to:
(1) Calculate an effect size for each comparison
(2) Weight the effect sizes
(3) Calculate a summary effect size
(4) Calculate the heterogeneity, and the extent to which the study design characteristics explain this heterogeneity
4.1. Calculating effect size (standardised mean difference)
For each comparison—where outcome in a cohort of animals receiving treatment is presented, along with that for a control cohort—an effect size will be calculated (correction for multiple uses of control group: where a single control group serves multiple treatment groups, the size of the control group entered to the meta-analysis will be adjusted by division by the number of treatment groups served.). The Hedge's G method will be used to calculate the standardised mean difference. The difference in group means will be divided by a measure of the pooled variance to convert all outcome measures to a standardised scale with units of SDs. This allows for data where different measurement scales are reported for the same outcome measure. This method also includes a correction factor for small sample biases. Small samples are considered groups of less than 10 animals.12
4.2. Weighting effect sizes
Weights are attributed to each study to reflect the contribution of individual studies to the total effect estimate and this is done according to the precision of the study. The weights will be calculated using the inverse variance method; individual effect sizes are multiplied by the inverse of their squared standard error.
4.3. The statistical model of analysis
A random-effects model will be used. This considers both the within-study (sampling error) and between-study (differences in the true effect size) variance. The distribution of effect sizes has a weighted mean (the summary estimate), a weighted sum of the square of the deviations from that mean (the heterogeneity), and an estimate of the variance of the effect sizes beyond that expected by chance (tau-squared, τ2). From the standard error, the 95% confidence intervals can then be calculated.
4.4. Assessing heterogeneity
I2 will be used to estimate the between-study heterogeneity. It is defined as the proportion of total variance between studies that is due to true differences in effect sizes as opposed to chance. I2 lies between 0% (all variation due to chance) and 100% (all variation reflects real differences between the effect sizes between studies and does not depend on the number of comparisons in the meta-analysis). 0%–25% reflects low heterogeneity, 50%–75% moderate heterogeneity, and >75% high heterogeneity.
4.5. Examining sources of heterogeneity
Stratified meta-analysis will be performed according to study quality; components of study quality checklist; type of outcome measure; species, strain, sex, and age of animal used; outcome measure used; and interval to quantification of outcome. Rats and mice will be analysed separately. The heterogeneity between groups will be tested using the χ2 distribution.
4.6. Assessing publication bias
(1) Funnel plots will allow for visual identification of studies with small precision that overstate the effect size and are consistent with the presence of small study publication bias
(2) Trim and fill analysis nonparametrically will attempt to correct for funnel plot asymmetry by identifying and imputing theoretically missing studies. This enables a recalculation of the overall effect size in the absence of publication bias
(3) Egger's regression will allow for statistical assessment of the presence of publication bias from the funnel plot. It determines whether the regression line and its 95% confidence interval, for precision vs standardised effect size intersect at the origin of the graph.
5. Discussion
This systematic review and meta-analysis will form part of wider body of work instituted by the International Association for the Study of Pain. It will provide an overview of the available evidence in the field of cannabinoid preclinical research. It will allow for the quantification of the analgesic or antinociceptive effect of cannabinoids and CBMs in animals models of pathological or injury-related persistent pain as well as providing an appreciation of the rigour of the data. The empirical evidence gained will allow for practical recommendations for the improvement of future research; it will be used to identify knowledge gaps, factors influencing treatment efficacy, inform experimental design of new studies, and reduce waste in future research.
Disclosures
S. Haroutounian reports grants from Pfizer, Inc, and consulting fees from Medoc Ltd, outside the scope of the submitted work. K. Wever has nothing to declare. D.P. Finn reports grants from both public bodies and industry (Randox, Alkermes, and Shionogi) for research outside the scope of the submitted work. A.S.C. Rice is Chair of the task force and an IASP councillor. He reports consultancy and advisory board work for Imperial College Consultants–in the past 24 months, this has included remunerated work for Galapagos, Toray, Quartet, Lateral, Novartis, Pharmaleads, Cambridge University (Prof Peter McNaughton), Orion, Asahi Kasei, and Theranexus, outside the scope of the submitted work. In addition, A.S.C. Rice was the owner of share options in Spinifex Pharmaceuticals from which personal benefit accrued on the acquisition of Spinifex by Novartis in July 2015 and from which future milestone payments may occur. In addition, Dr Rice is named as an inventor on patents: A.S.C. Rice, Vandevoorde S., and Lambert D.M Methods using N-(2-propenyl)hexadecanamide and related amides to relieve pain. WO 2005/079771 and Okuse et al. Methods of treating pain by inhibition of vgf activity EP13702262.0/WO2013 110945.
Funded by International Association for Study of Pain via Presidential Task Force on Cannabinoid Analgesia. The work is also supported by the BBSRC (grant number BB/M011178/1).
Acknowledgements
The authors thank Dr Emily Sena and Dr Jing Liao of CAMARDES, The University of Edinburgh, for their guidance in how machine learning and text mining can be used to improve the efficiency of the review process.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Andrews NA, Latremoliere A, Basbaum AI, Mogil JS, Porreca F, Rice ASC, Woolf CJ, Currie GL, Dworkin RH, Eisenach JC, Evans S, Gewandter JS, Gover TD, Handwerker H, Huang W, Iyengar S, Jensen MP, Kennedy JD, Lee N, Levine J, Lidster K, Machin I, McDermott MP, McMahon SB, Price TJ, Ross SE, Scherrer G, Seal RP, Sena ES, Silva E, Stone L, Svensson CI, Turk DC, Whiteside G. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. PAIN 2016;157:901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med 2010;7:e1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cramond F, O'Mara-Eves A, Doran-Constant L, Rice AS, Macleod M, Thomas J. The development and evaluation of an online application to assist in the extraction of data from graphs for use in systematic reviews. [version 3; peer review: 3 approved]. Wellcome Open Res 2019;3:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Vries RBM, Hooijmans CR, Langendam MW, van Luijk J, Leenaars M, Ritskes-Hoitinga M, Wever KE. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid Based Preclinical Med 2015;2:e00007. [Google Scholar]
- [5].de Vries RBM, Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Updated version of the Embase search filter for animal studies. Lab Anim 2014;48:88. [DOI] [PubMed] [Google Scholar]
- [6].Franzoni C, Sauermann H. Crowd science: the organization of scientific research in open collaborative projects. Res Pol 2014;43:1–20. [Google Scholar]
- [7].Habib AM, Okorokov AL, Hill MN, Bras JT, Lee MC, Li S, Gossage SJ, van Drimmelen M, Morena M, Houlden H, Ramirez JD, Bennett DLH, Srivastava D, Cox JJ. Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity. Br J Anaesth 2019. 10.1016/j.bja.2019.02.019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim 2010;44:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hauser W, Finn DP, Kalso E, Krcevski-Skvarc N, Kress HG, Morlion B, Perrot S, Schafer M, Wells C, Brill S. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. European Journal of Pain 2018;22:1547–64. [DOI] [PubMed] [Google Scholar]
- [10].Liao J, Ananiadou S, Currie GL, Howard BE, Rice A, Sena ES, Thomas J, Varghese A, Macleod MR. Automation of citation screening in pre-clinical systematic reviews. bioRxiv 2018;8:1–23. [Google Scholar]
- [11].Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics 2009;6:713–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rooke EDM, Vesterinen HM, Sena ES, Egan KJ, Macleod MR. Dopamine agonists in animal models of Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2011;17:313–20. [DOI] [PubMed] [Google Scholar]
- [13].Sena ES, Currie GL, McCann SK, Macleod MR, Howells DW. Systematic reviews and meta-analysis of preclinical studies: why perform them and how to appraise them critically. J Cereb Blood Flow Metab 2014;34:737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med 2007;147:224–33. [DOI] [PubMed] [Google Scholar]
- [15].Starowicz K, Finn DP. Cannabinoids and pain: sites and mechanisms of action. Adv Pharmacol 2017;80:437–75. [DOI] [PubMed] [Google Scholar]
- [16].Tricco AC, Brehaut J, Chen MH, Moher D. Following 411 Cochrane protocols to completion: a retrospective cohort study. PLoS One 2008;3:e3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, Antonic A, Howells DW, Macleod MR. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 2014;221:92–102. [DOI] [PubMed] [Google Scholar]
- [18].Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V. The cannabinoid system and pain. Neuropharmacology 2017;124:105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]