Abstract
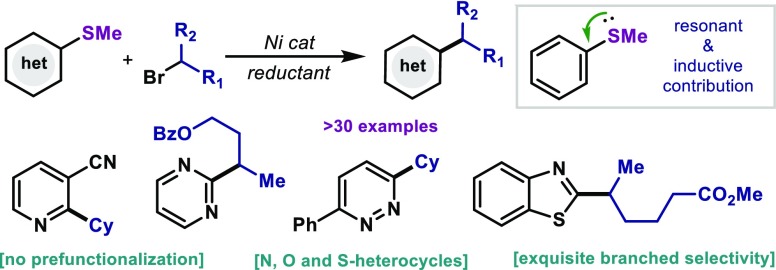
Herein we present a Ni-catalyzed alkylation of C–SMe with alkyl bromides for the decoration of heterocyclic frameworks. The protocol, reminiscent to the Liebeskind–Srogl coupling, makes use of simple C(sp2)–SMe to be engaged in a reductive coupling. The reaction is suitable for a preponderance of highly valuable heterocyclic motifs. In addition to cyclic bromides, noncyclic alkyl bromides are well accommodated with exquisite levels of retention over isomerization. The protocol is scalable and permits orthogonal couplings in the presence of other functionalization handles.
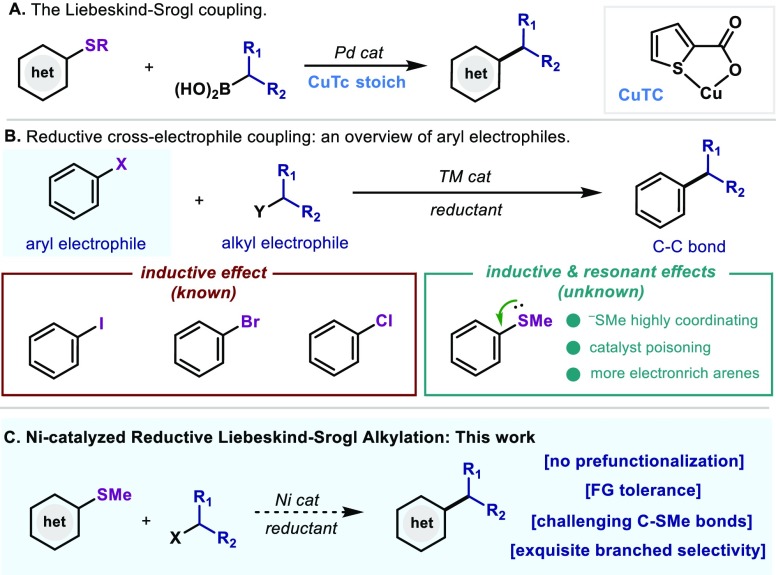
The functionalization of heterocycles via cross-coupling has become a powerful tool toward the diversification of biologically active compounds.1 In this context, the vast majority of electrophiles utilized rely primarily on the use of a heteroaryl halide as coupling partner due to its large availability.2 Nevertheless, in certain occasions, issues arising from stability and fast hydrolysis rates of heteroaryl halides had led to a reconsideration of such couplings and alternatives have been investigated.3 In this sense, the venerable Liebeskind–Srogl (L–S) coupling opened the door to the use of robust and stable C–SMe bonds as handles for C(sp2)–C(sp3) coupling utilizing boronic acids (Figure 1A).4 This approach has proven highly versatile in the derivatization of heteroaromatic groups as well as thioester derivatives.5 The widespread presence of thioether as modification handles has led to the development of a wide variety of cross-coupling strategies with a breadth of different organometallic reagents.6,7 Indeed, the majority of reported methods require the use of a prefunctionalized alkyl nucleophile, thus requiring several steps of synthesis for its preparation and, in some instances, the tolerance of functionality becomes a synthetic hurdle. Hence, alternatives to efficiently forge such bonds in a straightforward fashion would be highly desirable.
Figure 1.
(A) The Liebeskind–Srogl reaction. (B) Overview of the aryl counterparts in cross-electrophile coupling. (C) Ni-catalyzed reductive L–S alkylation of heteroaromatic thioethers.
Recently, reductive cross-couplings between two electrophiles have arisen as powerful, simple and practical strategies to circumvent the preparation of reactive organometallic reagents (Figure 1B).8 Albeit a plethora of methodologies have been reported in this area, the vast majority have focused on the use of aryl halides or pseudohalides where the C(sp2)–X bond is polarized due to the electronegative nature of the X element (inductive effect).9 On the other hand, reductive couplings with C(sp2)–X, where X is electronically contributing to the aryl ring via additional resonance effects through the lone pair pose a significant challenge and still remain elusive.10 Contrarily to the activation of simple aryl (pseudo)halides, C(sp2)–SMe bonds require highly nucleophilic catalysts for its activation. This results in chemoselectivity issues arising from the alkyl halide counterpart ultimately leading to undesired side-reactivity. Consequently, a fine compromise between reactivity and selectivity is crucial if this reductive coupling is to be realized. Additionally, the tendency of SMe anions to tightly bind to metal centers also poses a potential hurdle for achieving catalytic turnover without poisoning the metal catalyst.11 As part of our program on developing catalytic strategies for the modification of heterocyclic frameworks,12 we envisaged that a catalytic reductive cross-coupling between heteroaromatic thioethers and simple alkyl bromides would be highly beneficial for synthetic purposes.13 Herein, we report a practical and efficient Ni-catalyzed protocol based on the activation of C–SMe bonds, which are primed for reductive cross-coupling with a variety of secondary alkyl bromides (Scheme 1C). The method is characterized by the presence of a variety of aromatic heterocycles, thus permitting rapid decoration of pharmaceutically relevant scaffolds. The utility of this protocol is demonstrated by the facile scalability to gram-scale and the sequential modification of a heterocyclic framework via orthogonal couplings.
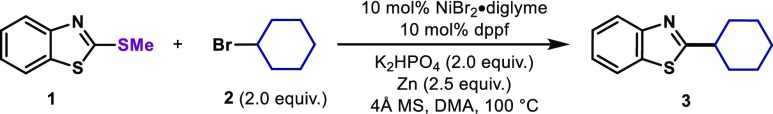
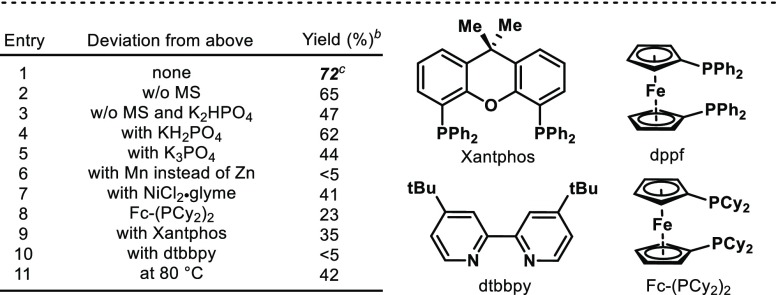
On the basis of their great catalytic activity in reductive cross-electrophile couplings, we started our investigations exploring the use of a Ni catalyst in the presence of a reducing agent. Initially, thiomethyl ether 1 and CyBr (2) were used as model substrates for the coupling.14 We anticipated that an electron-rich ligand for the Ni would be necessary for the activation of the strong C–SMe bond. Indeed, catalytic amounts of NiBr2·diglyme and dppf in the presence of Zn (2.5 equiv) and K2HPO4 (2.0 equiv) afforded the desired C–C product 3 in 72% isolated yield (Table 1, entry 1).
Table 1. Optimization of the Reactiona.
1 (1 equiv, 0.2 mmol), 2(2.0 equiv), NiBr2·diglyme (10 mol %), dppf (10 mol %), K2HPO4 (2.0 equiv), Zn (2.5 equiv), 4 Å MS in DMA (0.6 mL) at 100 °C, 6 h.
Yields calculated by GC-FID using dodecane as internal standard.
Isolated yield.
As highlighted in the optimization studies, omission of molecular sieves led to a slight decrease on the yield (Table 1, entry 2). Moreover, the basicity of the additive utilized seemed a crucial element as demonstrated by the lower yields obtained when tri- or monobasic phosphates of potassium were used (entries 4 and 5). Interestingly, the use of Mn in place of Zn as reducing agent completely suppressed the reactivity (entry 6). Other NiCl2 salts bearing glyme instead of diglyme also led to lower conversion and yield (entry 7). The low yields of 3 obtained when using Fc-P(Cy2)2 highlight the crucial electronic aspects of dppf for successful catalysis (entry 8). The use of a bidentate phosphine with a wider bite angle such as Xantphos also afforded the desired product 3 albeit in much lower yields, thus suggesting the need for a rigid cis-chelating phosphine (entry 9). As anticipated, the use of commonly utilized dinitrogenated ligands such as dtbpy led to traces of C–C formation (entry 10).8 Finally, lower temperatures reduced dramatically the amount of cross-coupling product (entry 11).
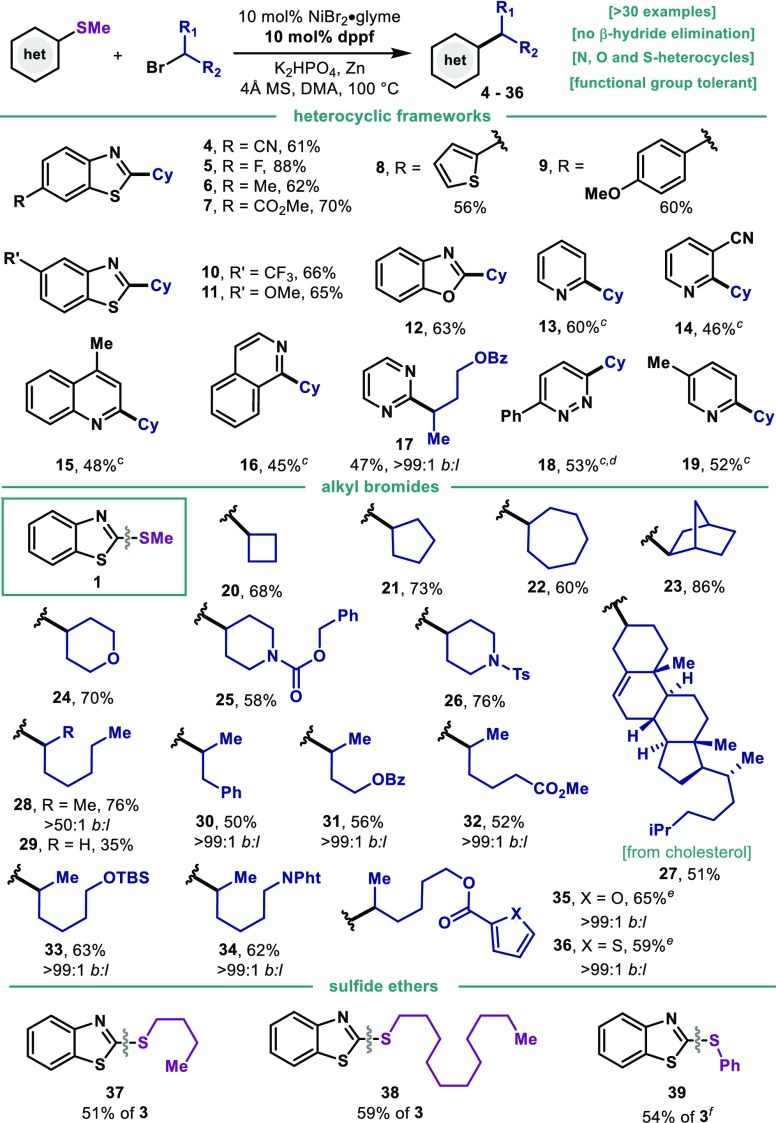
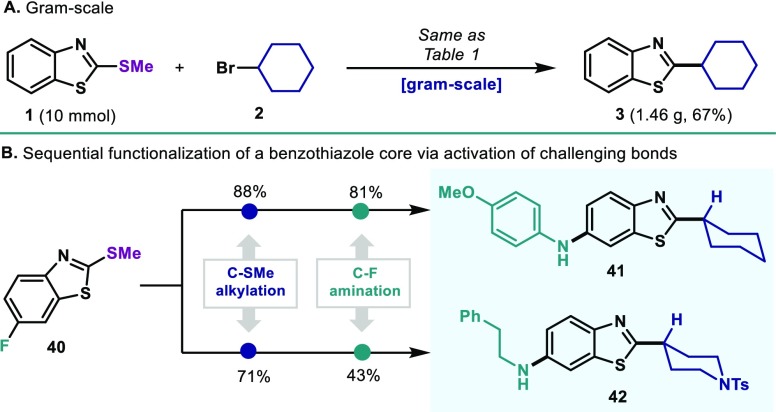
As shown in Table 2, the protocol was optimal for the coupling of benzothiazole derivatives bearing a variety of functionalities: nitriles (4), fluorides, (5), alkyl (6), esters (7), ethers (9, 11), trifluoromethyl (10) and thiophene groups (8) were all well accommodated. Ni salts have been shown to activate anisole and arylfluoride derivatives at high temperatures.15 However, no activation of the C–F (5) or C–OMe (9, 11) was observed and could serve as points for further derivatization (vide infra). Moreover, the reaction could be expanded to benzoxazole thioethers as exemplified by 12. With the aim of expanding the methodology to a wider chemical space, a variety of structurally distinct heterocyclic frameworks was surveyed. Gratifyingly, the protocol was applicable to 2-pyridines (13, 14, 19), 2-quinolines (15), 1-isoquinoline (16), 2-pyrimidine (17) and 3-pyridazine (18). The ability to forge C–C bonds in compounds bearing Lewis-basic N-containing motifs highlights the potential of the method when applied to complex drug-like settings. Compound 1 successfully coupled with a variety of alkyl bromides; 4-, 5- and 7- membered cycloalkyls (20–22) as well as bicyclic norbornyl (23) afforded good yields of coupling product. Heterocyclic bromides such as 4-tetrahydropyrane (24) and 4-piperidine (25 and 26) smoothly reacted in good yields. The polycyclic bromide derived from natural cholesterol was also amenable for coupling under the reaction conditions (27). At this point, we investigated the ability of the catalytic system to accommodate challenging open-chain secondary alkyl bromides. Catalytic systems featuring Ni in combination with bidentate electron-rich phosphines has traditionally led to deleterious isomerization events through degenerated Ni(II) intermediates, thus affording synthetically unviable branched and linear mixtures.16 However, despite the use of dppf as ligand, when 2-bromoheptane was subjected to the reaction conditions, branched product was obtained as single isomer (28). This observation points out to a mechanism involving different species than the canonical (dppf)Ni(II)(aryl)(alkyl) intermediate (vide infra). Despite the effort to accommodate primary alkyl bromides, their reactivity lead to substantially lower yields (29). A variety of noncyclic bromides was explored and revealed the possibility of coupling secondary bromides bearing aromatic groups (30), esters (32), protected alcohols (31, 33) and amines (34). Alkyl bromides bearing heterocyclic furan (35) and thiophene esters (36) smoothly coupled with good yields. Unfortunately, tertiary alkyl bromides could not be accommodated. The use of other thioethers bearing longer alkyl chains in place of Me also could be accommodated as highlighted by the reaction of 37 and 38. It is important to mention that when a bis-aryl thioether is utilized (39), high regioselectivity in the C–S cleavage event was observed in favor of the benzothiazole unit. The scalability of the process was investigated as exemplified in Figure 2A: at 10 mmol scale, 1 and 2 successfully afforded gram-quantities of the desired C–C bond with minimal erosion of the yield.
Table 2. Scope of the Reductive Liebeskind–Srogl Alkylationa,b.
Thioether (1 equiv, 0.2 mmol), alkyl bromide (2.0 equiv), NiBr2·diglyme (10 mol %), dppf (10 mol %), K2HPO4 (2.0 equiv), Zn (2.5 equiv), 4 Å MS, DMA (0.6 mL) at 100 °C, 6 h.
Isolated yields.
Alkyl bromide (3.0 equiv).
50 °C, 24 h.
12 h.
Traces of alkylation at Ph–S cleavage observed by GC-MS.
Figure 2.
(A) Scalability; (B) Decoration of the benzothiazole core via sequential activation of challenging bonds.
To test the translational potential of our method in pharmaceutically relevant contexts, we applied the reductive protocol to the modification of the benzothiazole core, a prevalent motif in a wide variety of biologically active compounds.17 As shown in Figure 2B, after successful reductive alkylation (88%), benzothiazole thioether 40 could be further modified at its C(sp2)–F through Sawamura’s Ni-catalyzed amination to afford 81% of the drug-like scaffold 41.18 In the same manner, piperidyl derivative selectively reacted with 40 to afford excellent yields of C–C coupling (71%). Subsequently, a more nucleophilic primary amine could also be incorporated through C–F amination to afford the complex target 42 in 43% without further optimization.
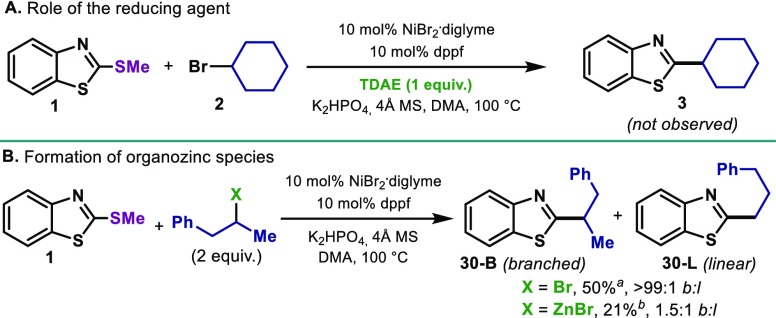
Intrigued by the high levels of retention over isomerization with the use of dppf, a series of mechanistic investigations were performed. Albeit the low reactivity of primary alkyl bromides, we conducted the coupling of cyclopropylmethyl bromide to explore possible ring-opening events (Figure 3A). Indeed, 43-D and 43-O were obtained in 1:2 ratio, respectively. This result suggests the involvement of carbon-centered alkyl radical species during the course of the reaction. Additionally, the presence of radical scavengers such as TEMPO and 1,1-diphenylethylene was also investigated (Figure 3B). Whereas the presence of 1 equiv of TEMPO led to a dramatic decrease in yield of 3, 2 equiv of TEMPO completely suppressed the reactivity. This inhibition could be the result of the interaction with Ni or Zn. However, 1,1-diphenylethylene was used instead, formation of product (3, 19%) was accompanied by the formation of 44 and 44-H2. Although these results might point out to noncage events of the alkyl radical, experiments with a 5-exo-trig cyclization suggest otherwise. If a radical-chain process is operating, the formation of uncyclized product should augment when increasing the amount of Ni.9f However, the ratio of uncyclized (46-U) and cyclized (46-C) cross-coupled product remained unaffected at higher concentrations of catalyst (ca. 1:1.5). Interestingly, when Zn was replaced by the common organic reducing agent TDAE (tetrakis(dimethylamino)ethylene), no product was obtained (Figure 4A). Due to the unique reactivity of Zn in this system, we speculated whether an organozinc reagent was formed in situ.19 Precedents in Pd-catalyzed Negishi aryl-alkyl cross-coupling clearly demonstrated a strong effect of the phosphine ligands in the isomerization of the nucleophile.16,20 Similarly, reports on the use of Ni as catalyst for Negishi couplings are restricted to di- or triamine based ligands to obtain high levels of selectivity.21
Figure 3.
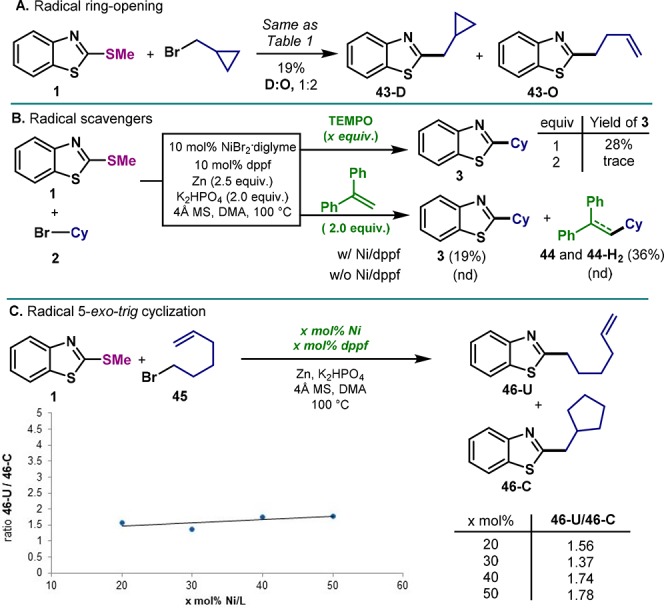
Mechanistic experiments. (A) Ring-opening of methylencyclopropyl radical; (B) Presence of radical scavengers; (C) Influence of [Ni] in radical cyclization.
Figure 4.
(A) Organic reducing agent. (B) Involvement of organozinc species. a2.5 equiv of Zn was added. bGC yield.
On the basis of these precedents, we speculated that in the event of forming a well-defined organozinc reagent during the reaction the ratios of branched and linear products should be highly dependent on the ancillary ligand used. To test this hypothesis, we subjected 1 to the coupling with (2-bromopropyl)benzene and the homologue zinc reagent in the presence of dppf ligand. Interestingly, the coupling of (2-bromopropyl)benzene under reductive conditions afforded exclusively the branched product 30-B independently of the phosphine (Figure 4B). On the contrary, the reaction of (1-phenylpropan-2-yl)zinc(II) bromide under the same conditions afforded mixtures of 30-B and 30-L(1.5:1), thus ruling out well-defined organozinc halides as intermediates. Taken together, these preliminary investigations suggest that the Ni is responsible for the radical formation and fast cage-rebound occurs.22 Additionally, the no-isomerization observed with open chain secondary centers point out to a fast reductive elimination from higher oxidation states of a Ni/phosphine complex.23 Efforts to elucidate these intriguing phosphine-Ni intermediate species are currently under investigation.
In summary, we have developed a Ni-catalyzed protocol for the direct alkylation of thiomethyl ethers (C(sp2)–SMe bonds) derived from heterocycles, which represent important handles commonly encountered in medicinal chemistry routes. This protocol has a wide substrate scope in both coupling partners and a high functional group tolerance. The high selectivity obtained toward the branched isomer with the use of simple dppf reveal interesting mechanistic scenarios which might differ from the canonical reductive cross-electrophile couplings. The successful coupling of strong C(sp2)–SMe bonds in cross-electrophile couplings opens the door to the use of other challenging partners with strong bonds to be included in the palette of electrophiles.
Acknowledgments
Financial support for this work was provided by Max-Planck-Gesellschaft, Max-Planck-Institut für Kohlenforschung and Fonds der Chemischen Industrie (FCI-VCI). We thank Prof. Dr. A. Fürstner for discussions and generous support. We also thank Elitenetzwerk Bayern (SYNCAT, fellowship J.C.).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b13534.
Experimental procedures and analytical data (1H, 19F and 13C NMR, HRMS) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a de Meijere A.; Bräse S.; Oestreich M.. Metal Catalyzed Cross-Coupling Reactions and More; Wiley-VCH: Weinheim, 2014. [Google Scholar]; b Patonay T.; Kónya K.. Synthesis and Modification of Heterocycles by Metal-Catalyzed Cross-coupling Reactions; Topics in Heterocyclic Chemistry; Springer International Publishing: Cham, 2016; Vol. 45. [Google Scholar]
- Petrone D. A.; Ye J.; Lautens M. Modern Transition-Metal-Catalyzed Carbon–Halogen Bond Formation. Chem. Rev. 2016, 116, 8003. 10.1021/acs.chemrev.6b00089. [DOI] [PubMed] [Google Scholar]
- Chandregowda V.; Rao G. V.; Reddy G. C. Improved Synthesis of Gefitinib and Erlotinib Hydrochloride-Anticancer Agents. Synth. Commun. 2007, 37, 3409. 10.1080/00397910701483761. [DOI] [Google Scholar]
- For seminal reports, see:; a Liebeskind L. S.; Srogl J. Thiol Ester-Boronic Acid Coupling. A Mechanistically Unprecedented and General Ketone Synthesis. J. Am. Chem. Soc. 2000, 122, 11260. 10.1021/ja005613q. [DOI] [Google Scholar]; b Yu Y.; Liebeskind L. S. Copper-Mediated, Palladium-Catalyzed Coupling of Thiol Esters with Aliphatic Organoboron Reagents. J. Org. Chem. 2004, 69, 3554. 10.1021/jo049964p. [DOI] [PubMed] [Google Scholar]; c Liebeskind L. S.; Srogl J. Heteroaromatic Thioether-Boronic Acid Cross-Coupling under Neutral Conditions. Org. Lett. 2002, 4, 979. 10.1021/ol0200091. [DOI] [PubMed] [Google Scholar]
- For reviews, see:; a Prokopcová H.; Kappe C. O. The Liebeskind–Srogl C–C Cross-Coupling Reaction. Angew. Chem., Int. Ed. 2009, 48, 2276. 10.1002/anie.200802842. [DOI] [PubMed] [Google Scholar]; b Cheng H.-G.; Chen H.; Liu Y.; Zhou Q. The Liebeskind–Srogl Cross-Coupling Reaction and Its Synthetic Applications. Asian J. Org. Chem. 2018, 7, 490. 10.1002/ajoc.201700651. [DOI] [Google Scholar]
- For recent reviews on C–S bond activation, see:; a Wang L.; He W.; Yu Z. Transition-Metal Mediated Carbon–Sulfur Bond Activation and Transformations. Chem. Soc. Rev. 2013, 42, 599. 10.1039/C2CS35323G. [DOI] [PubMed] [Google Scholar]; b Modha S. G.; Mehta V. P.; Van der Eycken E. V. Transition Metal-Catalyzed C–C Bond Formation via C–S Bond Cleavage: an Overview. Chem. Soc. Rev. 2013, 42, 5042. 10.1039/c3cs60041f. [DOI] [PubMed] [Google Scholar]; c Pan F.; Shi Z.-J. Recent Advances in Transition-Metal-Catalyzed C–S Activation: From Thioester to (Hetero)aryl Thioether. ACS Catal. 2014, 4, 280. 10.1021/cs400985m. [DOI] [Google Scholar]; d Otsuka S.; Nogi K.; Yorimitsu H. C–S Bond Activation. Top. Curr. Chem. 2018, 376, 13. 10.1007/s41061-018-0190-7. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Itami K.; Yamazaki D.; Yoshida J.-i. Pyrimidine-Core Extended π-Systems: General Synthesis and Interesting Fluorescent Properties. J. Am. Chem. Soc. 2004, 126, 15396. 10.1021/ja044923w. [DOI] [PubMed] [Google Scholar]; b Denmark S. E.; Cresswell A. J. Iron-Catalyzed Cross-Coupling of Unactivated Secondary Alkyl Thio Ethers and Sulfones with Aryl Grignard Reagents. J. Org. Chem. 2013, 78, 12593. 10.1021/jo402246h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Murakami K.; Yorimitsu H.; Osuka A. Practical, Modular, and General Synthesis of Benzofurans through Extended Pummerer Annulation/Cross-Coupling Strategy. Angew. Chem., Int. Ed. 2014, 53, 7510. 10.1002/anie.201403288. [DOI] [PubMed] [Google Scholar]; d Begouin J.-M.; Rivard M.; Gosmini C. Cobalt-Catalyzed C–SMe Bond Activation of Heteroaromatic Thioethers. Chem. Commun. 2010, 46, 5972. 10.1039/c0cc01055c. [DOI] [PubMed] [Google Scholar]; e Metzger A.; Melzig L.; Despotopoulou C.; Knochel P. Pd-Catalyzed Cross-Coupling of Functionalized Organozinc Reagents with Thiomethyl-Substituted Heterocycles. Org. Lett. 2009, 11, 4228. 10.1021/ol9017003. [DOI] [PubMed] [Google Scholar]; f Melzig L.; Metzger A.; Knochel P. Room Temperature Cross-Coupling of Highly Functionalized Organozinc Reagents with Thiomethylated N-Heterocycles by Nickel Catalysis. J. Org. Chem. 2010, 75, 2131. 10.1021/jo1001615. [DOI] [PubMed] [Google Scholar]; g Melzig L.; Metzger A.; Knochel P. Pd- and Ni-Catalyzed Cross-Coupling Reactions of Functionalized Organozinc Reagents with Unsaturated Thioethers. Chem. - Eur. J. 2011, 17, 2948. 10.1002/chem.201002850. [DOI] [PubMed] [Google Scholar]; h Otsuka S.; Fujino D.; Murakami K.; Yorimitsu H.; Osuka A. Palladium-Catalyzed Cross-Coupling of Unactivated Aryl Sulfides with Arylzinc Reagents under Mild Conditions. Chem. - Eur. J. 2014, 20, 13146. 10.1002/chem.201404380. [DOI] [PubMed] [Google Scholar]; i Liebeskind L. S.; Srogl J. Heteroaromatic Thioether-Boronic Acid Cross-Coupling under Neutral Reaction Conditions. Org. Lett. 2002, 4, 979. 10.1021/ol0200091. [DOI] [PubMed] [Google Scholar]; j Hooper J. F.; Young R. D.; Pernik I.; Weller A. S.; Willis M. C. Carbon–Carbon Bond Construction Using Boronic Acids and Aryl Methyl Sulfides: Orthogonal Reactivity in Suzuki-Type Couplings. Chem. Sci. 2013, 4, 1568. 10.1039/c3sc00052d. [DOI] [Google Scholar]; k Pan F.; Wang H.; Shen P.-X.; Zhao J.; Shi Z.-J. Cross Coupling of Thioethers with Aryl Boroxines to Construct Biaryls via Rh-Catalyzed C–S Activation. Chem. Sci. 2013, 4, 1573. 10.1039/c3sc22242j. [DOI] [Google Scholar]
- For recent reviews, see:; a Knappke C. E. I.; Grupe S.; Gärtner D.; Corpet M.; Gosmini C.; Jacobi von Wangelin A. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. - Eur. J. 2014, 20, 6828. 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]; b Everson D. A.; Weix D. J. Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem. 2014, 79, 4793. 10.1021/jo500507s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Moragas T.; Correa A.; Martin R. Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. - Eur. J. 2014, 20, 8242. 10.1002/chem.201402509. [DOI] [PubMed] [Google Scholar]; d Weix D. J. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res. 2015, 48, 1767. 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Gu J.; Wang X.; Xue W.; Gong H. Nickel-Catalyzed Reductive Coupling of Alkylhalides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411. 10.1039/C5QO00224A. [DOI] [Google Scholar]; f Wang X.; Dai Y.; Gong H. Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem. 2016, 374, 43. 10.1007/s41061-016-0042-2. [DOI] [PubMed] [Google Scholar]
- For selected examples of C(sp2)–C(sp3) reductive cross-coupling, see:; a Krasovskiy A.; Duplais C.; Lipshutz B. H. Zn-Mediated, Pd-Catalyzed Cross-Couplings in Water at Room Temperature Without Prior Formation of Organozinc Reagents. J. Am. Chem. Soc. 2009, 131, 15592. 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bhonde V. R.; O’Neill B. T.; Buchwald S. L. An Improved System for the Aqueous Lipshutz–Negishi Cross-Coupling of Alkyl Halides with Aryl Electrophiles. Angew. Chem., Int. Ed. 2016, 55, 1849. 10.1002/anie.201509341. [DOI] [PubMed] [Google Scholar]; c Czaplik W. M.; Mayer M.; Jacobi von Wangelin A. Domino Iron Catalysis: Direct Aryl–Alkyl Cross-Coupling. Angew. Chem., Int. Ed. 2009, 48, 607. 10.1002/anie.200804434. [DOI] [PubMed] [Google Scholar]; d Everson D. A.; Shrestha R.; Weix D. J. Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc. 2010, 132, 920. 10.1021/ja9093956. [DOI] [PubMed] [Google Scholar]; e Amatore M.; Gosmini C. Direct Method for Carbon–Carbon Bond Formation: The Functional Group Tolerant Cobalt-Catalyzed Alkylation of Aryl Halides. Chem. - Eur. J. 2010, 16, 5848. 10.1002/chem.201000178. [DOI] [PubMed] [Google Scholar]; f Everson D. A.; Jones B. A.; Weix D. J. Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc. 2012, 134, 6146. 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Wang S.; Qian Q.; Gong H. Nickel-Catalyzed Reductive Coupling of Aryl Halides with Secondary Alkyl Bromides and Allylic Acetate. Org. Lett. 2012, 14, 3352. 10.1021/ol3013342. [DOI] [PubMed] [Google Scholar]; h Biswas S.; Weix D. J. Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2013, 135, 16192. 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Molander G. A.; Traister K. M.; O’Neill B. T. Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides. J. Org. Chem. 2014, 79, 5771. 10.1021/jo500905m. [DOI] [PubMed] [Google Scholar]; j Molander G. A.; Traister K. M.; O’Neill B. T. Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides. J. Org. Chem. 2015, 80, 2907. 10.1021/acs.joc.5b00135. [DOI] [PubMed] [Google Scholar]; k Hu L.; Liu X.; Liao X. Nickel-Catalyzed Methylation of Aryl Halides with Deuterated Methyl Iodide. Angew. Chem., Int. Ed. 2016, 55, 9743. 10.1002/anie.201604406. [DOI] [PubMed] [Google Scholar]; l Zhang P.; Le C. C.; MacMillan D. W. C. Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc. 2016, 138, 8084. 10.1021/jacs.6b04818. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Wang X.; Wang S.; Xue W.; Gong H. Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2015, 137, 11562. 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]; n Wang X.; Wang S.; Xue W.; Gong H. Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2015, 137, 11562. 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]; o Wang X.; Ma G.; Peng Y.; Pitsch C. E.; Moll B. J.; Ly T. D.; Wang X.; Gong H. Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2018, 140, 14490. 10.1021/jacs.8b09473. [DOI] [PubMed] [Google Scholar]
- For a single example using in situ Grignard formation with π-extended C–OMe systems, see:; a Cao Z.-C.; Luo Q.-Y.; Shi Z.-J. Practical Cross-Coupling between O-Based Electrophiles and Aryl Bromides via Ni Catalysis. Org. Lett. 2016, 18, 5978. 10.1021/acs.orglett.6b02656. [DOI] [PubMed] [Google Scholar]; For homocoupling, see:; b Nakamura K.; Tobisu M.; Chatani N. Nickel-Catalyzed Formal Homocoupling of Methoxyarenes for the Synthesis of Symmetrical Biaryls via C–O Bond Cleavage. Org. Lett. 2015, 17, 6142. 10.1021/acs.orglett.5b03151. [DOI] [PubMed] [Google Scholar]
- Hegedus L. L.; McCabe R. W.. Catalyst Poisoning; Marcel Dekker: New York, 1984. [Google Scholar]
- a O’Neill M. J.; Riesebeck T.; Cornella J. Thorpe-Ingold Effect for Branch-Selective Alkylation of Unactivated Aryl Fluorides. Angew. Chem., Int. Ed. 2018, 57, 9103. 10.1002/anie.201804479. [DOI] [PubMed] [Google Scholar]; b Moser D.; Duan Y.; Wang F.; Ma Y.; O’Neill M. J.; Cornella J. Selective Functionalization of Aminoheterocycles by a Pyrylium Salt. Angew. Chem., Int. Ed. 2018, 57, 11035. 10.1002/anie.201806271. [DOI] [PubMed] [Google Scholar]
- For activation of 2-thiopyridine esters, see:; Wotal A. C.; Weix D. J. Synthesis of Functionalized Dialkyl Ketones from Carboxylic Acid Derivatives and Alkyl Halides. Org. Lett. 2012, 14, 1476. 10.1021/ol300217x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See Supporting Information for details.
- For selected reviews, see:; a Cornella J.; Zarate C.; Martin R. Metal-Catalyzed Activation of Ethers via C–O Bond Cleavage: a New Strategy for Molecular Diversity. Chem. Soc. Rev. 2014, 43, 8081. 10.1039/C4CS00206G. [DOI] [PubMed] [Google Scholar]; b Ahrens T.; Kohlmann J.; Ahrens M.; Braun T. Functionalization of Fluorinated Molecules by Transition-Metal-Mediated C–F Bond Activation To Access Fluorinated Building Blocks. Chem. Rev. 2015, 115, 931. 10.1021/cr500257c. [DOI] [PubMed] [Google Scholar]
- For recent review, see:; O’Neill M. J.; Cornella J. Retaining Alkyl Nucleophile Regiofidelity in Transition-Metal-Mediated Cross-Couplings to Aryl Electrophiles. Synthesis 2018, 50, 3974. 10.1055/s-0037-1609941. [DOI] [Google Scholar]
- Kamal A.; Syed M. A. H.; Mohammed S. M. Therapeutic Potential of benzothiazoles: a patent review (2010–2014). Expert Opin. Ther. Pat. 2015, 25, 335. 10.1517/13543776.2014.999764. [DOI] [PubMed] [Google Scholar]
- Harada T.; Ueda Y.; Iwai T.; Sawamura M. Nickel-Catalyzed Amination of Aryl Fluorides with Primary Amines. Chem. Commun. 2018, 54, 1718. 10.1039/C7CC08181B. [DOI] [PubMed] [Google Scholar]
- One of the potential roles of the Zn is aiding the capture of the SMe leaving group. For an excellent overview of the role of the Zn in a Liebeskind–Srogl reaction, see:; Liebeskind L. S.; Srogl J.; Savarin C.; Polanco C. Bioinspired Organometallic Chemistry. Pure Appl. Chem. 2002, 74, 115. 10.1351/pac200274010115. [DOI] [Google Scholar]
- a Hayashi T.; Konishi M.; Kobori Y.; Kumada M.; Higuchi T.; Hirotsu K. Dichloro[l, l’-bis(diphenylphosphino)ferrocene]palladium-(II): An Effective Catalyst for Cross-Coupling of Secondary and Primary Alkyl Grignard and Alkylzinc Reagents with Organic Halides. J. Am. Chem. Soc. 1984, 106, 158. 10.1021/ja00313a032. [DOI] [Google Scholar]; b Han C.; Buchwald S. L. Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2009, 131, 7532. 10.1021/ja902046m. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yang Y.; Niedermann K.; Han C.; Buchwald S. L. Highly Selective Palladium-Catalyzed Cross-Coupling of Secondary Alkylzinc Reagents with Heteroaryl Halides. Org. Lett. 2014, 16, 4638. 10.1021/ol502230p. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Atwater B.; Chandrasoma N.; Mitchell D.; Rodriguez M. J.; Organ M. G. Pd-PEPPSI-IHeptClA General-Purpose, Highly Reactive Catalyst for the Selective Coupling of Secondary Alkyl Organozincs. Chem. - Eur. J. 2016, 22, 14531. 10.1002/chem.201603603. [DOI] [PubMed] [Google Scholar]; e Zhang K.-F.; Christoffel F.; Baudoin O. Barbier–Negishi Coupling of Secondary Alkyl Bromides with Aryl and Alkenyl Triflates and Nonaflates. Angew. Chem., Int. Ed. 2018, 57, 1982. 10.1002/anie.201711990. [DOI] [PubMed] [Google Scholar]
- a Phapale V. B.; Guisan-Ceinos M.; Bunuel E.; Cardenas D. J. Nickel-Catalyzed Cross-Coupling of Alkyl Zinc Halides for the Formation of C(sp2)-C(sp3) Bonds: Scope and Mechanism. Chem. - Eur. J. 2009, 15, 12681. 10.1002/chem.200901913. [DOI] [PubMed] [Google Scholar]; b Joshi-Pangu A.; Ganesh M.; Biscoe M. R. Nickel-Catalyzed Negishi Cross-Coupling Reactions of Secondary Alkylzinc Halides and Aryl Iodides. Org. Lett. 2011, 13, 1218. 10.1021/ol200098d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hu X. Nickel-Catalyzed Cross Coupling of Non-activated Alkyl Halides: a Mechanistic Perspective. Chem. Sci. 2011, 2, 1867. 10.1039/c1sc00368b. [DOI] [Google Scholar]; For examples of cross-coupling including cage-rebound process, see:; b Jones G. D.; Martin J. L.; McFarland C.; Allen O. R.; Hall R. E.; Haley A. D.; Brandon R. J.; Konovalova T.; Desrochers P. J.; Pulay P.; Vicic D. A. Ligand Redox Effects in the Synthesis, Electronic Structure, and Reactivity of an Alkyl-Alkyl Cross-Coupling Catalyst. J. Am. Chem. Soc. 2006, 128, 13175. 10.1021/ja063334i. [DOI] [PubMed] [Google Scholar]; c Wilsily A.; Tramutola F.; Owston N. A.; Fu G. C. New Directing Groups for Metal-Catalyzed Asymmetric Carbon–Carbon Bond-Forming Processes: Stereoconvergent Alkyl–Alkyl Suzuki Cross-Couplings of Unactivated Electrophiles. J. Am. Chem. Soc. 2012, 134, 5794. 10.1021/ja301612y. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Dudnik A. S.; Fu G. C. Nickel-Catalyzed Coupling Reactions of Alkyl Electrophiles, Including Unactivated Tertiary Halides, To Generate Carbon–Boron Bonds. J. Am. Chem. Soc. 2012, 134, 10693. 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Xu H.; Zhao C.; Qian Q.; Deng W.; Gong H. Nickel-Catalyzed Cross-Coupling of Unactivated Alkyl Halides Using Bis(pinacolato)diboron as Reductant. Chem. Sci. 2013, 4, 4022. 10.1039/c3sc51098k. [DOI] [Google Scholar]
- For an excellent study on the formation and reactivity of (dppf)NiAr complexes, see:; Bajo S.; Laidlaw G.; Kennedy A. R.; Sproules S.; Nelson D. J. Oxidative Addition of Aryl Electrophiles to a Prototypical Nickel(0) Complex: Mechanism and Structure/Reactivity Relationships. Organometallics 2017, 36, 1662. 10.1021/acs.organomet.7b00208. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.