Abstract
Highlights
There are multiple modes of activating PDGFRs
Once activated, PDGFRs contribute to various pathological conditions
The PDGF/PDGFR family constitutes largely untapped therapeutic targets
The precise role of PDGFR in pathology along with receptor-specific agonists will enable the development of such therapies
The platelet-derived growth factor (PDGF)/PDGFR receptor (PDGFR) family is essential for a vast array of physiological processes such as migration and proliferation of percityes that contribute to the formation and proper function of blood vessels. While ligand-dependent de-repression of the PDGFR’s kinase activity is the major mode by which the PDGFR is activated, there are additional mechanisms to activate PDGFRs. Deregulated PDGFR activity contributes to various pathological conditions, and hence the PDGF/PDGFR family members are viable therapeutic targets. An increased appreciation of which PDGFR contributes to pathology, biomarkers that indicate the amplitude and mode of activation, and receptor-specific antagonists are necessary for the development of next-generation therapies that target the PDGF/PDGFR family.
The PDGF family and their relationships
There are four platelet-derived growth factor (PDGF) genes (PDGFA, PDGFB, PDGFC and PDGFD) that reside on chromosomes 7, 22, 4 and 11 in humans, and chromosomes 5, 15, 3 and 9 in mice, respectively. Biologically active (able to activate a PDGF receptor (PDGFR)) PDGF is a dimer of two PDGF protein chains. Extracellular, proteolytic processing is required for activation of some isoforms of PDGF (PDGF-C and PDGF-D), while this step occurs intracellularly for PDGF-A, PDGF-B and PDGF-AB [1]. Thus while all PDGFs are produced by cells and secreted, some of them are secreted in a latent form and become active only after being processed by proteases such as tissue plasminogen, urokinase plasminogen activator, plasmin and matriptase [1, 2].
There are a total of 5 biologically active PDGF proteins because in addition to the four homodimers (PDGF-A, PDGF-B, PDGF-C and PDGF-D) of PDGF, there is one heterodimer, PDGF-AB. Thus some cells co-express PDGF-A and PDGF-B, which assemble into a heterodimer that is proteolytically processed prior to secretion. Other types of PDGF heterodimers have not been reported. In addition to being secreted by cells (such as endothelial cells, macrophages and epithelial cells), PDGFs are present in platelets (hence their name), and released upon degranulation.
PDGFs are extraordinarily stable molecules; PDGF-AB retains biological activity after being heated to 100°C [3]. Heat-induced denaturation of proteins commences at 42°C, and many restriction enzymes can be completely inactivated by heating to 65°C. The exceptional stability of PDGFs is in part due to intra and inter disulfide bonds, which are required for the biological activity of PDGFs. PDGF-AB is the most resistant to high temperature, followed by PDGF-A and PDGF-B, which are more stable than PDGF-C and PDGF-D. The settings in which PDGFs function are typically physiological (i.e. 37°C), and hence it remains a mystery why PDGFs are so stable. In light of the fact that PDGFRs can be engaged by both PDGFs and vascular endothelial cells growth factor A (VEGF-A) [4, 5], it is tempting to speculate that the stability of PDGF is a reflection of a very precise structure, which may be necessary for PDGFRs to distinguish between PDGF, which binds and activates PDGFR, from VEGF, which binds but fails to dimerize or efficiently activate PDGFRs [5].
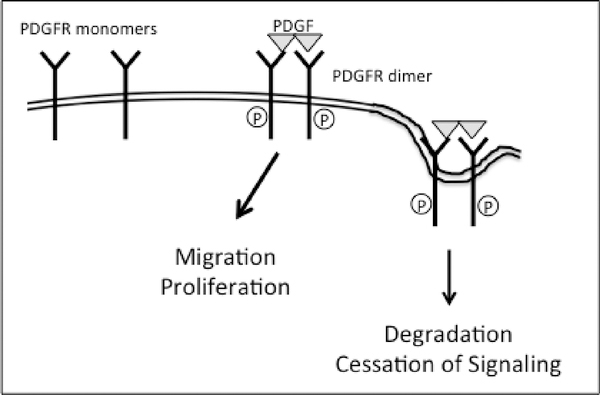
There are two PDGFR genes (PDGFRA and PDGFRB), and they reside on chromosome 4 and 5 in humans and 5 and 18 in mice, respectively. In both cases they are typical receptor tyrosine kinases (RTKs) that encode a transmembrane protein with an extracellular ligand binding domain and an intracellular tyrosine kinase domain. Each of the two PDGF molecules within a PDGF dimer bind one molecule of PDGFR. Thus ligand binding dimerizes PDGFRs, which are monomeric prior to exposure to PDGF (Fig 1).
Figure 1. PDGF-dependent activation of PDGFRs; the direct mode of activation.
PDGF drives assembly of monomeric PDGFRs into dimers, which de-represses the receptor’s intrinsic kinase activity. The activated PDGFRs initiates signal pathways that instruct cells to migrate and proliferate. Activated PDGFR dimers are internalized and degraded, which terminates signaling.
PDGF binding de-represses and activates the receptor’s kinase activity. Tyrosine phosphorylation of the receptor itself and other substrates triggers intracellular signaling cascades that are essential for evoking cellular responses such as migration and proliferation [6, 7]. Ligand mediated dimerization and activation of the receptor’s kinase also hastens internalization and degradation of PDGFRs, which terminate the PDGFRs output (Fig 1). The half-life of the PDGFR is greater than 2 hrs in resting cells, where it is only 5 min and 30 min for the PDGF-activated PDGFRα and PDGFRβ, respectively [8].
The specificity of interactions with the PDGF/PDGFR family is summarized in Table 1. PDGFs assemble all three possible dimeric combinations of the two PDGFRs (PDGFRα, PDGFRβ and PDGFRαβ). Only two PDGF isoforms (PDGF-B and PDGF-D) bring together a PDGFRβ homodimer, whereas four PDGFs can assemble PDGFRα homodimers or PDGFRαβ heterodimers. The response when a receptor is activated is typically irrespective of the PDGF isoform [9], although exceptions have been reported [10].
Table 1. Multiple PDGFs engage each of the PDGFRs.
Four PDGF isoforms dimerize PDGFRα homodimers or PDGFRαβ heterodimers, whereas only two of the PDGFs assemble homodimers of PDGFRβ. PDGF-D has been placed in parentheses because not all investigators report that it activates PDGFRαβ.
| LIGANDS | ||
| PDGF-A | ||
| PDGF-B | PDGF-B | PDGF-B |
| PDGF-AB | PDGF-AB | |
| PDGF-C | PDGF-C | |
| PDGF-D | (PDGF-D) | |
| RECEPTORS TO WHICH THE LIGANDS BIND | ||
| PDGFRα | PDGFRβ | PDGFRαβ |
The presence of two PDGFR genes begs the question of whether they have distinct functions. Mice that lack either one of the PDGFR genes display profound developmental defects, and the nature of the phenotype depends on which PDGFR gene is deleted [11, 12]. While the expression patterns of the two PDGFR genes is not identical, this is an insufficient explanation for why PDGFRA null mice do not resemble mice lacking PDGFRB. If the two PDGFRs functioned identically, then interchanging their intracellular domains would be expected to have no impact. Yet this is not what happens; interchanging the intracellular domains of the two PDGFRs also results in a phenotype [13]. Furthermore, mice harboring a single activated allele of PDGFRA display a distinct spectrum of abnormalities as compared with the corresponding mice expressing an activated allele of PDGFRB [14, 15]. Taken together, these findings reveal that the two PDGFR genes have distinct functions, which are probably not simply due to difference in their patterns of expression.
While manipulating PDGFR genes in mice indicates that the two PDGFR genes have distinct functions, the relative contribution of PDGFR homo versus heterodimers remains less well resolved because of technical limitations. Deleting one of the PDGFR genes eliminates both homo and hetero dimeric receptors. Thus the phenotype of PDGFRα null mice could be due to the lack of PDGFRα, PDGFRαβ or the presence of only PDGFRβ. The alternative approach of manipulating ligands to address the relative contribution of homo and heterodimeric PDGFRs is also not helpful because none of the PDGFs activate only heterodimeric receptors (Table 1). Novel experimental approaches are needed to address this long-standing question.
Modes to activate PDGFRs
PDGF-mediated
The best-known mechanism to activated PDGFRs is the PDGF-mediated (direct) mode of activation (Fig 1). PDGF assembles two PDGFR molecules and thereby intensely activates the receptor’s intrinsic tyrosine kinase activity. The activated PDGFR tyrosine phosphorylates substrates and thereby engages signaling cascades that drive subsequent cellular responses. For instance, both PDGFRα and PDGFRβ autophosphorylate and thereby create a docking sites for SH2 domain-containing proteins such as phosphoinositide 3 kinase (PI3K). The relocalization of PI3K to the plasma membrane increases its access to its lipid substrates (phosphoinositide and phosphoinositide 4,5 bisphosphate) and to co-activators such as activated Ras [16–18]. In addition to initiating signaling cascades, PDGF-dependent activation of PDGFRs hastens their internalization and degradation. This is how the signal emanating from directly activated PDGFRs is quenched. Thus intense and self-limiting activation of dimeric PDGFRs are quintessential features of the direct mode of activation. The concordance of the phenotype of PDGF and PDGFR null mice indicates that this is how PDGFRs are activated during development [19].
PDGF-independent
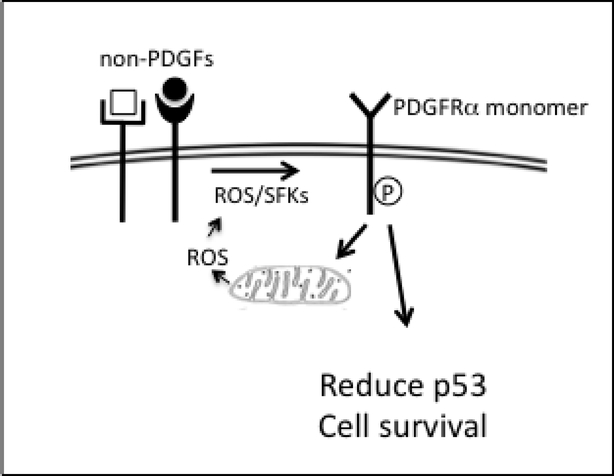
There are also PDGF-independent modes to activate PDGFRs, which were first detected in pathological settings. For instance, indirect activation of PDGFRα is driven by growth factors outside of the PDGF family (non-PDGFs) [8] (Fig 2). These non-PDGFs engage their own receptors and thereby trigger an intracellular signaling cascade that enduringly activates monomeric PDGFRα. The indirect mode of activation is persistent because internalization/degradation of monomeric receptors is slow as compared with dimeric receptors. Furthermore, indirect activation induces a feed-forward loop that promotes the activity of PDGFRα even after the initial cue, generated by receptors that were activated by non-PDGFs, has been extinguished [20]. Thus enduring and self-perpetuating activation of monomeric PDGFRα are quintessential features of the indirect mode of activation. Indirect activation of PDGFRα allows survival of cells mislocalized to the vitreous of the eye, and thereby results in a blinding condition called proliferative vitreoretinopathy (PVR) [8, 21].
Figure 2. The indirect mode of activating PDGFRα.
Growth factors outside of the PDGF family (non-PDGFs bind to their own receptors and initiate the indirect mode of activating PDGFRα. Reactive oxygen species (ROS) generated by NADPH oxidases stimulate Src family kinases (SFKs) to phosphorylate monomeric PDGFRα. In contrast to dimeric PDGFRs, activated monomeric PDGFRαs do not hasten their own internalization and degradation. This feature of the indirect mode of activating PDGFRα, together with an increase in mitochondrial ROS (due to suppression of autophagy/mitophagy) engages a feed-forward loop that enables the activity of PDGFRα to persist even after non-PDGF-dependent signaling has been extinguished. Indirect activation of PDGFRα results in a reduction in the level of p53, which promotes survival of cells.
In contrast to PDGFRα, PDGFRβ does not undergo indirect activation [22, 23]. This is because PDGFRβ engages RasGAP (PDGFRα does not), which promotes inactivation of Ras and thereby prevents establishment of the feed forward loop that is required for enduring activation of PDGFRα [23]. PDGFRβ has the capacity to undergo indirect activation; PDGFRβ mutants that do not associated with RasGAP undergo indirect action [23]. This capability of PDGFRβ may be utilized, although such scenarios have not yet been identified. These scenarios could include mutations of PDGFRβ that prevent it from binding RasGAP (GWAS studies indicate that such mutants exist). Recent proteomic studies demonstrate that the availability of adaptor proteins limits the output of receptor tyrosine kinases [24]. These conceptual advances suggest that a change in the relative expression level of signaling enzymes that associate with PDGFRβ (e.g. a relative decline in the abundance of RasGAP) would be another setting permissive for indirect activation of PDGFRβ.
Antibodies against PDGFRα are present in the serum of patients with scleroderma, and these autoantibodies activate PDGFRs by binding to their extracellular domain [25–27]. This mode of activating the PDGFRs results in increased type I collagen expression and conversion of fibroblasts to myofibrobalsts [25]. Such studies implicate this autoantibody-mediated mode of activation as a contributor to pathogenesis, which was confirmed by subsequent studies [28–30]. Even though not all groups detect autoantibodies or find that they are activating [31–34], subsequent clinical trials indicate a beneficial effect in some of the patients who were treated with tyrosine kinase inhibitors (TKIs) that antagonize PDGFRs [35].
E5 is a bovine papilloma virus protein that dimerizes PDGFRβ and thereby activates its kinase activity [36]. It is a short transmembrane protein that directly interacts with the transmembrane domain of PDGFRβ [37, 38]. Certain mutants of E5 not only fail to activate PDGFRβ, but prevent PDGF-dependent activation of PDGFRβ as well [38]. Since E5 does not interact with or activate PDGFRα [39, 40] such E5 mutants may be a unique opportunity to antagonize PDGFRβ.
Genetic lesions
There are 3 types of genetic lesions known to activate PDGFRs: point mutations, rearrangements and amplification. Point mutations within the kinase domain that activate the PDGFRα’s kinase activity are found in gastrointestinal stromal tumor cells [41]. In chronic monomyelocytic leukemia rearrangements involving PDGFB and TEL (which encodes a transcription factor) result in expression of a fusion protein between PDGFRβ and tel [42]. Un-mutated PDGFRA is amplified in glioblastoma [43], and this may contribute to pathogenesis since overexpression of wild type RTKs is sufficient for activation [44].
In summary, there are a variety of ways that PDGFRs can be activated. As outlined in the section below, distinct activation mechanisms result in non-identical biological consequences.
Consequences of activating the PDGFRs
PDGF-mediated
As mentioned above, similarities between certain PDGF and PDGFR knock out mice indicate that PDGF-dependent activation of PDGFRs is essential for normal development. Eliminating either PDGFRB, or PDGF-B reduces the number of pericytes and vascular smooth muscle cells, and thereby compromises the integrity and/or functionality of the vasculature in multiple organs, including the brain, heart, kidney, skin and eye [11, 45–47]. A distinct set of abnormalities manifest in mice lacking the PDGFRA gene, which are largely phenocopied when both PDGF-A, and PDGF-C genes are deleted [19, 48–50]. Taken together, these studies indicate that the PDGF-mediated mode to activate PDGFRs dominates embryogenesis.
In addition to physiological settings such as development, PDGF-dependent activation of PDGFRs can drive pathogenesis. The EWS/ETS chimeric transcription factor boosts expression of PDGF-C, which promotes the growth of Ewing tumors [51, 52].
Recent studies implicate PDGF-mediated activation of PDGFRs in neurodegenerative diseases [2]. The relevant anatomical location is the neurovascular unit (NVU), which governs the integrity of the blood brain barrier (BBB). Endothelial cells prevent the contents of the vasculature from leaking into the tissue by forming close-fitting seams amongst themselves. Pericytes, which reside perivascularly and share a basement membrane with the endothelium, enforce barrier function by preventing intracellular vesicular transport of plasma proteins. Recent studies investigating how pericytes accomplish this feat suggest that they direct vesicles to the lysosome instead of to the perivascular surface of the endothelial cell [53, 54]. Activation of PDGFRs, which are expressed by NVU pericytes and astrocytes, breaches the BBB [55]. In the context of stroke, the trigger for activation of PDGFRs appears to be PDGF-C, which is proteolytically processed as a result of a stroke-induced increase in the level of tPA (tissue plasminogen activator) [55–57]. Furthermore, chronic BBB dysfunction results in unmitigated inflammation, which is likely to contribute to neurodegeneration. Activation of the PDGF-C pathway prior to the onset of ALS (amyotropic lateral sclerosis) symptoms accelerates the onset of neurodegeneration [58]. Furthermore, blocking PDGF-C or inhibiting RTKs including PDGFRs protects from BBB breakdown in animal models of ischemic stroke, spinal cord injury, traumatic brain injury, multiple sclerosis and seizures [55, 59–62]. These findings reveal that PDGF-mediated activation of PDGFRs can breach the BBB, and thereby contribute to neurodegenerative diseases. Additional studies are required to determine how the PDGFR-expressing cell types of the NVU (astrocytes and pericytes) communicate with the endothelium to relax the barrier.
PDGF-independent
Like the PDGF-mediated mode of activating the PDGFRs (Fig 1), the PDGF-independent mode occurs in both pathological and physiological settings. The best-studied form of PDGF-independent activation of PDGFRs is the indirect mode, which is driven by non-PDGFs (Fig 2). It was discovered in the context of experimental PVR, and provides a plausible explanation for how cells survive and proceed to drive pathogenesis in the absence of a nurturing, pro-survival niche [8, 21]. Under these conditions PDGFRα persistently activates Akt to reduce the level of p53 [22]. Suppressing p53 enables cells to endure stressful conditions, which would otherwise trigger apoptosis and/or senescence. The scenario depends on prolonged activation of PDGFRα, for which there are two reasons. First, indirect activation involves monomeric PDGFRαs, which do not trigger self-destruction as do activated dimeric PDGFRαs. Second, indirect activation of PDGFRα engages a feed-forward loop that perpetuates activation of monomeric PDGFRα. These principles, which emerged from work with cultured cells and animal models of PVR, appear to be relevant to patients as well. Activated PDGFRs are found in membranes that develop in patients with PVR [63], and single nucleotide polymorphisms in the p53 pathway increase the likelihood of developing PVR [64, 65].
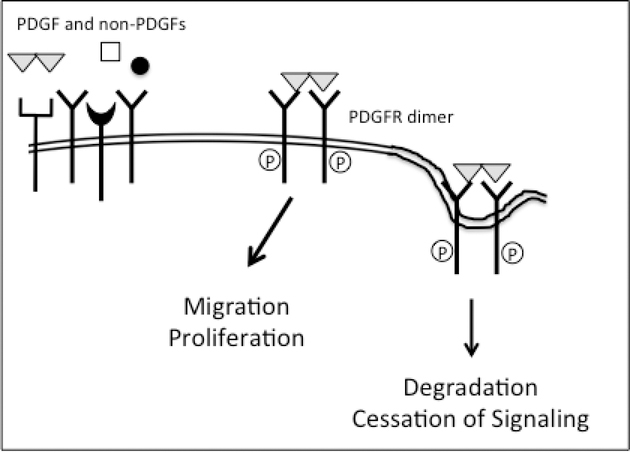
The relevant anatomical location for PVR is the vitreous, which abounds with growth factors, both PDGFs and non-PDGFs. This scenario begs the question of what happens when both types of PDGFR agonists are present. As illustrated in Fig 3, PDGF wins. This is because PDGF dimerizes PDGFR monomers and thereby makes them poor substrates for ROS-activated SFKs [66]. Furthermore, PDGF-activated PDGFR dimers are destined for degradation. This outcome is concentration dependent; there must be sufficient molecules of PDGF to engage enough PDGFRs that the indirect mode of activation would be diminished.
Figure 3. In the presence of both PDGF and non-PDGFs the PDGF-mediated mode of activation predominates.
Provided that there is enough PDGF to assemble PDGFRs into dimers, the PDGF-dependent mode of activation predominates even though non-PDGFs are present. This is the case because dimerized receptors are poor substrates for ROS activated SFKs. Furthermore, PDGF-mediated dimerization stimulates the internalization and degradation of PDGFRs; the half-life decreases from over 2 hours to 5 minutes.
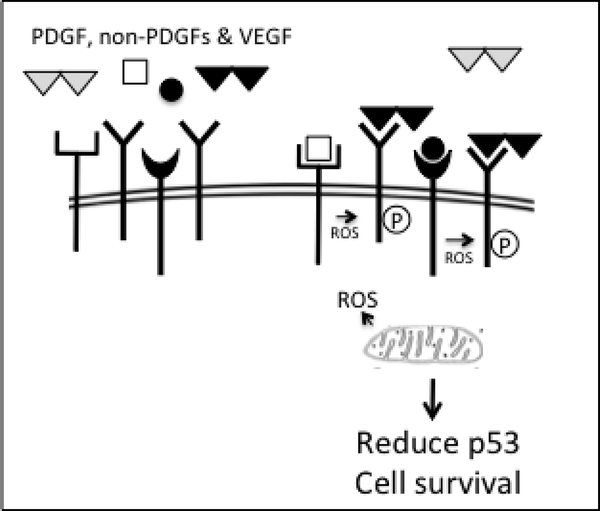
VEGF is elevated in vitreous of patients with PVR, even though this condition typically does not involve angiogenesis. This observation, reported by numerous investigators [67–70] suggested the VEGF functions beyond angiogenesis. Indeed, VEGF enables indirect activation of PDGFRα by antagonizing PDGF-dependent activation of PDGFRα (Fig 4). VEGF inhibits PDGF, which antagonize non-PDGFs. This conceptual insight guided efforts to develop approaches to prevent PVR. Instead of neutralizing many of the growth factors that are present in vitreous, targeting VEGF is sufficient [66, 71]. This is because removing VEGF allows PDGF to block the non-PDGFs, which are responsible for indirectly activating PDGFRα and thereby enabling survival of cells displaced into the vitreous (Fig 4).
Figure 4. The indirect mode of activating PDGFRα predominates when all three classes of growth factors that engage PDGFRα are present.
VEGF competes with PDGF for binding to PDGFRs [5]. Because VEGF does not dimerize PDGFRs, it sustains a population of monomeric PDGFRs in the face of PDGF. It is this population that can undergo indirect activation. This outcome is concentration dependent; there must be enough VEGF to overcome the PDGF, which dimerizes PDGFRs and thereby prevents indirect activation (Fig 3).
Thus in the context of PVR, where vitreal growth factors contribute to pathogenesis, there is a hierarchy amongst the three classes of growth factors that engage PDGFRα (Fig 5).
Figure 5. Hierarchy amongst the classes of growth factors that engage PDGFRα and are present in vitreous of patients afflicted by PVR.
VEGF competes with PDGF for binding to PDGFRs. PDGFs prevent non-PDGF-mediated activation of PDGFRα (the indirect mode) by dimerizing PDGFRs and accelerating their internalization and degradation. VEGF enables indirect activation of PDGFRα in the face of PDGF. These relationships are concentration dependent. Indirect activation of PDGFRα promotes PVR because it reduces the level of p53 and thereby enhances the viability of cells displaced into vitreous.
Subsequent studies revealed that indirect activation of PDGFRα also plays a role in physiological settings such as hypoxia. Hypoxia triggers many changes, including an increase in the level of VEGF, which provides immediate benefit for VEGFR-expressing cells. In addition to promoting survival of endothelial cells, VEGF also instructs them to generate new blood vessels, which alleviate hypoxia and thereby provides eventual benefit for even the VEGFR-negative cell types within the affected tissue. Since the time frame for angiogenesis is hours or even days, VEGFR-negative cell types within the hypoxic zone must be able to endure this period of stress. Surprisingly, VEGF also provides immediate benefit to some of the VEGFR-negative cell types. It does so by enabling indirect activation of PDGFRα, which, as mentioned above, promotes survival by lowering the level of p53 [72]. The way that VEGF promotes indirect activation of PDGFRα is by competing with PDGF for binding to PDGFRα [5]. While PDGF dimerizes PDGFRs, VEGF does not and hence maintains a supply of monomeric receptors, which are susceptible to indirect activation (Fig 4) [5]. Thus VEGF boost the survival of VEGFR-negative/PDGFRα-expressing cells within tissue (such as fibroblasts, pericytes, glial, epithelial and smooth muscle) by enabling indirect activation of PDGFRα. This particular example points to a beneficial consequence of indirect activation of PDGFRα that is likely during development and wound healing. Detrimental outcomes could result when PDGFRα positive cells use this same approach to endure the hypoxic stress within a solid tumor.
Genetic lesions
Genetic lesions that constitutively activate the kinase activity of PDGFRs cause receptor-specific phenotypes. PDGFRα mutant mice that harbor point mutations, which are present in some GIST (gastrointestinal stromal tumor) patients develop progressive fibrosis in multiple organs [14]. This is likely due to increased proliferation of fibroblasts and/or production of extracellular matrix proteins [19].
The phenotype of the PDGFRβ mutant mice reveals the PDGFRβ signaling influences progenitor cell fates [15]. Such mice exhibit reduced differentiation of aortic vascular smooth muscle cells and brain pericytes. Similarly, differentiation of adipose from pericytes and mesenchymal cells is suppressed. As discussed below, the distinct functionality of the two PDGFRs underscores the importance of developing approaches to selectively target each of the PDGFRs.
Therapeutic options
Existing ligand-directed therapies include aptamers and antibodies that selectively recognize a desired PDGF isoform [2, 50, 73]. They are typically highly selective, i.e. do not target growth factors outside of the PDGF family, and distinguish amongst the PDGF isoforms. The disadvantage of targeting a specific PDGF isoform is that it may not prevent activation of PDGFRs because multiple isoforms activate a given PDGFR (Table 1). For example, it is not possible to prevent PDGFRα activation by neutralizing PDGF-A because PDGF-B and PDGF-C also activate PDGFRα. Neutralizing several PDGFs overcomes this limitation, however this requires multiple reagents, and does not prevent the PDGF-independent modes of activating the PDGFRs.
The other approach to inhibit the PDGF/PDGFR family is by antagonizing the kinase activity of PDGFRs. The existing options include neutralizing PDGFR antibodies and tyrosine kinase inhibitors (TKIs). Many clinical trials with TKIs have been completed or are still ongoing, and their outcomes are encouraging [2, 50, 73, 74]. Unfortunately, it is challenging to interpret data generated with TKIs because they fail to distinguish between PDGFRs, and typically hit additional targets within the concentration necessary to block the kinase activity of PDGFRs. An increased appreciation of which PDGFR is contributing to pathology, biomarkers of its mode of activation, and receptor-specific antagonists are necessary to develop next generation therapeutic options for the PDGF/PDGFR family. Mutants of E5 that specifically block activation of PDGFRβ are an example of how this goal can be achieved [75]. Similarly, the PDGF-B/PDGFRβ interface can be selectively antagonized with a naturally occurring cyclopeptide termed destruxin A5 [76].
The recent failure of a clinical trial highlights our incomplete appreciation of PDGF’s role in vascular biology/pathology. Patients with the neovascular form of age-related macular degeneration rapidly lose vision because of macular edema that is caused by leakage of newly formed, immature blood vessels. Intravitreal injection of anti-VEGF has been tremendously successful because it restores vision, which constitutes a dramatic improvement in the quality of life in this elderly patient population. Unfortunately, the response to anti-VEGF is heterogeneous; ranging from a continued decline in vision to restoration of sight to 20/25 or better. The reason for the varied response to anti-VEGF therapy is unknown.
One of the ongoing strategies to improve the current standard of care (anti-VEGF monotherapy) is combo therapy that targets an additional growth factor that governs angiogenic homeostasis. PDGF was the focus of the Fovista Phase 3 clinical trial because neutralizing PDGF was expected to potentiate the effect of anti-VEGF. Antagonizing PDGF would prevent maturation of nascent blood vessels because pericyte recruitment, which drives maturation, is dependent on PDGF. Immature vessels are more dependent on VEGF than mature vessels. This was the rationale to expect that as compared with anti-VEGF mono therapy, the response of patients treated with anti-VEGF/anti-PDGF combo therapy would be more homogeneous, or a greater percentage of patients would achieve maximal restoration of vision, or a smaller percentage of patients would experience a continued decline in vision. There was no difference in the response of patients to mono versus combo therapy. These clinical results indicate that if combo therapy is to be effective, then VEGF needs a partner other than PDGF. Furthermore, they bring to light the possibility that anti-VEGF monotherapy includes a previously unappreciated benefit. When VEGF is abundant, PDGF is antagonized (Fig 4 and 5). Consequently, anti-VEGF activates PDGF, and thereby drives maturation, which stops the leakage of the newly formed blood vessels.
Conclusions
We currently appreciate that the PDGF/PDGFR family makes multiple contributions to physiology in distinct anatomical locations, and that the two PDGFRs have distinct functions. Furthermore, while ligand-driven activation is the major mode of de-repressing the kinase activity of PDGFRs, there are additional mechanisms. Activated PDGFRs trigger signaling events that direct a spectrum of cellular responses that underlie both physiology and pathology. Two types of advances will set the stage for a PDGF/PDGFR therapeutic era. First, a precise understanding of the role of the PDGF/PDGFR family in health and disease. Second, development of tools to monitor the amplitude and mode of each of the PDGFRs kinase activity, along with receptor-specific antagonists.
Acknowledgements
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series--a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE.
Footnotes
The corresponding Gene Wiki entry for this review can be found here:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fredriksson L, Li H and Eriksson U (2004) The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 15:197–204. [DOI] [PubMed] [Google Scholar]
- 2.Lewandowski SA, Fredriksson L, Lawrence DA and Eriksson U (2016) Pharmacological targeting of the PDGF-CC signaling pathway for blood-brain barrier restoration in neurological disorders. Pharmacol Ther. doi: 10.1016/j.pharmthera.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniades HN, Scher CD and Stiles CD (1979) Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A 76:1809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball SG, Shuttleworth CA and Kielty CM (2007) Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol 177:489–500. doi: jcb.200608093 [pii] 10.1083/jcb.200608093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennock S and Kazlauskas A (2012) Vascular Endothelial Growth Factor A Competitively Inhibits Platelet-Derived Growth Factor (PDGF)-Dependent Activation of PDGF Receptor and Subsequent Signaling Events and Cellular Responses. Mol Cell Biol 32:1955–66. doi: 10.1128/MCB.06668-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazlauskas A (1994) Receptor tyrosine kinases and their targets. Current Opinion in Genetics and Development 4:5–14. [DOI] [PubMed] [Google Scholar]
- 7.Heldin CH, Ostman A and Ronnstrand L (1998) Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1378:F79–113. [DOI] [PubMed] [Google Scholar]
- 8.Pennock S, Haddock LJ, Eliott D, Mukai S and Kazlauskas A (2014) Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res 40:16–34. doi: 10.1016/j.preteyeres.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Kazlauskas A, Bowen-Pope DF, Seifert R, Hart CE and Cooper JA (1988) Different effects of homo- and heterodimers of platelet-derived growth factor A and B chains on human and mouse fibroblasts. EMBO J. 7:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najy AJ, Won JJ, Movilla LS and Kim HR (2012) Differential tumorigenic potential and matriptase activation between PDGF B versus PDGF D in prostate cancer. Mol Cancer Res 10:1087–97. doi: 10.1158/1541-7786.MCR-12-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano P (1994) Abnormal kidney development and hematological disorders in PDGF b-receptor mutant mice. Genes Dev 8:1888–1896. [DOI] [PubMed] [Google Scholar]
- 12.Soriano P (1997) The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124:2691–2700. [DOI] [PubMed] [Google Scholar]
- 13.Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M and Soriano P (2001) The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol Cell 7:343–54. [DOI] [PubMed] [Google Scholar]
- 14.Olson LE and Soriano P (2009) Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16:303–13. doi: 10.1016/j.devcel.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson LE and Soriano P (2011) PDGFRbeta signaling regulates mural cell plasticity and inhibits fat development. Dev Cell 20:815–26. doi: 10.1016/j.devcel.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman JA, Luo J and Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–19. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD and Downward J (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras [see comments]. Nature 370:527–32. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD and Downward J (1996) Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. Embo J 15:2442–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Andrae J, Gallini R and Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22:1276–312. doi: 10.1101/gad.1653708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei H and Kazlauskas A (2014) A Reactive Oxygen Species-Mediated, Self-Perpetuating Loop Persistently Activates Platelet-Derived Growth Factor Receptor alpha. Mol Cell Biol 34:110–22. doi: 10.1128/MCB.00839-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei H, Rheaume MA and Kazlauskas A (2010) Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res 90:376–81. doi: S0014–4835(09)00321–2 [pii] 10.1016/j.exer.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei H, Velez G and Kazlauskas A (2011) Pathological signaling via platelet-derived growth factor receptor {alpha} involves chronic activation of Akt and suppression of p53. Mol Cell Biol 31:1788–99. doi: 10.1128/MCB.01321-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei H, Qian CX, Lei J, Haddock LJ, Mukai S and Kazlauskas A (2015) RasGAP Promotes Autophagy and Thereby Suppresses Platelet-Derived Growth Factor Receptor-Mediated Signaling Events, Cellular Responses, and Pathology. Mol Cell Biol 35:1673–85. doi: 10.1128/MCB.01248-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi T, Niepel M, McDermott JE, Gao Y, Nicora CD, Chrisler WB, Markillie LM, Petyuk VA, Smith RD, Rodland KD, Sorger PK, Qian WJ and Wiley HS (2016) Conservation of protein abundance patterns reveals the regulatory architecture of the EGFR-MAPK pathway. Sci Signal 9:rs6. doi: 10.1126/scisignal.aaf0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, Fraticelli P, Sambo P, Funaro A, Kazlauskas A, Avvedimento EV and Gabrielli A (2006) Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 354:2667–76. [DOI] [PubMed] [Google Scholar]
- 26.Moroncini G, Grieco A, Nacci G, Paolini C, Tonnini C, Pozniak KN, Cuccioloni M, Mozzicafreddo M, Svegliati S, Angeletti M, Kazlauskas A, Avvedimento EV, Funaro A and Gabrielli A (2015) Epitope Specificity Determines Pathogenicity and Detectability of Anti-Platelet-Derived Growth Factor Receptor alpha Autoantibodies in Systemic Sclerosis. Arthritis Rheumatol 67:1891–903. doi: 10.1002/art.39125 [DOI] [PubMed] [Google Scholar]
- 27.Svegliati S, Amico D, Spadoni T, Fischetti C, Finke D, Moroncini G, Paolini C, Tonnini C, Grieco A, Rovinelli M and Gabrielli A (2017) Agonistic Anti-PDGF Receptor Autoantibodies from Patients with Systemic Sclerosis Impact Human Pulmonary Artery Smooth Muscle Cells Function In Vitro. Front Immunol 8:75. doi: 10.3389/fimmu.2017.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, Moroncini G, Bacigalupo A, Leoni P, Avvedimento EV and Gabrielli A (2007) Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood 110:237–41. doi: 10.1182/blood-2007-01-071043 [DOI] [PubMed] [Google Scholar]
- 29.Kavian N, Servettaz A, Marut W, Nicco C, Chereau C, Weill B and Batteux F (2012) Sunitinib inhibits the phosphorylation of platelet-derived growth factor receptor beta in the skin of mice with scleroderma-like features and prevents the development of the disease. Arthritis Rheum 64:1990–2000. doi: 10.1002/art.34354 [DOI] [PubMed] [Google Scholar]
- 30.Luchetti MM, Moroncini G, Jose Escamez M, Svegliati Baroni S, Spadoni T, Grieco A, Paolini C, Funaro A, Avvedimento EV, Larcher F, Del Rio M and Gabrielli A (2016) Induction of Scleroderma Fibrosis in Skin-Humanized Mice by Administration of Anti-Platelet-Derived Growth Factor Receptor Agonistic Autoantibodies. Arthritis Rheumatol 68:2263–73. doi: 10.1002/art.39728 [DOI] [PubMed] [Google Scholar]
- 31.Balada E, Simeon-Aznar CP, Ordi-Ros J, Rosa-Leyva M, Selva-O’Callaghan A, Pardos-Gea J, Fonollosa-Pla V and Vilardell-Tarres M (2008) Anti-PDGFR-alpha antibodies measured by non-bioactivity assays are not specific for systemic sclerosis. Ann Rheum Dis 67:1027–9. doi: 10.1136/ard.2007.085480 [DOI] [PubMed] [Google Scholar]
- 32.Classen JF, Henrohn D, Rorsman F, Lennartsson J, Lauwerys BR, Wikstrom G, Rorsman C, Lenglez S, Franck-Larsson K, Tomasi JP, Kampe O, Vanthuyne M, Houssiau FA and Demoulin JB (2009) Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum 60:1137–44. doi: 10.1002/art.24381 [DOI] [PubMed] [Google Scholar]
- 33.Loizos N, Lariccia L, Weiner J, Griffith H, Boin F, Hummers L, Wigley F and Kussie P (2009) Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor alpha in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum 60:1145–51. doi: 10.1002/art.24365 [DOI] [PubMed] [Google Scholar]
- 34.Kurasawa K, Arai S, Owada T, Maezawa R, Kumano K and Fukuda T (2010) Autoantibodies against platelet-derived growth factor receptor alpha in patients with systemic lupus erythematosus. Mod Rheumatol 20:458–65. doi: 10.1007/s10165-010-0310-x [DOI] [PubMed] [Google Scholar]
- 35.Spiera RF, Gordon JK, Mersten JN, Magro CM, Mehta M, Wildman HF, Kloiber S, Kirou KA, Lyman S and Crow MK (2011) Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, phase IIa, single-arm, open-label clinical trial. Ann Rheum Dis 70:1003–9. doi: 10.1136/ard.2010.143974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai CC, Henningson C and DiMaio D (1998) Bovine papillomavirus E5 protein induces oligomerization and trans-phosphorylation of the platelet-derived growth factor beta receptor. Proc Natl Acad Sci U S A 95:15241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards AP, Xie Y, Bowers L and DiMaio D (2013) Compensatory mutants of the bovine papillomavirus E5 protein and the platelet-derived growth factor beta receptor reveal a complex direct transmembrane interaction. J Virol 87:10936–45. doi: 10.1128/JVI.01475-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Windisch D, Ziegler C, Grage SL, Burck J, Zeitler M, Gor’kov PL and Ulrich AS (2015) Hydrophobic Mismatch Drives the Interaction of E5 with the Transmembrane Segment of PDGF Receptor. Biophys J 109:737–49. doi: 10.1016/j.bpj.2015.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein DJ, Li W, Wang LM, Heidaran MA, Aaronson S, Shinn R, Schlegel R and Pierce JH (1994) The bovine papillomavirus type 1 E5 transforming protein specifically binds and activates the beta-type receptor for the platelet-derived growth factor but not other related tyrosine kinase-containing receptors to induce cellular transformation. J Virol 68:4432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staebler A, Pierce JH, Brazinski S, Heidaran MA, Li W, Schlegel R and Goldstein DJ (1995) Mutational analysis of the beta-type platelet-derived growth factor receptor defines the site of interaction with the bovine papillomavirus type 1 E5 transforming protein. J Virol 69:6507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD and Fletcher JA (2003) PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299:708–10. [DOI] [PubMed] [Google Scholar]
- 42.Golub TR, Barker GF, Lovett M and Gilliland DG (1994) Fusion of PDGF receptor b to a novel ets-like gene, tel, in chronic myelomoncytic leukemia with t(5;12) chromosomal translocation. Cell 77:1–20. [DOI] [PubMed] [Google Scholar]
- 43.Fleming TP, Saxena A, Clark WC, Robertson JT, Oldfield EH, Aaronson SA and Ali IU (1992) Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res 52:4550–3. [PubMed] [Google Scholar]
- 44.Kokai Y, Myers JN, Wada T, Brown VI, LeVea CM, Davis JG, Dobashi K and Greene MI (1989) Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell 58:287–92. [DOI] [PubMed] [Google Scholar]
- 45.Lindahl P, Johansson BR, Leveen P and Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277:242–5. [DOI] [PubMed] [Google Scholar]
- 46.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P and Betsholtz C (1998) Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125:3313–22. [DOI] [PubMed] [Google Scholar]
- 47.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E and Betsholtz C (1994) Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes and Development 8:1875–1887. [DOI] [PubMed] [Google Scholar]
- 48.Ding H, Wu X, Bostrom H, Kim I, Wong N, Tsoi B, O’Rourke M, Koh GY, Soriano P, Betsholtz C, Hart TC, Marazita ML, Field LL, Tam PP and Nagy A (2004) A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet 36:1111–6. doi: 10.1038/ng1415 [DOI] [PubMed] [Google Scholar]
- 49.Tallquist M and Kazlauskas A (2004) PDGF signaling in cells and mice. Cytokine Growth Factor Rev 15:205–13. [DOI] [PubMed] [Google Scholar]
- 50.Heldin CH (2014) Targeting the PDGF signaling pathway in the treatment of non-malignant diseases. J Neuroimmune Pharmacol 9:69–79. doi: 10.1007/s11481-013-9484-2 [DOI] [PubMed] [Google Scholar]
- 51.Zwerner JP and May WA (2001) PDGF-C is an EWS/FLI induced transforming growth factor in Ewing family tumors. Oncogene 20:626–33. doi: 10.1038/sj.onc.1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwerner JP and May WA (2002) Dominant negative PDGF-C inhibits growth of Ewing family tumor cell lines. Oncogene 21:3847–54. [DOI] [PubMed] [Google Scholar]
- 53.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR and Betsholtz C (2010) Pericytes regulate the blood-brain barrier. Nature 468:557–61. doi: 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- 54.Villasenor R, Ozmen L, Messaddeq N, Gruninger F, Loetscher H, Keller A, Betsholtz C, Freskgard PO and Collin L (2016) Trafficking of Endogenous Immunoglobulins by Endothelial Cells at the Blood-Brain Barrier. Sci Rep 6:25658. doi: 10.1038/srep25658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U and Lawrence DA (2008) Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med 14:731–7. doi: 10.1038/nm1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK and Lawrence DA (2003) Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest 112:1533–40. doi: 10.1172/JCI19212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y and Rosenberg GA (2011) Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42:3323–8. doi: 10.1161/STROKEAHA.110.608257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewandowski SA, Nilsson I, Fredriksson L, Lonnerberg P, Muhl L, Zeitelhofer M, Adzemovic MZ, Nichterwitz S, Lawrence DA, Hedlund E and Eriksson U (2016) Presymptomatic activation of the PDGF-CC pathway accelerates onset of ALS neurodegeneration. Acta Neuropathol 131:453–64. doi: 10.1007/s00401-015-1520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrams MB, Nilsson I, Lewandowski SA, Kjell J, Codeluppi S, Olson L and Eriksson U (2012) Imatinib enhances functional outcome after spinal cord injury. PLoS One 7:e38760. doi: 10.1371/journal.pone.0038760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adzemovic MV, Zeitelhofer M, Eriksson U, Olsson T and Nilsson I (2013) Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PLoS One 8:e56586. doi: 10.1371/journal.pone.0056586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharp KG, Yee KM and Steward O (2014) A re-assessment of treatment with a tyrosine kinase inhibitor (imatinib) on tissue sparing and functional recovery after spinal cord injury. Exp Neurol 254:1–11. doi: 10.1016/j.expneurol.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 62.Su EJ, Fredriksson L, Kanzawa M, Moore S, Folestad E, Stevenson TK, Nilsson I, Sashindranath M, Schielke GP, Warnock M, Ragsdale M, Mann K, Lawrence AL, Medcalf RL, Eriksson U, Murphy GG and Lawrence DA (2015) Imatinib treatment reduces brain injury in a murine model of traumatic brain injury. Front Cell Neurosci 9:385. doi: 10.3389/fncel.2015.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui J, Lei H, Samad A, Basavanthappa S, Maberley D, Matsubara J and Kazlauskas A (2009) PDGF receptors are activated in human epiretinal membranes. Exp Eye Res 88:438–44. doi: S0014–4835(08)00362-X [pii] 10.1016/j.exer.2008.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pastor-Idoate S, Rodriguez-Hernandez I, Rojas J, Fernandez I, Garcia-Gutierrez MT, Ruiz-Moreno JM, Rocha-Sousa A, Ramkissoon Y, Harsum S, Maclaren RE, Charteris D, van Meurs J, Gonzalez-Sarmiento R and Pastor JC (2013) The p53 codon 72 polymorphism (rs1042522) is associated with proliferative vitreoretinopathy: the Retina 4 Project. Ophthalmology 120:623–8. doi: 10.1016/j.ophtha.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 65.Pastor-Idoate S, Rodriguez-Hernandez I, Rojas J, Fernandez I, Garcia-Gutierrez MT, Ruiz-Moreno JM, Rocha-Sousa A, Ramkissoon Y, Harsum S, Maclaren RE, Charteris D, Vanmeurs JC, Gonzalez-Sarmiento R and Pastor JC (2013) The T309G MDM2 Gene Polymorphism Is a Novel Risk Factor for Proliferative Vitreoretinopathy. PLoS One 8:e82283. doi: 10.1371/journal.pone.0082283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pennock S, Kim D, Mukai S, Kuhnle M, Chun DW, Matsubara J, Cui J, Ma P, Maberley D, Samad A, Van Geest RJ, Oberstein SL, Schlingemann RO and Kazlauskas A (2013) Ranibizumab is a potential prophylaxis for proliferative vitreoretinopathy, a nonangiogenic blinding disease. Am J Pathol 182:1659–70. doi: 10.1016/j.ajpath.2013.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banerjee S, Savant V, Scott RA, Curnow SJ, Wallace GR and Murray PI (2007) Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Invest Ophthalmol Vis Sci 48:2203–7. [DOI] [PubMed] [Google Scholar]
- 68.Citirik M, Kabatas EU, Batman C, Akin KO and Kabatas N (2012) Vitreous vascular endothelial growth factor concentrations in proliferative diabetic retinopathy versus proliferative vitreoretinopathy. Ophthalmic Res 47:7–12. doi: 10.1159/000324200 [DOI] [PubMed] [Google Scholar]
- 69.Pennock S, Rheaume MA, Mukai S and Kazlauskas A (2011) A novel strategy to develop therapeutic approaches to prevent proliferative vitreoretinopathy. Am J Pathol 179:2931–40. doi: 10.1016/j.ajpath.2011.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dieudonne SC, La Heij EC, Diederen RM, Kessels AG, Liem AT, Kijlstra A and Hendrikse F (2007) Balance of vascular endothelial growth factor and pigment epithelial growth factor prior to development of proliferative vitreoretinopathy. Ophthalmic Res 39:148–54. doi: 10.1159/000103234 [DOI] [PubMed] [Google Scholar]
- 71.Pennock S, Haddock LJ, Mukai S and Kazlauskas A (2014) Vascular endothelial growth factor acts primarily via platelet-derived growth factor receptor alpha to promote proliferative vitreoretinopathy. Am J Pathol 184:3052–68. doi: 10.1016/j.ajpath.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pennock S, Kim LA and Kazlauskas A (2016) Vascular Endothelial Cell Growth Factor A Acts via Platelet-Derived Growth Factor Receptor alpha To Promote Viability of Cells Enduring Hypoxia. Mol Cell Biol 36:2314–27. doi: 10.1128/MCB.01019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heldin CH (2013) Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 11:97. doi: 10.1186/1478-811X-11-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borkham-Kamphorst E and Weiskirchen R (2016) The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev 28:53–61. doi: 10.1016/j.cytogfr.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 75.Petti LM, Talbert-Slagle K, Hochstrasser ML and DiMaio D (2013) A single amino acid substitution converts a transmembrane protein activator of the platelet-derived growth factor beta receptor into an inhibitor. J Biol Chem 288:27273–86. doi: 10.1074/jbc.M113.470054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, Wu X, Zhang A, Wang S, Hu C, Chen W, Shen Y, Tan R, Sun Y and Xu Q (2016) Targeting the PDGF-B/PDGFR-beta Interface with Destruxin A5 to Selectively Block PDGF-BB/PDGFR-betabeta Signaling and Attenuate Liver Fibrosis. EBioMedicine 7:146–56. doi: 10.1016/j.ebiom.2016.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]