Abstract
Studies of human memory have implicated a “parietal memory network” in the recognition of familiar stimuli. However, the automatic vs. top-down nature of information processing within this network is not yet understood. If the network processes stimuli automatically, one can expect repetition-related changes both when familiarity is central to an ongoing task and when it is task-irrelevant. Here, we tested this prediction in a group of 40 human subjects using fMRI. Subjects initially named 100 objects aloud in the scanner. They then repeated the same task with novel and previously-named objects intermixed (where familiarity was not task-relevant) and separately were asked to make old/new recognition decisions in response to pictures of novel and previously-named objects (where familiarity was central to task completion). Accuracy was matched across conditions, and voice reaction times reflected typical behavioral priming effects. Repetition enhancement effects were restricted primarily to parietal cortex—and in particular, the parietal memory network—and were task-general in nature, whereas repetition suppression effects were task-dependent and occurred primarily in frontal and ventral temporal cortex. Task context effects were also present in the parietal memory network and impacted responses to both novel and familiar items. We conclude by discussing implications of these findings with respect to current hypotheses regarding parietal contributions to memory retrieval.
Keywords: FMRI, Parietal cortex, Recognition memory, Repetition enhancement, Repetition suppression
1. Introduction
Researchers studying human memory have repeatedly demonstrated the impact of retrieval conditions on test performance. Within the realm of cognitive psychology, the principle of encoding specificity (Thomson and Tulving, 1970; Tulving, 1983) stressed that one’s ability to retrieve information is dependent upon the match between the stored engram and the type of retrieval cue utilized. A related concept of transfer-appropriate processing (Morris et al., 1977) effectively broadened this idea to suggest that performance depends on the degree to which processes engaged during retrieval match those engaged during encoding. Within the field of neuropsychology, studies have found that behavioral priming (e.g., improvements in response times [RTs] or decision accuracies) is evident in amnesic patients when they repeatedly encounter an item in the context of a single task, but these same subjects show gross memory impairments if retrieval is instead explicit, such as during a recognition memory test (e.g., Warrington and Weiskrantz, 1970). More recently, cognitive neuroscientists have also highlighted the importance of retrieval conditions with the use of neuroimaging tools. For example, it has been shown that the cortical locations of subsequent memory effects (i.e., observable differences in activity during encoding that can predict later memory performance) change as a function of different testing conditions (Otten, 2007). It has also been shown that repetition suppression (repetition-related reductions in neural activity, thought to reflect implicit memory retrieval; see e.g., Wiggs and Martin, 1998; Buckner et al., 1998) is quite sensitive to changes in task conditions, and can be reduced or entirely eliminated even when altering task conditions in very minor ways (Dobbins et al., 2004; Schacter et al., 2004).
In the context of such specificity, the identification of task-general effects is of broad theoretical interest. Recently, researchers have proposed that a sparse network of cortical regions is sensitive to stimulus familiarity (i.e., the recency with which one encountered a particular stimulus) irrespective of task conditions. These regions, consisting of the precuneus (PCU), mid-cingulate cortex (MCC), and posterior inferior parietal lobule/dorsal angular gyrus (pIPL/dAG), have been described as a “parietal memory network” (PMN; Gilmore et al., 2015). PMN regions reliably exhibit patterns of repetition enhancement in recognition tasks (e.g., Wagner et al., 2005; McDermott et al., 2009; Kim, 2013), with increases (rather than reductions) in activity accompanying repeated stimulus presentations (typically referred to as “retrieval success” or “old/new” effects within the memory literature).
An outstanding question is the degree to which PMN regions continue to exhibit similar sensitivity to familiarity outside the realm of explicit memory task conditions. Regions of parietal cortex have been observed to exhibit general repetition-related increases in activity in prior reports (Phillips et al., 2009; Elman and Shimamura, 2011; Kim, 2017), but the specific network membership of regions exhibiting such effects remains unclear. Proper localization of distinct functions has become increasingly important to inform and guide theories of parietal contributions to memory and its relation to other cognitive functions (Wagner et al., 2005; Cabeza et al., 2008, 2012; Shimamura, 2011; Nelson et al., 2012; Rugg and King, 2018; Sestieri et al., 2017), particularly because many of these were articulated before the mosaic nature of parietal cortex was fully appreciated (Nelson et al., 2010, 2013b; Seghier et al., 2010; Seghier, 2013; Sestieri et al., 2017). Thus, clearly identifying the neuroanatomic locations of different task effects is necessary for arbitrating between theories of and advancing ideas related to parietal cortex and memory.
In this report, we tested for task-generality by directly comparing—within a single group of forty subjects—conditions in which item familiarity was either orthogonal to the ongoing task or was central to it. Subjects initially named aloud 100 images while undergoing fMRI. They were then asked to repeat the same naming task with novel and repeated stimuli being intermixed (wherein one’s history with an item was not directly related to the decision at hand) and were separately asked to overtly verbalize recognition memory judgments for novel or repeated stimuli (where stimulus familiarity was the relevant dimension) (Fig. 1). Task conditions (including response accuracy) were matched with the exception of the specific retrieval instruction, to ensure that differences could be attributed to task instructions and not other features that differed between tasks (Schacter et al., 1989). Object naming was selected for the orthogonal task condition because it is known to elicit robust priming and repetition suppression effects (e.g., van Turennout et al., 2000). The inclusion of widely spaced trials and an ICA-based multi-echo denoising technique known as ME-ICA (Kundu et al., 2012) were implemented to reduce artifacts that might have been associated with in-scanner speech. Of primary interest was whether or not effects of stimulus familiarity would be observed within the PMN, and how these differed across task conditions.
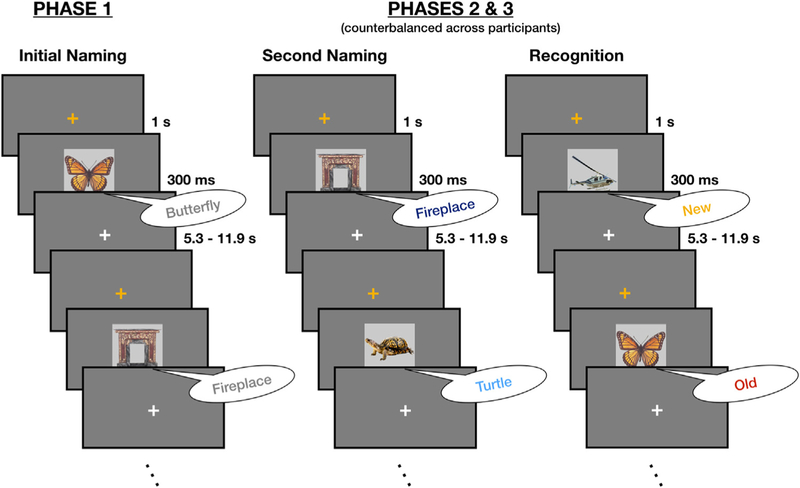
Fig. 1.
In an Initial Naming phase, subjects overtly named 100 images. In two subsequent phases, subjects named another set of 100 images (50 novel, 50 presented in Phase 1) and made old/new recognition judgments on a separate set of 100 images (50 novel, 50 presented in Phase 1). The sequence of Second Naming and Recognition phases was counter-balanced across participants. All phases were collected while subjects underwent fMRI.
2. Materials and methods
2.1. Subjects
Forty-four subjects were recruited from the NIH community and the DC metro area. Of these, one subject was excluded due to excessive inscanner motion, one was excluded upon disclosing that they had difficulty seeing stimuli in the scanner, and two subjects were excluded for falling asleep during scanning. The remaining 40 subjects (23 female) had a mean age of 24.6 years (range: 18–35), were right-handed, neurologically healthy native English speakers with normal or corrected-to-normal vision. Informed consent was obtained from all participants, and the experiment was approved by an NIH Institutional Review Board (protocol 93-M-0170, clinical trials number ). All subjects were monetarily compensated for their participation.
2.2. Stimuli
Task stimuli consisted of 200 photographic images of animals, plants, and man-made objects. These images were sorted into 4 lists of 50 images each and were constructed such that the lexical properties of the object names within each list were equated (omnibus F statistics all < 1). These list average properties included mean word length (5.46–6.08 letters) and log HAL frequency (8.42–8.64) as determined by querying the English Lexicon Project database (Balota et al., 2007). In addition, pilot data collected from a separate sample of 60 subjects provided a normed estimate of picture naming reaction time (list means: 811–840 ms). This property also did not vary across lists, F(3, 196) = 0.736, p = 0.532.
Images were resized to 600 × 600 pixels and presented in the center of a 100 Hz MR-compatible monitor (screen resolution: 1920 × 1080 pixels) located at the head of the scanner bore and viewed through a mirror attached to the head coil. Images subtended approximately 8° of the visual field. A fixation cross (48-point Arial type) separated image presentations, and all stimuli were presented against a gray background (RGB value: 75, 75, 75). Stimuli were presented using Presentation software (Neurobehavioral Systems) from an HP desktop computer running Windows 10.
2.3. Initial Naming phase
In this task, participants overtly named images presented on the screen (Fig. 1, left). Each image was preceded by a 1 s orange fixation cross, which served as an onset cue for the upcoming stimulus. The image itself was presented for 300 ms and was replaced by a white fixation cross for a variable period of 5.3–11.9 s. Participants were instructed to name aloud each image as quickly, accurately, and clearly as possible. Responses were spoken into an MR-compatible microphone that was seated next to the head coil and placed with its receiver approximately 3–5 cm from the subject’s mouth. Participants viewed and named 100 images in this phase, across two 50-image scan runs. This was always the first task completed in the experiment; the ordering of the following two tasks was counterbalanced evenly across subjects.
2.4. Second Naming phase
For half of the subjects, a Second Naming task immediately followed the Initial Naming phase. For the other half, this was the third experimental phase, with a Recognition phase interposed between the two naming tasks (see below). The Second Naming task was identical to the Initial Naming phase task, with one important exception: half of the images in this phase had been presented in the Initial Naming phase, and the other half were being presented for the first time. Regardless of whether or not an item was presented previously, participants were instructed to name the stimulus as quickly, accurately, and clearly as possible. New and old stimuli were presented pseudo-randomly, with the constraint that no more than three images in a row could be novel or repeated. Participants viewed 100 images in total across two 50-image scan runs, and each run had an equal number of new and old stimuli.
2.5. Recognition phase
Subjects performed the Recognition task either directly after the Initial Naming Phase or following the Second Naming phase. Stimulus presentation timing and trial durations were identical to the Initial Naming and Second Naming phases. During this task phase, subjects were asked to orally identify each item as “old” or “new” in the context of the experiment (speaking “old” if it had been presented previously, or “new” if it was being seen for the first time), rather than provide a specific name for each stimulus. As with the Second Naming task, half of the images had been seen in the Initial Naming phase, and the other half were novel items. Stimuli were pseudo-randomly intermixed as described previously. Participants made recognition judgments on 100 images in total, across two 50-image scan runs, and each run had an equal number of new and old stimuli.
2.6. Audio recording equipment
Subjects spoke all responses into an Opto-Acoustics FOMRI-III NC MR-compatible microphone with built-in noise cancellation. Audio signals from this microphone were routed into an M-Audio FastTrack Ultra 8-R USB audio interface, which in turn was connected to a Dell Precision M4400 laptop. Responses were recorded using Adobe Audition. In addition to a spoken audio recording, the stimulus presentation computer sent out a square wave pulse at the onset of each stimulus that was captured on a parallel audio track by the recording laptop. A Matlab program (written by SJG) calculated the time difference between the square pulse onset and the voice response in each trial, allowing for the calculation of voice response reaction times (RTs).
2.7. Behavioral task design and statistical analysis
Analyses of behavioral data focused on the Second Naming and Recognition conditions. A mixed-effects ANOVA was initially employed, with within-subject factors of Item History (Old, New) and Task context (Second Naming, Recognition), and a between-subjects factor of Counterbalance order (Second Naming first, Recognition first). No significant interactions with Counterbalance order were observed for either the response accuracy or RT comparisons (for response accuracy: Task context x Counterbalance F(1,38) = 3.79, p = 0.059, partial η2 = 0.091; all other ps > 0.1 for both accuracy and RT). We therefore collapsed across Counterbalance and report behavioral results in terms of two-way ANOVAs with factors of Item History and Task context. Additionally, planned comparisons were conducted between the Initial Naming task accuracy and accuracy in all other task conditions. These were analyzed as paired-samples, two-tailed t-tests. Effect sizes for t-tests are reported using Cohen’s d.
2.8. fMRI data acquisition
All images were acquired with a General Electric Discovery MR750 3.0 T scanner, using a 32-channel head coil. A high-resolution T1 structural image was obtained for each participant (TE = 3.47 ms, TR = 2.53 s, TI = 900 ms, flip angle = 7°, 172 slices with 1 × 1 × 1 mm voxels). Functional images were acquired using a BOLD-contrast sensitive multi-echo echo-planar sequence (Array Spatial Sensitivity Encoding Technique [ASSET] acceleration factor = 2, TEs = 12.5, 27.7, and 42.9 ms, TR = 2200 ms, flip angle = 75°, 64 × 64 matrix, inplane resolution = 3.2 × 3.2 mm). Whole brain EPI volumes (MR frames) of 33 interleaved, 3.5 mm-thick oblique slices (manually aligned to the AC-PC axis) were obtained every 2.2 s.
Foam pillows helped stabilize head position for all participants, and foam earplugs attenuated scanner noise. A sensor was placed on each participant’s left middle finger to record heart rate, and a respiration belt monitored breathing for each subject.
2.9. fMRI data preprocessing
fMRI data were preprocessed to reduce noise and facilitate across-subject registration. Initial preprocessing steps included a frame-by-frame rigid-body realignment to the first volume of each run, slice-timing correction, and despiking. The first four frames of each run were also discarded to ensure that net magnetization had reached a steady state. After these preliminary steps, data from all three acquired echoes was used to remove additional thermal and physiological noise with multi-echo independent component analysis (ME-ICA; Kundu et al., 2012, 2013, 2017). Briefly, this procedure initially uses a weighted-averaging of the different echo times to reduce thermal noise, and subsequently uses spatial ICA and the known linear properties of T2* signal decay to separate putatively neuronal BOLD components from those thought to be artefactual in nature (e.g. related to motion or hardware). Retained components are those that fit strongly to a model that assumes temporal dependence (i.e., are “BOLD-like” in nature) and that fit poorly with a model that assumes temporal independence (i.e., that are “non BOLD-like” in nature) (Kundu et al., 2012). Components were identified and classified using the default options present in AFNI’s tedana.py function. Following ME-ICA processing, data from each scan run were aligned across runs and registered to each individual’s T1 image. Data from each participant were then resampled into 3-mm isotropic voxels and linearly transformed into Talairach atlas space (Talairach and Tournoux, 1988).
2.10. GLM-based fMRI data analysis
Functional scans for each task phase consisted of 237 MR frames (233 after initial frames were discarded), with all task scans lasting 8 min 42 s in duration. Six runs were collected from each participant, two for each of the three task phases. For one subject, a single run was excluded because they misunderstood the instructions for the recognition task (this was corrected prior to the second recognition scan for this participant). Overall motion summaries for each run were obtained using the AFNI program @1dDiffMag, which estimates the average of first differences in frame-to-frame motion across each scan run. Any runs with DiffMag scores exceeding 0.3 mm/TR were excluded. This resulted in the exclusion of a single subject (noted also at the beginning of the Methods), for whom four of the six task scan runs had to be excluded. No other data were discarded based on this requirement.
Functional data for each subject were smoothed using a 3 mm FWHM Gaussian kernel, normalized by the mean signal intensity of each voxel, and detrended using a series of fourth-order polynomials. Analysis was conducted using a general linear model (GLM), in which the data at each time point are treated as the sum of all effects thought to be present at that time point, plus an error term. Responses associated with each condition were modeled using TENT functions, which is analogous to SPM’s finite impulse response (FIR) model. This approach assumes that all responses for a given condition share the same response shape but makes no assumption as to what the shape of that response might be. All conditions were modeled over six time points.
Five conditions of interest were modeled. These corresponded to items named during the Initial Naming phase, novel items from the Second Naming phase (“Second Naming-New”), repeat items from the Second Naming phase (“Second Naming-Old”), novel items from the Recognition phase (“Recognition-New”), and repeat items from the Recognition phase (“Recognition-Old”). Two additional regressors of non-interest coded for error trials which occurred during the Naming and Recognition periods, and both omission and commission errors were included in each case. For the purposes of statistical testing, response magnitudes were estimated for each condition by averaging the 3rd and 4th time points of the TENT function, reflecting activity 4.4–8.8 s following stimulus onset.
2.11. Correction for multiple comparisons
Correction for multiple comparisons was accomplished using AFNI’s 3dClustSim and its updated non-Gaussian autocorrelation function (Cox et al., 2017a, 2017b), which was released following false-positive findings by Eklund et al. (2016). First, the residual time series (concatenated across all runs) was used to estimate the smoothness of the data within each subject. These smoothness estimates were entered into 3dClustSim, with monte carlo simulations empirically determining the minimum cluster extents (k) to achieve a whole-brain p < 0.05. Following these simulations, a voxelwise p < 0.001 resulted in cluster minimums of k ≥ 20 for two tailed (t) tests, and k ≥ 25 for one-tailed (F) tests.
2.12. fMRI task design and statistical analysis
2.12.1. Task, item history, and counterbalance order ANOVA
Of primary interest was BOLD activity during the Second Naming and Recognition phases. However, there was a concern that our across-subject counterbalancing of task order (Second Naming first vs. Recognition first) may have impacted neural responses in some way, even if no behavioral interactions were observed (see above). Using AFNI’s 3dMVM program, we first employed a mixed effects ANOVA with within-subject factors of Task (Second Naming, Recognition) and Item History (Old, New), and with a between-subjects factor of Counterbalance Order (Second Naming first, Recognition first). No clusters exhibiting significant two- or three-way interactions were observed between Counterbalance Order and any other factors, and no main effect was observed for this factor. This held at the fully corrected p < 0.05 level as well as at a relaxed p < 0.10 whole-brain level. We therefore collapsed across Counterbalance Order for subsequent analyses, as was done with the behavioral response data.
2.12.2. Identification of PMN regions using Recognition task data
After an initial examination of potential order effects, we compared activity during the Recognition phase for Old and New trials. This was intended to identify putative regions within the PMN that could then be carried forward as regions of interest (ROIs) in subsequent analyses. Only correct trials were included in the analysis. Regions sensitive to Old/New effects were defined using a paired-samples, two-tailed t-test, comparing response magnitudes for the Recognition-Old and Recognition-New trials. Identified clusters were then used as a basis for ROI definition. This was accomplished by drawing 10-mm diameter spheres around the center of mass for identified pIPL/dAG, PCU, and MCC regions. The use of spherical ROIs allowed for consistency in the number of voxels associated with each identified region.
2.12.3. Analysis of Second Naming activity for Old and New items
A central question in this work relates to the sensitivity of PMN regions to stimulus novelty and familiarity across different task conditions. To address this question, we took the PMN ROIs identified in the Recognition task and examined their BOLD activity during Second Naming-Old and Second Naming-New trials. Activity was averaged across all voxels within each ROI and was subsequently contrasted between conditions. As these voxels were independently identified in the Recognition task data, there is no circularity concern for the analysis of Second Naming task data within these ROIs. Although no significant interactions of Counterbalance order with any other factors were observed at the whole brain level, we nonetheless examined possible effects of Counterbalance within these Recognition-defined ROIs. As before, no significant interactions were found (largest F: Task context × Counterbalance F(1,38) = 1.807, p = 0.187, partial η2 = 0.045).
In addition to examining putative PMN ROIs, a secondary analysis was conducted to address this question at the whole-brain level. Regions displaying different activity during Second Naming-Old and Second Naming-New trials were defined using a paired-samples, two- tailed t-test.
2.12.4. Task-related differences in PMN activity
After establishing the generality of Item History effects in PMN regions, we turned to examine the question of task effects on BOLD activity that could be associated with the Second Naming and Recognition conditions. First, we conducted a two-way ANOVA with within-subject factors of Task (Second Naming, Recognition) and Item History (old, new). Regions exhibiting main effects of Task and Item History were identified at the whole-brain level. A conjunction image of the binarized main effect maps of Task and Item History was computed to identify voxels that were—by definition—sensitive to both factors. This resulted in the identification of six distinct clusters, each with cluster extents of at least 21 voxels. The centers of mass for each cluster were used as the basis for ROI generation, as described previously.
2.12.5. Verification of network identity using prior connectivity data
In order to verify the network membership of voxels and regions we putatively associated with the PMN, we utilized previously-published network masks and examined activity for Old and New items during the Second Naming and Recognition tasks. The masks are publicly available (https://findlab.stanford.edu/functional_ROIs.html) and are based on networks identified by Shirer et al. (2012). The Shirer et al. “precuneus network” corresponds to the PMN discussed here (but antedates the network’s identification). For comparative purposes, we selected components of 4 other cortical networks that encompass most of lateral and medial parietal cortex. These included the default mode (DMN) and contextual association networks (described by Shirer et al. as the “dorsal” and “ventral” default network, respectively), dorsal attention network regions, and executive control regions; cortex subsumed by these masks has been associated with explicit retrieval success effects in prior literature (see e.g., Wagner et al., 2005; Spaniol et al., 2009; Nelson et al., 2010). Only network components within lateral or medial parietal cortex (and those along the cingulate gyrus) were included. Additionally, voxels associated with multiple networks were excluded from analysis.
We separately created a binary conjunction image of the Old > New effects in the Second Naming and Recognition tasks. Voxels within this image thus exhibited “task-general” repetition enhancement effects. The degree of overlap between this conjunction image and each of the network templates was assessed in two complementary ways to help localize the task-general effects within parietal cortex. First, the proportion of each Shirer network template that contained general Old > New effects was calculated (i.e., number of Old > New voxels in a network’s mask/total voxels in that network mask). A second, similar analysis was conducted, but this time using as the denominator the total number of Old > New voxels in the conjunction mask, and the number of each network’s voxels that contained elements of the conjunction image as the numerator (i.e., Old > New voxels in each network’s mask/total number of Old > New voxels).
2.13. Analysis and visualization software
fMRI data processing was conducted using AFNI (Cox, 1996). For the purposes of visualization, statistical maps were sampled from the volume to a partially-inflated representation of the cortical surface using SUMA (Saad and Reynolds, 2012). All coordinates listed in this report have been converted to MNI152 space.
3. Results
3.1. Subjects were accurate in all task conditions
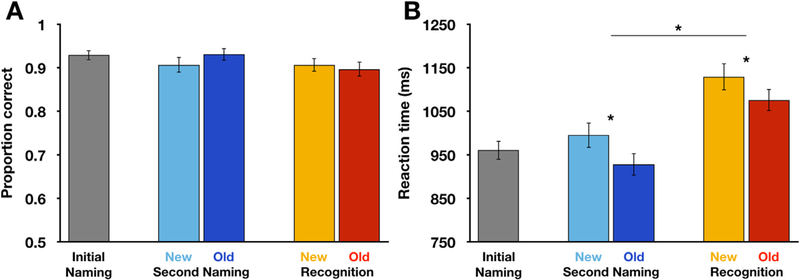
Across all task conditions, subjects were quite accurate in their responses, with approximately 90% of items being identified or recognized correctly in all cases (Fig. 2A). Little numeric variation was observed across conditions (range = 89.7–93.1%), suggesting that this was likely the performance ceiling based on the task design. Condition accuracies for the Second Naming and Recognition conditions were compared using an ANOVA with within-subject factors of Item History (Old, New) and Task context (Second Naming, Recognition). We did not observe main effects of Item History, F(1,39) = 0.64, p = 0.430, partial η2 = 0.017, or Task context, F(1,39) = 3.34, p = 0.075, partial η2 = 0.086. However, the interaction of these factors was significant, F (1,39) = 4.42, p = 0.042, partial η2 = 0.102; the numerical differences in accuracy between new and old items were in opposite directions for the two task conditions.
Fig. 2.
Response accuracy and reaction times. A) Subjects accurately identified stimuli in the Initial Naming and Second Naming conditions, as well as during the Recognition period. B) Reaction times were faster for Old than for New items and were also shorter for the Second Naming than Recognition phases. Error bars denote SEM. Second Naming: Second Naming phase. Recognition: Recognition phase. * denotes p < 0.001.
3.2. Subjects were faster to respond when naming than recognizing, and for old rather than new items
A second repeated measures ANOVA was conducted for reaction times across the different task conditions, again including factors of item history and task phase. Overall, responses were faster for Old than for New items, as well as for the Second Naming relative to the Recognition task conditions (Fig. 2B). This was reflected in significant main effects of Item History, F(1,39) = 26.54, p < 0.001, partial η2 = 0.405, and Task context, F(1,39) = 59.30, p < 0.001, partial η2 = 0.603. These factors did not interact, F(1,39) = 0.579, p = 0.451, partial η2 = 0.015. Thus, although overall response times differed across conditions, they did not appear to do so in a manner that would compromise our interpretation of the neuroimaging findings we present in the sections that follow.
3.3. PMN regions were identified in a voxelwise analysis of the recognition memory data
A primary goal of this experiment was to determine the generality of PMN responses to familiar stimuli—specifically, if regions within this network would exhibit their expected Old > New/repetition enhancement effects both when familiarity was a task-relevant stimulus dimension (i.e., the recognition memory condition) and when it was not. To accomplish this, we first conducted a voxelwise analysis of fMRI data collected during the Recognition task, contrasting activity for successfully identified Old and New items. This would enable us to identify PMN regions in a manner consistent with prior literature. A paired-samples, two-tailed t-test revealed Recognition-Old > Recognition-New effects in left pIPL/dAG, MCC and in bilateral regions of the PCU (Fig. 3, Table 1). In addition, small clusters exhibiting Recognition-Old > Recognition-New effects emerged bilaterally in regions near the supramarginal gyrus. No significant clusters were identified exhibiting the opposite response pattern.
Fig. 3.
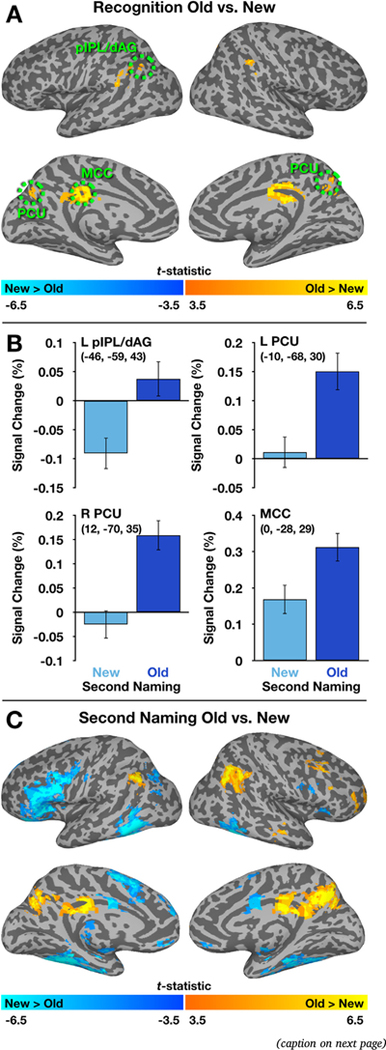
Differences in activity for Old and New items during the Recognition and Second Naming tasks. A) A voxelwise contrast of Old and New items during the recognition task identified PMN regions (circled in green), all of which exhibited a pattern of Old > New activity. B) The statistical map defined in A was used to generate ROIs, which were tested for activity differences between Old and New items in the independent Second Naming task. All ROIs continued to exhibit Old > New effects, even in the absence of explicit recognition memory judgments. Coordinates are listed in MNI152 space. Error bars denote SEM. C) A voxelwise analysis of the Second Naming data also identified PMN regions among those exhibiting Old > New effects, with regions showing the opposite pattern falling in locations that frequently display repetition suppression effects (e.g., Kim, 2017). For display purposes, data have been projected onto an inflated cortical surface using SUMA (Saad and Reynolds, 2012). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Table 1.
Regions exhibiting differential activity during the Recognition task for familiar relative to novel items (Recognition-Old vs. Recognition-New; paired samples t-test, two-tailed).
| Region Name | X | Y | Z | Size (voxels) | Peak t-stat |
|---|---|---|---|---|---|
| Recognition Old > New | |||||
| Mid-cingulate Cortex | 0 | −28 | 29 | 142 | 5.32 |
| Right Supramarginal Gyrus | 52 | − 45 | 48 | 44 | 4.45 |
| Left Supramarginal Gyrus | − 59 | − 50 | 30 | 32 | 4.51 |
| Left Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | − 46 | − 59 | 43 | 24 | 4.99 |
| Left Precuneus | − 10 | − 68 | 30 | 32 | 4.70 |
| Right Precuneus | 12 | − 70 | 35 | 71 | 4.49 |
| Recognition New > Old | |||||
| None |
The left hemisphere pIPL, MCC, and PCU locations were consistent with those reported previously in the literature, and the Euclidian distance between the current centers of mass and meta-analytically-derived coordinates reported by McDermott et al. (2017) differed by an average of 6.6 mm (and with both pIPL/dAG and PCU regions falling < 5 mm from those reported previously). No such meta-analytic coordinates have been reported for the right PCU, but we note here that the center of mass for our obtained region is 6.3 mm from coordinates reported by Chen et al. (2017a) and 7.5 mm from those reported by Rosen et al. (2016).
3.4. PMN regions also exhibited enhancement effects in the Second Naming task
After identifying group-averaged PMN clusters with the Recognition task data, we created ROIs around their centers of mass with the intention of applying them to the Second Naming data. We then asked if activity for Old and New items also differed under incidental retrieval conditions, which would be consistent with an automatic role for PMN regions the processing stimulus familiarity. In all four of our independently-defined PMN ROIs, we found significantly greater activity during the presentation of Second Naming-Old as compared to Second Naming-New items (least significant difference: t(39) = 6.09, p < 0.001, d = 0.962, obtained for the MCC ROI; see Supplementary Table 1 for complete results). Response estimates for PMN regions during the Second Naming task are provided in Fig. 3B.
Although our initial results did not suggest any interaction of the Counterbalance order with any other factors, we nevertheless wished to ensure that no interactions were present at the ROI level. We therefore computed ANOVAs for the Second Naming data within each ROI using factors of Item History and Counterbalance Order. No significant effects of Counterbalance were found in any ROI (all Fs ≤ 1), nor were there any interactions (largest F-stat: F(1,38) = 2.75, p = 0.106, obtained for left PCU; additional results of Counterbalance Order are reported in Supplementary Table 1).
3.5. A whole-brain analysis of Second Naming task data provides converging evidence of broad PMN sensitivity to Old and New items
After finding evidence for a general sensitivity to novelty and familiarity within the Recognition-defined ROIs, we conducted a voxel-wise analysis (paired-samples, two-tailed t-test) of the Second Naming task data to determine the specificity of the Second Naming-Old > Second Naming-New effect. Results of this analysis are summarized in Fig. 3C and Table 2. We found repetition enhancement effects (Second Naming-Old > Second Naming-New) in bilateral pIPL/ dAG clusters and a large midline cluster that included MCC, PCU, and posterior-cingulate cortex (Fig. 3C, warm colors), in regions consistent with those observed in the (explicit) Recognition task. We also observed several additional clusters within right frontal and temporal cortex exhibiting repetition enhancement effects. Clusters exhibiting the opposite pattern (repetition suppression) were found in regions previously associated with perceptual/conceptual processing, task control, and language production (Fig. 3C, cool colors), including fusiform cortex, anterior insular cortex, and left inferior frontal cortex, respectively. Thus, repetition enhancement was observed in regions thought to be associated with familiarity-related processing in the explicit memory literature, and repetition suppression was observed in regions associated with the naming of visually-presented objects.
Table 2.
Regions exhibiting differential activity during the Second Naming task for familiar relative to novel items (Second Naming-Old vs. Second Naming-New; paired samples t-test, two-tailed).
| Region Name | X | Y | Z | Size (voxels) | Peak t-stat |
|---|---|---|---|---|---|
| Second Naming Old > New | |||||
| Right Anterior Prefrontal Cortex | 31 | 59 | 2 | 162 | 5.55 |
| Right Middle Frontal Gyrus | 44 | 26 | 37 | 87 | 6.53 |
| Right Superior Frontal Sulcus | 31 | 22 | 49 | 44 | 5.29 |
| Right Superior Temporal Sulcus/Middle Temporal Gyrus | 62 | − 23 | − 14 | 38 | 5.39 |
| Mid-cingulate/Posterior Cingulate/Precuneus | 3 | − 54 | 37 | 673 | 8.96 |
| Left Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | − 44 | − 58 | 41 | 91 | 7.13 |
| Right Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | 50 | − 58 | 41 | 333 | 7.24 |
| Second Naming New > Old | |||||
| Left Dorsomedial Prefrontal Cortex | − 8 | 57 | 29 | 113 | 5.54 |
| Ventral Medial Prefrontal Cortex | −1 | 47 | − 17 | 66 | 5.74 |
| Right Anterior Insula | 37 | 27 | 0 | 28 | 5.24 |
| Left Inferior Frontal Gyrus/Anterior Insula | −46 | 22 | 10 | 1235 | 8.58 |
| Dorsal Anterior Cingulate/Pre-supplementary Motor Area | −4 | 16 | 53 | 291 | 6.64 |
| Right Inferior Frontal Junction | 50 | 10 | 26 | 53 | 5.12 |
| Anterior/Mid-cingulate Cortex | 0 | 7 | 28 | 49 | 6.69 |
| Left Amygdala | −17 | − 4 | − 12 | 25 | 5.24 |
| Right Amygdala | 18 | − 6 | − 16 | 26 | 5.74 |
| Left Thalamus | − 8 | − 15 | 2 | 102 | 5.34 |
| Left Fusiform Gyrus | − 44 | −48 | − 15 | 834 | 11.41 |
| Right Fusiform Gyrus | 37 | −54 | − 20 | 669 | 8.47 |
| Left Posterior Intraparietal Sulcus | − 28 | −72 | 36 | 68 | 4.78 |
| Left Transverse Occipital Sulcus | − 40 | − 79 | 25 | 25 | 4.53 |
3.6. Both stimulus familiarity and task context modulate activity within PMN regions
Having established evidence of a task-general sensitivity to stimulus familiarity, we sought to answer the question of how different task contexts (and their associated differences in attentional allocation) might impact activity in the PMN (and elsewhere). To address this question, we conducted an analysis to simultaneously account for both stimulus familiarity, and the task- (or, perhaps, attentional-) relevance of that familiarity: that is, we conducted a two-way ANOVA, with factors of Item History (Old, New) and Task context (Second Naming, Recognition).
Interactions of these two factors were present across regions of left inferior frontal cortex, left fusiform gyrus, left posterior intraparietal sulcus, midline prefrontal and premotor regions, the thalamus, and the right PCU (Supplementary Figure 1A; Supplementary Table 2). These regions overwhelmingly aligned with those found in the Second Naming-Old vs. Second Naming-New contrast to exhibit repetition suppression (cf. Fig. 3C), with a notable exception in the right PCU region, which exhibited enhancement effects in the same contrast. Response magnitudes in clusters displaying the interaction mirror this similarity, with regions generally showing clear suppression effects in the Second Naming task and little to no change in the Recognition task, but with the right PCU exhibiting enhancement in both cases (Supplementary Figure 1B).
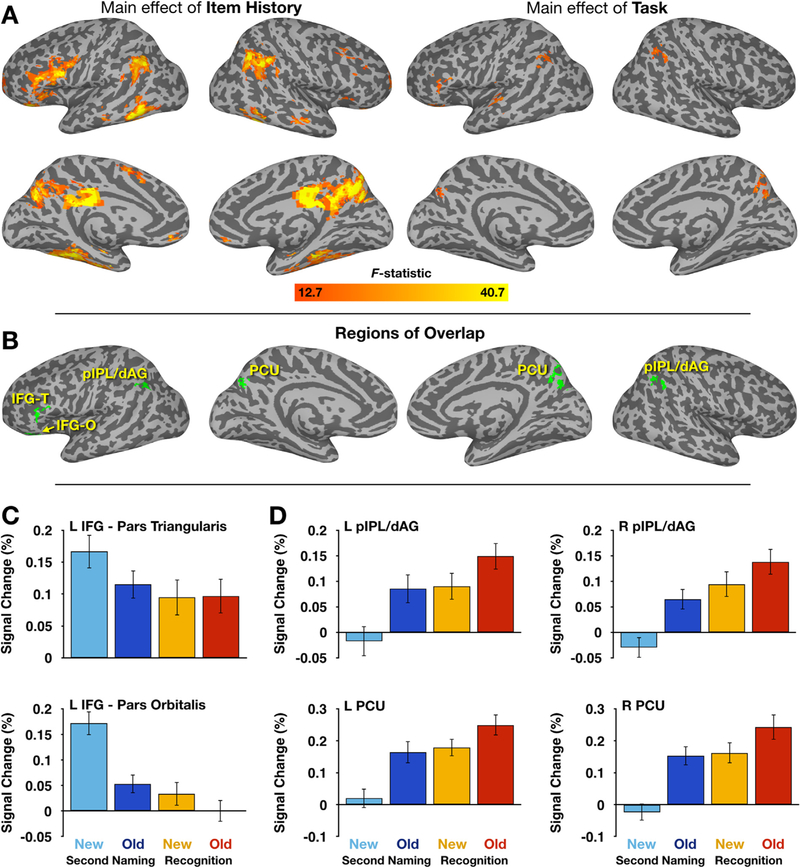
Of additional interest were the two main effect images, as well as their intersection. To focus first on the factor of Item History, a wholebrain analysis identified effectively the same regions as those identified in contrast of Second Naming-Old and Second Naming-New trials (Fig. 4A, left; Table 3). A much sparser map emerged when examining regions exhibiting a significant main effect of Task context, with significant differences being observed in the left inferior frontal gyrus, left superior temporal sulcus, and bilateral regions of pIPL/dAG and PCU (but not MCC; Fig. 4A, right; Table 4). A conjunction analysis found six overlapping clusters, with two in left IFG and four falling in the pIPL/ dAG and PCU regions (Fig. 4B; Table 5). The posterior regions aligned well with the PMN regions identified in the earlier contrasts. The six clusters served as the basis for ROIs from which magnitude estimates were extracted.
Fig. 4.
PMN region responses are sensitive to both item history and task condition. A) Main effect maps for factors of Item History (left) and Task context (right) include regions within and beyond the PMN. B) A conjunction image of the two main effect maps, with regions of overlap shown in green. This resulted in 6 identifiable clusters, two in inferior frontal cortex, and 4 within the PMN. IFG-T refers to pars triangularis; IFG-O to pars orbitalis. C) Inferior frontal regions exhibit repetition suppression in the Second Naming task, but little difference during the Recognition task. Error bars denote SEM. D) PMN regions are consistently more active when responding to Old rather than New items (light vs. dark bars) and are more active during the Recognition than Second Naming task (warm vs. cool bars), highlighting their sensitivity to both Item History and Task conditions.
Table 3.
Regions exhibiting a significant main effect of Item History.
| Region Name | X | Y | Z | Size (voxels) | Peak F-stat |
|---|---|---|---|---|---|
| Right Anterior Prefrontal Cortex | 26 | 62 | 3 | 94 | 34.96 |
| Left Anterior Prefrontal Cortex | −23 | 60 | 0 | 54 | 24.01 |
| Ventral Medial Prefrontal Cortex | − 1 | 48 | − 17 | 49 | 29.46 |
| Right Anterior Insula/Frontal Operculum | 35 | 31 | − 7 | 33 | 31.89 |
| Right Middle Frontal Gyrus | 41 | 29 | 37 | 62 | 26.04 |
| Left Inferior Frontal Gyrus/Anterior Insula | − 45 | 23 | 12 | 585 | 55.87 |
| Left Pre-supplementary Motor Area | − 5 | 16 | 49 | 63 | 29.33 |
| Right Posterior Middle Temporal Gyrus | 67 | − 27 | − 13 | 26 | 30.41 |
| Left Posterior Middle Temporal Gyrus | − 65 | − 29 | − 11 | 79 | 25.95 |
| Right Fusiform Gyrus/Parahippocampal Cortex | 39 | − 45 | − 18 | 385 | 66.53 |
| Left Fusiform Gyrus/Parahippocampal Cortex | − 42 | − 47 | − 16 | 579 | 85.07 |
| Mid-cingulate/Posterior Cingulate/Precuneus | 2 | − 51 | 34 | 923 | > 100 |
| Right Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | 51 | − 55 | 43 | 424 | 76.12 |
| Left Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | − 48 | − 57 | 38 | 248 | 51.12 |
Table 4.
Regions exhibiting a significant main effect of Task context.
| Region Name | X | Y | Z | Size (voxels) | Peak F-stat |
|---|---|---|---|---|---|
| Left Inferior Frontal Gyrus, pars triangularis | −50 | 32 | 13 | 39 | 22.90 |
| Left Inferior Frontal Gyrus, pars orbitalis | −40 | 30 | −17 | 30 | 25.91 |
| Left Superior Temporal Gyrus | − 61 | − 15 | 4 | 33 | 23.00 |
| Left Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | − 41 | − 56 | 44 | 35 | 22.35 |
| Right Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | 40 | − 56 | 45 | 48 | 23.73 |
| Right Precuneus | 8 | − 68 | 41 | 55 | 25.11 |
| Left Precuneus | − 9 | − 73 | 38 | 25 | 19.86 |
Table 5.
Regions associated with main effects of both Item History and Task context.
| Region Name | X | Y | Z | Size (voxels) |
|---|---|---|---|---|
| Left Inferior Frontal Gyrus, pars orbitalis | −37 | 29 | −8 | 21 |
| Left Inferior Frontal Gyrus, pars triangularis | −47 | 28 | 16 | 35 |
| Left Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | −38 | − 56 | 38 | 21 |
| Right Posterior Inferior Parietal Lobule/Dorsal Angular Gyrus | 40 | − 57 | 40 | 36 |
| Left Precuneus | −8 | − 71 | 32 | 22 |
| Right Precuneus | 9 | − 67 | 36 | 52 |
Both of the identified IFG regions exhibited a similar overall pattern of activity (Fig. 4C). Significant repetition suppression was observed for difference was present between Recognition-Old and Recognition-New items. This manifested in an Item History × Task con-text interaction for both regions: for the pars triangularis ROI, F (1,39) = 6.93, p = 0.012, partial η2 = 0.151, and for the pars orbitalis ROI, F(1,39) = 11.32, p = 0.002, partial η2 = 0.225.
A qualitatively distinct pattern was observed in the remaining four PMN ROIs (Fig. 4D). No repetition suppression was observed in these regions; instead, both tasks exhibited patterns of repetition enhancement, with an increase in activity associated with the Recognition compared to the Second Naming tasks. In all four regions, significant Item History × Task context interactions were again observed but manifested in forms quite distinct from the IFG regions. Here, the differences observed between Second Naming-Old and Second Naming-New responses was larger than the difference between Recognition-Old vs. Recognition-New responses (least significant ROI: Right pIPL/dAG, F(1,39) = 4.99, p = 0.031, partial η2 = 0.113; see Supplementary Table 3 for complete list of region results), but all exhibited a basic Old > New pattern. Thus, whereas the IFG regions were marked by patterns of repetition suppression, the PMN regions displayed their typical patterns of repetition enhancement, while also showing clear sensitivity to Task context.
3.7. Task-general Old > New effects are associated with the PMN as defined using independent network templates
The results thus far have identified a consistent group of regions that we identified as the PMN. However, to more confidently make this claim, we turned to prior literature to independently identify the network. This was done using publicly available network maps initially described by Shirer et al. (2012) and included a PMN network template (termed the “precuneus network” by Shirer and colleagues) as well as four comparison templates (Fig. 5A). Cortical regions associated with each of the non-PMN templates has been associated with retrieval success effects in (explicit) recognition memory tasks in prior literature (e.g., Nelson et al., 2010), and thus they appeared an appropriate comparison point in this investigation.
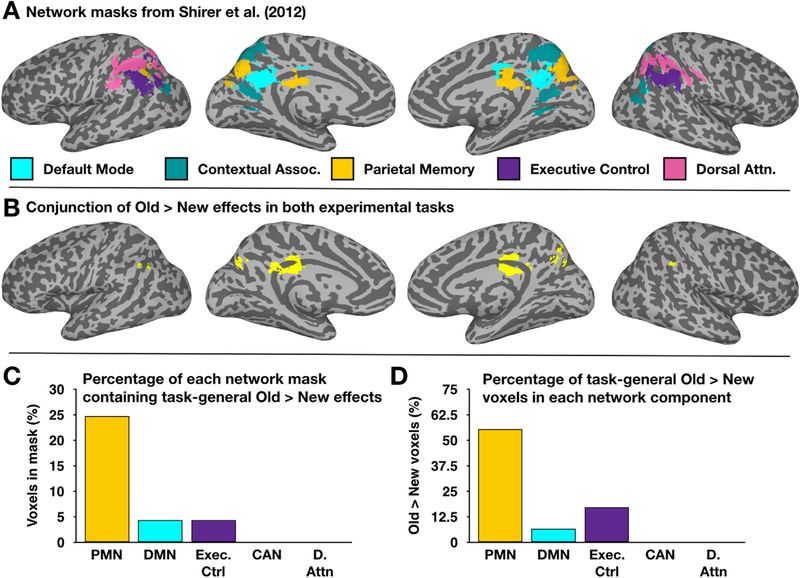
Fig. 5.
Independent network templates associate task-general repetition enhancement with the PMN. A) Aspects of 5 networks covering lateral and medial parietal cortex were adapted from Shirer et al. (2012). These include aspects of the default mode network (DMN), the contextual association network (CAN), the PMN, and elements of “executive control” and dorsal attention networks. B) Task-general enhancement effects were identified by creating a conjunction map of Old > New effects in the Second Naming and Recognition memory tasks. C) Overlap extents between each network in A and the conjunction in B were calculated. There is a six-fold increase in the proportion of the PMN template as compared to the DMN or executive control templates, and no overlap with the remaining two templates. D) Similar results are obtained when calculating the proportion of the Old > New mask in each network.
We then created a conjunction image of the Old > New effects in our Second Naming and Recognition memory tasks (Fig. 5B) in order to identify the location and quantify the extent of task-general repetition enhancement effects within our experiment. After creating this image, we assessed its overlap with each of the 5 network templates adapted from Shirer et al. (2012). This was conducted in two different ways. First, the proportion of each network that included Old > New voxels from the conjunction image was computed. This revealed at least some overlap in 3 of the 5 templates: PMN (24.7% of the template voxels), DMN (4.3% of the template voxels), and “executive control” network (4.3% of the voxels)(Fig. 5C). This analysis was re-conducted, this time examining the distribution of Old > New conjunction voxels that fell within each network template. This analysis revealed that 55.5% of task-general Old > New voxels fell within the PMN, 17.0% fell within the executive control network, and 6.6% fell within the DMN (Fig. 5D). Thus, irrespective of how the analysis was conducted, repetition enhancement effects were strongly linked with the PMN and only weakly associated with other nearby functional networks.
4. Discussion
In this report, we examined the robustness of familiarity-related PMN responses to differences in retrieval task conditions. By comparing BOLD responses during conditions in which familiarity was or was not task-relevant, we found a small collection of regions—independently identifiable as the PMN—to exhibit repetition enhancement irrespective of task context. This stands in contrast to repetition suppression effects in other cortical regions, which exhibited typical task-specificity. Implications of our findings are discussed below.
4.1. Informing repetition enhancement with repetition suppression
Behavioral priming and repetition suppression are thought to reflect implicit retrieval processes, and their underlying mechanisms remain matter of ongoing discussion (see e.g., Schacter et al., 2004; Gotts et al., 2012). One prominent hypothesis has suggested that response learning may underlie the observed behavioral and neural effects (Dobbins et al., 2004; see also Saggar et al., 2010). Our data were consistent with a response mapping account, with Old (relative to New) items in the Second Naming condition being associated with reduced neural responses in relevant regions (e.g., left IFG, bilateral fusiform regions), but without any such effects being observed in the Recognition task context (Figs. 3 and 4).
Considerably less theoretical discussion has been dedicated to repetition enhancement effects (Segaert et al., 2013). When these have been discussed, it is often in terms of the maintenance of information in working memory (e.g., Desimone, 1996) or linked with stimulus recognition effects (both voluntary and involuntary; Donaldson et al., 2001; Segaert et al., 2013; Kim, 2017). The latter case appears to be of particular relevance to the current discussion. Such characterizations would suggest that enhancement should be tied to a specific item, but it is not clear that a response needs to be both item- and response-specific (as is repetition suppression). The results of the current study are consistent with an item-only specificity: enhancement—not suppression—effects were observed in both Recognition and Second Naming conditions, with similar enhancement effects present within parietal cortex for Old stimuli regardless of task context (Fig. 3). These findings converge with those of previous studies that have examined implicit and explicit retrieval conditions (Phillips et al., 2009; Elman and Shimamura, 2011) and cannot easily be accounted for by simple RT differences, as Old items were associated with faster RTs but greater BOLD responses in both the Second Naming and Recognition tasks. The regions most reliably exhibiting enhancement fell within the PMN, as determined both by comparing coordinates in the current study to those reported in prior studies and by the use of independent network-level masking derived from separate functional connectivity data (Fig. 5). Thus, we can now clearly place the locations of previously-observed enhancement effects within the broader expanse of parietal cortex, an important outcome given the increasing number of distinct modules that have been identified on the lobe’s lateral and medial surfaces (Nelson et al., 2010, 2012, 2013b; Seghier et al., 2010; Seghier, 2013; Bzdok et al., 2015; Cha et al., 2017). That the effects were primarily associated with the PMN is consistent with prior hypotheses regarding the automaticity of familiarity processing within the network (Gilmore et al., 2015; Chen et al., 2017a).
The degree to which repetition enhancement effects necessarily reflect conscious recognition remains unclear, particularly in light of their apparently obligatory (or automatic) nature. Certainly, conscious awareness would be consistent with prior discussions of such effects (Donaldson et al., 2001; Nelson et al., 2013a; Kim, 2017) and of course with the explicit Recognition task data in the current experiment. Further, the high accuracy of subjects in the Recognition task would suggest that stimuli were highly memorable (and thus might be expected to elicit high levels of involuntary explicit memory retrieval in the Second Naming task; Schacter, 1987). At the same time, the present data suggest that a more nuanced account may be required. This is due to the combination of repetition suppression and repetition enhancement effects observed in the Second Naming condition (Fig. 3C). One could reason that when subjects named stimuli that they had seen before, they also engaged in explicit recognition of that stimulus. If so, then that would mean that subjects were processing the items differently during the Second Naming condition (as compared to Initial Naming). As a result, one would expect the suppression effects to be markedly reduced, if not completely absent, in the Second Naming task (Dobbins et al., 2004; Schacter et al., 2004). However, this was not the case. Thus, the suppression data suggested that even if subjects were, in fact, aware of their recent prior history with specific stimuli, they were not processing these items in a manner that impacted behavioral or fMRI data. This appears to have implications for the long-standing concerns of the degree to which explicit recognition might impact performance in nominally implicit tests of human memory (Schacter, 1987; Roediger, 1990; Roediger and McDermott, 1993).
4.2. Both task- and familiarity-related effects are observable within PMN regions
The current work adds to a growing literature on the impact of attentional effects within the PMN, and additionally speaks to several prominent hypotheses regarding the role of parietal cortex in memory and cognition. One of the first questions raised about PMN function was the degree to which the network’s responses were impacted by top-down attentional control and the degree to which they were automatic or task-invariant in nature (Gilmore et al., 2015). Earlier hypotheses were unclear on this point: The PMN sits at the border of regions that are thought to direct “top-down” attention to memory signals or are captured by “bottom up” recollective processes according to several popular hypotheses (Cabeza et al., 2008, 2012), and the pIPL/dAG component of the PMN appears to fall outside of the ventral lateral parietal regions associated with the “cortical binding of relational activity” hypothesis (Shimamura, 2011, 2014). Thus, although these hypotheses are in general quite fruitful for guiding memory research, they do not make clear predictions about what might be observed within PMN regions.
The presence of repetition enhancement effects outside of explicit retrieval conditions suggested that the posterior parietal responses to familiarity might be largely automatic, although at least some top-down effects were observable within PMN-like regions in prior experiments (O’Connor et al., 2010; Jaeger et al., 2013; Rosen et al., 2016, 2018). Importantly, the current data suggest that elements of both positions appear necessary to describe responses within the network. Familiarity—defined simply as one’s experimental history with a stimulus—cannot explain the full range of PMN responses we observed in this report, even if it is sufficient to capture the basic presence of “old/new” responses observed within PMN regions. In particular, it fails to account for the large differences in response amplitude present between task conditions (Figs. 3 and 4), such that one observes qualitatively similar responses for Old items during Second Naming and New items during Recognition. This seems unlikely to be a simple coincidence, as a prior experiment conducted by Elman and Shimamura (2011) also observed similar activation patterns for intentionally-recognized new items and incidentally familiar old items in a region very close to pIPL.
The presence of such clear task-related differences across item types strongly suggests a role for top-down attentional processing, and is consistent with recent suggestions that the PMN may be critical in integrating information across mnemonic and attentional control domains (Rosen et al., 2016, 2018; for related discussion, see Kim, 2018). Thus, both attentional (or in the current experiment, “task context”) and item history information seems to be reflected by activity within PMN regions, even if a basic pattern of enhancement is automatic in nature.
4.3. Laterality of the PMN
Although the PMN was initially associated with the left hemisphere (Gilmore et al., 2015; Rosen et al., 2018), an early question was raised regarding whether or not the network might be present bilaterally. This was raised by a disconnect between the network as it was identified in meta-analyses—which suggested a left hemisphere advantage (e.g., Kim, 2013)—and the network as it was defined using functional connectivity (e.g. Shirer et al., 2012)—which suggested a bilateral PMN. In the current data, we observed generally consistent bilateral effects within the network (albeit with sub-threshold effects present in right pIPL/dAG during the recognition task that became significant in subsequent analyses, as shown in Figs. 3B and 4). In addition, other recent work examining Old/New effects within the PMN has also observed bilateral effects (Chen et al., 2017a; McDermott et al., 2017; Rosen et al., 2018; Gilmore et al., submitted). These recent studies have primarily used picture stimuli, but the right pIPL/dAG region identified by McDermott and colleagues was observed in a task that used word stimuli exclusively. Thus, it seems unlikely that stimulus properties alone can explain the recent observations of PMN bilaterality, although previous studies have found links between properties of to-be-remembered stimuli and hemispheric involvement during retrieval more generally (e.g., McDermott et al., 1999; Klostermann et al., 2009). Instead, evidence appears to be building that supports a bilateral PMN. The observations of bilateral PMN region presence raise questions regarding the differential contributions of homotopic regions in the processing of familiar stimuli, and answers to these questions may in turn clarify why right pIPL/dAG appeared to be less frequently associated with the PMN in earlier work.
4.4. Spoken responses are “MR-compatible”
Conventional wisdom would suggest that speaking within the scanner is strictly verboten. This relates to concerns of subject motion, which has numerous deleterious effects on BOLD data quality (e.g., Friston et al., 1996; Bullmore et al., 1999; Lund et al., 2005). However, in-scanner speech can be used to ask questions that are beyond the scope of simple button press responses, and can do so without the need for additional verification steps as is appropriate in situations of covert cued recall (e.g., Wing et al., 2013; Gilmore et al., 2018). For example, experimenters can investigate processes associated with free recall of items within a list (Kragel and Polyn, 2013, 2016; Kragel et al., 2015), or examine effects related to continuous recall and description of scenes from video clips (e.g., Chen et al., 2017b; Baldassano et al., 2017).
Here, we utilized widely-spaced trials such that spoken responses which occurred within ~1 s of stimulus onset in all conditions would have minimal impact on the peak response periods associated with each trial. We also employed a multi-echo based denoising technique, ME-ICA (Kundu et al., 2013, 2012, 2017), to further improve data quality. Prior work has indicated that ME-ICA greatly reduces thermal noise, and its ICA-based identification of noise components further reduces possible motion-related artifacts in the time series (Power et al., 2018).
4.5. Limitations and future directions
The current report highlights an apparent robustness of the PMN to retrieval task conditions. However, numerous questions remain about the information being processed within the PMN that enables it to exhibit its characteristic sensitivity to stimulus familiarity.
A potential limitation of our conclusions comes from a similar experiment reported by Elman and Shimamura (2011), in which the authors observed similar effects in explicit and incidental recognition conditions comparable to those observed here, but failed to detect significant Old > New effects in pIPL (or elsewhere) during a color discrimination task. This led the authors to conclude Old > New effects were to some degree constrained by task conditions, which is inconsistent with the generality we have suggested here. However, during Elman & Shimamura’s discrimination task, visual properties of stimuli were altered from those initially studied (i.e., the font color was modified). Thus, although these data certainly suggest that task effects can modify the amplitudes of responses to novel and familiar stimuli (an observation in line with our current results), it may also suggest that the nature of the “source” of the familiarity signal requires further clarification; lacking this information, it is unclear if Elman & Shimamura’s conclusions of task relevance is indeed critical for the presence of enhancement effects, or if the match between recognition cue and memory trace is of more consequence (which would be consistent with an encoding specificity-type account, see e.g., Tulving, 1983).
Another outstanding question concerns the degree to which enhancement effects may rely upon inputs from the medial temporal lobe or elsewhere. The literature is unclear on this point: fMRI studies of declarative memory in amnesic patients are infrequent, usually tapping information that is semantic or temporally remote in nature (e.g., Maguire et al., 2005; Rosenbaum et al., 2007), or focusing on amnesia from other sources (e.g., Westmacott et al., 2008). Experiments with lesion patients would be useful in elucidating the potential signal sources supporting repetition enhancement within the PMN. In turn, this will likely provide novel insights into the nature of what enhancement effects represent, and the degree to which such signals reflect conscious or nonconscious stimulus recognition.
An additional question relates to activity observed in response to novel items in both the Second Naming and Recognition conditions. Prior work has suggested that the PMN activates in response to familiar items but that responses to novelty typically result in deactivations (Gilmore et al., 2015; Gilmore et al., submitted). Responses in our Second Naming task are generally consistent with these reports (Fig. 3B), but no deactivation was observed in the Recognition task (Fig. 4D). The cause of this discrepancy is unclear. One possibility may relate to our task design—widely spaced trials are not common in recent fMRI studies, and the inter-trial delay may produce relatively strong trialwise reorientation effects that impact observe BOLD responses. Directly comparing rapid and slow event-related task conditions in recognition testing may address this issue and could inform our understanding of what underlying processes are being captured by observable PMN response dynamics.
4.6. Conclusions
In this report, we examined BOLD responses associated with novel and familiar items, both when familiarity was task-relevant and when it was not, focusing in particular on a sparse functional network located primarily in parietal cortex. Consistent with prior reports, we found the PMN to be more active in response to familiar than to novel items during recognition memory decisions. A similar pattern was observed in the same regions when comparing responses to familiar and novel items during an object naming task, suggesting the response to be automatic in nature. At the same time, separable effects associated with item history and task context were observed in PMN regions. Collectively, these results emphasize an apparent task-generality of responses within the PMN, highlighting an automatic role for the network in the detection of stimulus familiarity.
Supplementary Material
Acknowledgements
We thank Vinai Roopchansingh for assistance with multi-echo sequence optimization and Javier Gonzalez-Castillo for assistance with implementing ME-ICA. We also thank Steve Nelson and Michal Ramot for thoughtful comments and discussions throughout the project.
Funding
This work was supported by the National Institute of Mental Health at the National Institutes of Health, Division of Intramural Research (ZIAMH002920).
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neuropsychologia.2018.12.023.
References
- Baldassano C, Chen J, Zabood A, Pillow JW, Hasson U, Norman KA, 2017. Discovering event stricture in continuous narrative perception and memory. Neuron 95, 709–721 (e705). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Corsese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Trieman R, 2007. The English lexicon project. Behav. Res. Methods 39, 445–459. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter DL, Rosen BR, Dale AM, 1998. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron 20, 285–296. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SC, Sharma T, McGuire PK, 1999. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum. Brain Mapp 7, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Heeger A, Langner R, Laird AR, Fox PT, Palomero-Gallagher N, Vogt BA, Zilles K, Eickhoff SB, 2015. Subspecialization in the human posterior medial cortex. Neuroimage 106, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M, 2012. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cognit. Sci 16, 338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M, 2008. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci 9, 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Jo HJ, Gibson WS, Lee J-M, 2017. Functional organization fo the human posterior cingulate cortex, revealed by multiple connectivity-based parcellation methods. Hum. Brain Mapp 38, 2808–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-Y, Gilmore AW, Nelson SM, McDermott KB, 2017a. Are there multiple kinds of episodic memory? An fMRI investigation comparing autobiographical and recognition memory tasks. J. Neurosci 37, 2764–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Leon gYC, Honey CJ, Yong CH, Norman KA, Hasson U, 2017b. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci 20, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance images. Comput. Biomed. Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017a. fMRI clustering and false-positive rates. Proc. Natl. Acad. Sci. USA 114, E3370–E3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017b. FMRI clustering in AFNI: false-positive rates redux. Brain Connect 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, 1996. Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. USA 93, 13494–13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL, 2004. Cortical activity reductions during repetition priming can result from rapid response learning. Nature 428, 316–319. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL, 2001. Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron 31, 1047–1059. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Shimamura AP, 2011. Task relevance modulates successful retrieval effects during explicit and implicit memory tests. Neuroimage 56, 345–353. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R, 1996. Movement-related effects in fMRI time-series. Magn. Reson. Med 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, Laumann TO, Gordon EM, Berg JJ, Greene DJ, Gratton C, Nguyen AL, Ortega M, Coalson RS, Schlaggar BL, Petersen SE, Dosenbach NUF, McDermott KB (submitted). High-fidelity mapping of implicit memory retrieval in the parietal memory network [DOI] [PMC free article] [PubMed]
- Gilmore AW, Nelson SM, McDermott KB, 2015. A parietal memory network revealed by multiple MRI methods. Trends Cognit. Sci 19, 534–543. [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, Naaz F, Shaffer RA, McDermott KB, 2018. BOLD activity during correct-answer feedback in cued recall predicts subsequent retrieval performance: an fMRI investigation using a partial trial design. Cereb. Cortex 28, 4008–4022. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Chow CC, Martin A, 2012. Repetition priming and repetition suppression: a case for enhanced efficiency through neural synchronization. Cogn. Neurosci 3, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger A, Konkel A, Dobbins IG, 2013. Unexpected novelty and familiarity orienting respones in lateral parietal cortex during recognition judgment. Neuropsychologia 51, 1061–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, 2013. Differential neural activity in the recognition of old versus new events: an activation likelihood estimation meta-analysis. Hum. Brain Mapp 34, 814–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, 2017. Brain regions that show repetition suppression and enhancement: a meta-analysis of 137 neuroimaging experiments. Hum. Brain Mapp 38, 1894–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, 2018. Parietal control network activation during memory tasks may be associated wtih the co-occurrence of externally and internally directed cognition: a crossfunction meta-analysis. Brain Res 1683, 55–66. [DOI] [PubMed] [Google Scholar]
- Klostermann EC, Louis P, Shimamura AP, 2009. Activation of right parietal cortex during memory retrieval of nonlinguistic auditory stimuli. Cogn. Affect. Behav. Neurosci 9, 242–248. [DOI] [PubMed] [Google Scholar]
- Kragel JE, Morton NW, Polyn SM, 2015. Neural activity in the medial temporal lobe reveals the fidelity of mental time travel. J. Neurosci 35, 2914–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel JE, Polyn SM, 2013. Functional interactions between large-scale networks during memory search. Cereb. Cortex 25, 667–679. [DOI] [PubMed] [Google Scholar]
- Kragel JE, Polyn SM, 2016. Decoding episodic retrieval processes: frontoparietal and medial temporal lobe contributions to free recall. J. Cognit. Neurosci 28, 125–139. [DOI] [PubMed] [Google Scholar]
- Kundu P, Brenowitz ND, Voon V, Worbe Y, Vértes PE, Inati SJ, Saad ZS, Bandettini PA, Bullmore ET, 2013. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc. Natl. Acad. Sci. USA 110, 16187–16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh W-M, Bandettini PA, 2012. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 60, 1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Voon V, Balchandani P, Lombardo MV, Poser BA, Bandettini PA, 2017. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage 154, 59–80. [DOI] [PubMed] [Google Scholar]
- Lund TE, Nørgaard MD, Rostrup E, Rowe JB, Paulson OB, 2005. Motion or activity: their role in intra- and inter-subject variation in fMRI. Neuroimage 26, 960–964. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Rudge P, Cipolotti L, 2005. The effect of adult-acquired hippocampal damage on memory retrieval: an fMRI study. Neuroimage 27, 146–152. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Buckner RL, Petersen SE, Kelley WM, Sanders AL, 1999. Set- and code-specific activation in the frontal cortex: an fMRI study of encoding and retrieval of faces and words. J. Cognit. Neurosci 11, 631–640. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Gilmore AW, Nelson SM, Watson JM, Ojemann JG, 2017. The parietal memory network activates similarly for true and associative false recognition elicited via the DRM procedure. Cortex 87, 96–107. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ SE, 2009. Laboratory-based and auto-biographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia 47, 2290–2298. [DOI] [PubMed] [Google Scholar]
- Morris CD, Bransford JD, Franks JJ, 1977. Levels of processing versus transfer appropriate processing. J. Verb. Learn Verb. Behav 16, 519–533. [Google Scholar]
- Nelson SM, Arnold KM, Gilmore AW, McDermott KB, 2013a. Neural signatures of test-potentiated learning in parietal cortex. J. Neurosci 33, 11754–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, Petersen SE, 2010. A parcellation scheme for human left lateral parietal cortex. Neuron 67, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, McDermott KB, Petersen SE, 2012. In favor of a ‘fractionation’ view of ventral parietal cortex: comment on Cabeza et al. Trends Cognit. Sci 16, 399–400. [DOI] [PubMed] [Google Scholar]
- Nelson SM, McDermott KB, Wig GS, Schlaggar BL, Petersen SE, 2013b. The critical roles of localization and physiology for understanding parietal contributions to memory retrieval. Neuroscientist 19, 578–591. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG, 2010. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J. Neurosci 30, 2924–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, 2007. Fragments of a larger whole: retrieval cues constrain observed neural correlates of memory encoding. Cereb. Cortex 17, 2030–2038. [DOI] [PubMed] [Google Scholar]
- Phillips JS, Velanova K, Wolk DA, Wheeler ME, 2009. Left posterior parietal cortex participates in both task preparation and episodic retrieval. Neuroimage 46, 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Plitt M, Gotts SJ, Kundu P, Voon V, Bandettini PA, Martin A, 2018. Ridding fMRI data of motion-related influences: removal of signals with distinct spatial and physical bases in multiecho data. Proc. Natl. Acad. Sci. USA 115, E2105–E2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, 1990. Implicit memory: retention without remembering. Am. Psychol 45, 1043–1056. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB, 1993. Implicit memory in normal human subjects. In: Boller F, Grafman J (Eds.), Handbook of Neuropsychology Elsevier, Amsterdam, pp. 63–131. [Google Scholar]
- Rosen ML, Stern CE, Devaney KJ, Somers DC, 2018. Cortical and subcortical cotributions to long-term memory-guided visuospatial attention. Cereb. Cortex 28, 2935–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Stern CE, Michalka SW, Devaney KJ, Somers DC, 2016. Cognitive control network contributions to memory-guided visual attention. Cereb. Cortex 26, 2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Grady CL, Ziegler M, Moscovitch M, 2007. Memory for familiar environments learned in the remote past: fMRI studies of healthy people and an amnesic person with extensive bilateral hippocampal lesions. Hippocampus 17, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Rugg MD, King DR, 2018. Ventral lateral parietal cortex and episodic memory retrieval. Cortex 107, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, 2012. SUMA. Neuroimage 62, 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggar M, Miikkulainen R, Schnyer DM, 2010. Behavioral, neuroimaging, and computational evidence for perceptual caching in repetition priming. Brain Res 1315, 75–91. [DOI] [PubMed] [Google Scholar]
- Schacter DL, 1987. Implicit memory: history and current status. J. Exp. Psychol. Learn Mem. Cogn 13, 501–518. [Google Scholar]
- Schacter DL, Bowers J, Booker J, 1989. Intention, awareness and implicit memory: the retrieval intentionality criterion. In: Lewandowsky S, Dunn JC, Kirsner K (Eds.), Implicit Memory: Theoretical Issues Erlbaum, Hillsdale, NJ, pp. 47–65. [Google Scholar]
- Schacter DL, Dobbins IG, Schnyer DM, 2004. Specificity of priming: a cognitive neuroscience perspective. Nat. Rev. Neurosci 5, 853–862. [DOI] [PubMed] [Google Scholar]
- Segaert K, Weber K, de Lange FP, Petersson KM, Hagoort P, 2013. The suppression of repetition enhancement: a review of fMRI studies. Neuropsychologia 51, 59–66. [DOI] [PubMed] [Google Scholar]
- Seghier ML, 2013. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ, 2010. Functional subdivisions in left angular gyrus where the semantic system meets and diverges from the default network. J. Neurosci 30, 16809–16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M, 2017. The contribution of the human posterior parietal cortex to episodic memory. Nat. Rev. Neurosci 18, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A, 2014. Remembering the past: neural substrates underlying episodic encoding and retrieval. Curr. Dir. Psychol. Sci 23, 257–263. [Google Scholar]
- Shimamura AP, 2011. Episodic retrieval and the cortical binding of relational activity. Cogn. Affect. Behav. Neurosci 11, 277–291. [DOI] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD, 2012. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL, 2009. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47, 1765–1779. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-Planar Stereotaxic Atlas of the Human Brain Thieme Medical Publishers, Inc, New York. [Google Scholar]
- Thomson DM, Tulving E, 1970. Associative encoding and retrieval: strong and weak cues. J. Exp. Psychol 86, 255–262. [Google Scholar]
- Tulving E, 1983. Elements of Episodic Memory Oxford University Press, New York. [Google Scholar]
- van Turennout M, Ellmore T, Martin A, 2000. Long-lasting cortical plasticity in the object naming system. Nat. Neurosci 12, 1329–1334. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL, 2005. Parietal lobe contributions to episodic memory retrieval. Trends Cognit. Sci 9, 445–453. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L, 1970. Amnesic syndrome: consolidation or retrieval? Nature 228, 628–630. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Silver FL, McAndrews MP, 2008. Understanding medial temporal activation in memory tasks: evidence from fMRI of encoding and recognition in a case of transient global amnesia. Hippocampus 18, 317–325. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A, 1998. Properties and mechanisms of perceptual priming. Curr. Opin. Neurobiol 8, 227–233. [DOI] [PubMed] [Google Scholar]
- Wing EA, Marsh EJ, Cabeza R, 2013. Neural correlates of retrieval-based memory enhancement: an fMRI study of the testing effect. Neuropsychologia 51, 2360–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.