Abstract
Since early work attempting to characterize the brain's role in pain, it has been clear that pain is not generated by a specific brain region, but rather by coordinated activity across a network of brain regions, the “neuromatrix.” The advent of noninvasive whole-brain neuroimaging, including functional magnetic resonance imaging, has provided insight on coordinated activity in the pain neuromatrix and how correlations in activity between regions, referred to as “functional connectivity,” contribute to pain and its modulation. Initial functional connectivity investigations assumed interregion connectivity remained stable over time, and measured variability across individuals. However, new dynamic functional connectivity (dFC) methods allow researchers to measure how connectivity changes over time within individuals, permitting insights on the dynamic reorganization of the pain neuromatrix in humans. We review how dFC methods have been applied to pain, and insights afforded on how brain connectivity varies across time, either spontaneously or as a function of psychological states, cognitive demands, or the external environment. Specifically, we review psychophysiological interaction, dynamic causal modeling, state-based dynamic community structure, and sliding-window analyses and their use in human functional neuroimaging of acute pain, chronic pain, and pain modulation. We also discuss promising uses of dFC analyses for the investigation of chronic pain conditions and predicting pain treatment efficacy and the relationship between state- and trait-based pain measures. Throughout this review, we provide information regarding the advantages and shortcomings of each approach, and highlight potential future applications of these methodologies for better understanding the brain processes associated with pain.
Keywords: Pain, Neuroimaging, Dynamic brain connectivity, Functional connectivity, Time-varying connectivity, Psychophysiological interaction, Dynamic causal modeling
1. Introduction
Neuroimaging is a powerful tool that provides insights on the brain mechanisms of pain and its modulation. Studies of pain-related brain activation focus on identifying which regions are influenced by painful stimulation or show alterations with pain conditions. Noninvasive human imaging studies16,27,28,134 have confirmed animal models indicating that nociceptive neurons and pain-related regions are highly distributed throughout the brain (for meta-analyses and reviews, see Refs. 2, 35, 59, and 99), and provided unique insights on how pain is modulated in humans. Yet, it has long been understood that the brain is functionally integrated129; considering regions in isolation will ignore the important role of communication between regions. Evidence from lesion patients and cortical stimulation studies suggests that individual brain regions are insufficient for the production of pain.42,46,53,97 Although the brain regions that respond to pain are anatomically linked,40,47,88,90 only by studying how activation across regions covaries systematically can we begin to understand how networks within the brain construct and modulate pain.
To understand the communication between brain regions within the pain neuromatrix in humans, neuroscientists have combined noninvasive whole-brain neuroimaging with analytic techniques to measure correlations between brain regions, referred to collectively as functional connectivity (FC). Functional connectivity techniques do not measure physical connections (eg, axonal projections), but instead assess functional coupling between 2 or more spatially or anatomically distinct regions of the brain, ie, whether activity in those regions correlates over time. Initial FC approaches assumed that connectivity is stable over time within individuals. Researchers used static FC to characterize functional networks (eg, intrinsic networks in the absence of a task, referred to as “resting-state”137), to map basic pain processing,117 and to measure whether the strength of communication between relevant brain regions varied across individuals in meaningful ways (eg, differences between patients and controls61,65,92,131,141; or in relation to a relevant behavioral dimension92,122,137). However, recent work on “dynamic functional connectivity” (dFC) assumes that functional coupling between brain regions can actually change over time within individuals.5,20 Indeed, FC can vary both spontaneously and as a function of psychological processes and experimental demands,22,63,104 and longitudinal changes in FC have been linked to the development of persistent pain7,91 (for a review, see Ref. 14). In this review, we provide an overview of recent dFC approaches that have been applied to the study of pain using functional magnetic resonance imaging (fMRI), which balances high spatial and temporal resolution, thereby permitting investigations of communication between multiple brain regions over time (for a review of general approaches to dFC, see Refs. 5, 20, 63, and 104).
1.1. Studying dynamic functional connectivity can enhance the study of pain
Understanding how FC varies dynamically across time and as a function of external factors can enhance the study of the brain processes of pain. The experience of pain varies greatly between individuals,26,94 even among patients with chronic pain who are ascribed the same medical diagnosis.130 Pain is influenced by many processes that fluctuate over time in both healthy individuals and individuals with chronic pain disorders, including attention,19,71 emotion,19,138 and treatment or treatment context.3,68 Indeed, the experience of pain varies spontaneously over seconds, days, months, and years.6,76,96,105 Pain can also cause variations in cognitive processes: pain is a salient stimulus that automatically captures attention36 and can impair performance on complex cognitive tasks.10,89 This suggests that pain is not a stable qualia but rather a highly variable experience that exhibits a complex relationship with brain processes that engender, enhance, and diminish it. Standard FC measures treat communication between brain regions as static, and therefore cannot account for these fluctuations across time, within individuals. Dynamic functional connectivity approaches instead systematically account for this variance by analyzing how coordinated neural activity between different regions evolves and varies across time.
Because interregional covariance in activity varies dynamically across time and as a function of external factors, particularly in the context of pain, dFC approaches will strengthen our ability to understand how networks of brain regions dynamically communicate, construct, and modulate acute and chronic pain.
1.2. The present review
In this review, we use the term “dynamic functional connectivity” (dFC) to refer broadly to measures of interregional coordinated activity that vary across time, either spontaneously (sometimes referred to as “time-varying connectivity”84) or as a function of psychological states, cognitive demands, or the external environment. We first review research on FC patterns in acute pain that vary as a function of experimentally controlled conditions, as identified with psychophysiological interactions (PPI) models and dynamic causal modeling (DCM). We next survey data-driven dFC methods that assess how connectivity varies across different brain states (ie, different patterns of brain network organization), including how it varies across time within a task-free (ie, “resting”) state, and how these methods are being used to understand acute pain perception and coping. Finally, we discuss how dFC may vary between individuals, and highlight clinically relevant observations that have the potential to inform diagnosis and treatment of chronic pain conditions. We examine the insights these approaches have produced for our understanding of pain perception and modulation, while identifying limitations and potential confounds of each method. Finally, we discuss future directions for research using dFC approaches to study the brain processes of pain and pain modulation.
2. Modeling task-induced changes in functional connectivity
Many pain neuroimaging studies combine fMRI measurement with experimental manipulations to measure how cognitive factors influence brain responses to noxious stimulation. For example, fMRI studies of placebo analgesia might compare brain responses to noxious stimulation during placebo administration relative to a control condition without an analgesic. Researchers then often use statistical regression to measure whether brain activation within a region varies as a function of the experimental manipulation (eg, whether activation within pain-related brain regions is reduced with placebo; whether responses differ between patients and controls). For a thorough primer on basic approaches to pain neuroimaging, please see Ref. 87. Task-based fMRI studies also offer an additional opportunity: to test not only whether activity within regions changes based on experimental manipulations, but also whether the experimental manipulation influences between-region FC. We consider 2 dFC approaches that have been applied to task-based fMRI in pain research: PPI and DCM.
2.1. Psychophysiological interactions
Some of the earliest neuroimaging work examining dFC in pain was conducted by modeling PPI. Psychophysiological interactions measure whether and how FC between brain regions varies with context, typically some “psychological” variable that is experimentally manipulated50 (eg, placebo vs control). Psychophysiological interactions measure the relationship between FC and known, observable, or assumed features of the experimental design. An assumption of PPI analysis is that if 2 brain regions interact, then activity in those regions will correlate over time, even when controlling for the effect of the task on activation in both regions.95 Psychophysiological interactions test formally whether this correlation differs in different experimental conditions (Fig. 1), ie, whether the correlation can be statistically predicted by a Region × Condition interaction. Psychophysiological interactions can test how context changes both functional coupling between regions (ie, a positive correlation) and functional decoupling between regions (ie, a negative correlation). Importantly, PPI can be applied to measure both static FC (where the modulating contextual factor varies between individuals, for example, the presence of a chronic pain condition61,131) and dynamic FC (where the modulating contextual factor varies within individuals, for example, attention toward or away from pain); here, we focus on the latter. Although the standard approach to PPI analyses in early applications was limited to studying contexts that varied with only 2 levels, a more recent approach (referred to as generalized PPI85) can account for more variable contexts (eg, comparing connectivity that differs under multiple task conditions) than the standard PPI approach. For a thorough description of generalized PPI, see Ref. 85.
Figure 1.
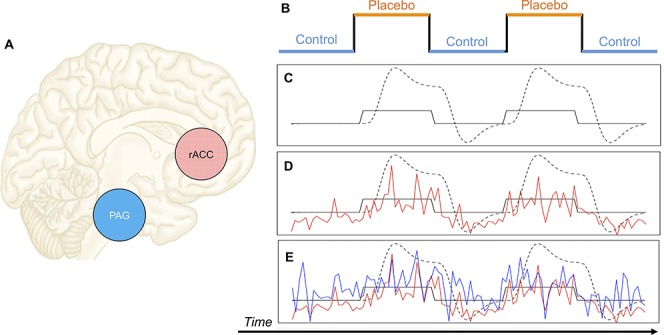
Psychophysiological interaction. Here we present a cartoon example of how PPI can be used to measure task-based changes in connectivity. A) PPI has been used to measure placebo-induced changes in connectivity between the rACC and PAG11. In PPI analysis, a variable that represents 2 levels of an experimental condition (B) is convolved with a canonical hemodynamic response function (the dotted line in C). Then, the time series of the BOLD signal of an ROI is extracted (eg, the red time series in D could represent the time series of the rACC), which will correspond roughly to the onsets of the experimental condition if that ROI is responsive to the experimental condition. Finally, those 2 time series are multiplied together to create a time series that can be used to predict activity in other brain regions (eg, the time series of the PAG, represented by the blue time series in E). Regions of interest whose time series correlate with the red time series in D can be said to exhibit FC with the ROI that generated the red time series, and if the correlation varies as a function of experimental condition (eg, is enhanced under placebo, relative to control), then FC is associated with experimental condition. Adapted from Ref. 95. FC, functional connectivity; PAG, periaqueductal gray; PPI, psychophysiological interactions; rACC, rostral anterior cingulate cortex; ROI, region of interest.
In the context of pain research, PPI has played a pivotal role in enhancing our understanding of the neural basis of placebo analgesia,11,38,39,111 cognitive and emotional function in patients with chronic pain,86,123 and other forms of acute pain modulation.83,100,107 In an early use of PPI in the study of placebo analgesia, Bingel et al. (2006) evaluated the relationship between the rostral anterior cingulate cortex (rACC) and the antinociceptive descending pain modulatory network in healthy volunteers.11 Consistent with previous work,98,135 rACC activation in response to noxious stimulation was higher during placebo relative to control (eg, as in Fig. 1D). Bingel et al.11 then used PPI analysis to measure whether rACC connectivity with subcortical regions differed as a function of the placebo manipulation. Rostral anterior cingulate cortex covaried more with activity in the periaqueductal gray (PAG) and amygdala under placebo than control.11 Given the role for the PAG and amygdala in descending antinociceptive control and affective responses to pain,43,62,119,132 one interpretation of these results is that the rACC may play a role in transforming cognitive factors (ie, expectations) into experienced analgesia through its association with subcortical antinociceptive networks. These PPI findings were later replicated and extended by Eippert et al.,38 who found not only that rACC-PAG connectivity is modulated by placebo, but also that this connectivity is abolished with opioid-antagonist naloxone, demonstrating that the functional coupling between these regions is opioid dependent.
As demonstrated here, PPI is a useful tool for testing whether the correlation between the time series of evoked brain activity in multiple regions varies as a function of psychological context. Although most applications of PPI use experimental manipulations, such as in the examples above, PPI can also be used flexibly with psychological regressors of many different types and sources. For example, one can test whether connectivity covaries with behavior within individuals, such as decisions about pain (eg, painful or not painful, as in Ref. 101) or reaction time (similar to Ref. 73). However, PPI analyses are fundamentally limited because they can only measure changes in connectivity that are associated with known or observable contextual factors. Thus, researchers must assume that their experimental manipulations induce dynamic changes in brain activity at specific, known time points, which requires assumptions about the dynamics of the cognitive processes affected by experimental manipulations. We discuss approaches that use latent methods and data-driven techniques to identify shifts in connectivity below. Another limitation of PPI analysis is that it rests on the fundamental assumption that if brain activity in 2 regions is correlated, the regions are interacting or communicating. Indeed, this is arguably a limitation to all dFC approaches that measure FC as a statistical dependency (eg, correlation) between brain regions, and we return to the need to validate fMRI measures of FC with methods that directly manipulate or measure brain activity in our discussion below. This assumption leaves open the possibility that 2 brain regions may exhibit task-dependent correlated activity not because they actually interact but because both regions share connectivity with some third active region. In other words, PPI cannot measure “effective connectivity,” where “effective connectivity” is defined as a directional influence of one neuronal system over another neuronal system.49 We turn now to DCM as a method to estimate effective, rather than functional, connectivity.
2.2. Dynamic causal modeling
Similar to PPI, DCM is a method that investigates how contextual factors modulate coordinated activity between discrete brain regions. Although DCM analysis methods for resting-state fMRI have been proposed recently,52,80 to the best of our knowledge, resting-state DCM has yet to be applied to the study of pain. Thus, for the purposes of this review, we focus on DCM of task-related brain activity. Dynamic causal modeling is a framework for generating models of “hidden” (ie, unobservable) neuronal states and estimating parameters of those models using observed data (eg, blood-oxygen level dependent, or BOLD, signals). Dynamic causal modeling assumes that the brain is a dynamic system that is perturbed by deterministic inputs (eg, experimental stimuli, contextual factors) and produces measurable outputs (eg, hemodynamic responses51). In DCM, researchers design multiple competing statistical models (Fig. 2A–C) regarding how predefined anatomical regions of interest (ROIs) interact and how observable external perturbations (eg, sensory inputs) modulate those interactions (Fig. 2). Dynamic causal modeling analyses use Bayesian model selection to test which of the competing models (ie, hypotheses) best explains the observed data (ie, the BOLD signal, Fig. 2D). Unlike PPI, which measures correlations in the BOLD signal, DCM estimates underlying neuronal activity by statistically modeling time-varying hidden parameters that affect the transformation of neuronal activity into a hemodynamic response that comprises BOLD signal.124 By modeling these time-varying parameters, DCM attempts to estimate the temporal precedence of one neuronal system over another,125 which is why DCM is considered a model of effective connectivity. In other words, DCM assesses how changes in one system linearly and nonlinearly influence another (or how changes in multiple systems influence each other) across time, and how those interactions are influenced by external perturbations (eg, sensory inputs124). Dynamic causal modeling can be used to compare directional models that describe how observed neural networks that exhibit an evoked response to pain are organized, how the neural systems influence each other, and how those influences change under different conditions. Although these directional statistical analyses have been argued to provide evidence of causal influences between regions,48,49 this point is still under debate.31,109 Factors such as variation in the hemodynamic response function across regions of the brain, for example, may lead to spurious conclusions in any analyses that assess effective connectivity.57,106,110 Stronger inferences about directionality that do not depend on hemodynamic response function may be possible with advances in fMRI temporal resolution through new sequences such as multiband imaging acquisition,41 as discussed in more detail below (see “Summary and Future Directions”). Although methods that directly perturb brain circuits (eg, lesions, electrophysiology, and transcranial magnetic stimulation [TMS]) are necessary to truly demonstrate causality, the noninvasive nature of DCM makes it a useful method for approximating dynamic effective connectivity.
Figure 2.
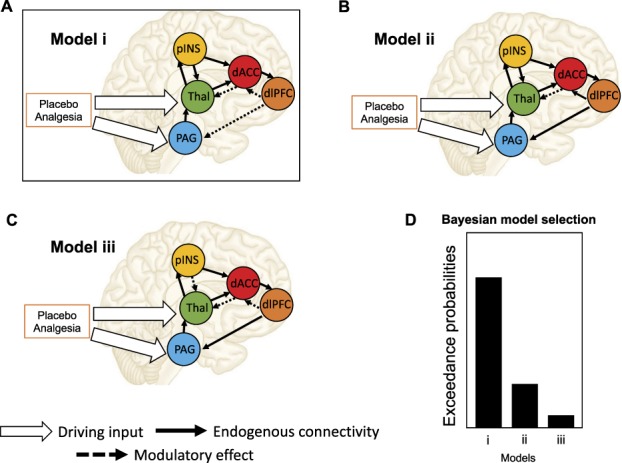
Dynamic causal modeling. Here we present a cartoon example of how DCM can be used to model placebo-related modulation of descending pain pathways. In DCM analysis, models are generated to predict BOLD activity of ROIs (eg, the dlPFC, PAG, and other regions, adapted from Ref. 114). Example models (A, B, and C), shown here, comprise endogenous connectivity (the black arrows), driving inputs (the white arrows), and modulatory effects (dotted lines) of the driving input on the endogenous connectivity but can vary on hypothesized connectivity between regions (for example, in A, B, and C, dlPFC connectivity varies). Placebo analgesia could thus be a driving input that can directly influence the activity of certain regions or modulate the intrinsic connectivity between regions. Models are compared using Bayesian model selection (BMS) to find which best explains the observed BOLD signal (D). For more information, see Ref. 51. DCM, dynamic causal modeling; dlPFC, dorsolateral prefrontal cortex; PAG, periaqueductal gray; ROI, region of interest; pINS, posterior insula; Thal, thalamus; dACC, dorsal anterior cingulate cortex.
Dynamic causal modeling has been used in acute pain research to test both the analgesic effects of pharmacological136 and nonpharmacological treatments for pain,114,115 as well as basic pain processing mechanisms.66,81 Sevel et al.,114 for instance, observed that descending effective connectivity from the dorsolateral prefrontal cortex (dlPFC) to the PAG was significantly diminished during placebo analgesia (Fig. 2A), and that modulatory connectivity between these regions during pain stimulation (relative to rest) was associated with future placebo analgesic responses in healthy participants over 2 weeks later.115 These results indicate a directional pain modulation under placebo analgesia (ie, the dlPFC influences the PAG, rather than bidirectional correlations between the 2 regions). These directional findings can be confirmed using direct manipulations of brain activity, such as TMS, to truly support causal claims; indeed, TMS to dlPFC has been associated with reduced placebo analgesia,70 although downstream effects on PAG were not measured in this study. In other cases, however, directionality inferred based on DCM model solutions may be more complex. For example, 2 studies used DCM to investigate whether painful and nonpainful stimuli are processed in the primary and secondary somatosensory cortex (SI and SII, respectively) in serial or parallel66,81 and came to very different conclusions. One group observed that connectivity between the thalamus, SI, and SII was modulated by both nociceptive and nonnociceptive somatosensory inputs in a parallel fashion,81 suggesting no serial organization between SI and SII when processing somatosensory information. However, another group observed that sensory inputs go directly to contralateral SI, and modulatory effects of the inputs were observed from contralateral SI to contralateral SII, and from contralateral SII to ipsilateral SII,66 suggesting sequential rather than parallel processing of noxious and innocuous stimuli. These studies tested slightly different models using DCM, so it is impossible to reconcile the different conclusions on the basis of DCM alone. Furthermore, although DCM can be used to approximate effective connectivity, research questions that demand high temporal precision (eg, whether processing occurs in serial or parallel) may be better addressed with methodologies that provide greater temporal resolution (eg, electroencephalography or magnetoencephalography; EEG/MEG), or by pairing such methodologies with fMRI data collection (eg, simultaneous fMRI/EEG). This is especially true in the case where cortical regions are the primary ROIs.
Together, these studies clearly demonstrate both advantages and disadvantages of using DCM analysis to study dFC. The strengths of DCM include its use of model comparison to produce statistical inferences about neuronal activity and to identify the best directional effective connectivity model for the observed data. On the other hand, model testing is also an inherent pitfall of DCM analyses—the model that “best fits” the data is inherently constrained to the selection of models that researchers test, and the types of modulatory parameters they consider. Thus, even if studies are testing the same research question, as were the 2 studies described above.66,81 it is challenging to directly compare between studies unless they test identical models. Furthermore, it is possible in model testing that no single model is significantly superior to all others, or there could be different winning models between different hemispheres, as was the case in Ref. 115. Dynamic causal modeling also does not permit tests of a null hypothesis; that is, a way to validate whether effective connectivity actually exists. In summary, DCM analysis is a powerful method that provides directional information on how pain-related information is received and processed in a given network. However, DCM requires clear hypotheses because it is neither a data-driven nor a data-exploring analysis method, and is highly influenced by modeling decisions. We return to these considerations below (see “Summary and Future Directions”).
3. Modeling network-based dynamic functional connectivity
The methods reviewed above focus on changes in connectivity that depend on known or observable events that are specified during analysis, and require that researchers make assumptions regarding the relationship between experimental timing and potential functional reorganization in brain networks. These approaches are particularly useful in the context of task-based fMRI experiments that deliver stimuli at known intervals determined by the experimenter, such as most acute pain experiments. Other methods for studying FC circumvent the requirement to make assumptions about the timing of behavior or cognitive processes and concomitant changes in connectivity, and instead use data-driven approaches to identify shifts in connectivity and characterize spontaneous fluctuations within networks in the absence of experimental manipulations. These methods may be more appropriate for pain studies that involve uncertainty about task timing, such as pharmacological experiments, and for resting-state fMRI experiments. Below, we review data-driven and network-based dFC approaches that have been applied to pain neuroimaging.
3.1. Combining graph theory with dynamic functional connectivity: state-based dynamic community structure
New approaches to FC measure communication within large networks of brain regions by using graph theory (for reviews, see Refs. 8 and 18). Graph theory–based network analysis represents the brain as a network of interacting “nodes” (eg, brain regions) that are linked by “edges” (eg, measures of interregional connectivity), and quantitatively describes the topological properties of brain network connectivity.127 In the past decade, graph theory–based analysis has been used to investigate human brain connectivity by illuminating the architecture in brain structure and function and the organization of dynamic behavior over time in resting state, during different tasks, and across the lifespan.33,140 Recently, several researchers have introduced new dFC approaches that combine graph theory–based approaches with methods to identify latent shifts in brain connectivity state.30,63,108,120 One such method that has been applied to pain neuroimaging is state-based dynamic community structure (SDCS108; Fig. 3). State-based dynamic community structure evaluates connectivity between an a priori set of regions/nodes (Fig. 3I), and uses data-driven statistical analysis (ie, stochastic block model combined with hidden Markov modeling; see Ref. 108 for complete details) to identify timepoints of change in connectivity in network structure (Fig. 3II). Unlike many other dFC approaches, SDCS formally compares models that involve changes in FC with the null alternative that connectivity within the network does not change over time. This direct comparison is an important advantage of SDCS relative to other dFC approaches.
Figure 3.
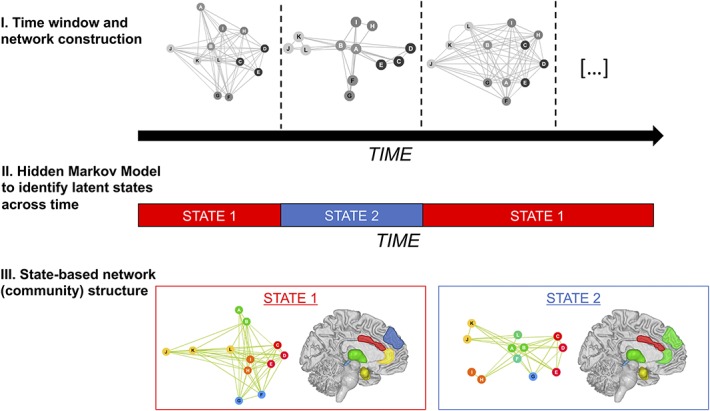
State-based dynamic community structure. (I) In SDCS analysis, networks are first identified and features of the network are estimated in nonoverlapping time windows. (II) A hidden Markov model is then used to determine network states and when the network shifts between states. (III) Finally, Markov Chain Monte Carlo is used to construct the network structure for each state, including the strength of within-network connectivity. Adapted from Ref. 108. SDCS, state-based dynamic community structure.
State-based dynamic community structure was used to examine dFC during noxious heat administration under open-label administration of the opioid analgesic remifentanil.108 Initial task-based univariate analyses4 tested whether the magnitude of the heat-evoked BOLD response differed as a function of drug or expectancy, but did not measure how these factors influenced connectivity between brain regions. In a follow-up analysis,108 SDCS analysis was applied within 3 separate a priori functional networks (a pain-related network, an emotion network, and a working memory network), to determine whether the networks changed over time and whether network reorganization corresponded to known experimental dynamics (eg, changes in drug concentration and expectations about drug infusion).
State-based dynamic community structure revealed distinct dynamics of network reorganization within the 3 networks.108 The structure of connectivity within the emotion network remained stable across the task, although univariate analyses had revealed strong effects of instructions on heat-evoked responses within these regions.4 There was significant reorganization, however, of network structure within a pain network and working memory network. Each of those networks transitioned between 2 states, with state shifts occurring when drug concentration was high. For example, the pain network shifted between one state (before and after the drug), characterized by the highest within-community coupling in a network that included ACC and insula, to a second state (present at peak drug concentration) in which the ACC was part of a different community characterized by low coupling. This suggests that remifentanil might decouple the insula and ACC at peak concentrations. Such conclusions would not be possible in FC analyses that assume connectivity remains stable over time, or that require strong assumptions about event timing, because remifentanil pharmacokinetics lead to slow changes over time.
State-based dynamic community structure and other graph-based measures require relatively long periods to capture meaningful network structure. For instance, Robinson et al.108 found stable estimations when they measured intervals 150 seconds in duration, but shorter intervals were less stable. Thus, SDCS and other graph-based dFC approaches might be more appropriate for pharmacological fMRI and block designs, but less useful in cases where connectivity is likely to shift over shorter timescales. We note that SDCS and related dFC methods assume that dFC is consistent across individuals. The approaches we turn to next may be better suited to determine how dFC differs as a function of individual differences (eg, patient status, behavioral response, etc.).
3.2. Approaches for measuring time-varying connectivity in resting-state analyses
The approaches we have examined thus far have focused on dFC during pain perception or pain-related tasks and focused on within-participant fluctuations in connectivity. However, it is also possible to study spontaneous brain connectivity dynamics in a task-free (or “resting”) state. Indeed, static FC analyses have demonstrated much utility for elucidating the network dynamics of the brain at rest in the study of pain and pain modulation.69,128 Yet, even at “rest,” the brain displays spontaneous fluctuations in FC, thought to largely reflect intrinsic physiological operations.17 Over the timescale of several minutes or longer, remote brain regions display highly organized spatiotemporal patterns of spontaneous activity9,13 that topographically resemble the networks that are commonly observed to coactivate/deactivate during active task performance or in response to external stimulation.121 For example, the “salience network,” including subregions within the insular and cingulate cortex that commonly coactivate during pain perception,34,64 also displays coordinated activity during resting states.113
It is now widely appreciated that resting-state network organization remains relatively stable within individuals across long timescales.45,56,77 However, emerging evidence suggests that spontaneous FC dynamics may vary on the order of tens of seconds or even shorter,63,104 motivating the development of nascent methodologies to analyze resting-state dFC. Similar to SDCS described above, resting-state analyses are not guided by known or observable events. A popular approach is sliding-window correlation analysis (Fig. 4), in which FC is repeatedly calculated within subjects across multiple distinct temporal windows (Fig. 4; top panel) within a single resting-state session. The resulting dFC time course can then be summarized in various ways. A simple example is that how much FC varies across windows (ie, FC variability) can be used as an index of the flexibility or stability of the connectivity between a given pair of regions71,74 (Fig. 4; bottom panel). Alternatively, to summarize dFC more globally across the whole brain, a “dFC matrix” can be computed, consisting of pairwise FC between all predefined ROIs for each temporal window. Network “states” can then be defined based on clustering analyses of the dFC matrix1 or graph theoretical metrics similar to those described above126,142 computed across windows. Another important analysis decision concerns how to define windows and FC within each window. Although using windows with fixed lengths and computing FC with interregional Pearson correlation is a common approach, this method can lead to spuriously inflated estimates of FC, especially if window lengths are short or windows are overlapping.60,78,84 Furthermore, window length can affect the frequency components that a given dFC analysis can identify.78 Given that resting state networks are typically identified in slow components of the BOLD signal,29 short windows (eg, 20–30 seconds) for analysis may capture only a single cycle of activity fluctuation or even less. Thus, research conclusions can be biased by decisions during analysis (eg, shorter window lengths may be biased to identify high-frequency, rather than low-frequency, components of dynamic connectivity). Proposed metrics that aim to address shortcomings of the sliding-window correlation approach can offer more time-resolved dFC estimates, including dynamic conditional correlation (DCC)82 and multiplication of temporal derivatives,118 but can be more computationally intensive, and their adoption for the study of resting-state dFC has been slow. Guidelines for best practices in resting-state dFC analysis are beginning to emerge,84 but open questions remain, and—as for task-based dFC analyses described above—analysis choices must be tailored for specific research questions.
Figure 4.
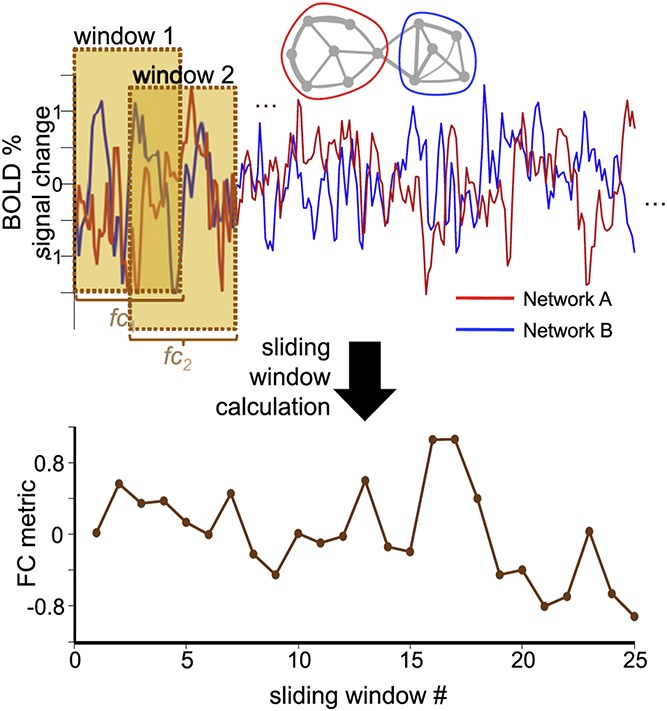
Sliding-window analysis of dynamic functional connectivity in resting-state fMRI. In sliding-window analyses, the BOLD time series from 2 distinct regions or networks are extracted, and then the correlation between the time series over a set “window” of time is computed as a metric of FC, a process that is repeated for N windows (in this case, N = 25) to determine the time series of FC. Reproduced with permission from Ref. 63. FC, functional connectivity; fMRI, functional magnetic resonance imaging.
Example applications of such tailored analyses to pain are found in a handful of existing studies linking individual variability in resting-state dFC with aspects of pain perception and coping. Thus, resting-state dFC analyses provide a means of relating within-subjects fluctuations in connectivity with individual differences across participants. For instance, initially using task-based PPI analysis, Kucyi et al.74 showed that FC between the PAG and medial prefrontal cortex (mPFC) was increased within individuals when they reported that they were mind-wandering away from a painful stimulus. Subsequent analyses across individuals revealed that greater PAG–mPFC resting-state dFC variability was associated with the tendency to mind-wandering away from pain.74 Cheng et al.23 also applied resting-state dFC analysis to test the hypothesis that pain-induced changes in cognitive task performance relate to coupling among nodes of the executive control and salience networks during resting state. Functional connectivity variability within and between these networks during resting state was associated with individual differences in the capacity to prioritize task performance over pain.
Importantly, in the aforementioned studies, brain-behavior relationships for resting-state dFC were found, but static connectivity between the same brain regions during resting state was not significantly related to the same behavior. These studies therefore highlight the possibility that spontaneous changes in dFC at rest may capture unique, behaviorally relevant aspects of pain perception and coping across individuals. Further supporting this idea, recent studies demonstrate that there is marked variability in dFC across individuals, which is consistent across multiple tasks but remains stable within individuals, including across both rest and task.33,116 Together, these studies suggest that resting-state dFC could be a useful tool for studying how intrinsic network dynamics differ between individuals, and whether those differences are associated with pain-related experiences or behavior.
4. Using dynamic brain connectivity to characterize chronic pain patients
Throughout this review, we have examined different methods for studying dFC that have primarily been applied to the study of acute pain. Here, we consider how dFC approaches can provide insight into chronic pain and its treatment. Patients with chronic pain, including those ascribed the same medical diagnosis, are heterogeneous in their experience of pain,130 and this heterogeneity can contribute to interindividual variability in treatment outcomes.37 Recent findings suggest promising applications of dFC to characterize pain pathophysiology and predict treatment outcomes in patients with chronic pain.21,58,67
As mentioned above, one approach to assess individual differences in dFC is analysis of the brain at rest. A promising new dFC method is DCC.82 Briefly, time-varying variances for time series from multiple regions are each first estimated using a statistical model for time series data (specifically, a generalized autoregressive conditional heteroscedastic, or GARCH, model), and then used to derive standardized residuals for each time series. The dynamic correlation between these standardized residuals is then calculated using exponentially weighted moving average windows followed by a rescaling step. A key feature of the DCC approach is that it produces a summary measure, the variance of the estimated dynamic connectivity between 2 brain regions over time, that has been shown to be scan–rescan reliable and can be performed on an individual basis.25 Correlations between this measure and behavior can then be performed across individuals to investigate individual differences, or contrasted between patients and controls to determine group differences.
One illustrative example of the application of dFC to chronic pain focused on patients with ankylosing spondylitis, an inflammatory arthritis that affects the axial skeleton.24 A multivariate regression approach was used to determine whether multivariate patterns of dFC computed using DCC, combined with measures of static FC, were related to levels of chronic pain. Importantly, this study also measured pain on multiple timescales—both state pain (pain during the day of the study) and trait pain (average pain in the past 4 weeks)—affording the opportunity to directly compare both static and dynamic pain and FC. When the multivariate weights were used as a proxy of the importance of the features in the model, dFC features were generally more important than the static FC features, demonstrating that accounting for the temporal dynamics of FC may explain important variance in clinical outcomes.
Furthermore, multivariate patterns of brain connectivity were more highly related to measures of trait pain (average pain in the past 4 weeks) than measures of state pain (pain during the day of the study, both measures assessed with a single item24). This latter finding may be driven by the fact that the dFC measure used (the variance of DCC) is scan–rescan reliable (as mentioned above), and thus was better associated with stable measures (eg, trait pain) that capture pain on average than with measures that might be related more to fluctuations in the external environment (eg, state pain). Whether dFC maps onto dynamic fluctuations in current pain among patients with chronic pain, however, remains to be established. In future investigations, one method to address this question could entail regressing continuous fluctuations in pain collected throughout the scan with raw DCC estimates, rather than summarizing dFC using the variance of DCC.
Dynamic FC methods may also be useful in predicting treatment outcomes. For example, a recent study of neuropathic pain patients showed that dFC between the default-mode network and descending antinociceptive system before ketamine infusion distinguished between those who responded (≥30% pain relief) and did not respond to the infusion.15 Notably, pretreatment temporal summation of pain (TSP) also distinguished between responders and nonresponders. In a mediation analysis, it was determined that the relationship between TSP and treatment response was mediated by the dFC between the default-mode network and the antinociceptive system,15 suggesting that dFC explained some variability in the link between TSP and treatment outcome. Although the study of dFC, as it pertains to treatment outcomes or individual differences across patients, is still nascent and further work in this area is necessary, early research in this domain demonstrates promise for future clinical applications of this method.
5. Summary and Future Directions
We have reviewed and highlighted dFC methods that have been applied to pain neuroimaging research and provided new insights not possible with standard approaches or static connectivity. Pain is modulated by many psychological and biological factors that vary dynamically across time, both spontaneously and with changing environmental contexts and demands. Patterns of connectivity in the brain are also dynamic, fluctuating over both very short and longer timescales, and these fluctuations have utility for predicting cognition and behavior. Dynamic functional connectivity analyses permit us to investigate the neural basis of pain processing and pain modulation with greater nuance, and to approach understanding pain as a process encoded by a “pain connectome,” a “spatiotemporal signature of brain network communication that represents the integration of all cognitive, affective, and sensorimotor aspects of pain.”71 Psychophysiological interaction analyses demonstrate that the time courses of activity in key pain-responsive brain regions correspond more under certain contexts (eg, placebo analgesia) than others. Dynamic causal modeling can further investigate the dynamics of brain activity in each node using Bayesian modeling to test for putative directional pathways between nodes, with utility in predicting future pain-related outcomes. Methodologies such as SDCS that use graph theory–based network analyses combined with data-driven change point detection can identify shifts in brain network organization and characterize both the timing and structure of shifts in connectivity. Most recently, the study of dynamic resting-state FC has revealed a role for spontaneous dFC in predicting differences in pain processing and coping between individuals. In particular, resting-state dFC measures seem to capture individual differences in stable, trait measures across individuals, including patients with chronic pain. Although work in this area is still in its infancy, dFC offers great promise as a tool for predicting treatment outcomes and characterizing patients, including those with chronic pain, and may explain greater variance in clinical outcomes than static FC.24
As dFC analytical approaches continue to be applied to the study of pain, it will be important to remain cognizant of the fact that dFC methods are constantly evolving. There are still a number of open questions and methodological concerns regarding dFC analytical approaches. Although some of the approaches for studying dFC have been around since the early days of pain neuroimaging (eg, PPI and DCM), others are relatively new (eg, SDCS, sliding windows, DCC), and the comprehensive application of all these methodologies to the study of experimental pain and pain in patient populations is yet to be fully established. One methodological concern to consider when investigating brain dynamics in pain is the susceptibility of dFC analyses to influence by researcher decisions during experimental design and analyses. Methods for studying task-evoked changes in FC are of course influenced by decisions about task design, such as decisions pertaining to sample size and the number of trials to ensure adequate power and choices regarding task timing. We encourage researchers interested in task-evoked changes in FC to consult comprehensive reviews on neuroimaging acquisition, including a recent report from the Organization for Human Brain Mapping Committee on Best Practices in Data Analysis and Sharing (COBIDAS93) and a recent primer that specifically focuses on pain neuroimaging.87
Yet, even fully data-driven methods such as resting-state dFC analyses also require careful decisions before data acquisition. For example, recent research suggests that test–retest reliability metrics of resting-state static connectivity are strongly related to the amount of time participants spend in the scanner, with 30 minutes of resting-state data nearly doubling the reliability obtained at shorter scan times,44,116 and at least 10 minutes of data collection having been recommended previously for the measurement of whole-brain connectivity.12,55 However, these recommendations must be tempered with practical concerns regarding feasibility, especially when working with patient populations who may not be able to withstand the physical demands of scanning for extensive periods. Indeed, fMRI FC analyses are particularly sensitive to motion artifacts,102 which is a further point researchers must consider when designing studies to evaluate contextual effects that might vary in the extent to which they evoke motion (eg, pain vs nonpain) or when comparing populations who might exhibit differences in motion (eg, chronic back pain patients forced to lie on a scanner bed might exhibit more motion from discomfort than pain-free controls). Scan sequences that enhance the temporal resolution and reduce the acquisition time of fMRI, such as using multiband/multislice imaging (where multiple slices are acquired in parallel41), are worth considering as potential solutions to these concerns because they reduce the length of time participants are in the scanner without necessarily decreasing signal quality103,112 or limiting the types of dFC approaches that can be measured (for further discussion on selection of scan sequences in pain imaging, see Ref. 87). Furthermore, multiband approaches can improve the sensitivity of dFC measures for capturing small connectivity changes and higher-frequency signals (eg, greater than 0.2 Hz),79 overcoming typical weaknesses associated with assessing dFC with fMRI. However, because the improved reliability of connectivity measures derived from longer scan lengths is due to not only the increased number of volumes acquired but also the increased total duration of the scan,12 multiband imaging sequences may not be a fully adequate solution to the practical concerns that can come with acquiring neuroimaging data in pain studies, particularly when working with pain populations, and multiple short scans with parallel multislice sequences could be more practical. Ultimately, more research is necessary to establish guidelines for dynamic, rather than static, measures (although see Refs. 54 and 84 for emerging recommendations and open questions).
At a more theoretical level, another important and challenging theoretical question for pain researchers to consider is whether the timescales assessed with dFC can be matched to the timescales at which pain varies, especially in clinical pain states. Pain can vary over timescales ranging from seconds to days and months to years,6,76,96,105 and even within the same noxious stimulus, different features of pain (eg, prickling, aching sensations) display distinct temporal profiles.32 It remains to be established how these variations in subjective experience can be best measured using dFC. Some studies have previously used continuous reports of patients' spontaneous pain to measure brain activation.96 This approach could easily be modified to measure how interregional fluctuations in FC, rather than activation in a single region, correspond to continuous pain ratings. It may also be possible to determine the frequency bands at which certain pain features are most likely to fluctuate, and to select time windows for resting-state analyses that can capture fluctuations at these frequencies. This latter recommendation, however, would be limited by the temporal resolution of the imaging methodology used. As discussed previously, fMRI may be limited in its ability to detect high-frequency fluctuations, and approaches with enhanced temporal resolution (eg, EEG/MEG) could be better suited for detecting dFC corresponding to rapid changes in clinical pain states.
Dynamic functional connectivity approaches also require careful decisions during the analysis stage. A critical decision for all dFC approaches, for instance, is also the selection of brain parcellations and definition of ROIs, particularly when defining nodes for building brain networks. Although brain nodes and ROIs are often defined using anatomical boundaries (ie, brain atlas–based ROIs), simulations demonstrate that data-driven methods (such as independent component analysis) for defining nodes may be less susceptible to artifacts and more accurately reflect true network structure.139 Thus, selection of any methodology for analyzing dFC should be guided by research questions and specific hypotheses, in tandem with an understanding of the limitations of each technique. These methodological concerns and open questions should not preclude the use of dFC analytical approaches to understanding pain, but do suggest that continued research efforts toward addressing these concerns are necessary to enhance their applicability to pain research.
We are still in the infancy of our understanding of fMRI and dFC approaches and their application to pain, and work in this area is replete with both challenges and opportunities for enhancing our understanding of the functional dynamics of pain and its modulation. A difficult challenge in this field will concern determining whether dFC approaches can be leveraged to track intraindividual spontaneous dynamics of pain as they occur in the real world, involving continuous, ongoing interactions with attention and emotion.71,72 Furthermore, it remains an open question whether dFC detected with fMRI could have utility for tracking more ecologically valid fluctuations in pain or whether electrophysiological techniques with greater temporal resolution and the ability to capture a greater spectrum of frequency- and phase-specific network dynamics75,133 could be needed. Although other neuroimaging methodologies may be able to better characterize rapid fluctuations in FC because of their superior temporal resolution, these methods may not provide the spatial resolution and insights on whole-brain connectivity provided by fMRI. Furthermore, it is still not clear whether correlations between regions reflect actual communication between networks or other physiological influences that lead to shared fluctuations. Validating the fMRI-based dFC approaches reviewed here by measuring brain connections and communication directly using intracranial recordings, perturbations (TMS and lesion studies), or confirmation in animal models will be important next steps. Going forward, research using dFC methodologies has promise for enhancing our understanding of the neural mechanisms of pain and pain modulation, for clarifying how network dynamics are linked to pain both within and across individuals, and for providing new paths forward for the clinical diagnosis and treatment of pain.
Disclosures
The authors have no conflict of interest to declare.
This work was supported in part by funding from the Intramural Program of the National Center for Complementary and Integrative Health.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 2014;24:663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Apkarian AV, Bushnell MC, Treede R, Zubieta J. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463–84. [DOI] [PubMed] [Google Scholar]
- [3].Atlas LY, Wager TD. How expectations shape pain. Neurosci Lett 2012;520:140–8. [DOI] [PubMed] [Google Scholar]
- [4].Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci 2012;32:8053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nat Rev Neurosci 2018;19:17–33. [DOI] [PubMed] [Google Scholar]
- [6].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006;26:12165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15:1117–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist 2006;12:512–23. [DOI] [PubMed] [Google Scholar]
- [9].Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 2005;360:1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. PAIN 2013;154:1181–96. [DOI] [PubMed] [Google Scholar]
- [11].Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. PAIN 2006;120:8–15. [DOI] [PubMed] [Google Scholar]
- [12].Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 2013;83:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41. [DOI] [PubMed] [Google Scholar]
- [14].Borsook D, Youssef AM, Barakat N, Sieberg CB, Elman I. Subliminal (latent) processing of pain and its evolution to conscious awareness. Neurosci Biobehav Rev 2018;88:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bosma RL, Cheng JC, Rogachov A, Kim JA, Hemington KS, Osborne NR, Raghavan LV, Bhatia A, Davis KD. Brain dynamics and temporal summation of pain predicts neuropathic pain relief from ketamine infusion. Anesthesiology 2018;129:1015–24. [DOI] [PubMed] [Google Scholar]
- [16].Brodersen KH, Wiech K, Lomakina EI, Lin CS, Buhmann JM, Bingel U, Ploner M, Stephan KE, Tracey I. Decoding the perception of pain from fMRI using multivariate pattern analysis. Neuroimage 2012;63:1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 2013;16:832–7. [DOI] [PubMed] [Google Scholar]
- [18].Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–98. [DOI] [PubMed] [Google Scholar]
- [19].Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Calhoun VD, Miller R, Pearlson G, Adali T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 2014;84:262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Čeko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp 2015;36:2075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 2010;50:81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheng JC, Bosma RL, Hemington KS, Kucyi A, Lindquist MA, Davis KD. Slow-5 dynamic functional connectivity reflects the capacity to sustain cognitive performance during pain. Neuroimage 2017;157:61–8. [DOI] [PubMed] [Google Scholar]
- [24].Cheng JC, Rogachov A, Hemington KS, Kucyi A, Bosma RL, Lindquist MA, Inman RD, Davis KD. Multivariate machine learning distinguishes cross-network dynamic functional connectivity patterns in state and trait neuropathic pain. PAIN 2018;159:1. [DOI] [PubMed] [Google Scholar]
- [25].Choe AS, Nebel MB, Barber AD, Cohen JR, Xu Y, Pekar JJ, Caffo B, Lindquist MA. Comparing test-retest reliability of dynamic functional connectivity methods. Neuroimage 2017;158:155–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clark JW, Bindha D. Individual differences in pain thresholds. Can J Psychol 1956;10:69–76. [DOI] [PubMed] [Google Scholar]
- [27].Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 2017;82:1934–43. [DOI] [PubMed] [Google Scholar]
- [28].Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci 1994;14:4095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- [30].Cribben I, Haraldsdottir R, Atlas LY, Wager TD, Lindquist MA. Dynamic connectivity regression: determining state-related changes in brain connectivity. Neuroimage 2012;61:907–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Daunizeau J, David O, Stephan KE. Dynamic causal modelling: a critical review of the biophysical and statistical foundations. Neuroimage 2011;58:312–22. [DOI] [PubMed] [Google Scholar]
- [32].Davis KD, Pope GE. Noxious cold evokes multiple sensations with distinct time courses. PAIN 2002;98:179–85. [DOI] [PubMed] [Google Scholar]
- [33].Davison EN, Turner BO, Schlesinger KJ, Miller MB, Grafton ST, Bassett DS, Carlson JM. Individual differences in dynamic functional brain connectivity across the human lifespan. PLoS Comput Biol 2016;12:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage 2003;20:1540–51. [DOI] [PubMed] [Google Scholar]
- [35].Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp 2011;34:109–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 1999;125:356–66. [DOI] [PubMed] [Google Scholar]
- [37].Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, Brell J, Bujanover S, Burke LB, Carr D, Chappell AS, Cowan P, Etropolski M, Fillingim RB, Gewandter JS, Katz NP, Kopecky EA, Markman JD, Nomikos G, Porter L, Rappaport BA, Rice ASC, Scavone JM, Scholz J, Simon LS, Smith SM, Tobias J, Tockarshewsky T, Veasley C, Versavel M, Wasan AD, Wen W, Yarnitsky D. Patient phenotyping in clinical trials of chronic pain treatments. PAIN 2016;157:1851–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009;63:533–43. [DOI] [PubMed] [Google Scholar]
- [39].Ellingsen D, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc Natl Acad Sci U S A 2013;10:17993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ezra M, Faull OK, Jbabdi S, Pattinson KTS. Connectivity-based segmentation of the periaqueductal gray matter in human with brainstem optimized diffusion MRI. Hum Brain Mapp 2015;36:3459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Feinberg DA, Setsompop K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J Magn Reson 2013;229:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Feinstein JS, Khalsa SS, Salomons TV, Prkachin KM, Frey-Law LA, Lee JE, Tranel D, Rudrauf D. Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct Funct 2016;221:1499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 2004;5:565–75. [DOI] [PubMed] [Google Scholar]
- [44].Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, Constable RT. Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage 2017;160:140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 2015;18:1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Foltz EL, White LE. Pain “relief” by frontal cingulumotomy. J Neurosurg 1961;19:89–100. [DOI] [PubMed] [Google Scholar]
- [47].Friedman DP, Murray EA. Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the macaque. J Comp Neurol 1986;252:348–73. [DOI] [PubMed] [Google Scholar]
- [48].Friston K. Causal modelling and brain connnectivity in functional magnetic resonance imaging. PLos Biol 2009;7:e100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Friston KJ. Functional and effective connectivity: a review. Brain Connect 2011;1:13–36. [DOI] [PubMed] [Google Scholar]
- [50].Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997;6:218–29. [DOI] [PubMed] [Google Scholar]
- [51].Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage 2003;19:1273–302. [DOI] [PubMed] [Google Scholar]
- [52].Friston KJ, Kahan J, Biswal B, Razi A. A DCM for resting state fMRI. Neuroimage 2014;94:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Geschwind N. Disconnexion syndromes in animals and man: Part I. Neuropsychol Rev 2010;20:128–57. [DOI] [PubMed] [Google Scholar]
- [54].Gonzalez-Castillo J, Bandettini PA. Task-based dynamic functional connectivity: recent findings and open questions. Neuroimage 2017;180:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gonzalez-Castillo J, Handwerker DA, Robinson ME, Hoy CW, Buchanan LC, Saad ZS, Bandettini PA. The spatial structure of resting state connectivity stability on the scale of minutes. Front Neurosci 2014;8:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, Nelson SM, Coalson RS, Snyder AZ, Schlaggar BL, Dosenbach NUF, Petersen SE. Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron 2018;98:439–52 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Handwerker DA, Gonzalez-Castillo J, D'Esposito M, Bandettini PA. The continuing challenge of understanding and modeling hemodynamic variation in fMRI. Neuroimage 2012;62:1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. PAIN 2012;153:2393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Heinricher MM, Fields HL. Central nervous system mechanisms of pain modulation. In: Wall & Melzack's textbook of pain. Philadelphia: Elsevier Health Sciences, 2013. p. 129–42. [Google Scholar]
- [60].Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, Deco G. Can sliding-window correlations reveal dynamic functional connectivity in resting- state fMRI? Neuroimage 2016;127:242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hiramatsu T, Nakanishi K, Yoshimura S, Yoshino A, Adachi N, Okamoto Y, Yamawaki S, Ochi M. The dorsolateral prefrontal network is involved in pain perception in knee osteoarthritis patients. Neurosci Lett 2014;581:109–14. [DOI] [PubMed] [Google Scholar]
- [62].Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 1977;197:183–6. [DOI] [PubMed] [Google Scholar]
- [63].Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 2013;80:360–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back). Exp Brain Res 2010;205:1–12. [DOI] [PubMed] [Google Scholar]
- [65].Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SCR, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain 2012;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Khoshnejad M, Piché M, Saleh S. Serial processing in primary and secondary somatosensory cortex: a DCM analysis of human fMRI data in response to innocuous and noxious electrical stimulation. Neurosci Lett 2014;577:83–8. [DOI] [PubMed] [Google Scholar]
- [67].Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol 2014;192:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kirsch I. Response expectancy and the placebo effect. Int Rev Neurobiol 2018;138:81–93. [DOI] [PubMed] [Google Scholar]
- [69].Kong J, Jensen K, Loiotile R, Cheetham A, Wey H, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. PAIN 2013;154:459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. PAIN 2010;148:368–74. [DOI] [PubMed] [Google Scholar]
- [71].Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci 2015;38:86–95. [DOI] [PubMed] [Google Scholar]
- [72].Kucyi A, Davis KD. The neural code for pain: from single-cell electrophysiology to the dynamic pain connectome. Neuroscientist 2017;23:397–414. [DOI] [PubMed] [Google Scholar]
- [73].Kucyi A, Hove MJ, Esterman M, Hutchison RM, Valera EM. Dynamic brain network correlates of spontaneous fluctuations in attention. Cereb Cortex 2017;27:1831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A 2013;110:18692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kucyi A, Schrouff J, Bickel S, Foster BL, Shine JM, Parvizi J. Intracranial electrophysiology reveals reproducible intrinsic functional connectivity within human brain networks. J Neurosci 2018;38:4230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology 2005;65:1268–77. [DOI] [PubMed] [Google Scholar]
- [77].Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen MY, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NU, Schlaggar BL, Mumford JA, Poldrack RA, Petersen SE. Functional system and areal organization of a highly sampled individual human brain. Neuron 2015;87:657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Leonardi N, Van De Ville D. NeuroImage on spurious and real fl uctuations of dynamic functional connectivity during rest. Neuroimage 2015;104:430–6. [DOI] [PubMed] [Google Scholar]
- [79].LeVan P, Akin B, Hennig J, LeVan P, Akin B, Hennig J. Fast imaging for mapping dynamic networks. Neuroimage 2018;180:547–58. [DOI] [PubMed] [Google Scholar]
- [80].Li B, Daunizeau J, Stephan KE, Penny W, Hu D, Friston K. Generalised filtering and stochastic DCM for fMRI. Neuroimage 2011;58:442–57. [DOI] [PubMed] [Google Scholar]
- [81].Liang M, Mouraux A, Iannetti GD. Parallel processing of nociceptive and non-nociceptive somatosensory information in the human primary and secondary somatosensory cortices: evidence from dynamic causal modeling of functional magnetic resonance imaging data. J Neurosci 2011;31:8976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lindquist MA, Xu Y, Nebel MB, Caffo BS. Evaluating dynamic bivariate correlations in resting- state fMRI: a comparison study and a new approach. Neuroimage 2014;101:531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Longo MR, Iannetti GD, Mancini F, Driver J, Haggard P. Linking pain and the Body : neural correlates of visually induced analgesia. J Neurosci 2012;32:2601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lurie DJ, Kessler D, Bassett DS, Betzel RF, Breakspear M, Keilholz S. On the nature of resting fMRI and time-varying connectivity. bioRxiv Prepr 2018:1–33. [Google Scholar]
- [85].McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 2012;61:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Meier ML, Stämpfli P, Humphreys BK, Vrana A, Seifritz E, Schweinhardt P. The impact of pain- related fear on neural pathways of pain modulation in chronic low back pain. Pain Rep 2017;2:e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Moayedi M, Salomons TV, Atlas LY. Pain neuroimaging in humans: a primer for beginners and non-imagers. J Pain 2018;19:961.e1–961.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabaté JM. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain 2010;14:142–8. [DOI] [PubMed] [Google Scholar]
- [89].Moore DJ, Keogh E, Eccleston C. The interruptive effect of pain on attention. Q J Exp Psychol 2012;65:565–86. [DOI] [PubMed] [Google Scholar]
- [90].Mufson EJ, Mesulam MM. Insula of the old world monkey. 111: efferent cortical output and comments on function. J Comp Neurol 1982;212:23–37. [DOI] [PubMed] [Google Scholar]
- [91].Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 2014;111:1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, Kriegeskorte N, Milham MP, Poldrack RA, Poline JB, Proal E, Thirion B, Van Essen DC, White T, Yeo BTT. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci 2017;20:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nielsen CS, Staud R, Price DD. Individual differences in pain sensitivity: measurement, causation, and consequences. J Pain 2009;10:231–7. [DOI] [PubMed] [Google Scholar]
- [95].O'Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci 2012;7:604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Parks EL, Geha PY, Baliki MN, Katz J, Schnitzer TJ, Apkarian AV. Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain 2011;15:843.e1–843.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937;60:389–443. [Google Scholar]
- [98].Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science 2002;295:1737–40. [DOI] [PubMed] [Google Scholar]
- [99].Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Clin Neurophysiol 2000;30:263–88. [DOI] [PubMed] [Google Scholar]
- [100].Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex 2011;21:719–26. [DOI] [PubMed] [Google Scholar]
- [101].Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A 2010;107:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Preibisch C, Castrillón G JG, Bührer M, Riedl V. Evaluation of multiband EPI acquisitions for resting state fMRI. PLoS One 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Preti MG, Bolton TA, Van De Ville D. The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage 2017;160:41–54. [DOI] [PubMed] [Google Scholar]
- [105].Rahman QA, Janmohamed T, Pirbaglou M, Clarke H, Ritvo P, Heffernan JM, Katz J. Defining and predicting pain volatility in users of the manage my pain app: analysis using data mining and machine learning methods. J Med Internet Res 2018;20:e12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C. Six problems for causal inference from fMRI. Neuroimage 2010;49:1545–58. [DOI] [PubMed] [Google Scholar]
- [107].Reicherts P, Wiemer J, Gerdes ABMM, Schulz SM, Pauli P, Wieser MJ. Anxious anticipation and pain—the influence of instructed versus conditioned threat on pain. Soc Cogn Affect Neurosci 2017;12:544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Robinson LF, Atlas LY, Wager TD. Dynamic functional connectivity using state-based dynamic community structure: method and application to opioid analgesia. Neuroimage 2015;108:274–91. [DOI] [PubMed] [Google Scholar]
- [109].Roebroeck A, Formisano E, Goebel R. The identification of interacting networks in the brain using fMRI: model selection, causality and deconvolution. Neuroimage 2011;58:296–302. [DOI] [PubMed] [Google Scholar]
- [110].Roebroeck A, Ivanov D, Gardumi A, Uludag K, Havlicek M, Friston KJ. On the importance of modeling fMRI transients when estimating effective connectivity: a dynamic causal modeling study using ASL data. Neuroimage 2017;155:217–33. [DOI] [PubMed] [Google Scholar]
- [111].Schenk LA, Sprenger C, Onat S, Colloca L, Büchel C. Suppression of striatal prediction errors by the prefrontal cortex in placebo hypoalgesia. J Neurosci 2017:1101–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Schulz J, Boyacioʇlu R, Norris DG. Multiband multislab 3D time-of-flight magnetic resonance angiography for reduced acquisition time and improved sensitivity. Magn Reson Med 2016;75:1662–8. [DOI] [PubMed] [Google Scholar]
- [113].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sevel LS, Craggs JG, Price DD, Staud R, Robinson ME. Placebo analgesia enhances descending pain-related effective connectivity: a dynamic causal modeling study of endogenous pain modulation. J Pain 2015;16:760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sevel LS, O'Shea AM, Letzen JE, Craggs JG, Price DD, Robinson ME. Effective connectivity predicts future placebo analgesic response: a dynamic causal modeling study of pain processing in healthy controls. Neuroimage 2015;110:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Shah LM, Cramer JA, Ferguson MA, Birn RM, Anderson JS. Reliability and reproducibility of individual differences in functional connectivity acquired during task and resting state. Brain Behav 2016;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, Becerra L, Borsook D. Mapping pain activation and connectivity of the human habenula. J Neurophysiol 2012;107:2633–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shine JM, Koyejo O, Bell PT, Gorgolewski KJ, Gilat M, Poldrack RA. Estimation of dynamic functional connectivity using multiplication of temporal derivatives. Neuroimage 2015;122:399–407. [DOI] [PubMed] [Google Scholar]
- [119].Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 2014;35:527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sizemore AE, Bassett DS. Dynamic graph metrics: tutorial, toolbox, and tale. Neuroimage 2018;180:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 2009;106:13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sprenger C, Finsterbusch J, Buchel C. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical fMRI study. J Neurosci 2015;35:4248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Stankewitz A, Sorg C, Kalckreuth A, Schulz E, Valet M, Neufang S, Zimmer C, Henningsen P, Gündel H, Wohlschläger AM, Tölle TR, von Kalckreuth A. Fronto-insular connectivity during pain distraction is impaired in patients with somatoform pain. J Neuroimaging 2018;28:621–8. [DOI] [PubMed] [Google Scholar]
- [124].Stephan KE, Friston KJ. Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip Rev Cogn Sci 2010;1:446–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Stephan KE, Penny WD, Moran RJ, den Ouden HEM, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. Neuroimage 2010;49:3099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Front Hum Neurosci 2012;6:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Telesford QK, Simpson SL, Burdette JH, Hayasaka S, Laurienti PJ. The brain as a complex system: using network science as a tool for understanding the brain. Brain Connect 2011;1:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tétreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN. Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol 2016;14:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tononi G, Edelman GM, Sporns O. Complexity and the integration of information in the brain. Trends Cogn Sci 1998;2:474–84. [DOI] [PubMed] [Google Scholar]
- [130].Turk DC. The potential of treatment matching for subgroups of patients with chronic pain. Clin J Pain 2005;21:44–55. [DOI] [PubMed] [Google Scholar]
- [131].Vachon-Presseau E, Martel M, Roy M, Caron E, Marin M, Plante I, Sullivan MJ, Lupien SJ, Rainville P. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci 2013;33:6826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 2013;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Vidaurre D, Hunt LT, Quinn AJ, Hunt BAE, Brookes MJ, Nobre AC, Woolrich MW. Spontaneous cortical activity transiently organises into frequency specific phase-coupling networks. Nat Commun 2018;9:2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013;368:1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 2004;303:1162–7. [DOI] [PubMed] [Google Scholar]
- [136].Walter C, Oertel BG, Felden L, Kell CA, Nöth U, Vermehren J, Kaiser J, Deichmann R, Lötsch J. Brain mapping-based model of Δ 9-tetrahydrocannabinol effects on connectivity in the pain matrix. Neuropsychopharmacology 2016;41:1659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wiech K, Jbabdi S, Lin CS, Andersson J, Tracey I. Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. PAIN 2014;155:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage 2009;47:987–94. [DOI] [PubMed] [Google Scholar]
- [139].Yu Q, Du Y, Chen J, He H, Sui J, Pearlson G, Calhoun VD. Comparing brain graphs in which nodes are regions of interest or independent components: a simulation study. J Neurosci Methods 2017;291:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yu Q, Du Y, Chen J, Sui J, Adali T, Pearlson GD, Calhoun VD. Application of graph theory to assess static and dynamic brain connectivity: approaches for building brain graphs. Proc IEEE 2018;106:886–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin 2014;6:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. Time-resolved resting-state brain networks. Proc Natl Acad Sci U S A 2014;111:10341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


