Abstract
Background
Studies have shown that D-dimer levels are significantly correlated with the differential diagnosis and clinicopathological features of breast cancer. However, the results are currently limited and controversial. Therefore, we performed this meta-analysis to evaluate the relationship between D-dimer levels and breast cancer.
Materials and methods
The PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, Chinese Biomedical Literature, and Wanfang databases were searched to find studies that assessed the association of D-dimer with clinicopathological features of breast cancer and its usefulness in aiding with differential diagnosis. The standardized mean difference (SMD) was applied as the correlation measure.
Results
A total of 1244 patients with breast cancer from 15 eligible studies were included in the meta-analysis. D-dimer levels were higher in the breast cancer group than in the benign (SMD = 1.02; 95% confidence interval [CI] = 0.53–1.52) and healthy (SMD = 1.27; 95% CI = 0.85–1.68) control groups. In addition, elevated D-dimer levels were associated with progesterone receptor-negative tumors (SMD = -0.25; 95% CI = -0.44–-0.05). Similarly, there was a significant correlation between D-dimer levels and tumor node metastasis staging (n = 11, SMD = 0.82; 95% CI = 0.57–1.06) and lymph node involvement (n = 8, SMD = 0.79; 95% CI = 0.50–1.09). In contrast, other clinicopathological factors, including estrogen receptor expression and human epidermal growth factor receptor 2 expression, were not associated with D-dimer levels.
Conclusion
The results of this meta-analysis indicate that plasma D-dimer levels can be used as an important reference for the early identification and staging of breast cancer.
Introduction
Breast cancer is the leading cause of death in women aged between 20 and 59 years and is estimated to account for 30% of all new cancer diagnoses in women in 2019[1]. Breast cancer has multiple levels of tumor heterogeneity. Clinical pathological conditions such as tumor node metastasis (TNM) stage, hormone receptor expression, human epidermal growth factor 2 (HER2) expression, and metastasis lead to different prognoses of breast cancer[2]. From 1990 to 2016, the mortality rate of female breast cancer decreased by 40% [1], but it still threatens women’s health. Early diagnosis and treatment are key to improving the survival rates of breast cancer[3]. In addition to clinically and widely used tumor markers, such as carcinoembryonic antigen[4] and cancer antigen 15–3[5], other clinical laboratory indicators are urgently needed to assist in differential diagnosis and predict prognosis.
Tumor-induced coagulation is closely related to tumorigenesis and tumor development. Malignant disease can show signs of venous thromboembolism years before the patient has any obvious clinical symptoms[6]. By promoting neovascularization and metastasis, a vicious cycle is formed between procoagulant proteins and malignant tumor cells[7]. There is evidence that activated fibrinogens prevent NK cell-mediated tumor cell elimination, improve circulating tumor cell survival, increase tumor metastasis potential, and lead to poor prognosis[8]. Therefore, D-dimer, which is the end product of fibrinogen hydrolysis, has certain clinical value for the differential screening of benign and malignant tumors[9] and prediction of the prognosis of tumors[10–12]. Studies have shown that D-dimer has a significant correlation with the diagnosis and prognosis of a variety of malignant tumors (e.g., colorectal cancer and ovarian cancer), and D-dimer levels can be used as a diagnostic marker to design more individualized and effective treatment strategies [13].
However, Research on evaluating the association of D-dimer levels with breast cancer are currently limited, and the results have been controversial. Therefore, this meta-analysis was performed to assess the association between D-dimer levels and breast cancer-associated differential diagnosis and clinicopathological features.
Materials and methods
Literature search
The literature search was performed using the PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure, Chinese Biomedical Literature, and Wanfang databases. We included articles published from the establishment of the database to March 19, 2019. We included only studies published in English or Chinese. The keywords used for the search can be found in S1 Table. We also performed a supplementary search for references included in the studies identified in the original search.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) the study group consisted of patients with breast cancer with a definite diagnosis; 2) the control group consisted of healthy women or patients with benign breast tumors; 3) the D-dimer test method in the study was clear; 4) the study results contained or had sufficient data to calculate the mean and standard deviation, defined here as more than 20 patients; and 5) the study showed a correlation between D-dimer levels and diagnostic and/or clinicopathological features of breast cancer. The exclusion criteria were as follows: 1) case reports or reviews; 2) studies describing animal experiments; 3) repeated publications; and 4) articles with a low Newcastle-Ottawa scale (NOS) score (≤4).
Data extraction and quality assessment
Data extraction and quality evaluation of the literature were performed independently by two authors. We extracted the following information: first author’s last name, year of publication, country, method used to assess D-dimer levels, type of anticoagulant used, number of experimental groups included in the study, number of healthy controls and benign tumor controls, and number of patients with TNM stage I-II and III-IV disease. The NOS standard[14] was used as a research quality assessment standard. Studies with a score ≤ 4 were considered low quality. When there was a difference in opinion on a document, the two authors resolved the problem through mutual discussion and requested help from a third author if necessary.
Statistical analysis
All data analyses were performed using the Review Manager software version 5.3 (Cochrane Collaboration, London, UK) and STATA software version 14.0 (Stata Corporation, College Station, TX, USA). The standardized mean difference (SMD) was used as a measure of the association between D-dimer levels and breast cancer, and the results are presented in the form of forest plots. Inter-study heterogeneity was assessed using the Q test and I2 statistic. When P > 0.10 or I2 < 50% indicated that there was no obvious heterogeneity[15], a fixed effects model was used; otherwise, a random effects model was used[16]. In addition, when the heterogeneity was significant, we performed subgroup analyses, followed by a sensitivity analysis. We used a funnel plot and an Egger test to assess publication bias[17]. P < 0.05 was considered statistically significant.
Results
Study search
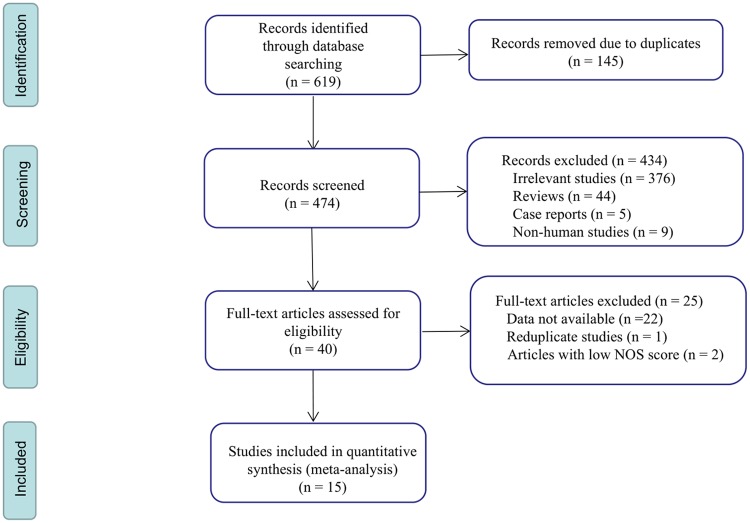
Through our database search, we found 619 studies, of which 474 remained after duplicates were excluded. Based on the title and abstract, we excluded 434 articles that were not related to the research content and evaluated the remaining 40 articles in full. After full-text articles were assessed for eligibility, we included 15 studies that could be used for meta-analysis. A flow chart of the screening process is shown in Fig 1.
Fig 1. Flow diagram of the study selection process.
NOS: Newcastle-Ottawa scale.
Characteristics of eligible studies
Table 1 summarizes the basic information of the 15 eligible studies. The included studies were published between 2000 and 2018. D-dimer detection methods included enzyme-linked immunosorbent assay, immunoturbidimetry, and enzyme-linked immunofluorescence.
Table 1. Characteristics of included studies.
| Author | Year | Country | Detection Method/Anticoagulant |
Breast cancer patients | Benign controls | Healthy controls | TNM stage I-II | TNM stage III-IV | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Blackwell[18] | 2000 | USA | ELISA/sodium citrate | 95 | NR | NR | 69 | 26 | 7 |
| Hua[19] | 2004 | China | ELISA/ethylenediamine tetra-acetic acid | 51 | 10 | 42 | 40 | 11 | 7 |
| Kim[20] | 2004 | Korea | ITM/sodium citrate | 93 | 27 | 29 | 77 | 10 | 8 |
| Khangarot[21] | 2010 | India | ELISA/NR | 50 | NR | NR | 20 | 30 | 6 |
| Zhao[22] | 2011 | China | ITM/NR | 43 | 43 | 43 | 32 | 11 | 7 |
| Xie[23] | 2011 | China | ITM/sodium citrate | 95 | 80 | NR | 58 | 37 | 7 |
| Huang[24] | 2012 | China | ITM/sodium citrate | 149 | 89 | 82 | 87 | 62 | 8 |
| Zhou[25] | 2012 | China | ELISA/NR | 48 | 40 | 40 | 36 | 12 | 7 |
| Liu[26] | 2013 | China | ITM/sodium citrate | 142 | NR | 150 | NR | NR | 7 |
| Chaari[27] | 2014 | France | ELFA/sodium citrate | 62 | NR | 30 | NR | NR | 6 |
| Yang[28] | 2014 | China | ITM/sodium citrate | 59 | NR | 50 | 29 | 31 | 7 |
| Feng[29] | 2014 | China | ELFA/NR | 189 | NR | NR | 95 | 94 | 7 |
| Chai[30] | 2015 | China | ITM/sodium citrate | 73 | 36 | 50 | NR | NR | 7 |
| Bai[31] | 2017 | China | ITM/sodium citrate | 35 | 37 | NR | NR | NR | 5 |
| S.H.[32] | 2018 | India | ITM/sodium citrate | 60 | NR | NR | 40 | 20 | 7 |
ITM: immunoturbidimetry; ELISA: enzyme-linked immunosorbentassay; ELFA: enzyme-linked immunofluorescence assay; NOS: Newcastle-Ottawa Scale; NR: not reported
Outcomes
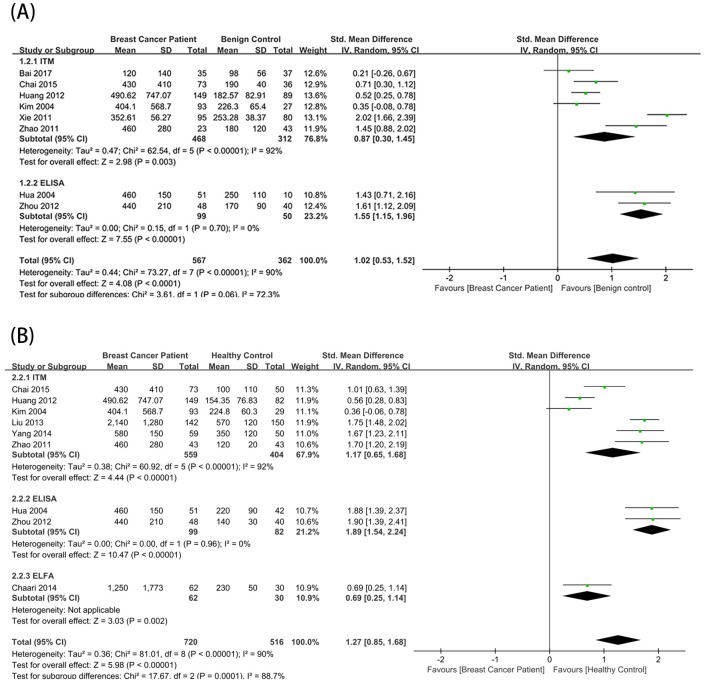
We first compared the breast and benign control groups. The benign control groups from 8 studies were stratified using the D-dimer test. The total effect rate showed that the D-dimer level was higher in the breast cancer group (SMD = 1.02; 95% confidence interval [CI] = 0.53–1.52; P < 0.0001)(Fig 2A).
Fig 2. Relationship between D-dimer levels and breast cancer diagnosis.
Forest plots depicting comparisons between breast cancer patients and (A) benign controls and (B) healthy controls. SD: standard deviation; CI: confidence interval; ELISA: enzyme-linked immunosorbent assay; ITM: immunoturbidimetry; ELFA: enzyme-linked immunofluorescence assay.
We next compared the breast and healthy control groups. After stratification using the D-dimer test, nine articles evaluating a healthy control group were divided into three subgroups. Using a random effects model, the total effect rate showed that the D-dimer level was significantly higher in the breast cancer group (SMD = 1.27; 95% CI = 0.85–1.68; P < 0.00001) (Fig 2B).
We also examined the correlation between D-dimer levels and clinical pathological parameters of breast cancer. Four studies examined the relationship between D-dimer levels and progesterone receptor (PR) expression, and there was no significant heterogeneity (P = 0.38, I2 = 3%) (Fig 3A). Using a fixed effects model, we observed that elevated D-dimer levels were associated with PR-negative tumors (SMD = -0.25; 95% CI = -0.44–-0.05; P = 0.01). There was also a significant correlation between D-dimer levels and TNM stage (n = 11, SMD = 0.82; 95% CI = 0.57–1.06; P < 0.00001) and lymph node involvement(n = 8, SMD = 0.79; 95% CI = 0.50–1.09, P < 0.00001) (Fig 3B and 3C). Here, we used a random effects model and combined subgroup analyses due to significant heterogeneity (TNM stage: P = 0.005, I2 = 60%; lymph node involvement: P = 0.0003, I2 = 74%). In contrast, other clinicopathological factors were not associated with D-dimer levels, including estrogen receptor (ER) expression (n = 4, SMD = -0.28; 95% CI = -0.62–0.07; P = 0.12) and HER2 expression (n = 3, SMD = -0.21; 95% CI = -0.42–0.00; P = 0.05) (Fig 3D and 3E). Due to the heterogeneity, the correlation between D-dimer levels and ER (P = 0.05, I2 = 62%) was based on a random effects model, and the correlation between D-dimer levels and HER2 (P = 0.62, I2 = 0%) used a fixed effects model.
Fig 3. Relationship between D-dimer levels and clinicopathological characteristics of breast cancer.
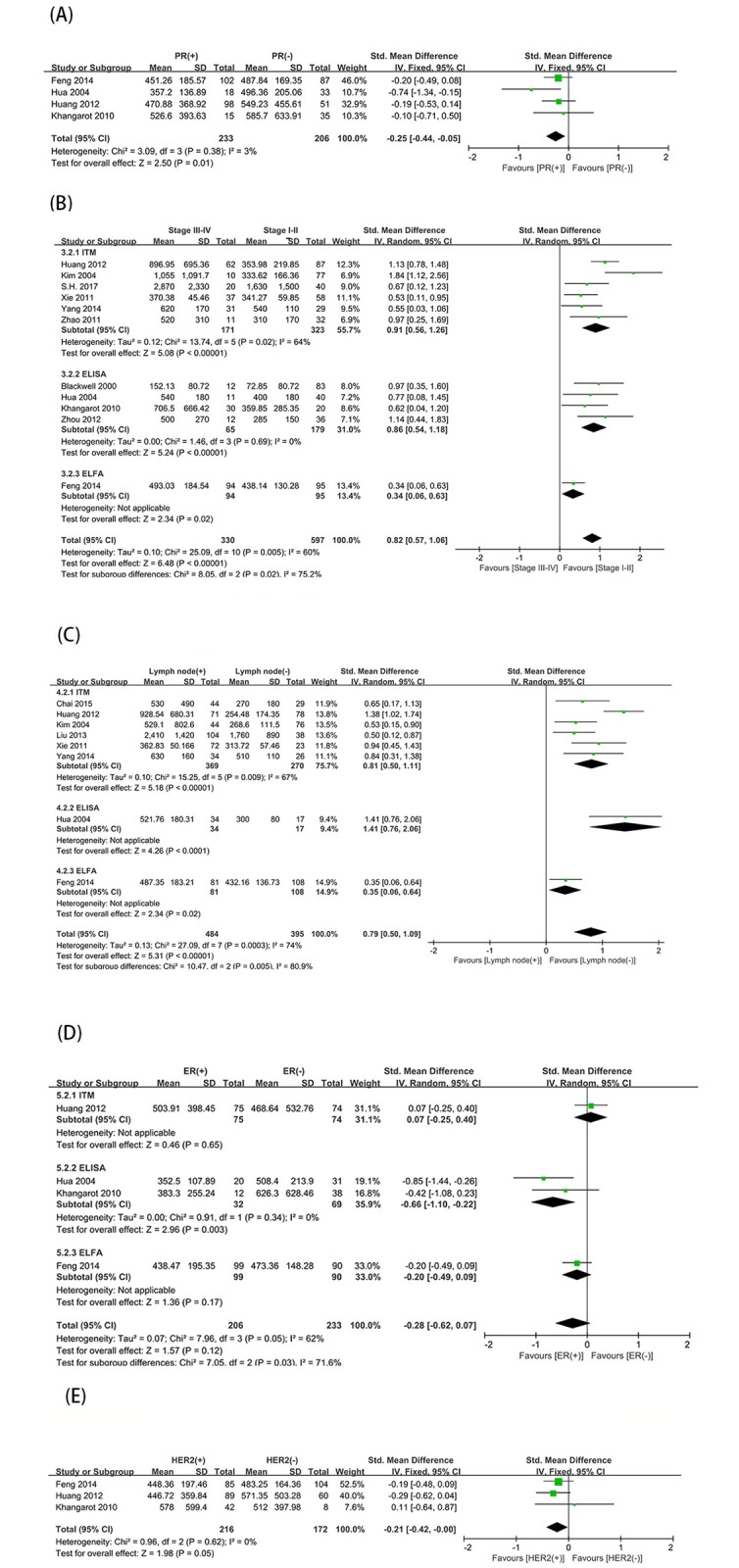
Forest plots of SMDs for the association between D-dimer and (A) progesterone receptor (PR) status (positive vs. negative), (B) tumor node metastasis (TNM) stage (stage III-IV vs. stage I-II), (C) lymph node status (positive vs. negative),(D)estrogen receptor (ER) status (positive vs. negative), and (E) human epidermal growth factor receptor (HER2) status (positive vs. negative). SMD: standardized mean difference; SD: standard deviation; CI: confidence interval.
Heterogeneity
As shown in Fig 3, the subgroup analysis based on the differences in D-dimer detection methods found significant differences between the subgroups (benign controls, I2 = 72.3%; healthy controls, I2 = 88.7%; TNM, I2 = 75.2%; lymph node status, I2 = 80.9%; ER, I2 = 71.6%).
Additionally, most of the literature was obtained from China, and the sample sizes were smaller in other countries. The subgroup analysis was also used to examine the source of heterogeneity based on region. In addition to the benign control group (I2 = 79.2%) (Fig 4A), the results showed that there were no significant differences between the subgroups of the other groups with significant heterogeneity. (Fig 4B, 4C, 4D and 4E).
Fig 4. Subgroup analysis of D-dimer levels and breast cancer-associated differential diagnosis and clinicopathological features according to region.
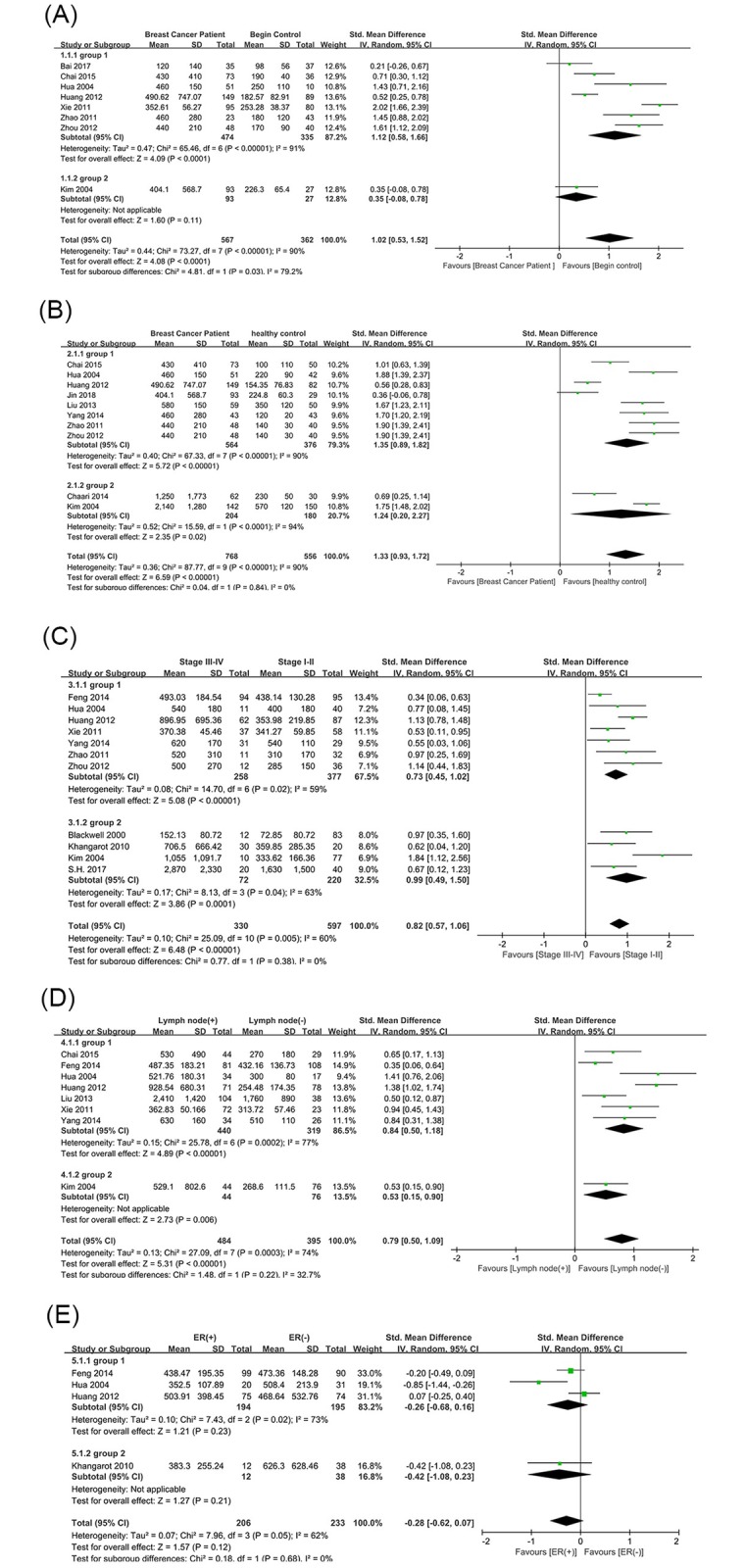
Plots depicting comparisons between breast cancer patients and (A) benign controls, (B) healthy controls, (C) tumor node metastasis (TNM) stage (stage III-IV vs. stage I-II), (D) lymph node status (positive vs. negative), and (E)estrogen receptor (ER) status (positive vs. negative). Group1: China; Group2: Regions outside China.
Publication bias and sensitivity analysis
The symmetry of the funnel plot and results of the Egger’s test (benign controls, P = 0.470; healthy controls, P = 0.545; TNM, P = 0.093; lymph node status, P = 0.204; PR, P = 0.495; ER, P = 0.272; HER2, P = 0.408) indicated that there was no publication bias. Sensitivity analysis was used to test the effect of a single study on the results. No significant differences were found when we removed any of the studies included in the analysis, indicating that the conclusions were stable.
Discussion
To the best of our knowledge, this is the first meta-analysis on the role of D-dimer in the differential diagnosis and clinicopathological characteristics of breast cancer. As early as 1991, Mitter[33] found that D-dimer levels were elevated in patients with breast cancer. With the deepening of research in recent years, more links between D-dimer and the clinical pathology of breast cancer have been proposed.
The role of D-dimer in the differential diagnosis of breast cancer
The results showed that the D-dimer level in the breast cancer group was significantly higher than those in the benign and healthy control groups. Increased plasma D-dimer levels reflect increased activation of the coagulation system in patients with breast cancer, suggesting that the plasma D-dimer level could have an auxiliary value for the differential diagnosis of breast cancer. Studies have shown that the sensitivity and specificity of D-dimer is higher than that of the existing tumor markers cancer antigen 15–3 and carcinoembryonic antigen [34]. Unfortunately, most of the research data did not allow to calculate the sensitivity and specificity of the effect indicator of D-dimer level for the diagnosis of breast cancer.
The relationship between D-dimer and clinical pathology of breast cancer
Despite advances in breast cancer treatment, patients with metastatic breast cancer have a poor prognosis, with a low median survival of at most 2 to 3 years [2]. Plasma D-dimer levels in patients with TNM stage III-IV disease were significantly different from those in patients with stage I-II disease. Plasma D-dimer levels were also significantly higher in patients with lymph node metastasis than in patients without metastasis. Elevated D-dimer levels suggest a worsening of the disease, a later clinical stage, and a greater likelihood of tumor metastasis. The plasma D-dimer levels can be used as an auxiliary index for the diagnosis and staging of breast cancer. Furthermore, in this study, the D-dimer level was not related to the ER or HER2 status of patients with breast cancer, and it was increased in patients with PR-negative tumors. Due to the limitations of the literature, the role of D-dimer in the clinical pathology and prediction of prognosis of breast cancer still needs to be studied in a large number of patients.
Limitations
The existence of heterogeneity is a potential problem when interpreting the results of this meta-analysis. To this end, we performed a subgroup analysis based on the differences in D-dimer detection methods. The results indicated that the difference in D-dimer detection methods is one of the main sources of heterogeneity. Because our meta-analysis is based on published research, the fact that most of the data coming from China may lead to regional bias. Therefore, the subgroup analysis was also used to examine the source of heterogeneity based on region with only significant differences in the benign control group. However, after excluding the study by Kim et al.[20] of Korea from the benign control group, the heterogeneity between the eight studies from China did not reduce, indicating that the regional differences cannot explain the heterogeneity between benign control groups. In addition, there may be other sources of heterogeneity. For example, this meta-analysis only included English and Chinese literature, which leads to language bias. Fortunately, although heterogeneity existed, the sensitivity analysis was stable, and no publication bias was found.
At present, there is no uniform standard for the methods and units used to detect D-dimer levels, and the consistency between the results of the same test items in each laboratory is not strong. In this paper, the unified D-dimer unit was ng/mL, and the standardized mean difference was used as the effect combination index. However, inconsistent detection methods, reagents and type of anticoagulant may cause the absolute D-dimer value to differ greatly, leading to high heterogeneity among the literature results. Therefore, a uniform methodological standard should be established for D-dimer detection so that the data between different laboratories can be interoperable or comparable.
Conclusion
In this meta-analysis, plasma D-dimer levels were elevated in patients with breast cancer and correlated with PR expression, TNM stage, and metastasis in breast cancer. This evidence suggests that D-dimer has potential in the differential diagnosis and staging of breast cancer. However, the current results are somewhat restrictive, and we recommend further big data research and development of unified D-dimer detection methods in multiple regions.
Supporting information
(DOCX)
(XLSX)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer Journal for Clinicians. 2019; 69(1): 7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Cabuk D, Basaran G, Teomete M, Dane F, Korkmaz T, Seber S, et al. Clinical outcome of Turkish metastatic breast cancer patients with currently available treatment modalities—single center experience. Asian Pacific journal of cancer prevention: APJCP. 2014; 15(1): 117–22. 10.7314/apjcp.2014.15.1.117 . [DOI] [PubMed] [Google Scholar]
- 3.Wu TY, Lee J. Promoting Breast Cancer Awareness and Screening Practices for Early Detection in Low-Resource Settings. 2019; 15(1): 18–25. 10.5152/ejbh.2018.4305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosaka Y, Minatani N, Tanaka Y, Shida A, Kikuchi M, Nishimiya H, et al. Lymph node metastasis and high serum CEA are important prognostic factors in hormone receptor positive and HER2 negative breast cancer. Molecular and clinical oncology. 2018; 9(5): 566–74. 10.3892/mco.2018.1716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shering SG, Sherry F, McDermott EW, O’Higgins NJ, Duffy MJ. Preoperative CA 15–3 concentrations predict outcome of patients with breast carcinoma. Cancer. 1998; 83(12): 2521–7. . [PubMed] [Google Scholar]
- 6.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. The Lancet Oncology. 2005; 6(6): 401–10. 10.1016/S1470-2045(05)70207-2 . [DOI] [PubMed] [Google Scholar]
- 7.Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best practice & research Clinical haematology. 2009; 22(1): 49–60. 10.1016/j.beha.2008.12.009 . [DOI] [PubMed] [Google Scholar]
- 8.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005; 105(1): 178–85. 10.1182/blood-2004-06-2272 . [DOI] [PubMed] [Google Scholar]
- 9.Ryu SH, Min SW, Kim JH, Jeong HJ, Kim GC, Kim DK, et al. Diagnostic Significance of Fibrin Degradation Products and D-Dimer in Patients With Breast Cancer-Related Lymphedema. Annals of rehabilitation medicine. 2019; 43(1): 81–6. 10.5535/arm.2019.43.1.81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Japanese journal of clinical oncology. 2001; 31(8): 388–94. 10.1093/jjco/hye075 . [DOI] [PubMed] [Google Scholar]
- 11.Inal T, Anar C, Polat G, Unsal I, Halilcolar H. The prognostic value of D-dimer in lung cancer. The clinical respiratory journal. 2015; 9(3): 305–13. 10.1111/crj.12144 . [DOI] [PubMed] [Google Scholar]
- 12.Stender MT, Larsen AC, Sall M, Thorlacius-Ussing O. D-Dimer predicts prognosis and non-resectability in patients with pancreatic cancer: a prospective cohort study. Blood Coagul Fibrinolysis. 2016; 27(5): 597–601. 10.1097/MBC.0000000000000559 . [DOI] [PubMed] [Google Scholar]
- 13.Zsuzsanna N. Biomarkers in solid tumors. Magyar Onkologia. 2013; 57(1): 56–62. [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010; 25(9): 603–5. 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP. Cochrane handbook for systematic reviews of interventions version 5.0. 1. The Cochrane Collaboration. http://www.cochrane-handbook.org. 2008.
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary clinical trials. 2015; 45(Pt A): 139–45. 10.1016/j.cct.2015.09.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997; 315(7109): 629–34. 10.1136/bmj.315.7109.629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell K, Haroon Z, Broadwater G, Berry D, Harris L, Iglehart JD, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000; 18(3): 600–8. 10.1200/JCO.2000.18.3.600 [DOI] [PubMed] [Google Scholar]
- 19.Hua D. Clinical Value of Determining of plasma D-dimer in patients with breast cancer. Chinese Modern Medicine. 2004; 2(7). [Google Scholar]
- 20.Kim HK, Song KS, Lee KR, Kang YH, Lee YJ, Lee ES. Comparison of plasma D-dimer and thrombus precursor protein in patients with operable breast cancer as a potential predictor of lymph node metastasis. Blood Coagul Fibrinolysis. 2004; 15(1): 9–13. [DOI] [PubMed] [Google Scholar]
- 21.Khangarot SS, Gupta N, Goswami B, Hadke NS, Lal P, Gupta N, et al. Correlation of D dimer and factor VIII levels with histopathology in patients with breast carcinoma. Cancer Biomark. 2010; 7(6): 305–14. 10.3233/CBM-2010-0196 [DOI] [PubMed] [Google Scholar]
- 22.Zhao R. Correlation between plasma D-dimer levels and breast cancer. Journal of Modern laboratory medicine. 2011; 3(26). [Google Scholar]
- 23.XIE B, Xian W, Yidi H. The Level of Plasma D-dimer Pre-and Post-Treatment in Patients with Breast Cancer and its Clinical Significance. Journal of Chinese Oncology. 2011; 17(06): 469–70. [Google Scholar]
- 24.Huang Y, Chen J, Yu N, Song S. Clinical significance of plasma D-dimeride detection in patients with breast cancer. Clinical Misdiagnosis And Mistherapy. 2012; 25(09): 79–81. [Google Scholar]
- 25.Zhou Z. Clinical significance of plasma D-dimer level in diagnosis and prognosis of breast cancer. Pharmaceutical Biotechnology. 2012; 19(02): 162–4. [Google Scholar]
- 26.Liu Y, Liu H. Determination and clinical significance of CA153 and D-dimer in patients with primary breast cancer. China Health Industry. 2013; 10(23): 161+3. [Google Scholar]
- 27.Chaari M, Ayadi I, Rousseau A, Lefkou E, Van Dreden P, Sidibe F, et al. Impact of breast cancer stage, time from diagnosis and chemotherapy on plasma and cellular biomarkers of hypercoagulability. BMC Cancer. 2014; 14: 991 10.1186/1471-2407-14-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang P. Clinical significance of fibrinogen, D-dimer and antithrombin-III in patients with breast cancer. J Clin Hematol (China). 2014; 27(01): 106–7+9. [Google Scholar]
- 29.Feng H, Cui l, Li z, Han y, Zhang p. Relationship between plasma D-D level and clinical pathologic characterstics in breast cancer. Shandong Medical Journal. 2014; 54(42): 8–10. [Google Scholar]
- 30.CHAI Y, WANG J, LIU B. Plasma D-dimer levels in breast cancer patients correlate with axillary lymph node status. J Clin Pathol Res. 2015; 35(07): 1323–8. [Google Scholar]
- 31.Bai Y, Shuai S, Li X. Relationship between coagulation parameters and clinicopathological features in patients with breast cancer. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine. 2017; 17(10): 110–1. [Google Scholar]
- 32.S H, Sringeri RR, Chandra PS. Role of Plasma D-Dimer Levels in Breast Cancer Patients and Its Correlation with Clinical and Histopathological Stage. Indian J Surg Oncol. 2018; 9(3): 307–11. 10.1007/s13193-017-0682-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitter CG, Zielinski CC. Plasma levels of D-dimer: a crosslinked fibrin-degradation product in female breast cancer. Journal of cancer research and clinical oncology. 1991; 117(3): 259–62. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neises M, Schafer T, Strittmatter HJ, Wischnik A, Dettmar P, Melchert F. [D-dimer and plasminogen activator of the urokinase type: personal experiences with breast cancer]. Geburtshilfe und Frauenheilkunde. 1993; 53(7): 455–60. 10.1055/s-2007-1022913 . [DOI] [PubMed] [Google Scholar]