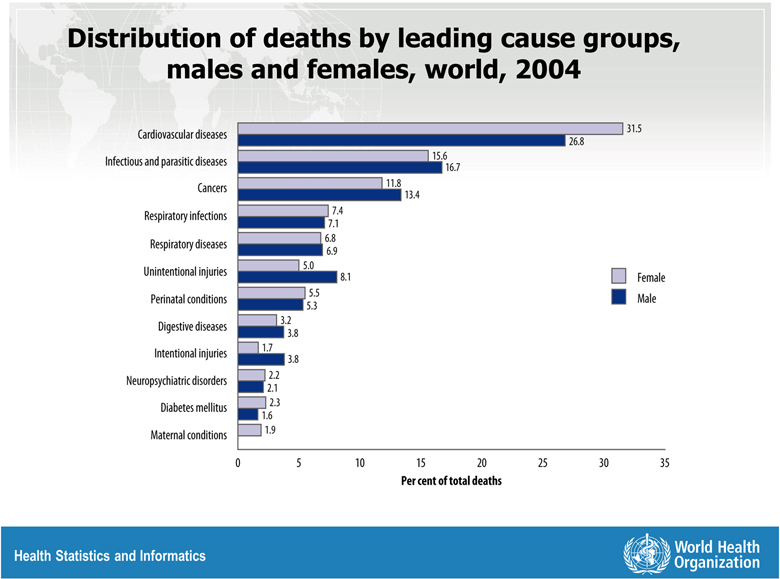
It has become evident that there are sex-differences in coronary heart disease (CHD) presentation, treatment, and outcomes, and there is excessive CHD mortality in women compared to men now observed worldwide (Figure 1)1. Given the high global burden of CHD, it is imperative to gain an understanding of sex-differences in risk factor control in contemporary practices in various regions of the world. Zhao et al2 report on risk factor management for secondary prevention of CHD in three regions (Europe, Asia, and the Middle East) from 2012–2013 in the SUrvey of Risk Factors (SURF) study. They found that risk factor management is less in women than men, and that sex differences varied by region. Overall cardiovascular health index score (CHIS), age-adjusted, was better in Asia, and modestly worse in Europe and Middle East in women compared to men. The SURF extends existing literature on sex-differences in CHD risk factor control to women in Asia and the Middle East, although the results may not be representative of a region. For instance, Saudi Arabia alone represented the Middle East, while 60% overall of participants were from Europe. Nevertheless, this study documents the need for region/country specific data to shape specific policy and priorities.
Figure 1.
Distribution of deaths by leading cause groups, males and females, world, 2004
Women comprised less than one-third of the participants in the current registry, comparable to other international registries of stable and unstable ischemic heart disease (IHD), despite the fact that the absolute number of deaths in due to cardiovascular disease (CVD) is higher in women compared to men1. The “sex-death-diagnosis gap” may be attributable to several factors. The current registry results included patients only with “objectively confirmed” CHD, yet women are more likely to be under-diagnosed with current male-pattern diagnostic criteria3. New classifications that incorporates IHD phenotypes more common to women is warranted to address this gap, including stroke as a major contributor to CVD mortality in women worldwide4.
Further phenotyping demonstrated that stable angina and acute coronary syndrome (ACS) was dominant (73%) in women compared to Coronary Artery Bypass Grafting (CABG) and Percutaneous Coronary Intervention (PCI) dominance (72%) in men. While angiographic obstructive Coronary Artery Disease (CAD) was not reported, these phenotypic sex differences suggest differences in obstructive CAD pertinent to revascularization. Indeed, prior data demonstrate that women have more ischemia with no obstructive CAD (INOCA), which is associated with elevated major adverse cardiac events at 5–7 year follow-up5. These combined lines of evidence support that the acronym “CHD”, commonly understood as obstructive CAD that impacts the myocardium, should be replaced with “IHD” to include the multiple mechanisms that contribute to adverse outcomes, including anatomical atherosclerosis and functional coronary vasomotor dysfunction. Currently, there are no specific evidence-based management guidelines for INOCA subjects, contributing to the relatively low use of optimal medical therapy in women. Research to address knowledge gaps in phenotype, pathogenesis and clinical trials of existing and novel therapies is needed.
Geographical and sex variability of body mass index (BMI) and unadjusted waist circumference was reported, with an average overweight status and range that included obesity (mean BMI of 28±5). Overweight and obesity variables are not well understood as IHD risk factors, as attested by the “obesity paradox”, and the lack of inclusion in primary or secondary prevention risk scores. It is therefore difficult to know what to do with these international data with regard to sex differences in risk factor management. More detailed phenotyping of body composition known to differ by sex6 relative to IHD outcome is needed to understand if these variables are justified as treatment targets.
Notably, in this international registry, women universally reported more “adequate” physical inactivity compared to men, in contrast to prior gender-biased questionnaire surveys where domestic activities such as cooking, cleaning and childcare were not included and women appeared more sedentary. Sex-specific work in women has demonstrated that CHD incidence decreased across increasing levels of activity. Most accurately measured exercise capacity, also known as physical fitness, is a strong independent predictor of all-cause mortality in women using a sex-specific normative value7. The risk of death among asymptomatic and symptomatic women whose exercise capacity was less than 85% of the predicted value for age and sex was at least twice that of women whose exercise capacity was at least 85% of their age-predicted value7. Physical inactivity or poor physical fitness are appropriately guidelines criteria for identifying women “at risk” when sex-specific tools are used.
Current international cardiovascular guidelines recommend the use of statin for secondary prevention of IHD irrespective of age and sex8. Despite this, achievement of treatment targets for total cholesterol and low-density lipoprotein (LDL) in this study were 34–76% lower in women than men and varied by country; only 50% of women in Asia were treated with statin therapy. The failure to receive evidence-based therapies in women is sadly a recurrent theme increasingly documented worldwide. Young women (<45 years) are less likely to be told they are at risk and treated prior a myocardial infarction, but are more often told to lose weight, compared to men6. In the current registry, younger women (<65 years) were less likely to meet goals as opposed to older women., however, the mean age of the women was 67.5±10.9 years, well past the age of menopause, suggesting these age-disparities sustain into mid-life when reproduction concerns are no longer relevant.
While women in the registry were less likely to achieve cholesterol targets, they were 1.3 times more likely to achieve target blood pressure, achieved in both older and younger women, despite a higher prevalence of hypertension (80.8% vs 71.9%) and higher systolic blood pressure (SBP) (133.7 vs 130.5 mmHg) in women compared to men., respectively This finding of superior blood pressure control despite higher numbers in women is difficult to explain, but suggests that there may be sex differences in antihypertensive selection and dosing, medication response, or medication compliance. Men were more likely to be prescribed beta-blockers and angiotensin converting enzyme inhibitors, while women were more likely to be prescribed calcium channel blockers and angiotensin renin blockers. In addition, while traditional risk factors were evaluated, history of pregnancy-related hypertensive disorders was not assessed in the women, potentially a missed opportunity for sex-specific risk stratification in women9.
In summary, the current registry adds important international data regarding sex differences in IHD risk factor management. Knowledge gaps remain, which can be addressed by careful phenotyping of the increasingly available digital medical records and ambulatory monitoring technology, including proteomics, metabolomics, and genomics. Investigation addressing if large sex differences in risk factor management are due to lower treatment of women/higher treatment of men (USA and Europe), vs if lower sex differences are due to lower treatment of both women and men (Asia and Middle East). The need for country and region-specific IHD data stratified by sex is needed to optimize personalized medicine (Figure 2)10.
Figure 2.
Sex- and gender-specific medicine is the most ready-for-translation approach among the genomic, proteomic, and metabolomic personalized medicine approaches
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, K23-HL127262-01A1, K23-HL125941-01A1, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR000064, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., The Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California.
References
- 1.Department WHOHSaI. Global Burden of Disease 2004 Update: Selected figures and tables. 2004.
- 2.Zhao M, Cooney MT, Klipstein-Grobusch K, Vaartjes I, De Bacquer D, De Sutter J, Reiner Z, Prescott E, Faggiano P, Vanuzzo D, AlFaleh H, Menown IB, Gait D, Posogova N, Sheu WH, Zhao D, Zuo H, Grobbee DE and Graham IM. Simplifying the audit of risk factor recording and control: A report from an international study in 11 countries. European journal of preventive cardiology. 2016;23:1202–10. [DOI] [PubMed] [Google Scholar]
- 3.Bairey Merz CN. Sex, death, and the diagnosis gap. Circulation. 2014;130:740–2. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PK, Minissian M and Bairey Merz CN. Stroke in women: Where are we in 2015? Trends in cardiovascular medicine. 2016;26:89–91. [DOI] [PubMed] [Google Scholar]
- 5.Bairey Merz CN, Pepine CJ, Walsh MN and Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bairey Merz CN, Andersen HS and Shufelt CL. Gender, Cardiovascular Disease, and the Sexism of Obesity. J Am Coll Cardiol. 2015;66:1958–60. [DOI] [PubMed] [Google Scholar]
- 7.Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, Marwick TH, Pandey DK, Wicklund RH and Thisted RA. The prognostic value of a nomogram for exercise capacity in women. The New England journal of medicine. 2005;353:468–75. [DOI] [PubMed] [Google Scholar]
- 8.Morris PB, Ballantyne CM, Birtcher KK, Dunn SP and Urbina EM. Review of Clinical Practice Guidelines for the Management of LDL-Related Risk. Journal of the American College of Cardiology. 2014;64:196–206. [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Minissian M and Bairey Merz CN. Pregnancy outcomes, reproductive history and cardiovascular disease risk in women: What do we know and what is needed? European journal of preventive cardiology. 2016;23:1860–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bairey Merz CN and Regitz-Zagrosek V. The case for sex- and gender-specific medicine. JAMA internal medicine. 2014;174:1348–9. [DOI] [PubMed] [Google Scholar]