Abstract
Regional anesthesia may prolong survival following surgery for different types of cancers. The mechanisms behind this are unclear but direct effects on cancer cells by local anesthetics (LA) have been suggested. The aim of this study was to investigate if lidocaine or ropivacaine have a dose-dependent effect on the cell viability and proliferation of a primary and a secondary colon carcinoma cell line in vitro. The colon cancer cell lines SW480 derived from primary tumor and SW620 from a metastatic site in the same patient were exposed to increasing concentrations of lidocaine and ropivacaine (5–1,000 µM). Cell viability was measured using CellTiter-Blue® and cell proliferation by PKH67 after exposure for up to 72 h. Cell viability was significantly reduced by ropivacaine at the highest concentration (1,000 µM) after 48 and 72 h in the cell line SW480 and at 72 h in SW620. Exposure to lidocaine did not show any significant reduction in cell viability. Notably, low concentrations of both lidocaine and ropivacaine significantly increased cell viability after 48 and 72 h in SW620. Cell proliferation was significantly reduced by 1,000 µM lidocaine in SW480 and by 1,000 µM ropivacaine in SW620. In summary, both lidocaine and ropivacaine showed an anti-proliferative effect in the colon cancer cell lines at high concentrations and after prolonged exposure to LA in vitro. Our findings also indicate that lower concentrations promote cell viability in the metastatic cell line.
Keywords: local anesthetics, colon cancer, SW480, SW620, in vitro, cell viability, proliferation
Introduction
The surgical procedure is the mainstay of treatment for colorectal and other solid cancers. However, it may itself promote cancer growth and metastasis (1) since tumor cells can disseminate during surgery. Perioperative immunosuppression may facilitate the spread and survival of malignant cells in the body (2). Several retrospective studies have suggested that the use of regional anesthesia in cancer surgery might improve survival (3–5). The precise mechanisms underlying the possible effects of regional anesthesia have not been fully elucidated but an impact on reduction in stress response, postoperative inflammation and prevention of immunosuppression has been proposed (6,7). Local anesthetics (LA) act by blocking voltage-gated sodium channels (VGSC) in all cells and may also have direct inhibitory effects on cancer cells by inducing apoptosis (8), demethylating DNA (9), blocking metastatic cancer cell invasion in vitro (10) and may have direct cytotoxic (11) and anti-proliferative effects (12). While evolving from primary tumor cells to metastatic cells, cancer cells have to change phenotypes and properties (13). This transformation might also affect the response of cancer cells to LA.
LA administered by the epidural route are absorbed into the systemic circulation. Peak plasma concentrations of ropivacaine during an epidural infusion for 120 h ranged between 2.4 and 6.1 µg/ml, equivalent to approximately 10–22 µM (14). Systemic plasma levels for lidocaine have been found to lie in the same range (15). After local application of LA by intraperitoneal injection or tissue infiltration, the LA concentrations at the injection site are in the millimolar range, which is 1,000 times greater than that achieved following intravenous administration (16). Therefore, it is possible that LA may prevent cancer cell proliferation and micro-infiltration of cancer cells when injected locally into tissues as well as potentially inhibit imminent metastases during the perioperative period when immune modulation is sub-optimal.
In this study, we hypothesized that commonly used local anesthetic agents, lidocaine and ropivacaine, decrease cell viability and inhibit proliferation of colon cancer cells in vitro in a dose-dependent manner when used in clinically relevant concentrations. Furthermore, we investigated if there is a different effect of LA on primary colon cancer cells and cells derived from metastatic colon cancer.
Materials and methods
Cell culture and LA
Immortalized human colon cancer cells SW480 and SW620 were purchased from American Type Culture Collection (ATCC® CCL-228 and CCL-227). SW480 originates from an adenocarcinoma of the colon in a 50-year-old male and SW620 was derived from a lymph node metastasis in the same patient one year later (17). Cells were cultured in Dulbecco's modified Eagle medium (DMEM) with GlutaMAX, supplemented with 10% fetal bovine serum (FBS) and 1 µg/ml penicillin/streptomycin (all from Life technologies, Stockholm, Sweden) in a humidified incubator at 37°C and 5% CO2. Cells were cultured following standard microbiological practices and handled according to recommended seeding procedures. Lidocaine 10 mg/ml (Xylocaine hydrochloride; AstraZeneca, Södertälje, Sweden) and ropivacaine 2 mg/ml (Fresenius Kabi, Uppsala, Sweden) were diluted with Dulbecco's PBS (DPBS) to the desired concentrations used in the experiments (low concentrations = equivalent to systemic plasma concentration after intravenous or epidural application, high concentrations = equivalent to local concentration after tissue infiltration).
Cell viability assay
In 96-well plates, 4,000 cells per well were seeded in 100 µl supplemented medium and cultured for 24 h. The next day, 10 µl lidocaine or ropivacaine diluted in DPBS were added to the wells to reach the final concentrations 5, 10, 15, 20, 25, 50, 100 and 500 µM. In addition, a 20 µl solution was added to reach a final concentration of 1,000 µM of lidocaine and ropivacaine, respectively. As drug-free control, cells were cultured in supplemented medium and 10 µl DPBS for 5–500 µM (control 1) and 20 µl DPBS for 1,000 µM (control 2). Each concentration of anesthetics was run in quadruplicate wells and three independent experiments were performed. Cell viability was tested after 24, 48 and 72 h exposure using CellTiter-Blue® Cell Viability Assay (Promega Biotech AB, Stockholm, Sweden) according to manufacturer's protocol. Briefly, to estimate cell viability, 20 µl CellTiter-Blue was added to the 96-well cell culture plate and shaken for 10 sec. The plates were incubated for 2.5 h before fluorescence was measured with FLUOstar optima (BMG Labtech GmbH, Ortenberg, Germany) with a 544Ex/590Em filter set.
Cell proliferation assay
Cell Linker kit PKH67, MINI67 (Sigma-Aldrich, Stockholm, Sweden) was used to analyze cell proliferation with flow cytometry. The cells were dyed at time of seeding according to manufacturer's protocol. The cell linker kit stains lipid regions of the cell membrane with a green fluorochrome without impairing cellular functions. The amount of incorporated fluorochrome decreases as the cells divide during mitosis.
In 6-well plates, 120,000 PKH67 stained cells per well were seeded in 3 ml supplemented medium 24 h before drug exposure. Then, 330 µl lidocaine or ropivacaine diluted in DPBS were added to the cells to achieve the final concentrations: 10, 500 and 1,000 µM. The same volume DPBS was used as drug-free control. Each concentration of anesthetics was run in duplicate wells and three independent experiments were performed. After 72 h, cells were trypsinated and then analysed using a Gallios Flow Cytometer (Beckman Coulter, Indianapolis, IN, USA) equipped with blue laser (488 nm), yellow laser (561 nm) red laser (638 nm) and violet laser (405 nm). Data was collected with Kaluza for Gallios 1.0 and analyzed with Kaluza analysis 1.3 (Beckman Coulter). The amount of incorporated PKH67 was measured by median fluorescence intensity (MFI). A MFI ratio between drug-free control and each concentration was then calculated.
Statistical analysis
Results are presented as median (range) ratio between respective drug concentration and drug-free control. A ratio >1 indicates an increased cell viability or cell proliferation while a ratio <1 indicates decreased cell viability or inhibition of cell proliferation.
We used Shapiro-Wilks test for normal distribution. The results showed that the data for some of the experiments were not normally distributed. Therefore, non-parametric methods were used for statistical analysis. Cell viability data from three independent experiments in quadruplicate (n=12) were analyzed using Kruskal-Wallis test (5–500 µM) with Dunn's correction for multiple comparisons. Mann-Whitney-U test was used for the highest concentration (1,000 µM) in comparison with its drug-free control (control 2). Cell proliferation data from three independent experiments set in duplicate (n=6) were analyzed using Kruskal-Wallis test with Dunn's post hoc test. P<0.05 was considered to indicate a statistically significant difference. The statistical analysis was performed using GraphPad Prism version 7.03 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Cell viability
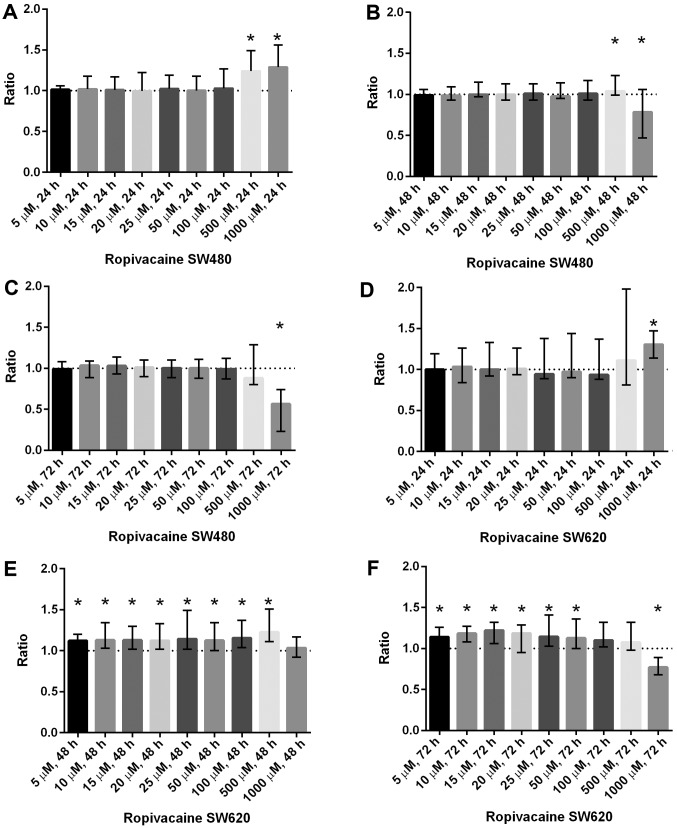
Ropivacaine: Increased cell viability was found at 500 µM [1.24 (1.06–1.49), P<0.0001] and 1,000 µM [1.29 (1.01–1.56), P<0.001] after 24 h in SW480. A significant increase in cell viability was also shown in SW620 at concentrations between 5–500 µM after 48 h exposure and at 5–50 µM after 72 h exposure. Ropivacaine 1,000 µM resulted in significantly reduced cell viability in SW480 after exposure for 48 h [0.78 (0.44–1.06), P=0.001] and 72 h [0.57 (0.23–0.74), P<0.0001]. In SW620, cell viability was only reduced by 1,000 µM [0.77 (0.68–0.89), P<0.0001] after 72 h exposure (Fig. 1).
Figure 1.
Cell viability in SW480 and SW620 following incubation with increasing concentrations of ropivacain are presented. (A) Ropivacaine increases cell viability in SW480 after 24 h at 500 and 1,000 µM. (B) Ropivacaine increases cell viability in SW480 after 48 h at 500 µM. A decrease was observed at 1,000 µM. (C) Ropivacaine reduces cell viability in SW480 after 72 h at 1,000 µM. (D) Ropivacaine increases cell viability in SW620 after 24 h at 1,000 µM. (E) After 48 h a significant increase in cell viability in SW620 is noted in all concentrations except 1,000 µM. (F) Ropivacaine increases cell viability in SW620 after 72 h at concentrations between 5 and 50 µM, a reduction in cell viability was observed at 1,000 µM. Results are presented as the median ratio and range of CellTiter Blue® values from three independent experiments in quadruplicate (n=12) between drug exposed cells and unexposed cells (control=1, dotted line). *P<0.05 vs. control.
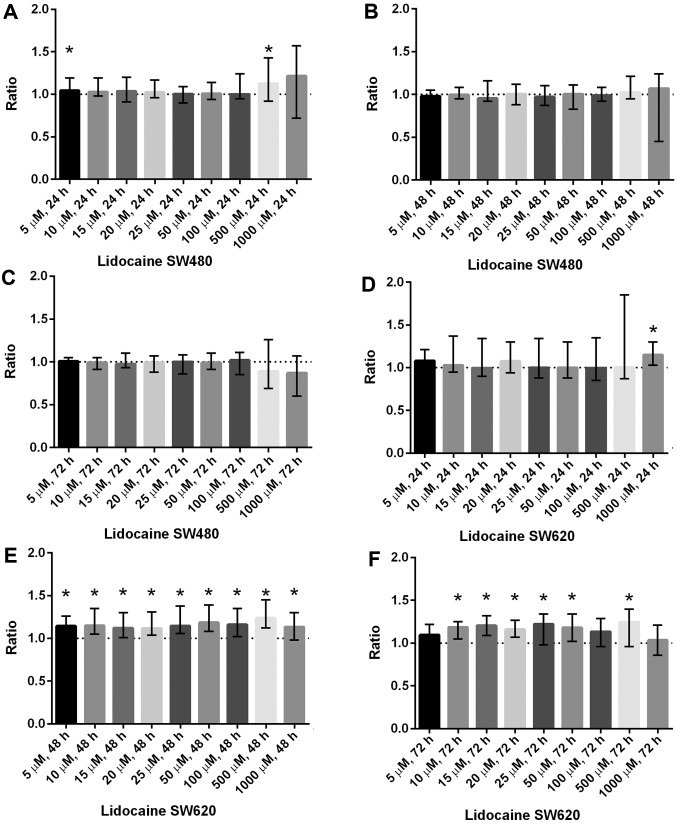
Lidocaine: there was a significant increase in cell viability after 24 h in SW480 at 500 µM [1.13 (0.92–1.43), P<0.05] and in SW620 at 1,000 µM [1.15 (1.03–1.30), P<0.001]. In the metastatic cell line SW620 (but not in SW480) cell viability was significantly increased even at lower concentrations after exposure for 48 and 72 h (Fig. 2). No significant reduction in cell viability was found after exposure to lidocaine at any concentration and at any time point for both cell lines SW480 and SW620.
Figure 2.
Cell viability in SW480 and SW620 after incubation with increasing concentrations of lidocaine is presented. (A) Lidocaine increases cell viability in SW480 after 24 h at 5 and 500 µM. (B and C) No significant change in cell viability in SW480 is seen after 48 and 72 h with lidocaine. (D) Lidocaine increases cell viability in SW620 after 24 h at 1,000 µM. (E) After 48 h an increase in cell viability in SW620 is noted at all tested concentrations. (F) Lidocaine increases cell viability in SW620 after 72 h at concentrations between 10 and 50 µM, and at 500 µM. Results are presented as the median ratio and range of Celltiter Blue® values from three independent experiments in quadruplicate (n=12) between drug exposed cells and unexposed cells (control=1, dotted line). *P<0.05 vs. control.
Cell proliferation
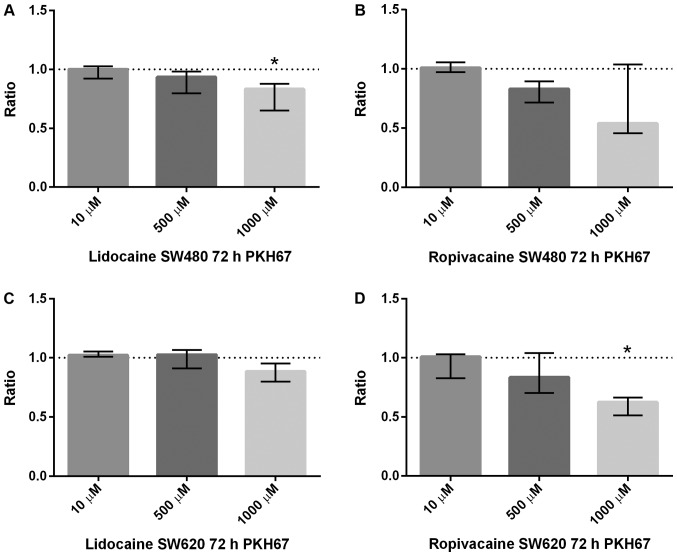
No significant effect on cell proliferation was found at lower concentrations (10 and 500 µM) of lidocaine or ropivacaine (Fig. 3). However, reduction in cell proliferation was found in both cell lines after exposure to the highest concentration (1,000 µM) of lidocaine and ropivacaine (Fig. 3). However, after correction for multiple comparisons, it was only statistically significant for lidocaine in SW480 and for ropivacaine in SW620. The anti-proliferative effect seems to be more pronounced for ropivacaine than for lidocaine (Fig. 4).
Figure 3.
Cell proliferation measured by PKH67 is presented. The results are presented as the median ratio and range of PKH67 values from three independent experiments in duplicate (n=6) between drug exposed cells and drug-free cells (control=1, dotted line). (A) Lidocaine significantly decreased cell proliferation in SW480 at 1,000 µM. (B) Ropivacaine did not significantly affect cell proliferation in SW480 at any concentration tested. (C) Lidocaine demonstrated no significant effect on cell proliferation in SW620 at any concentration tested. (D) Ropivacaine significantly decreased cell proliferation in SW620 at 1,000 µM. *P<0.05 vs. control.
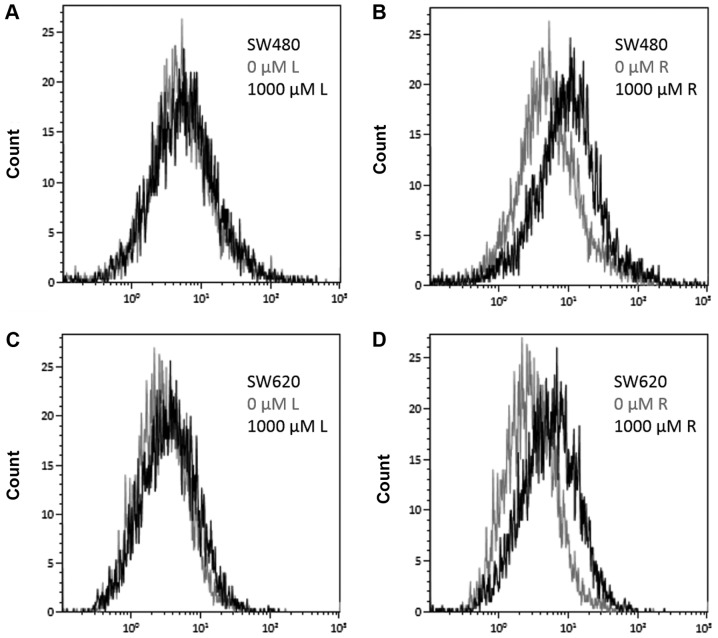
Figure 4.
Change in cell proliferation measured by PKH67 presented in overlays. The data are presented as an overlay histogram of PKH67 median fluorescence intensity compared with the drug-free control (0 µM) and 1,000 µM L or R. (A) Overlay of PKH67 median fluorescence intensity for SW480 cells comparing drug-free control (light grey) and 1,000 µM L (black). (B) Overlay of PKH67 median fluorescence intensity for SW480 cells comparing drug free control (light grey) and 1,000 µM R (black). (C) Overlay of PKH67 median fluorescence intensity for SW620 cells comparing drug-free control (light grey) and 1,000 µM L (black). (D) Overlay of PKH67 median fluorescence intensity for SW620 cells comparing drug free control (light grey) and 1,000 µM R (black). L, lidocaine; R, ropivacaine.
Discussion
Our hypothesis of reduced cell viability following LA exposure was only partially supported by our findings. Cell viability was not significantly reduced by lidocaine in any concentration tested or at any time point. However, a significant decrease in cell viability was observed for ropivacaine at 1,000 µM, when the exposure time was at least 48 h. Our results are based on estimating cell viability using an assay where a redox dye is converted into a fluorescent product by metabolically active, living cells. An increase in fluorescence is regarded to be proportional to increased cell count, while a reduction in fluorescence may accordingly be proportional to reduced cell count. The method has limitations and we cannot be sure that changes in fluorescence depend on changes in cell metabolism rather than cell count (18). However, the reduction in cell viability measured by CellTiter-Blue® for ropivacaine at 1,000 µM corresponds to a reduction in cell proliferation measured by PKH67 at the same concentration. Xuan et al reported similar findings in ovarian and prostate cancer cell lines (19). In their study, only bupivacaine at 1 mM decreased cell viability significantly, but not at lower concentrations. The effect was more pronounced when these cancer cells were exposed to bupivacaine for 72 h. Similarly, in a study by Le Gac et al, lidocaine and ropivacaine did not reduce cell viability in human hepatocellular carcinoma cell lines at low concentrations (1–10 µM). At higher concentrations (100 µM, 1 and 5 mM), a dose-dependent decrease in cell viability could be detected, which was more pronounced after 72 h compared to 24 h (20).
Retrospective studies in humans have demonstrated increased survival in patients having epidural analgesia with LA compared to intravenous analgesia with morphine in colon and rectal cancer (3,21). The precise mechanisms for this protective effect of epidural analgesia remain unclear but one hypothesis has been that absorption of LA from the epidural space may inhibit cell proliferation of circulating tumor cells released during surgery, thereby impeding perioperative cancer metastases (22). Cell proliferation is an essential process in the development of malignant tumors and metastasis (23). Based on our results and previous studies, we believe that LA do not exert an anti-proliferative effect on cancer cell lines in the range of systemic plasma concentrations achieved during epidural administration of LA. Other anti-metastatic mechanisms of LA in this low range of concentrations have been proposed. Piegeler et al have shown that lidocaine and ropivacaine inhibit Src tyrosine kinase when used in clinically relevant concentrations (24). Src tyrosine kinase is an important enzyme in tumor growth and metastasis and controls the activation of matrix-metalloproteinases (MMP), another important factor in the pathogenesis of metastasis. Lidocaine and ropivacaine inhibited MMP9-secretion by NCl-H838 lung adenocarcinoma cells with an IC50 of 3.3 and 1.5 µM, respectively (25). This effect of LA was independent of their primary mechanism of action, the blockade of VGSC. VGSC have been detected on a variety of different cancer cells and are presumed to play an important role in the process of metastasis (26). Cancer cell lines SW480 and SW620 used in our study express mainly the VGSC-isoform Nav1.5 (27). Baptista-Hon et al were able to show that metastatic cell invasion of SW620 is potently inhibited by ropivacaine in vitro with an IC50 value below 5 µM (10).
The effect of LA varies with different types of cancer cell lines and their properties. Our findings on inhibition of cell viability and proliferation are in line with Martinsson (28). They showed that ropivacaine reduced cell proliferation in colon cancer cell lines, HT-29 and Caco-2, in a dose-dependent manner with an IC50>250 and 430 µM, respectively. They also found that lidocaine had a less potent anti-proliferative effect than ropivacaine. HT-29, Caco-2 and SW480 all originate from human colon adenocarcinoma. There are many similarities but even distinct differences between these cell lines (29) justifying further research.
In contrast to our primary hypothesis, we noted a significant increase in cell viability especially in the metastatic cell line SW620 when exposed for at least 48 h to concentrations of lidocaine or ropivacaine equivalent to those systemically achievable in vivo. A comparable increase in cell viability could not be seen in the primary tumor cell line SW480. The cell lines SW480 and SW620 are unique as they originate from the same patient reflecting progression from primary to metastatic tumor cells. It is believed that tumor cells undergo distinctive changes in both morphologic and functional properties to metastasize to distant tissues (30). The process of metastasis is generally inefficient and not all cancer cells released into the circulation during a surgical procedure are able to develop into distant metastases (31). Cells that already have passed through the ‘epithelial-mesenchymal transition’ (EMT) and have acquired the ability for invasion and dissemination are likely to be able to form metastases (32). Hewitt et al showed that SW480 and SW620 have retained significant histological differences (33). SW620 cells have a more fibroblast-like appearance, a higher growth rate and are more invasive than SW480. The fact that SW620 respond with increased cell viability to LA is thus especially interesting, as it must be suspected that mainly cells that have undergone metastatic transformation are able to form distant metastases if released during the perioperative period. To the best of our knowledge there is no study published with focus on the effect of LA on primary and metastatic colon cancer cell lines. The observation that there is increased cell viability in the metastatic cell line is remarkable and Bundscherer et al found similar results in a previous study (34). Bupivacaine also significantly increased cell growth in PaTu8988t, a cell line originated from a liver metastasis of a pancreatic adenocarcinoma, in similar concentrations (0.1–100 µM). However, the increase in cell viability as measured by CellTiter-Blue® for both ropivacaine and lidocaine is not accompanied by an increase in cell proliferation as analyzed by PKH67. The cell viability assay used in this study cannot exclude that the increase of viability shown is a result of increased metabolism and not increased cell count. Ropivacaine has been shown to affect energy metabolism in cells by uncoupling of oxidative phosphorylation and a direct inhibitory effect on mitochondrial enzyme complexes (35,36). However, this should cause a reduction in cell viability not an increase. Thus, the mechanisms behind the increase in cell viability of the metastatic cell line SW620 when exposed to LA are still unclear, as is its clinical significance. This needs to be further investigated in future studies.
In conclusion, our findings show that exposure of colon cancer cell lines to lidocaine and ropivacaine results in increased cell viability at clinically relevant concentrations, specifically in the metastatic cancer cell line. Future studies should explore possible mechanisms for this observation. Reduced cell viability and proliferation were only seen at the highest concentration. These high concentrations can be achieved locally by intraperitoneal administration of LA for several days via a catheter following intra-abdominal surgery. This opens the window for in vivo studies investigating the clinical effectiveness of intraperitoneally administered LA in patients undergoing surgery for colorectal cancer.
Acknowledgements
An abstract of this study was presented previously at the 34th SSAI Congress 3017 in Malmö, Sweden. The authors would like to thank Dr Anna Göthlin Eremo (Örebro University, Örebro, Sweden) for statistical guidance.
Funding
Financial support was obtained from the Research Committee of the Örebro County Council.
Availability of data and materials
The datasets analyzed during the study are available from the corresponding author on reasonable request.
Authors' contributions
AG and ET designed the study. ET and AKVS conducted the laboratory research with assistance from WS. WS performed the statistical analysis and wrote the manuscript in close cooperation with all authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ceelen W, Pattyn P, Mareel M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit Rev Oncol Hematol. 2014;89:16–26. doi: 10.1016/j.critrevonc.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–768. doi: 10.1016/S1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Björnsson A, Fredriksson M, Hallböök O, Eintrei C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth. 2011;107:164–170. doi: 10.1093/bja/aer100. [DOI] [PubMed] [Google Scholar]
- 4.Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–332. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 5.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 6.Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, Bessler H. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97:822–827. doi: 10.1213/01.ANE.0000078586.82810.3B. [DOI] [PubMed] [Google Scholar]
- 7.Kurosawa S. Anesthesia in patients with cancer disorders. Curr Opin Anaesthesiol. 2012;25:376–384. doi: 10.1097/ACO.0b013e328352b4a8. [DOI] [PubMed] [Google Scholar]
- 8.Chang YC, Liu CL, Chen MJ, Hsu YW, Chen SN, Lin CH, Chen CM, Yang FM, Hu MC. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg. 2014;118:116–124. doi: 10.1213/ANE.0b013e3182a94479. [DOI] [PubMed] [Google Scholar]
- 9.Lirk P, Berger R, Hollmann MW, Fiegl H. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J Anaesth. 2012;109:200–207. doi: 10.1093/bja/aes128. [DOI] [PubMed] [Google Scholar]
- 10.Baptista-Hon DT, Robertson FM, Robertson GB, Owen SJ, Rogers GW, Lydon EL, Lee NH, Hales TG. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth. 2014;113(Suppl 1):i39–i48. doi: 10.1093/bja/aeu104. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Castro R, Patel S, Garavito-Aguilar ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ, Xu F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997–1007. doi: 10.1213/ane.0b013e31819385e1. [DOI] [PubMed] [Google Scholar]
- 12.Lucchinetti E, Awad AE, Rahman M, Feng J, Lou PH, Zhang L, Ionescu L, Lemieux H, Thébaud B, Zaugg M. Antiproliferative effects of local anesthetics on mesenchymal stem cells: potential implications for tumor spreading and wound healing. Anesthesiology. 2012;116:841–856. doi: 10.1097/ALN.0b013e31824babfe. [DOI] [PubMed] [Google Scholar]
- 13.Alizadeh AM, Shiri S, Farsinejad S. Metastasis review: from bench to bedside. Tumour Biol. 2014;35:8483–8523. doi: 10.1007/s13277-014-2421-z. [DOI] [PubMed] [Google Scholar]
- 14.Wiedemann D, Mühlnickel B, Staroske E, Neumann W, Röse W. Ropivacaine plasma concentrations during 120-hour epidural infusion. Br J Anaesth. 2000;85:830–835. doi: 10.1093/bja/85.6.830. [DOI] [PubMed] [Google Scholar]
- 15.Mayumi T, Dohi S, Takahashi T. Plasma concentrations of lidocaine associated with cervical, thoracic, and lumbar epidural anesthesia. Anesth Analg. 1983;62:578–580. doi: 10.1213/00000539-198306000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93:858–875. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- 17.Leibovitz A, Stinson JC, McCombs WB, III, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976;36:4562–4569. [PubMed] [Google Scholar]
- 18.Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel) 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xuan W, Zhao H, Hankin J, Chen L, Yao S, Ma D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci Rep. 2016;6:26277. doi: 10.1038/srep26277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gac G, Angenard G, Clément B, Laviolle B, Coulouarn C, Beloeil H. Local anesthetics inhibit the growth of human hepatocellular carcinoma cells. Anesth Analg. 2017;125:1600–1609. doi: 10.1213/ANE.0000000000002429. [DOI] [PubMed] [Google Scholar]
- 21.Vogelaar FJ, Abegg R, van der Linden JC, Cornelisse HG, van Dorsten FR, Lemmens VE, Bosscha K. Epidural analgesia associated with better survival in colon cancer. Int J Colorectal Dis. 2015;30:1103–1107. doi: 10.1007/s00384-015-2224-8. [DOI] [PubMed] [Google Scholar]
- 22.Votta-Velis EG, Piegeler T, Minshall RD, Aguirre J, Beck-Schimmer B, Schwartz DE, Borgeat A. Regional anaesthesia and cancer metastases: the implication of local anaesthetics. Acta Anaesthesiol Scand. 2013;57:1211–1229. doi: 10.1111/aas.12210. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, Minshall RD, Borgeat A. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012;117:548–559. doi: 10.1097/ALN.0b013e3182661977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piegeler T, Schläpfer M, Dull RO, Schwartz DE, Borgeat A, Minshall RD, Beck-Schimmer B. Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFα-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of Akt and focal adhesion kinase. Br J Anaesth. 2015;115:784–791. doi: 10.1093/bja/aev341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels (Austin) 2012;6:352–361. doi: 10.4161/chan.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinsson T. Ropivacaine inhibits serum-induced proliferation of colon adenocarcinoma cells in vitro. J Pharmacol Exp Ther. 1999;288:660–664. [PubMed] [Google Scholar]
- 29.Biazik JM, Jahn KA, Su Y, Wu YN, Braet F. Unlocking the ultrastructure of colorectal cancer cells in vitro using selective staining. World J Gastroenterol. 2010;16:2743–2753. doi: 10.3748/wjg.v16.i22.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tohme S, Simmons RL, Tsung A. Surgery for Cancer: a trigger for metastases. Cancer Res. 2017;77:1548–1552. doi: 10.1158/0008-5472.CAN-16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, Stamp GW, Stetler-Stevenson WG. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–454. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Bundscherer A, Malsy M, Gebhardt K, Metterlein T, Plank C, Wiese CH, Gruber M, Graf BM. Effects of ropivacaine, bupivacaine and sufentanil in colon and pancreatic cancer cells in vitro. Pharmacol Res 95–96. 2015:126–131. doi: 10.1016/j.phrs.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Sztark F, Malgat M, Dabadie P, Mazat JP. Comparison of the effects of bupivacaine and ropivacaine on heart cell mitochondrial bioenergetics. Anesthesiology. 1998;88:1340–1349. doi: 10.1097/00000542-199805000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Nouette-Gaulain K, Sirvent P, Canal-Raffin M, Morau D, Malgat M, Molimard M, Mercier J, Lacampagne A, Sztark F, Capdevila X. Effects of intermittent femoral nerve injections of bupivacaine, levobupivacaine, and ropivacaine on mitochondrial energy metabolism and intracellular calcium homeostasis in rat psoas muscle. Anesthesiology. 2007;106:1026–1034. doi: 10.1097/01.anes.0000265164.29630.b4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the study are available from the corresponding author on reasonable request.