Abstract
Dysbiosis of the gut microbiome in preterm infants predisposes the neonate to various major morbidities including neonatal necrotizing enterocolitis and sepsis in the neonatal intensive care unit (NICU), and adverse neurological outcomes later in life. There are parallel early developmental windows for the gut microbiota and the nervous system during prenatal to postnatal of life. Therefore, preterm infants represent a unique population in which optimization of initial colonization and microbiota development can affect brain development and enhance neurological outcomes. In this review, we will first discuss the factors affecting the assembly of neonatal gut microbiota and the contribution of dysbiosis in preterm infants to neuroinflammation and neurodevelopmental disorders. We then will discuss the emerging pathways connecting the gut microbiome and brain development. Further we will discuss the significance of current models for alteration of the gut microbiome (including humanized gnotobiotic models and exposure to antibiotics) to brain development and functions. Understanding the role of early optimization of the microbiome in brain development is of paramount importance for developing microbiome-targeted therapies and protecting infants from prematurity-related neurodevelopmental diseases.
Keywords: Microbiome, preterm, brain, neurodevelopment
1 |. INTRODUCTION
The impact of the microbiome on host development has been well documented with accumulating evidence suggesting an association between gut microbiota and brain function (Burokas, Moloney, Dinan, & Cryan, 2015); however, few studies have put emphasis on the trajectory of microbiome development and its impact on brain development in preterm infants. Preterm birth rates and the neurodevelopmental disabilities associated with preterm birth are rising despite collective efforts to improve both maternal and postnatal care (Bhutta, Cleves, Casey, Cradock, & Anand, 2002). Preterm infants, especially those born at less than 32 weeks gestational age and/or with a birth weight less than 1500g, are at increased risk for adverse neurological outcomes with later cognitive and behavioral deficits (Bhutta et al., 2002; Larroque et al., 2008; Woodward, Edgin, Thompson, & Inder, 2005). Preterm birth deprives preterm infants of a critical period of normal brain development and maturation in utero, since fundamental processes such as cortical and grey matter volumetric growth, neurogenesis, axonal and dendritic growth, synaptogenesis, and myelination begin as early as 20 weeks gestation (Borre, O’Keeffe, et al., 2014; Clouchoux, Guizard, Evans, du Plessis, & Limperopoulos, 2012; Huppi et al., 1998). The neurodevelopment of preterm infants is further confounded by the medical complications associated with preterm birth, such as hypoxia, intraventricular hemorrhages, periventricular leukomalacia, and metabolic and nutritional insults; as well as aspects of the rearing environment such as the child’s socioeconomic status (Duncan, Brooks-Gunn, & Klebanov, 1994; Evans & Schamberg, 2009). The gut microbiota composition at birth and the postnatal succession of microbial development differs between term and preterm infants (Koenig et al., 2011; La Rosa et al., 2014; Mackie, Sghir, & Gaskins, 1999; Palmer, Bik, DiGiulio, Relman, & Brown, 2007; Underwood & Sohn, 2017). Targeting the critical developmental windows of both microbiome and brain for potential neuroprotective strategies for preterm infants has potential therapeutic significance (Borre, Moloney, Clarke, Dinan, & Cryan, 2014; Borre, O’Keeffe, et al., 2014; Diaz Heijtz et al., 2011). However, the impact of early colonization and development of the preterm infants’ microbiome on later brain development is largely unknown. In this review, we will present the current knowledge of the factors influencing the assembly and development of the preterm infant gut microbiota, the risks for neurodevelopment deficits associated with dysbiosis in preterm infants and potential mechanistic pathways linking brain and microbiota development.
2 |. FACTORS AFFECTING THE ASSEMBLY OF NEONATAL GUT MICROBIOTA
Information generated by studies to describe the development of microbial communities is confounded by geographical and demographic differences, hospital environments, standard of care protocols, and sampling and sequencing regimens that contribute to a much more complex picture than initially anticipated. Nonetheless, the general consensus emerging from these studies is that there are differences between term and preterm infants in gut microbiota composition and the postnatal microbial development (Grier et al., 2017; Schwiertz et al., 2003).
2.1 |. Initial bacterial colonization and succession
Our early study demonstrated that the intestinal microbiome samples from preterm infants up through five weeks of life cluster distinctly from those of a full term breast-fed infant, and that the microbial patterns converge toward those of full term breast-fed infants only at or after six weeks of life (Claud et al., 2013). We and others further demonstrated that the microbiome of the preterm infant is characterized by low diversity and high inter-individual variation (Claud et al., 2013; Dahl et al., 2018; Kurokawa et al., 2007; Moles et al., 2013) with succession of bacterial classes from Bacilli to Gammaproteobacteria to Clostridia (Itani et al., 2017; Korpela et al., 2018; La Rosa et al., 2014; Underwood & Sohn, 2017). Postmenstrual age (PMA), which is the gestational age (GA) plus age of life, has been reported as the major driver in the development of the microbiome for preterm infants specifically for the rate of the assembly, with the slowest rate found in the most premature of infants (between 25 to 30 weeks of PMA) (Korpela et al., 2018; La Rosa et al., 2014). A recent study corroborated this finding, demonstrating that GA is the dominant factor in microbiome assembly; independent of confounders such as mode of delivery, breastfeeding duration and antibiotic exposure with preterm infant microbiota demonstrating lower diversity and more Proteobacteria and Enterococcus compared with full-term infants at 10 days postpartum (Dahl et al., 2018). However, the difference between preterm and term infants in bacteria diversity was not observed at four months or 12 months postpartum, consistent with our study observing a shift of preterm microbiome to full term infant microbiome patterns at six weeks of age and later (Claud et al., 2013) and confirming an age-dependent maturation of the preterm infant microbiome.
Preterm infants, however, might not necessarily differ from the term infant in the succession sequence of bacteria (Korpela et al., 2018; La Rosa et al., 2014). Initial colonizing genera are mostly facultative anaerobes including enterobacteria, coliforms, Lactobacilli and Streptococci, which are then replaced by anaerobic genera such as Bifidobacterium, Bacteroides, Clostridium and Eubacterium by the end of the first week of life (Jost, Lacroix, Braegger, & Chassard, 2012). The microbial community at PMA between 25 and 30 weeks is dominated by Staphylococcus, followed by Enterococcus dominance from 30 weeks to 35 weeks, and Enterobacteriaceae dominance (mainly genus Enterobacter in the preterm infants) at 35 weeks PMA. Bifidobacterium dominance, which is characteristic of healthy term-born infants, starts to develop gradually only after 30 weeks PMA (Korpela et al., 2018; La Rosa et al., 2014). Preterm infants have a delayed progression to a Bifidobacterium-dominated community compared to term infants because of a prolonged dominance of Enterobacteriaceae after 35 weeks PMA (Backhed et al., 2015; Butel et al., 2007; Korpela et al., 2018). These data demonstrate that while the succession of bacteria at the genus level is similar, dominance is different between preterm and term infants.
2.2 |. Factors influencing the assembly
There are several environmental determinants affecting the initial colonization and the course of development of the gut microbiota. Mode of delivery is known to play a significant role in the natural assembly of the neonate gut microbiota in term infants (Backhed et al., 2015; Dominguez-Bello et al., 2010). However, with PMA and GA as the major drivers for preterm infant microbiome development, and due to the low number of preterm spontaneous vaginal deliveries without any confounding factors such as antibiotic exposure recruited to most of the studies, the impact of mode of delivery in preterm infants is difficult to asses (Hill et al., 2017). Early studies of full term infants (Backhed et al., 2015; Dominguez-Bello et al., 2010; Penders et al., 2006) demonstrated that the pioneer colonizers of vaginally delivered full term infants resemble the mother’s vaginal microbiota (e.g. Lactobacillus, Prevotella, Sneathia spp.). Term infants delivered by cesarean section (c-section) are colonized by microbiota derived from their mother’s skin (e.g. Staphylococcus, Corynebacterium, and Propionibacterium spp.) and possibly healthcare workers and environment. Furthermore, infants delivered by c-section show decreased gut microbiota diversity and delayed colonization of Bacteroides and Bifidobacterium spp. (Dominguez-Bello et al., 2010). However, a recent larger cohort study found that differences in infant gut microbiome could not be significantly attributed to a c-section or vaginal mode of delivery (Chu et al., 2017). Additional analysis revealed that the association between mode of delivery and neonatal gut microbiota was only pronounced when comparing unlabored c-sectioned infants to vaginally delivered infants. Furthermore, low levels of Bacteroides and Bifidobacterium were equally observed in both c-section and vaginally-delivered neonates in disagreement with early studies (Chu et al., 2017; Yassour et al., 2016). For preterm infants, several studies have reported that the mode of delivery does not influence the bacteria diversity difference between preterm and term infants (Dahl et al., 2018; Hill et al., 2017). Furthermore, a more recent study of preterm infant gut microbiome supported the previously mentioned studies that only GA at birth but not antibiotic exposure in the first few days of life nor mode of delivery significantly impact the bacterial alpha-diversity among preterm infants (Chernikova et al., 2018). This study demonstrated that vaginal delivery was associated with greater Bacteroides abundance agreeing with most of the studies in term infants, but had no significant effects in preterm infants on bacterial abundance of Streptococcus, Bacteroides, and Lactobacillus (Chernikova et al., 2018). It’s worth emphasizing the low enrollment number of spontaneous vaginal delivery of preterm term infants in these studies thus emerging studies with more patients might yield more precise insight into the impact of delivery mode on preterm infant microbiota.
Whether the infants are breastfed or formula-fed also affects the microbiome in the gut (O’Sullivan, Farver, & Smilowitz, 2015). However, current studies do not allow us to fully assess the impact of own mother’s milk feeding to preterm infants on early microbiome assembly due to the low numbers of preterm infants exclusively breast fed and the various timings that formula feeding are introduced or added (Dahl et al., 2018). Therefore, data on the effect of breast milk vs formula on microbiome development is largely from studies in term infants. In term infants, exclusively breastfed infants have increased numbers of taxa from the protective bacterial class Actinobacteria (i. e. Bifidobacterium spp.) while formula-fed infants have a higher diversity and increased levels of Escherichia. coli, Clostridium difficile, Bacteroides fragilis and Lactobacilli (Backhed et al., 2015; Bezirtzoglou, Tsiotsias, & Welling, 2011; Favier, Vaughan, De Vos, & Akkermans, 2002; Penders et al., 2006). Even though the intestinal microbiota diversity is significantly lower (Backhed et al., 2015; Thompson, Monteagudo-Mera, Cadenas, Lampl, & Azcarate-Peril, 2015), in breast-fed infants, their microbial communities interact significantly more with host genes compared with formula-fed infants, and their transcriptomic activities are more associated with immune response and metabolic activities (Praveen, Jordan, Priami, & Morine, 2015; Schwartz et al., 2012). For example, there is enrichment for anti-inflammatory genes and genes required for the utilization of human milk oligosaccharides (HMOs) from breast milk in breast fed infant host epithelial cells (Schwartz et al., 2012) . Utilization of different HMOs, for example fucosylated oligosaccharides, can promote the growth of Bifidobacterium longum and several species of Bacteroides leading to a transition from proinflammatory bacteria such as Escherichia. coli and Clostridium perfringens (Marcobal et al., 2011; Yu et al., 2013).
The composition of gut microbiota is also affected by the timing, duration and type of antibiotic exposure in both preterm and term infants (Fouhy et al., 2012; Gasparrini et al., 2016; Gibson et al., 2016; Yassour et al., 2016). Intrapartum antibiotic prophylaxis is associated with reduced diversity and lower abundance of Lactobacilli and Bifidobacteria in the neonatal gut (Mueller, Bakacs, Combellick, Grigoryan, & Dominguez-Bello, 2015). In preterm infants, three broad-spectrum antibiotics (meropenem, cefotaxime, and ticarcillin-clavulanate) were significantly associated with decreased species richness of the preterm gut microbiota; whereas ampicillin, vancomycin and gentamicin were not associated with the diversity of the gut microbiota in preterm infants (Gibson et al., 2016). More interestingly, after antibiotic treatment, potential pathogenic species such as Klebsiella pneumonia, Escherichia coli, and Enterobacter cloacae harbored more antibiotic resistance genes than commensal Bifidobacterium spp. (Gibson et al., 2016), increasing the risk for opportunistic pathogenic bacteria dominance. In term infants, parenteral ampicillin and gentamicin administration (within 48 hours of birth) resulted in a significantly increased abundance of Proteobacteria and decreased abundance of Actinobacteria (particularly Bifidobacterium genus) and Lactobacillus four weeks after the treatment compared to the untreated controls (Fouhy et al., 2012). The levels of Actinobacteria, Bifidobacterium and Lactobacillus were similar, but the proportion of Proteobacteria remained significantly higher than those of the controls after eight weeks of treatment.
While post discharge environmental factors including social economic status in general change the development of microbiome (Bailey et al., 2011), for preterm infants the neonatal intense care unit (NICU) in which they might stay for up to months has been one of the early postnatal environments shaping the assembly of the preterm infant microbiota (Brooks et al., 2018). A strong link has been shown between NICU-specific Operational Taxonomic Units and the occupancy of these taxa in the gut microbiota of preterm infants, mostly mediated by healthcare providers and cleaning regimen (Brooks et al., 2018). This study demonstrated the unique room microbiome character of the NICU and its bidirectional interaction with preterm infants and suggests that approaches to change NICU microbiome might be an effective pathway to manipulating the early microbiome of the preterm infants.
To summarize, the early window for gut microbial establishment is critical. Host biology such as PMA and GA as well as environmental factors drive the assembly of neonatal gut microbiota with significant functional implications. Preterm infants differ from term infants and represent a unique population in which modifying initial colonization and microbiota development can potentially provide benefits to enhance maturation and offset the adverse morbidities associated with prematurity.
3 |. CONTRIBUTION OF DYSBIOSIS IN PRETERM INFANTS TO NEUROINFLAMMATION AND NEURODEVELOPMENTAL OUTCOMES
3. 1 |. Dysbiosis and neuroinflammation
Prematurity is associated with significant risk for neurologic complications including periventricular leukomalacia (Huang et al., 2017), cerebral palsy (Hirvonen et al., 2014), attention-deficit/hyperactivity disorder (Bhutta et al., 2002; Sucksdorff et al., 2015), and reduced cognitive performance (Bhutta et al., 2002). Prematurely born children have specific deficits in sustained attention, visuospatial processing, and spatial working memory when evaluated at 3–4 years of age compared to age-matched term infants (Vicari, Caravale, Carlesimo, Casadei, & Allemand, 2004). Dysbiosis, or altered gut bacterial composition, has been associated with the pathogenesis of inflammatory diseases and infections that have long term adverse neurological outcomes in preterm infants, such as necrotizing enterocolitis and sepsis (Underwood & Sohn, 2017).
Clinically, neonatal infections affect approximately 1% of all live births and 80% of the neonatal infections occur in infants born preterm, placing them at significantly higher risk of bacterial infection than term infants (Berardi et al., 2013; Hornik et al., 2012; Strunk et al., 2014). Neonatal sepsis is an independent risk factor for neurodevelopmental impairment in extremely preterm infants (Schlapbach et al., 2011). Current knowledge of the detrimental effects of neonatal infection has suggested a link with the release and circulation of pro-inflammatory bacteria-derived molecules that induce systemic inflammation resulting in brain injury and neurodevelopmental abnormalities (Strunk et al., 2014). Systemic inflammatory products can act on the blood-brain barrier (BBB) or across the BBB to trigger the activation of microglia, resulting in the white matter injury of periventricular leukomalacia (Volpe, 2005) . Chorioamnionitis is an important risk factor for both early-onset neonatal infection (Martius et al., 1999) and white matter injury (Chau, McFadden, Poskitt, & Miller, 2014) and is associated with increased incidence of periventricular leukomalacia and cerebral palsy (Schlapbach et al., 2011; Wu & Colford, 2000). Neonatal sepsis is also associated with an increased risk for bacterial meningitis from blood-borne distribution of bacteria through the blood–brain barrier (BBB) or the blood-CSF barrier into the brain (Kim, 2003; Koedel, Scheld, & Pfister, 2002) resulting in neuroinflammation evidenced by increased concentrations of TNF-α, IL-1β and IL-6 (Krebs, Okay, Okay, & Vaz, 2005).
Numerous animal experiments have modeled maternal infection, maternal and fetal chorioamnionitis, and neonatal infection to investigate the role of microbiota-related inflammation in brain development (Ginsberg, Khatib, Weiner, & Beloosesky, 2017; Hagberg et al., 2015; Strunk et al., 2014). The experimental designs have included intrauterine inflammation modeled by lipopolysaccharide (LPS) (Burd et al., 2010; Dada et al., 2014; Elovitz et al., 2011; Leitner et al., 2014), maternal systemic inflammation modeled by LPS (Beloosesky, Gayle, & Ross, 2006; Ginsberg, Khatib, Weiner, et al., 2017; Ginsberg, Khatib, Weiss, et al., 2017), neonatal meningitis modeled by injection of Streptococcus pneumoniae or Streptococcus agalactiae into neonatal pups (Barichello et al., 2011; Mittal, Krishnan, Gonzalez-Gomez, & Prasadarao, 2011), and neonatal infection modeled by toll-like receptor ligands and proinflammatory cytokines administrated both systematically and locally (Cai, Lin, Pang, & Rhodes, 2004; Du et al., 2011; Smith, Hagberg, Naylor, & Mallard, 2014; X. Wang et al., 2009). Studies using these models have all demonstrated neuroinflammation in the brain evidenced by local proinflammatory cytokine production including IL-1β, TNF, and IL-6. Additionally, these studies have demonstrated associated white matter damage evidenced by myelination deficits, and microglia activation (Hagberg et al., 2015).
To specifically study the impact of preterm infant dysbiosis on neuroinflammation, we used a fecal transfaunation model. We demonstrated that the microbiota of a preterm infant associated with a poor growth phenotype when transfaunated to germ free (GF) pregnant mice was associated with both systemic inflammation and neuroinflammation in the pups as evidenced by elevated pro-inflammatory mediators including IL-1β, TNF, and IFNγ in the circulation and increased NOS1 in the brain (J. Lu et al., 2018; L. Lu et al., 2015). Emerging studies using whole microbial communities associated with a phenotype instead of a single bacterial product, i.e. a cytokine or a bacterial component, shed new light on how dysbiosis of the gut microbiota impacts neurological development, raising the possibility of whole microbiota manipulation as a therapeutic target.
3.2 |. Dysbiosis and neurodevelopmental outcomes
3.2.1 |. Dysbiosis and attention deficit hyperactivity disorder
Attention deficit hyperactivity disorder (ADHD) is the most prevalent neurodevelopmental disorder, and is highly heritable with other contributing environmental factors such as diet and microbiome also influencing risk (David et al., 2014; Nigg, Lewis, Edinger, & Falk, 2012). In the first multi-site cohort study to compare the microbiome of ADHD patients and healthy controls (Aarts et al., 2017), ADHD individuals had an increase of Actinobacteria mainly at the expense of Firmicutes compared to the healthy controls. Bacteroidetes and other phyla did not differ significantly in relative abundance between healthy participants and those with ADHD. The genus Bifidobacterium within the phylum Actinobacteria was significantly increased in ADHD cases. Cyclohexadienyl dehydratase, an enzyme participating in the biosynthesis of phenylalanine (a dopamine precursor), was functionally predicted by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt, a bioinformatics software package designed to predict metagenome functional content) to be significantly more abundant in the microbiome of ADHD cases. Functional magnetic resonance imaging (fMRI), also predicted altered CDT levels that were negatively related to reward anticipation responses, which is a hallmark for ADHD (Aarts et al., 2017). Another study (Partty, Kalliomaki, Wacklin, Salminen, & Isolauri, 2015) with a smaller sample size treated seventy-five babies between 0–6 months with a probiotic (Lactobacillus rhamnosus) or placebo, and found that the placebo group had a higher diagnosis of ADHD at age thirteen and a lower abundance of Bifidobacterium at diagnosis. More studies are warranted to understand the link between ADHD and altered microbiome composition and potentially more importantly the functionality of the microbiome.
3.2.2 |. Dysbiosis and autism spectrum disorders (ASDs)
While many clinical studies show altered microbial profiles in ASD, there is a high heterogeneity in the findings (Cao, Lin, Jiang, & Li, 2013; Kelly, Minuto, Cryan, Clarke, & Dinan, 2017). A recent systematic review (Cao et al., 2013) validated the notion that there are alterations in the microbial communities in ASD but conflicting results are reported in the prevalence of the three main phyla of GI bacteria namely Firmicutes, Bacteroidetes and Proteobacteria (Finegold et al., 2010; Kang et al., 2013; Williams et al., 2011), as well as Clostridium genus bacteria (Finegold et al., 2010; Finegold et al., 2002; Parracho, Bingham, Gibson, & McCartney, 2005; Song, Liu, & Finegold, 2004), and Bifidobacterium (genus or species) (Adams, Johansen, Powell, Quig, & Rubin, 2011; Finegold et al., 2010; Gondalia et al., 2012; L. Wang et al., 2011) between children with ASD and controls. The inconsistent findings of these studies may be associated with the use of antibiotics and marked dietary variations in ASD patients, which confound the interpretation of microbiota profiles in ASD individuals. However, recent studies focusing on bacterial metabolites might bring another dimension to investigate the association between dysbiosis and ASD. Fecal total short chain fatty acids; namely acetic, butyric, isobutyric, valeric, isovaleric acid concentrations and fecal ammonia were significantly higher in children with ASD compared to controls (L. Wang et al., 2012). ASD individuals also had lower levels of urinary hippurate, phenylacetylglutamine and 4- cresol sulfate than the healthy controls (Yap et al., 2010). Their precursors, benzoic acid and phenylacetic acid, are produced by bacterial metabolism in the intestine (Yap et al., 2010). Therefore, these studies suggest that an approach to study changes in gut microbiota metabolism by metabolic profiling might provide additional information for the interaction between host and microbiome relevant to ASD.
3.2.3 |. Dysbiosis and schizophrenia spectrum disorder
The incidence of schizophrenia is positively correlated with preterm birth and associated with microbiota changes (Gibson et al., 2016) (Nosarti et al., 2012), suggesting that schizophrenia is a neuropsychiatric disorder where microbiome early in life might be involved in the disease process (Schwarz et al., 2018; Severance, Prandovszky, Castiglione, & Yolken, 2015). Limited clinical studies have investigated the gut microbial composition in schizophrenia (Schwarz et al., 2018) with a few studies also analyzing the oropharyngeal microbiome (Castro-Nallar et al., 2015; Yolken et al., 2015). According to the latest study (Schwarz et al., 2018), the gut microbiota in schizophrenia First Episode Psychosis (FEP) patients was different from healthy controls. At the family level, Lactobacillaceae, Halothiobacillaceae, Brucellaceae, and Micrococcineae were increased, whereas Veillonellaceae was decreased in FEP patients. At the genus level, Lactobacillus, Tropheryma, Halothiobacillus, Saccharophagus, Ochrobactrum, Deferribacter, and Halorubrum were increased, and Anabaena, Nitrosospira, and Gallionella were decreased in FEP. A small study (n = 32) of the oropharyngeal microbiome in schizophrenia showed an increased abundance of Lactobacillus in schizophrenia patients, in addition to, Bifidobacterium and Ascomycota, compared to healthy controls (Castro-Nallar et al., 2015).
There are complex and significant limitations in study design, methodologies and power in clinical studies investigating the link between the microbiome and specific neurodevelopmental disorders. Another challenge comes from the difficulty in defining a “healthy” microbiome. Gut microbiota profiles of healthy individuals have high interpersonal and population variation (Backhed et al., 2015; Falony et al., 2016; Zhernakova et al., 2016). Nonetheless, these emerging clinical studies, while largely cross sectional, begin to explore the potential link between microbial composition and functionality and neurodevelopmental diseases. Further studies are warranted to test the possibility of manipulating the microbiome in early life as a preventive strategy.
4 |. EMERGING PATHWAYS CONNECTING MICROBIOME AND BRAIN DEVELOPMENT
4.1 |. Current models of alteration of gut microbiome
The current experimental approaches to investigate the effect of microbiota on development include the use of the GF mouse model; “humanization” of the mouse gut microbiota, which is transplanting fecal microbiota from specific human conditions to germ free mice; and using antibiotics to manipulate gut microbiota.
GF mice have been crucial in establishing the role of the commensal gut microbiota in early brain development and behavior (Clarke et al., 2013; Neufeld, Kang, Bienenstock, & Foster, 2011). Pioneer studies have demonstrated that GF mice exhibit increased exploratory activities, reduced anxiety, exaggerated hypothalamic-pituitary response to stress, and impaired non-spatial and working memory (Diaz Heijtz et al., 2011; Gareau et al., 2011; Neufeld et al., 2011; Sudo et al., 2004). More interestingly, these studies have also emphasized that normalization of behaviors in GF mice can be only achieved when GF mice are reconstituted with specific-pathogen free (SPF) microbiota at an early stage of life, highlighting that early modification of the microbial community might be critical in shaping brain function.
While GF mice are a foundational tool, they poorly represent microbiota dysbiosis in animals or humans and might have compensatory mechanism in their physiology (Frohlich et al., 2016; Rogers et al., 2016). However, GF mice can be colonized with a single strain of bacteria/probiotics or a microbial community associated with defined conditions or disease phenotypes. The “humanized” GF model which is colonization of a microbial community representing a state or disease in humans has been used extensively to test the hypothesis that disease- or lifestyle-associated dysbiosis contributes or predisposes humans to certain physical conditions (Arrieta, Walter, & Finlay, 2016). For example, GF mice colonized with a bacterial component of obese co-twins’ fecal microbiota had significantly higher increases in body mass and adiposity than those colonized with microbiota from lean co-twins’ fecal samples (Ridaura et al., 2013). Transplanting microbiota from six- to 18-month old healthy or undernourished children into young germ-free mice revealed that immature microbiota from undernourished infants can transmit impaired growth phenotypes (Blanton et al., 2016). However, most studies have used adult GF mice and few studies of microbial-colonized GF mice have modeled the early life dysbiosis of preterm infants associated with early brain development. One approach to model the early effects on newborn development is to colonize GF pregnant dams with specific microbial communities and evaluate the offspring neurodevelopment. Using this model, we have demonstrated that maternal colonization with microbiota from a poor growth human infant resulted in altered neuronal development evidenced by decreased expression of the neuronal development marker NeuN and an altered myelination process evidenced by reduced expression of the myelination marker myelin basic protein at both pre-weaned and immediate post-weaned ages of the offspring (J. Lu et al., 2018). The preterm infant microbiota effects on brain development were mediated by local and systemic IGF-1 levels and neuroinflammation. Furthermore, in behavioral tests, offspring from GF dams transfaunated with human preterm infant fecal lysates from a patient who developed necrotizing enterocolitis (NEC), a disease known to have adverse long term neurological outcomes, had a slower spatial learning curve, decreased memory and locomotion deficits at post-weaned age (unpublished data, Claud lab).
Empiric antibiotic treatment, defined as treatment based solely on clinical suspicion of infection without a positive culture result, is a common practice in the NICU due to concerns about intrauterine infection as the cause of spontaneous premature labor, premature rupture of membranes, and chorioamnionitis (Clark, Bloom, Spitzer, & Gerstmann, 2006). However, studies on long term effects of antibiotic use in early life on infant development are lacking. Animal studies have demonstrated that antibiotic-induced short-term disruption of the intestinal microbial community (dysbiosis) impairs cognitive performance (Bercik et al., 2011; Frohlich et al., 2016). The majority of data on microbiome, antibiotics, and neurodevelopment is from mouse studies. Studies in C57BL/6N mice have shown a significantly different microbial community with reduced bacterial load and diversity in antibiotic-treated compared to vehicle-treated mice using principal coordinate analysis (PCoA) (beta diversity describing similarity between samples used to generate a distance coordinate plot). Antibiotic-treated mice also had altered circulating metabolites and a disrupted working and spatial memory by novel object recognition test (Frohlich et al., 2016). Oral, but not intraperitoneal, antimicrobial administration to specific-pathogen free (SPF) mice transiently altered the composition of the microbiota and increased exploratory behavior and hippocampal expression of Brain-derived neurotrophic factor (BDNF) (Bercik et al., 2011). A recent study (Jang, Lee, Jang, Han, & Kim, 2018) extended the previous findings demonstrating that ampicillin treatment resulted in dysbiosis and specifically noted increased Proteobacteria, decreased lactobacilli and increased fecal and blood LPS levels. Furthermore, the antibiotic treatment was associated with anxiety and neuroinflammation evidenced by increased recruitment of microglia, monocytes and dendritic cells to the hippocampus and activation of NF-κB in the brain (Jang et al., 2018).
Taken together, animal models have suggested that non-optimized microbial communities influence brain development. Studies focusing on the effects of microbial communities from antibiotic-treated preterm infants and preterm infants with specific brain function deficits will provide mechanistic insights for which early microbiome optimization may improve neurological outcomes associated with prematurity.
4.2 |. Potential mechanistic pathways
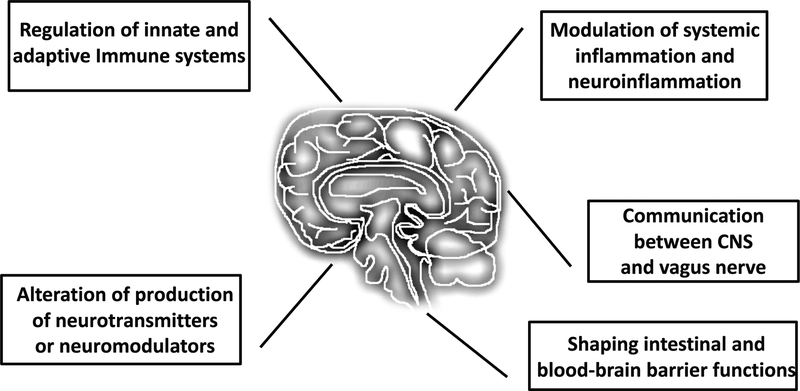
Despite the collective efforts to dissect the gut brain connection, the mechanistic nature of this bidirectional communication has yet to be fully elucidated. There are several proposed mechanisms that could potentially be relevant to the preterm infants, however, few studies have been designed to explore the mechanisms by which the microbiome specifically affects preterm infant brain development. To date, immune system modulation (Berer et al., 2011; Erny et al., 2015; Honda & Takeda, 2009; Lee, Menezes, Umesaki, & Mazmanian, 2011; Sampson & Mazmanian, 2015), microbial metabolites (Bourassa, Alim, Bultman, & Ratan, 2016) and vagus nerve activation (Bonaz, Bazin, & Pellissier, 2018) are the most studied pathways linking gut microbiota and neurological development (summarized in Figure 1).
Figure 1.
Potential mechanistic pathways by which the gut microbiome may influence maturation of the preterm infant brain. Potential communication/connections between gut microbiota and brain that might have implications in preterm infant brain development include immune system modulation, neurotransmitters and/or neuromodulators produced by gut microbiota, alteration of systemic inflammation and neuroinflammation, signaling between CNS and vagus nerve, and regulation intestinal and blood-brain barrier functions.
A key signaling pathway involves microbial modulation of both the innate and adaptive immune system (Levy, Kolodziejczyk, Thaiss, & Elinav, 2017; Thaiss, Zmora, Levy, & Elinav, 2016). Microbiota can induce epigenetic changes in innate lymphoid cells, referring to changes in DNA methylation and histone modification resulting in altered pattern of gene transcription (Gury-BenAri et al., 2016). The microbial metabolite n-butyrate affects gene expression by inhibiting the activity of histone deacetylases (HDACs) in bone marrow-derived macrophages and isolated colonic lamina propria macrophages (Chang, Hao, Offermanns, & Medzhitov, 2014). Microglia are the macrophages of the brain and play a critical role in brain development (Erny et al., 2015). GF mice have significantly more activated microglia and early myeloid cell activation markers expressed in the microglia in many regions of the brain (Erny et al., 2015). GF mice also have been shown to have impaired innate immune responses as intraperitoneal injections of LPS result in attenuated neuroinflammation (Erny et al., 2015), demonstrating the role of microbiota in regulating a potential link between systemic inflammation and neuroinflammation. Recently, bacterial peptidoglycan (PGN) derived from the commensal gut microbiota was shown to be translocated into the brain and sensed by specific pattern-recognition receptors (PRRs) of the innate immune system through PGN-sensing molecules such as TLR2 and PGN-recognition proteins 2–4 in the brain (Arentsen et al., 2017). This finding may be a novel signaling pathway to directly link microbial components to the brain.
The microbial and brain connection can also be mediated through the production of bacterial metabolites. Short chain fatty acids (SCFAs) are bacteria fermentation products that have been implicated in the pathogenesis of several neurologic conditions including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and autism (Bourassa et al., 2016). Butyrate is a HDAC inhibitor and in mouse models of Alzheimer’s disease, butyrate treatment restored histone acetylation and increased expression of learning-associated genes (Govindarajan, Agis-Balboa, Walter, Sananbenesi, & Fischer, 2011; Kilgore et al., 2010). The identification of the SCFA free fatty acid receptors (FFAR) 3 and 2 in the brain provide novel potential pathways linking the gut brain neural circuit (Brown et al., 2003). Recent studies have also shown that intracerebroventricular and peripheral injections of propionic acid result in autistic like behavior with increased reactive astrogliosis and activated microglia in the rat (MacFabe et al., 2007).
Microbiota can also produce specific neurotransmitter and neuromodulator compounds (Holzer & Farzi, 2014). Serotonin or 5-hydroxytrytamine (5-HT) can be produced by several genera of bacteria including Streptococcus, Escherichia, and Enterococcus (Cryan & Dinan, 2012; Yano et al., 2015) or generated through the precursor tryptophan (Gao et al., 2018). Brain 5-HT is involved in the serotonergic neurotransmission pathways regulating mood and cognition (Wrase, Reimold, Puls, Kienast, & Heinz, 2006). GF mice have increased hippocampal levels of 5-HT and increased plasma tryptophan concentrations which are normalized after colonizing the GF mice with commensal bacteria (Clarke et al., 2013). Dopamine, noradrenaline, acetylcholine, and gamma-aminobutyric acid (GABA) can also be produced by microbiota (Lyte, 2011). GF mice have lower total brain levels of tryptophan, tyrosine (precursor of dopamine and noradrenaline) and glutamine (precursor of GABA) than their conventionalized counterparts (Matsumoto et al., 2013). With the advance of metabolomic technology, more complete microbial metabolite profiles of microbial communities will be revealed that may provide more mechanistic insights into understanding the gut brain axis.
Next, the vagus nerve originates from the brainstem and vagal afferent terminals exist underneath the gut epithelium (Cawthon & de La Serre, 2018). Because they can receive signals produced by the gut microbiota, the vagus nerve may mediate the communication between gut microbiota and central nerve system (CNS). Lactobacillus rhamnosus has been shown to reduce stress-induced corticosterone and anxiety- and depression-related behavior through the regulation of GABA receptors expression in the brain. Vagotomy reduces anxiolytic and antidepressant effects, further suggesting that microbial effects on brain function may be relayed by the vagus nerve system (Bravo et al., 2011). These studies suggest that microbiota can indirectly impact the central nervous system via the enteric nervous system through the vagus nerve.
Last, studies have begun to investigate the effects of microbiota on the BBB, the interface between the circulation and brain. Preterm infants have an underdeveloped intestinal barrier (Rouwet et al., 2002; Saleem et al., 2017) and gut dysbiosis has been associated with altered intestinal barrier function in the disease models (Claud, 2009; Hamilton, Boudry, Lemay, & Raybould, 2015). The BBB is the vascular endothelium that tightly governs the interaction between the circulatory system and CNS through regulation of transport which allows for proper neuronal function and also protects the CNS from toxins, pathogens, and inflammation (Daneman & Prat, 2015). The BBB may be immature in preterm infants as well. Pre-clinical studies have suggested that microbiota-related systematic inflammation can modulate BBB integrity and multiple circulating cytokines, for example human IL-1α and IL-6, murine IL-1β, TNF, IL-6 and interferon gamma all cross the BBB (Banks, 2005). Systemic inflammation, modeled by LPS, increases permeability of the BBB (Varatharaj & Galea, 2017). There are several potential mechanisms for this finding including endothelial damage and alteration in beta-catenin, ZO-1 and claudin-5 expression (Cardoso et al., 2012); degradation of the glycocalyx mediated by TNF and ROS (Moseley, Waddington, & Embery, 1997; Wiesinger et al., 2013); astrocyte loss and structural changes (Biesmans et al., 2013); and astrocyte gene transcription with a proinflammatory profile (Zamanian et al., 2012). Interestingly, there seems to be a specific window in brain development when the BBB is susceptible to systemic inflammation since systemic inflammation, modeled by intraperitoneal injection of LPS, induced increased BBB permeability was only observed in rats before the postnatal age of 20 days (Stolp, Dziegielewska, Ek, Potter, & Saunders, 2005). Studies have further demonstrated that microbial communities can modulate BBB integrity (Braniste et al., 2014). GF mice display increased BBB permeability throughout the life course compared to pathogen-free mice with a normal gut flora. The difference in BBB development is seen as early as E17.5, indicating an early effect of microbiota on BBB development. The increased permeability in GF mice is associated with reduced expression of the tight junction proteins occludin and claudin-5. Studies have not looked at preterm development models, but have shown that exposure of adult GF adult mice to a commensal microbiota decreases BBB permeability and normalizes the expression of tight junction proteins. Additionally, monocolonization of the intestine of GF adult mice with SCFA-producing bacterial strains can normalize BBB permeability, and sodium butyrate treatment increases the expression of the tight junction protein occludin in the frontal cortex and hippocampus. Furthermore, antibiotic-induced gut dysbiosis reduces the expression of tight junction protein mRNA in the hippocampus but increases the expression of tight junction protein 1 and occludin mRNA in the amygdala (Frohlich et al., 2016). Together, these studies suggest that dysbiosis, dysbiosis related systematic inflammation and altered microbial metabolites can affect BBB integrity and function.
5 |. CONCLUSION
With the rising rate of preterm birth and healthcare cost for neurodevelopmental diseases, it is becoming increasingly urgent to understand the mechanisms mediating early life microbiome dysbiosis and brain development. Preterm infants differ from term infants in initial colonization and succession of microbiota due to host and environmental factors. There are multiple current gaps in knowledge including the effect of the prematurely developed preterm infant microbiome and its altered microbial metabolite profile on enteric nerve-CNS communication, brain immune function and neuroinflammation, the integrity and functions of BBB, brain development and long term neurological outcomes in preterm infants. While all the above-mentioned mechanisms interplay to contribute to the gut-brain axis, one should keep in mind that the key character of preterm infants is underdevelopment. Immature gut development might present specific challenges to preterm infants since the underdeveloped gut barrier might lose its frontline battle to defend against pathogenic bacteria in the gut, resulting in dysregulated responses by yet again an immature immune system. Furthermore, the underdeveloped blood brain barrier could be the next compromised defense line where microbiome-associated systemic components including immune cells, cytokines, metabolites and neurotransmitters might cross in a non-regulated manner to alter brain functions. Studies understanding preterm dysbiosis and its associated developmental deficits are required to develop more promising microbiota-modulating-based therapeutic interventions for neurodevelopmental disorders.
ACKNOWLEDGEMENT
The current work is supported by NIH R01 HD083481 (E. Claud).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, . . . Arias Vasquez A (2017). Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One, 12(9), e0183509. doi: 10.1371/journal.pone.0183509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Johansen LJ, Powell LD, Quig D, & Rubin RA (2011). Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol, 11, 22. doi: 10.1186/1471-230X-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentsen T, Qian Y, Gkotzis S, Femenia T, Wang T, Udekwu K, . . . Diaz Heijtz R (2017). The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry, 22(2), 257–266. doi: 10.1038/mp.2016.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta MC, Walter J, & Finlay BB (2016). Human Microbiota-Associated Mice: A Model with Challenges. Cell Host Microbe, 19(5), 575–578. doi: 10.1016/j.chom.2016.04.014 [DOI] [PubMed] [Google Scholar]
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, . . . Wang J (2015). Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe, 17(6), 852. doi: 10.1016/j.chom.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, & Lyte M (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun, 25(3), 397–407. doi: 10.1016/j.bbi.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA (2005). Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des, 11(8), 973–984. [DOI] [PubMed] [Google Scholar]
- Barichello T, Santos AL, Silvestre C, Generoso JS, Cipriano AL, Petronilho F, . . . Quevedo J (2011). Dexamethasone treatment reverses cognitive impairment but increases brain oxidative stress in rats submitted to pneumococcal meningitis. Oxid Med Cell Longev, 2011, 173035. doi: 10.1155/2011/173035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloosesky R, Gayle DA, & Ross MG (2006). Maternal N-acetylcysteine suppresses fetal inflammatory cytokine responses to maternal lipopolysaccharide. Am J Obstet Gynecol, 195(4), 1053–1057. doi: 10.1016/j.ajog.2006.06.081 [DOI] [PubMed] [Google Scholar]
- Berardi A, Rossi C, Lugli L, Creti R, Bacchi Reggiani ML, Lanari M, . . . Gbs Prevention Working Group, E.-R. (2013). Group B streptococcus late-onset disease: 2003–2010. Pediatrics, 131(2), e361–368. doi: 10.1542/peds.2012-1231 [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, . . . Collins SM (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology, 141(2), 599–609, 609 e591–593. doi: 10.1053/j.gastro.2011.04.052 [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, . . . Krishnamoorthy G (2011). Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature, 479(7374), 538–541. doi: 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- Bezirtzoglou E, Tsiotsias A, & Welling GW (2011). Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe, 17(6), 478–482. doi: 10.1016/j.anaerobe.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, & Anand KJ (2002). Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA, 288(6), 728–737. [DOI] [PubMed] [Google Scholar]
- Biesmans S, Meert TF, Bouwknecht JA, Acton PD, Davoodi N, De Haes P, . . . Nuydens R (2013). Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm, 2013, 271359. doi: 10.1155/2013/271359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, . . . Gordon JI (2016). Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science, 351(6275). doi: 10.1126/science.aad3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, & Pellissier S (2018). The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci, 12, 49. doi: 10.3389/fnins.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, Moloney RD, Clarke G, Dinan TG, & Cryan JF (2014). The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol, 817, 373–403. doi: 10.1007/978-1-4939-0897-4_17 [DOI] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, & Cryan JF (2014). Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med, 20(9), 509–518. doi: 10.1016/j.molmed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Bourassa MW, Alim I, Bultman SJ, & Ratan RR (2016). Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett, 625, 56–63. doi: 10.1016/j.neulet.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, . . . Pettersson S (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med, 6(263), 263ra158. doi: 10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, . . . Cryan JF (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A, 108(38), 16050–16055. doi: 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B, Olm MR, Firek BA, Baker R, Geller-McGrath D, Reimer SR, . . . Banfield JF (2018). The developing premature infant gut microbiome is a major factor shaping the microbiome of neonatal intensive care unit rooms. Microbiome, 6(1), 112. doi: 10.1186/s40168-018-0493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, . . . Dowell SJ (2003). The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem, 278(13), 11312–11319. doi: 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, . . . Elovitz MA (2010). Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res, 88(9), 1872–1881. doi: 10.1002/jnr.22368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A, Moloney RD, Dinan TG, & Cryan JF (2015). Microbiota regulation of the Mammalian gut-brain axis. Adv Appl Microbiol, 91, 1–62. doi: 10.1016/bs.aambs.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Butel MJ, Suau A, Campeotto F, Magne F, Aires J, Ferraris L, . . . Dupont C (2007). Conditions of bifidobacterial colonization in preterm infants: a prospective analysis. J Pediatr Gastroenterol Nutr, 44(5), 577–582. doi: 10.1097/MPG.0b013e3180406b20 [DOI] [PubMed] [Google Scholar]
- Cai Z, Lin S, Pang Y, & Rhodes PG (2004). Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr Res, 56(3), 377–384. doi: 10.1203/01.PDR.0000134249.92944.14 [DOI] [PubMed] [Google Scholar]
- Cao X, Lin P, Jiang P, & Li C (2013). Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry, 25(6), 342–353. doi: 10.3969/j.issn.1002-0829.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso FL, Kittel A, Veszelka S, Palmela I, Toth A, Brites D, . . . Brito MA (2012). Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS One, 7(5), e35919. doi: 10.1371/journal.pone.0035919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, . . . Crandall KA (2015). Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ, 3, e1140. doi: 10.7717/peerj.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon CR, & de La Serre CB (2018). Gut bacteria interaction with vagal afferents. Brain Res, 1693(Pt B), 134–139. doi: 10.1016/j.brainres.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, & Medzhitov R (2014). The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A, 111(6), 2247–2252. doi: 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau V, McFadden DE, Poskitt KJ, & Miller SP (2014). Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol, 41(1), 83–103. doi: 10.1016/j.clp.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Chernikova DA, Madan JC, Housman ML, Zain-Ul-Abideen M, Lundgren SN, Morrison HG, . . . Hoen AG (2018). The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr Res, 84(1), 71–79. doi: 10.1038/s41390-018-0022-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, & Aagaard KM (2017). Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med, 23(3), 314–326. doi: 10.1038/nm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Bloom BT, Spitzer AR, & Gerstmann DR (2006). Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics, 117(6), 1979–1987. doi: 10.1542/peds.2005-1707 [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, . . . Cryan JF (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry, 18(6), 666–673. doi: 10.1038/mp.2012.77 [DOI] [PubMed] [Google Scholar]
- Claud EC (2009). Neonatal Necrotizing Enterocolitis -Inflammation and Intestinal Immaturity. Antiinflamm Antiallergy Agents Med Chem, 8(3), 248–259. doi: 10.2174/187152309789152020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claud EC, Keegan KP, Brulc JM, Lu L, Bartels D, Glass E, . . . Antonopoulos DA (2013). Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome, 1(1), 20. doi: 10.1186/2049-2618-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouchoux C, Guizard N, Evans AC, du Plessis AJ, & Limperopoulos C (2012). Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am J Obstet Gynecol, 206(2), 173 e171–178. doi: 10.1016/j.ajog.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Cryan JF, & Dinan TG (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci, 13(10), 701–712. doi: 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- Dada T, Rosenzweig JM, Al Shammary M, Firdaus W, Al Rebh S, Borbiev T, . . . Burd I (2014). Mouse model of intrauterine inflammation: sex-specific differences in long-term neurologic and immune sequelae. Brain Behav Immun, 38, 142–150. doi: 10.1016/j.bbi.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C, Stigum H, Valeur J, Iszatt N, Lenters V, Peddada S, . . . Eggesbo M (2018). Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int J Epidemiol. doi: 10.1093/ije/dyy064 [DOI] [PubMed] [Google Scholar]
- Daneman R, & Prat A (2015). The blood-brain barrier. Cold Spring Harb Perspect Biol, 7(1), a020412. doi: 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, . . . Turnbaugh PJ (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, . . . Pettersson S (2011). Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A, 108(7), 3047–3052. doi: 10.1073/pnas.1010529108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, & Knight R (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A, 107(26), 11971–11975. doi: 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Fleiss B, Li H, D’Angelo B, Sun Y, Zhu C, . . . Wang X (2011). Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS One, 6(5), e19583. doi: 10.1371/journal.pone.0019583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Brooks-Gunn J, & Klebanov PK (1994). Economic deprivation and early childhood development. Child Dev, 65(2 Spec No), 296–318. [PubMed] [Google Scholar]
- Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, & Burd I (2011). Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci, 29(6), 663–671. doi: 10.1016/j.ijdevneu.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, . . . Prinz M (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci, 18(7), 965–977. doi: 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, & Schamberg MA (2009). Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci U S A, 106(16), 6545–6549. doi: 10.1073/pnas.0811910106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, . . . Raes J (2016). Population-level analysis of gut microbiome variation. Science, 352(6285), 560–564. doi: 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- Favier CF, Vaughan EE, De Vos WM, & Akkermans AD (2002). Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol, 68(1), 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, . . . Green JA 3rd (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe, 16(4), 444–453. doi: 10.1016/j.anaerobe.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, . . . Kaul A (2002). Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis, 35(Suppl 1), S6–S16. doi: 10.1086/341914 [DOI] [PubMed] [Google Scholar]
- Fouhy F, Guinane CM, Hussey S, Wall R, Ryan CA, Dempsey EM, . . . Cotter PD (2012). High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother, 56(11), 5811–5820. doi: 10.1128/AAC.00789-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich EE, Farzi A, Mayerhofer R, Reichmann F, Jacan A, Wagner B, . . . Holzer P (2016). Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun, 56, 140–155. doi: 10.1016/j.bbi.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, . . . Yin Y (2018). Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol, 8, 13. doi: 10.3389/fcimb.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, . . . Sherman PM (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut, 60(3), 307–317. doi: 10.1136/gut.2009.202515 [DOI] [PubMed] [Google Scholar]
- Gasparrini AJ, Crofts TS, Gibson MK, Tarr PI, Warner BB, & Dantas G (2016). Antibiotic perturbation of the preterm infant gut microbiome and resistome. Gut Microbes, 7(5), 443–449. doi: 10.1080/19490976.2016.1218584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MK, Wang B, Ahmadi S, Burnham CA, Tarr PI, Warner BB, & Dantas G (2016). Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat Microbiol, 1, 16024. doi: 10.1038/nmicrobiol.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg Y, Khatib N, Weiner Z, & Beloosesky R (2017). Maternal Inflammation, Fetal Brain Implications and Suggested Neuroprotection: A Summary of 10 Years of Research in Animal Models. Rambam Maimonides Med J, 8(2). doi: 10.5041/RMMJ.10305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg Y, Khatib N, Weiss B, Arison S, Ross MG, Weiner Z, & Beloosesky R (2017). Magnesium sulfate (MG) prevents maternal inflammation induced offspring cerebral injury evident on MRI but not via IL-1beta. Neuroscience, 353, 98–105. doi: 10.1016/j.neuroscience.2017.03.046 [DOI] [PubMed] [Google Scholar]
- Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, & Austin DW (2012). Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res, 5(6), 419–427. doi: 10.1002/aur.1253 [DOI] [PubMed] [Google Scholar]
- Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, & Fischer A (2011). Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis, 26(1), 187–197. doi: 10.3233/JAD-2011-110080 [DOI] [PubMed] [Google Scholar]
- Grier A, Qiu X, Bandyopadhyay S, Holden-Wiltse J, Kessler HA, Gill AL, . . . Gill SR (2017). Impact of prematurity and nutrition on the developing gut microbiome and preterm infant growth. Microbiome, 5(1), 158. doi: 10.1186/s40168-017-0377-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, . . . Amit I (2016). The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell, 166(5), 1231–1246 e1213. doi: 10.1016/j.cell.2016.07.043 [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, & Gressens P (2015). The role of inflammation in perinatal brain injury. Nat Rev Neurol, 11(4), 192–208. doi: 10.1038/nrneurol.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MK, Boudry G, Lemay DG, & Raybould HE (2015). Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol, 308(10), G840–851. doi: 10.1152/ajpgi.00029.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, . . . Stanton C (2017). Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome, 5(1), 4. doi: 10.1186/s40168-016-0213-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen M, Ojala R, Korhonen P, Haataja P, Eriksson K, Gissler M, . . . Tammela O (2014). Cerebral palsy among children born moderately and late preterm. Pediatrics, 134(6), e1584–1593. doi: 10.1542/peds.2014-0945 [DOI] [PubMed] [Google Scholar]
- Holzer P, & Farzi A (2014). Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol, 817, 195–219. doi: 10.1007/978-1-4939-0897-4_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, & Takeda K (2009). Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol, 2(3), 187–196. doi: 10.1038/mi.2009.8 [DOI] [PubMed] [Google Scholar]
- Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr., Smith PB, . . . Cohen-Wolkowiez M (2012). Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev, 88 Suppl 2, S69–74. doi: 10.1016/S0378-3782(12)70019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang L, Kang B, Zhu T, Li Y, Zhao F, . . . Mu D (2017). Association between perinatal hypoxic-ischemia and periventricular leukomalacia in preterm infants: A systematic review and meta-analysis. PLoS One, 12(9), e0184993. doi: 10.1371/journal.pone.0184993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, . . . Volpe JJ (1998). Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol, 43(2), 224–235. doi: 10.1002/ana.410430213 [DOI] [PubMed] [Google Scholar]
- Itani T, Ayoub Moubareck C, Melki I, Rousseau C, Mangin I, Butel MJ, & Karam Sarkis D (2017). Establishment and development of the intestinal microbiota of preterm infants in a Lebanese tertiary hospital. Anaerobe, 43, 4–14. doi: 10.1016/j.anaerobe.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Jang HM, Lee HJ, Jang SE, Han MJ, & Kim DH (2018). Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. doi: 10.1038/s41385-018-0042-3 [DOI] [PubMed] [Google Scholar]
- Jost T, Lacroix C, Braegger CP, & Chassard C (2012). New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One, 7(8), e44595. doi: 10.1371/journal.pone.0044595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, & Krajmalnik-Brown R (2013). Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One, 8(7), e68322. doi: 10.1371/journal.pone.0068322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Minuto C, Cryan JF, Clarke G, & Dinan TG (2017). Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front Neurosci, 11, 490. doi: 10.3389/fnins.2017.00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, & Rumbaugh G (2010). Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology, 35(4), 870–880. doi: 10.1038/npp.2009.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS (2003). Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci, 4(5), 376–385. doi: 10.1038/nrn1103 [DOI] [PubMed] [Google Scholar]
- Koedel U, Scheld WM, & Pfister HW (2002). Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis, 2(12), 721–736. [DOI] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, . . . Ley RE (2011). Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A, 108 Suppl 1, 4578–4585. doi: 10.1073/pnas.1000081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpela K, Blakstad EW, Moltu SJ, Strommen K, Nakstad B, Ronnestad AE, . . . de Vos W (2018). Intestinal microbiota development and gestational age in preterm neonates. Sci Rep, 8(1), 2453. doi: 10.1038/s41598-018-20827-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs VL, Okay TS, Okay Y, & Vaz FA (2005). Tumor necrosis factor-alpha, interleukin-1beta and interleukin-6 in the cerebrospinal fluid of newborn with meningitis. Arq Neuropsiquiatr, 63(1), 7–13. doi:/S0004-282X2005000100002 [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, . . . Hattori M (2007). Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res, 14(4), 169–181. doi: 10.1093/dnares/dsm018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, . . . Tarr PI (2014). Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A, 111(34), 12522–12527. doi: 10.1073/pnas.1409497111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroque B, Ancel PY, Marret S, Marchand L, Andre M, Arnaud C, . . . group, E. S. (2008). Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet, 371(9615), 813–820. doi: 10.1016/S0140-6736(08)60380-3 [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, & Mazmanian SK (2011). Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A, 108 Suppl 1, 4615–4622. doi: 10.1073/pnas.1000082107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner K, Al Shammary M, McLane M, Johnston MV, Elovitz MA, & Burd I (2014). IL-1 receptor blockade prevents fetal cortical brain injury but not preterm birth in a mouse model of inflammation-induced preterm birth and perinatal brain injury. Am J Reprod Immunol, 71(5), 418–426. doi: 10.1111/aji.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Kolodziejczyk AA, Thaiss CA, & Elinav E (2017). Dysbiosis and the immune system. Nat Rev Immunol, 17(4), 219–232. doi: 10.1038/nri.2017.7 [DOI] [PubMed] [Google Scholar]
- Lu J, Lu L, Yu Y, Cluette-Brown J, Martin CR, & Claud EC (2018). Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice. Sci Rep, 8(1), 5443. doi: 10.1038/s41598-018-23692-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Yu Y, Guo Y, Wang Y, Chang EB, & Claud EC (2015). Transcriptional modulation of intestinal innate defense/inflammation genes by preterm infant microbiota in a humanized gnotobiotic mouse model. PLoS One, 10(4), e0124504. doi: 10.1371/journal.pone.0124504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays, 33(8), 574–581. doi: 10.1002/bies.201100024 [DOI] [PubMed] [Google Scholar]
- MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, . . . Ossenkopp KP (2007). Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res, 176(1), 149–169. doi: 10.1016/j.bbr.2006.07.025 [DOI] [PubMed] [Google Scholar]
- Mackie RI, Sghir A, & Gaskins HR (1999). Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr, 69(5), 1035S–1045S. doi: 10.1093/ajcn/69.5.1035s [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, . . . Sonnenburg JL (2011). Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe, 10(5), 507–514. doi: 10.1016/j.chom.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martius JA, Roos T, Gora B, Oehler MK, Schrod L, Papadopoulos T, & Gross U (1999). Risk factors associated with early-onset sepsis in premature infants. Eur J Obstet Gynecol Reprod Biol, 85(2), 151–158. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, & Benno Y (2013). Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci, 7, 9. doi: 10.3389/fnsys.2013.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R, Krishnan S, Gonzalez-Gomez I, & Prasadarao NV (2011). Deciphering the roles of outer membrane protein A extracellular loops in the pathogenesis of Escherichia coli K1 meningitis. J Biol Chem, 286(3), 2183–2193. doi: 10.1074/jbc.M110.178236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles L, Gomez M, Heilig H, Bustos G, Fuentes S, de Vos W, . . . Jimenez E (2013). Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS One, 8(6), e66986. doi: 10.1371/journal.pone.0066986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley R, Waddington RJ, & Embery G (1997). Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim Biophys Acta, 1362(2–3), 221–231. [DOI] [PubMed] [Google Scholar]
- Mueller NT, Bakacs E, Combellick J, Grigoryan Z, & Dominguez-Bello MG (2015). The infant microbiome development: mom matters. Trends Mol Med, 21(2), 109–117. doi: 10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, & Foster JA (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil, 23(3), 255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Nigg JT, Lewis K, Edinger T, & Falk M (2012). Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry, 51(1), 86–97 e88. doi: 10.1016/j.jaac.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, . . . Hultman CM (2012). Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry, 69(6), E1–8. doi: 10.1001/archgenpsychiatry.2011.1374 [DOI] [PubMed] [Google Scholar]
- O’Sullivan A, Farver M, & Smilowitz JT (2015). The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants. Nutr Metab Insights, 8(Suppl 1), 1–9. doi: 10.4137/NMI.S29530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, & Brown PO (2007). Development of the human infant intestinal microbiota. PLoS Biol, 5(7), e177. doi: 10.1371/journal.pbio.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho HM, Bingham MO, Gibson GR, & McCartney AL (2005). Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol, 54(Pt 10), 987–991. doi: 10.1099/jmm.0.46101-0 [DOI] [PubMed] [Google Scholar]
- Partty A, Kalliomaki M, Wacklin P, Salminen S, & Isolauri E (2015). A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res, 77(6), 823–828. doi: 10.1038/pr.2015.51 [DOI] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, . . . Stobberingh EE (2006). Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics, 118(2), 511–521. doi: 10.1542/peds.2005-2824 [DOI] [PubMed] [Google Scholar]
- Praveen P, Jordan F, Priami C, & Morine MJ (2015). The role of breast-feeding in infant immune system: a systems perspective on the intestinal microbiome. Microbiome, 3, 41. doi: 10.1186/s40168-015-0104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, . . . Gordon JI (2013). Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science, 341(6150), 1241214. doi: 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, & Wesselingh S (2016). From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry, 21(6), 738–748. doi: 10.1038/mp.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwet EV, Heineman E, Buurman WA, ter Riet G, Ramsay G, & Blanco CE (2002). Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr Res, 51(1), 64–70. doi: 10.1203/00006450-200201000-00012 [DOI] [PubMed] [Google Scholar]
- Saleem B, Okogbule-Wonodi AC, Fasano A, Magder LS, Ravel J, Kapoor S, & Viscardi RM (2017). Intestinal Barrier Maturation in Very Low Birthweight Infants: Relationship to Feeding and Antibiotic Exposure. J Pediatr, 183, 31–36 e31. doi: 10.1016/j.jpeds.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, & Mazmanian SK (2015). Control of brain development, function, and behavior by the microbiome. Cell Host Microbe, 17(5), 565–576. doi: 10.1016/j.chom.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, . . . Follow-Up G (2011). Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics, 128(2), e348–357. doi: 10.1542/peds.2010-3338 [DOI] [PubMed] [Google Scholar]
- Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, Dahl DB, . . . Chapkin RS (2012). A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol, 13(4), r32. doi: 10.1186/gb-2012-13-4-r32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, . . . Suvisaari J (2018). Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res, 192, 398–403. doi: 10.1016/j.schres.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Schwiertz A, Gruhl B, Lobnitz M, Michel P, Radke M, & Blaut M (2003). Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res, 54(3), 393–399. doi: 10.1203/01.PDR.0000078274.74607.7A [DOI] [PubMed] [Google Scholar]
- Severance EG, Prandovszky E, Castiglione J, & Yolken RH (2015). Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep, 17(5), 27. doi: 10.1007/s11920-015-0574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Hagberg H, Naylor AS, & Mallard C (2014). Neonatal peripheral immune challenge activates microglia and inhibits neurogenesis in the developing murine hippocampus. Dev Neurosci, 36(2), 119–131. doi: 10.1159/000359950 [DOI] [PubMed] [Google Scholar]
- Song Y, Liu C, & Finegold SM (2004). Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol, 70(11), 6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB, Dziegielewska KM, Ek CJ, Potter AM, & Saunders NR (2005). Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur J Neurosci, 22(11), 2805–2816. doi: 10.1111/j.1460-9568.2005.04483.x [DOI] [PubMed] [Google Scholar]
- Strunk T, Inder T, Wang X, Burgner D, Mallard C, & Levy O (2014). Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis, 14(8), 751–762. doi: 10.1016/S1473-3099(14)70710-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, & Sourander A (2015). Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/ Hyperactivity Disorder. Pediatrics, 136(3), e599–608. doi: 10.1542/peds.2015-1043 [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, . . . Koga Y (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol, 558(Pt 1), 263–275. doi: 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, & Elinav E (2016). The microbiome and innate immunity. Nature, 535(7610), 65–74. doi: 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML, & Azcarate-Peril MA (2015). Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol, 5, 3. doi: 10.3389/fcimb.2015.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MA, & Sohn K (2017). The Microbiota of the Extremely Preterm Infant. Clin Perinatol, 44(2), 407–427. doi: 10.1016/j.clp.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A, & Galea I (2017). The blood-brain barrier in systemic inflammation. Brain Behav Immun, 60, 1–12. doi: 10.1016/j.bbi.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Vicari S, Caravale B, Carlesimo GA, Casadei AM, & Allemand F (2004). Spatial working memory deficits in children at ages 3–4 who were low birth weight, preterm infants. Neuropsychology, 18(4), 673–678. doi: 10.1037/0894-4105.18.4.673 [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2005). Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics, 116(1), 221–225. doi: 10.1542/peds.2005-0191 [DOI] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, & Conlon MA (2011). Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol, 77(18), 6718–6721. doi: 10.1128/AEM.05212-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, & Conlon MA (2012). Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci, 57(8), 2096–2102. doi: 10.1007/s10620-012-2167-7 [DOI] [PubMed] [Google Scholar]
- Wang X, Hellgren G, Lofqvist C, Li W, Hellstrom A, Hagberg H, & Mallard C (2009). White matter damage after chronic subclinical inflammation in newborn mice. J Child Neurol, 24(9), 1171–1178. doi: 10.1177/0883073809338068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesinger A, Peters W, Chappell D, Kentrup D, Reuter S, Pavenstadt H, . . . Kumpers P (2013). Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One, 8(11), e80905. doi: 10.1371/journal.pone.0080905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, . . . Lipkin WI (2011). Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One, 6(9), e24585. doi: 10.1371/journal.pone.0024585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Edgin JO, Thompson D, & Inder TE (2005). Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain, 128(Pt 11), 2578–2587. doi: 10.1093/brain/awh618 [DOI] [PubMed] [Google Scholar]
- Wrase J, Reimold M, Puls I, Kienast T, & Heinz A (2006). Serotonergic dysfunction: brain imaging and behavioral correlates. Cogn Affect Behav Neurosci, 6(1), 53–61. [DOI] [PubMed] [Google Scholar]
- Wu YW, & Colford JM Jr. (2000). Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA, 284(11), 1417–1424. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, . . . Hsiao EY (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161(2), 264–276. doi: 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, & Nicholson JK (2010). Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res, 9(6), 2996–3004. doi: 10.1021/pr901188e [DOI] [PubMed] [Google Scholar]
- Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, . . . Xavier RJ (2016). Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med, 8(343), 343ra381. doi: 10.1126/scitranslmed.aad0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, . . . Dickerson FB (2015). Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr Bull, 41(5), 1153–1161. doi: 10.1093/schbul/sbu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZT, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, . . . Newburg DS (2013). The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology, 23(2), 169–177. doi: 10.1093/glycob/cws138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, & Barres BA (2012). Genomic analysis of reactive astrogliosis. J Neurosci, 32(18), 6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, . . . Fu J (2016). Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science, 352(6285), 565–569. doi: 10.1126/science.aad3369 [DOI] [PMC free article] [PubMed] [Google Scholar]