Abstract
Objective
To describe the Klinefelter Syndrome(KS) phenotype during childhood in a large cohort.
Study design
Clinical assessment, measurement of hormonal indices of testicular function, and parent of origin of extra X chromosome were assessed in a cross-sectional study of 55 boys with KS, aged 2.0 to 14.6 years, at an outpatient center.
Results
Mean height and body mass index SD scores (SDS ± SD) were 0.9 ± 1.3 and 0.4 ± 1.4, respectively. Mean penile length and testicular volume SDS were −0.5 ± 0.9 and −0.9 ± 1.4. Testosterone levels were in the lowest quartile of normal in 66% of the cohort. Other features included clinodactyly (74%), hypertelorism (69%), elbow dysplasia (36%), high-arched palate (37%), hypotonia (76%), and requirement for speech therapy (69%). Features were similar in boys in whom diagnosis was made prenatally versus boys in whom the diagnosis was made postnatally. There was no evidence for a phenotypic effect of parent of origin of the extra X chromosome.
Conclusions
Boys with KS commonly have reduced penile length and small testes in childhood. The phenotype in boys with KS does not differ according to ascertainment or origin of the extra X chromosome. Boys with KS may be identified before puberty by tall stature, relatively decreased penile length, clinodactyly, hypotonia, and requirement for speech therapy.
Klinefelter syndrome (KS) is the most common disorder of sex chromosomes, affecting 1 in 500 to 1000 males of all races.1 In 1942, Klinefelter et al described a series of patients with gynecomastia, small testes, and hypogonadism.2 More than a decade later, KS was characterized by the abnormal karyotype 47,XXY.3 Since that time, the spectrum of the phenotype has been expanded to include tall stature and language-based learning disabilities.4 Advances in prenatal screening have increased the number of boys in whom the diagnosis is made antenatally,5–8 but it is not known whether the phenotype in this population is less severe than the phenotype described in boys diagnosed postnatally.
These questions about the development of boys with KS were formulated by the investigators at the onset of the study: What is the natural history of childhood testicular function in boys with KS? Does ascertainment bias influence the description of the phenotype? Although most boys with KS are identified on the basis of delayed puberty or oligospermia, many are currently identified with routine prenatal testing.5–7 The phenotype would be hypothesized to be mildest in boys in whom the diagnosis was ascertained prenatally as opposed to boys with a specific finding such as hypogonadism. Is the tall stature described frequently in KS present early in childhood, or does it develop later? Are weight and body mass index (BMI) relatively increased in addition to height, and if so, at what age do these changes occur? There has been some indication of reduced muscle tone9 in boys with KS, but little description of any dysmorphic features.10 Does the parent of origin of the extra X chromosome also contribute to variation in the KS phenotype?
Our goal in this study was to address some of these unanswered questions about childhood growth and development of boys with KS. We investigated auxologic measures, testicular function, and hormonal status. We also investigated the possible impact of parent of origin of the extra X chromosome and ascertainment bias on phenotype in a large cohort of boys with KS, aged 2 to 14 years.
METHODS
Subjects
Subjects were generally referred to the pediatric endocrine clinic at Thomas Jefferson University. All subjects had postnatal karyotypes confirming the diagnosis of KS. The study was approved by the Human Studies Committee at Thomas Jefferson University and UT Southwestern Medical School. Informed consent/assent was obtained in all cases. The clinical evaluation was performed at Thomas Jefferson University, and the genetic evaluation was performed at UT Southwestern Medical School.
Physical Features
Anthropometric measurements.
The clinical assessment included conversion of measurements to SD scores (SDS) by using age- and sex-specific norms of height (with stadiometer),11 lower and upper segments,12 arm span,13 weight,11 BMI,11 and head circumference.12 Measured or reported parental heights were recorded, and target height SDS were calculated from the National Center for Health Statistics data.11 The difference between measured height SDS and midparental target height SDS was also calculated. Gyneco-mastia was measured by means of palpation of breast tissue and assigned a Tanner stage accordingly.
Genitalia.
Penile length and testicular size were measured and converted to SDS by using published normative data.12 Penile length was measured from the pubic ramus to the tip of the glans.12 Testicular size was measured by using standard Prader orchidometer beads.12 When the 2 testes were of different size, the mean result was reported.
Muscle tone.
Muscle tone was evaluated clinically as normal, mildly decreased, severely decreased, or increased, by assessing the degree of resistance to passive movement at the elbow and knee, and the degree of pes planus and foot pronation.
Dysmorphic features.
Fifth finger clinodactyly was assessed by visual inspection of the angle of the fifth finger distal interphalangeal joint. Hypertelorism was defined as inner canthal distance ≥2 SD.12 Elbow dysplasia was assessed clinically by evaluating range of motion for supination of the wrist in the same horizontal plane as the antecubital fossa.
Bone age.
Bone age assessment was determined from a radiograph of the left hand, according to the standards of Greulich and Pyle.14
Genetic, laboratory testing.
Hormone levels were measured as reported previously.8,15 The reference values for age and pubic hair Tanner staging have been previously reported15 and are derived from the same laboratory used to analyze the hormone levels in this study.15 These reference values were established on the basis of data obtained from 651 healthy boys, sampled in the course of school population-based studies or for systematic investigation in siblings of children with allergic disease. All the referenced subjects were selected on a clinical basis: normal auxologic, bone age, and pubertal development for age, with no symptoms of an endocrine disorder.15 Serum testosterone and dehydroepiandrosterone sulfate (DHEAS) levels were measured by means of coated tube RIAs (CIS biointernational, Gif-sur-Yvette, France). Testosterone sensitivity was 0.01 ng/mL (0.05 nmol/L). Estradiol levels were measured by means of a double-antibody RIA (Diasorin, Anthony, France), and follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were measured by means of fluoroimmunometric assays (AutoDelfia reagents, Perkin-Elmer, Courtaboeuf, France). Antimullerian hormone (AMH) and inhibin-B were measured by means of enzyme-linked immunosorbent assays (DSL-France, Cergy-Pontoise, France), with respective sensitivities of 6 pg/mL and 0.35 ng/mL (2.5 pmol/L). Parent of origin of the extra X chromosome was determined by genotyping a panel of highly informative polymorphic X chromosome microsatellite markers with blood DNA from the KS proband and 1 or both parents as previously described.7 None of these results have been previously reported.
Statistics
All results are presented as mean SDS ± SD. Statistical comparisons included t tests, the Pearson correlations, and Fisher exact test comparisons. Results were considered statistically significant at a P value <.05.
RESULTS
Demographics
Our cohort included 55 boys, aged 2.0 to 14.6 years. The karyotype results included 51 boys; 47,XXY, 2 mosaic; 46,XY/47,XXY, and 2; 48,XXYY. The sample included 49 Caucasian boys, 5 African-American boys, and 1 Asian boy. 40 boys had received the diagnosis before the age of 2 years (35 for prenatal screening, 2 for hypotonia, 1 for developmental delay, and 2 for language delay). In the 35 boys in whom KS was diagnosed prenatally, 1 amniocentesis was performed for elevated alpha-fetoprotein level and the rest were performed for “advanced maternal age.” KS was diagnosed in 13 boys at ages 2 to 12 years (1 for tall stature, 6 because of behavior issues, 1 for hypotonia, 4 for language issues, and 1 at the mother’s request), and in 2 boys KS was diagnosed after age 12 years (1 for behavior and 1 for small testes). 38 of 55 boys (69%) had received speech therapy, 22 of 40 school-age boys (55%) had received school-based reading therapy, and 29 of 55 boys (53%) had received physical therapy, occupational therapy, or both. 3 boys, aged 13.8 to 14.3 years, received testosterone treatment during adolescence (duration, 2.0–2.3 years). These 3 boys were excluded from the penile measurements and all hormonal analyses.
Auxologic Measurements
The results are presented in Table I.
Table I.
Mean values (± SD) for auxologic and testicular measurements in boys with Klinefelter syndrome divided by age group
| Age 2.0–9.9 years | Age 10.0–14.9 years | P value | All | |
|---|---|---|---|---|
| N | 37 | 18 | 55 | |
| Height SDS | 0.8 ± 1.3 | 1.0 ± 1.2 | .54 | 0.9 ± 1.3 |
| Weight SDS | 0.7 ± 1.4 | 0.6 ± 1.3 | .57 | 0.7 ± 1.4 |
| Height SDS adjusted for midparental height SDS* | 0.7 ± 1.4 | 0.8 ± 1.3 | .68 | 0.7 ± 1.3 |
| BMI (kg/m2) | 18 ± 3 | 20 ± 5 | .07 | 18 ± 4 |
| BMI SDS | 0.5 ± 1.3 | 0.1 ± 1.4 | .25 | 0.4 ± 1.4 |
| Head Circumference SDS | 0.3 ± 1.7 | 1.0 ± 2.0 | .18 | 0.5 ± 1.8 |
| Upper segment/lower segment SDS | 1.3 ± 3.4 | 0.1 ± 3.0 | .22 | 0.8 ± 3.2 |
| Testicular volume (ml) | 1.2 ± 0.4 | 2.6 ± 1.3 | <.01 | 1.7 ± 1.1 |
| Testicular volume SDS | −1.2 ± 1.2 | −0.4 ± 1.6 | .04 | −0.9 ± 1.4 |
| Penile length (cm) | 4.0 ± 0.7 | 5.1 ± 2.2 | .01 | 4.4 ± 1.7 |
| Penile length SDS | −1.7 ± 0.6 | −1.4 ± 0.9 | .19 | −1.6 ± 0.8 |
P value younger versus older group.
If the height SDS adjusted for midparental height SDS = 0, then the child’s height = the midparental height for age.
Height.
The mean height SDS was increased (0.9 ± 1.3) without a significant correlation with age. 25 of 55 boys (45%) had height SDS >1. The mean height SDS of the 2 boys with 48,XXYY was 2.1, suggesting even taller height associated with this karyotype. There was a decreasing upper to lower segment ratio with age (r = −0.6, P < .001). The mean difference in height minus arm span was 2.2 ± 4.0 cm, suggesting relatively long legs in most boys. The mean height SDS adjusted for midparental target height SDS was 0.7 ± 1.3, indicating that the boys were generally taller than would be predicted by the midparental target height.
Body mass index and weight.
The mean weight and BMI SDS were increased, without any significant trend with age.
Head circumference.
The mean head circumference SDS was slightly greater than average (mean SDS, 0.5 ± 1.8) and did increase slightly with age (r = 0.31, P = .03).
Bone age.
Bone age was assessed in 27 boys, aged 3.6 to 14.6 years. The mean bone age minus chronologic age was −0.34 ± 1.7 years. The correlation of this difference with age was not significant, indicating that bone age was not systematically increased or decreased, relative to chronologic age.
Testicular Function Phenotype
Testicular volume.
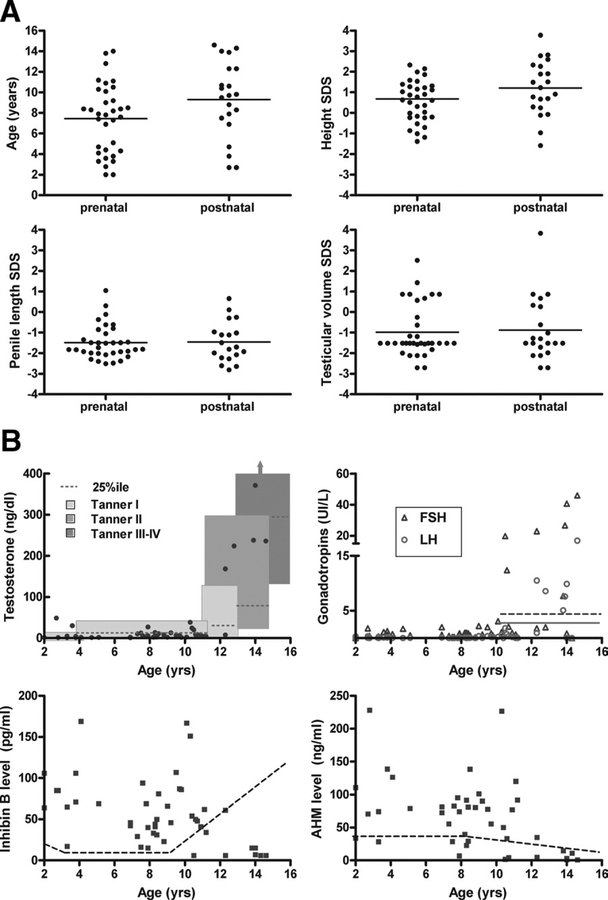
Small testes were observed in both the prepubertal- and pubertal-aged boys (Figure 1, Table I). Most boys (42/55, 76%) had testicular volume SDS <0, and 38 of 55 boys (69%) had testicular volume SDS < −1. Only 3 of 46 boys had testicular volume SDS >1 (age, 7.8, 10.3, and 10.7 years; Figure 1). In the older group, the 2 boys with testicular volume SDS >1 were, by hormone status, in early puberty. All boys older than 13 years (n = 6) had testicular volume SDS <0. Thus, small testes were observed in boys within the entire age range of this study, but 2 boys who had recently entered puberty had early testicular volumes closer to normal.
Figure 1.
A, Features by timing of diagnosis of KS: prenatal versus postnatal. The mean is indicated by the horizontal line in each group, age, height SDS, penile length SDS, and testicular volume SDS. B, Hormones in boys with KS in relationship to age. Testosterone values6: the boxes m ark the normal values for age and Tanner stage. G onadotropins6: upper threshold of norm al LH level during puberty marked by solid line, and for FSH marked by dotted line. Inhibin B levels: lower threshold of normal marked by dotted line. AMH levels: lower threshold of normal marked by dotted line.
Penile length.
Reduced penile length was observed in both the prepubertal- and pubertal-aged boys (Table I).
Gynecomastia.
8 of 18 boys (>10 years old) had gynecomastia (7 Tanner 2, 1 Tanner 3). In boys older than 10 years, there was a significant correlation between the presence of gynecomastia and increased BMI SDS (r = 0.47, P = .047), but not with penile length SDS, estradiol level, testosterone level, estradiol/testosterone ratio, or testicular volume SDS.
Ascertainment (Figure 1A).
Boys in whom the diagnosis was made prenatally were, on average, 2 years younger at the time of inclusion in this study. They had similar auxologic SDS scores. Small testes and reduced penile length were observed in many of the boys, regardless of their ascertainment.
Other clinical Features
Adrenarche.
Premature adrenarche (defined as onset of pubic hair before age 9 years) was observed in 1 of 32 boys (age, 8.3 years; Tanner 3). 12 of 17 boys aged 9.0 to 12.8 years had not yet achieved Tanner 2 or greater pubic hair, but all boys older than 12.8 years had Tanner 2 or greater pubic hair.
Other physical features (Table II).
Table II.
Frequency of physical and clinical characteristics in boys with Klinefelter syndrome
| Characteristic | Frequency in boys with KS |
|---|---|
| Penile length SDS <0 | 92% |
| Testicular volume SDS <0 | 76% |
| Height SDS >0 | 73% |
| BMISDS >0 | 62% |
| Gynecomastia in boys > 10 years | 44% |
| Clinodactyly | 74% |
| Hypertelorism | 69% |
| Elbow dysplasia (mild) | 36% |
| Abnormal palate | 37% |
| Hypotonia | 76% |
| Speech therapy | 69% |
| Reading therapy | 55% |
40 of 54 boys had fifth finger clinodactyly (Figure 2), 22 of 32 had hypertelorism, 20 of 54 had a high arched palate, 19 of 53 had mild elbow dysplasia, 4 of 53 (8%) had scoliosis, 1 had prominent veins in the lower extremities (age, 8.8 years), and none had cryptorchidism or hypertension.
Figure 2.
Phenotypic features in boys with KS. A, Boy with increased BMI, gynecomastia, and elbow dysplasia. B, Clinodactyly. C, High arched palate. D, Pes planus, appearance of decreased muscle mass in lower legs of boys with KS.
Muscle tone.
42 of 55 boys (76%) had hypotonia (8 severe and 34 mild; Figure 2). There was no significant correlation between testosterone levels and the presence of hypotonia (r = −0.005, P = .97).
Hormone levels
Testosterone (Figure 1B).
35 of 47 boys (75%) had testosterone levels <25th percentile for age and pubic hair Tanner stage.
DHEAS.
In nearly all the boys (26/30), DHEAS levels were within reference range for pubic hair stage.
Estradiol.
Estradiol levels were in the reference range in 46 of 46 boys (defined as undetectable—32 pg/mL at Tanner I-IV).
Gonadotropins (Figure 1B).
All the boys < 10 years old had prepubertal gonadotropins. Excluding those receiving testosterone treatment, 6 of 15 boys (40%) >10 years had elevated FSH levels (>4.8 IU/L). LH levels were elevated (>2 IU/L) in 5 of 15 boys (33%), most of whom (4/5) also had elevated FSH levels.
Inhibin B (Figure 1B).
All boys <10 years old had normal inhibin B levels. 7 of 16 boys >10 years old had a low inhibin B level, in contrast with the normal physiologic increase seen in puberty. The correlation between inhibin B and FSH levels was inverse (r = −0.35, P = .02).
Antimullerian hormone (Figure 1B).
AMH levels were low in 3 of 43 boys. There was an inverse correlation with age (r = −0.43, P = .004), FSH level (r = −0.40, P = .03), LH level (r = −0.37, P = .02), and testosterone level (r = −0.34, P = .03) and a positive correlation with inhibin B level (r = 0.74, P < .0001).
Genetics.
The parent of origin of the extra X chromosome was determined in 50 subjects: the extra X was of maternal origin in 30 boys and of paternal origin in 20 boys. There was no significant difference in the physical characteristics including penile length and testicular size.
DISCUSSION
This study describes the physical phenotype and hormonal and genetic findings in 55 boys with KS, aged 2 to14 years. We compared the phenotype of boys in whom the diagnosis was made prenatally through routine antenatal screening with that of boys in whom the diagnosis was made after birth, and we found no significant differences. Tall stature was observed at all ages within our cohort. Weight and BMI, on average, were also increased. There was a high prevalence of reduced penile length and reduced testicular size before puberty. Other notable findings included increased prevalence of hypotonia and dysmorphic features such as clinodactyly and hypertelorism and speech and reading therapy requirements. The previously described lack of association of parent of origin of the extra X chromosome with phenotype is confirmed in this larger cohort.5,7 Limitations of this study relate to the cross-sectional rather than longitudinal design. Some of the phenotypic description is subjective and therefore subject to bias.
Diagnosis of KS in childhood is typically delayed, with <10% of boys with KS receiving the diagnosis before puberty,1 and perhaps as many as 70% of male subjects with KS males in whom a diagnosis is never made.1 Parents have reported frustration with the consequences of a delay in diagnosis.16 The heterogeneity of this group is probably one factor contributing to this delay. Earlier diagnosis of KS would facilitate earlier interventions for these boys and better-informed pre-natal counseling.17
Studies are ongoing to evaluate whether there may be a benefit to low-dose non-aromatizing androgen therapy for cognitive and motor deficits. As more is known about potential fertility preservation, early knowledge of the diagnosis will become even more valuable. In addition, earlier intervention for speech and reading therapy in pediatrics improves out-come.
Although several earlier studies have included boys with KS ascertained both by means of screening and clinical referral,5,7,18 no comparison has been made in the 2 groups. One might expect that the phenotype would be less pronounced in boys in whom it is diagnosed incidentally, as is seen in Turner syndrome (45,X),19 so it is interesting that there were no differences in the two groups in this cohort. This is likely because of the subtler and less well-known phenotype of KS before mid-puberty.
The onset of childhood tall stature in KS has been described9,20 and is believed to become most apparent starting at 5 to 8 years of age.4 Boys in our cohort exhibited tall stature as early as age 2 years, without a significant correlation with age. As noted in previous studies,21 the boys’ tall stature exceeded that predicted by the midparental target height. This height increase may be related to the presence of an extra copy of the stature gene SHOX on the X and Y chromo-somes22 and delayed epiphyseal fusion on the basis of reduced testosterone and estradiol levels.
Increased weight and BMI in this cohort was not universal, but was more pronounced than in earlier descriptions of boys with KS.4,9,10,23 This finding may be related to the overall increase in childhood obesity in the United States.24 Head circumference has been reported to be decreased in infancy and childhood in KS20 or within reference range.25 We did not observe reduced head circumference. Boys with KS have previously been described as non-dysmorphic,10 but on careful inspection, we found an increased prevalence of hypertelorism, clinodactyly, high arched palate, and elbow dysplasia. This may prove to be helpful in diagnosis in childhood if clinicians become aware of the importance of looking for these features on routine examination. Hypotonia has previously been reported in childhood in smaller cohorts of boys26 and in infancy.8 Here we report hypotonia in 76% of the boys. In boys with KS, decreased tone is postulated to be part of a motor planning dyspraxia.27
On average, testicular volume was decreased throughout childhood in our cohort. Some early reports have indicated reduced testicular size in the prepubertal age group,18 and others indicate that testicular size in prepuberty is normal.4 It appears that the prepubertal reduced testicular size is caused by germ cell loss and some hyalinization and fibrosis of the interstitium and peritubular connective tissue.28 Leydig and Sertoli cells may be preserved until the onset of puberty, when Sertoli cells begin to degenerate and Leydig cells become hyperplastic.28
Penile length has been reported to be normal or reduced during prepuberty18,23 or reduced only starting in midpuberty.21 We observed reduced penile length throughout childhood in many of our cohort. As Caldwell and Smith noted, there is no convincing evidence of end-organ androgen insensitivity, because most boys with KS are born without hypospadius or cryptorchidism, and appropriate penile growth is seen in response to testosterone treatment.4,23 Penile length therefore serves as a biomarker of testosterone effect, and reduced penile length in these boys may reflect overall testosterone deficiency during childhood. There also may be some contribution of discrete genetic polymorphisms to penile growth in KS, because our earlier study showed an inverse correlation between the number of CAG repeats in the androgen receptor and penile length.7
We detected gynecomastia in 44% of boys older than 10 years, and its presence was associated only with increased BMI. Unlike adults with KS, this may not indicate an increased prevalence of gynecomastia during early adolescent boys with KS, because gynecomastia is common during puberty in the general male pediatric population.
It has been previously thought that testosterone deficiency does not manifest until midpuberty.18,29 In our cohort, testosterone levels were relatively low in boys as young as 2 to 8 years, with most values being <25th percentile for healthy subjects, despite LH values within reference range. The neonatal surge of testosterone is also attenuated in infants with KS.6,8 Together, this suggests androgen deficiency and a relative resistance of Leydig cells to LH during infancy and childhood. Wikstrom recently concluded that 14 adolescent boys with KS did not have unequivocal evidence of androgen deficiency.29 Their mean testosterone values were in the low reference range, and LH levels were elevated starting in midpuberty. Our data are similar to those of Wikstrom.29 It seems that the pubertal maturation of LH secretion is able to offset, at least temporarily, a relative Leydig cell deficiency.
There is conflicting data about androgen action in KS during childhood and adolescence.29 Penile length, an important biomarker of androgen action, was generally decreased in our cohort. Wikstrom et al found elevated leptin/BMI ratios and exaggerated responses of gonadotropins to gonadotropin-releasing hormone stimulation, also consistent with decreased androgen action,29 but they also found that sex hormone binding globulin and prostate specific androgen levels were normal, consistent with normal androgen action. These data may reflect differences in tissue responsiveness.
Inhibin B has also been of interest in male patients with hypogonadism because it is thought to reflect Sertoli cell number, spermatogenesis, or both.30 Inhibin B levels have been reported as normal in infancy in KS.6,17 We replicate earlier findings that inhibin B levels are normal until the beginning of puberty,28,31 consistent with normal Sertoli cell function before puberty. Inhibin B levels subsequently decrease as FSH levels increase, signaling further deterioration of Sertoli cell function with some possible contribution of decreased or absent spermatogenesis.
Another marker of Sertoli cell number and function is AMH. During embryogenesis, AMH is responsible for apoptosis of fetal Mullerian ducts. Its role in infancy and childhood is unclear. Levels are normally highest in the first year of life, decrease by adolescence, and are low by adulthood. Normal AMH secretion has been observed in infants and boys with KS6,28,29 and was observed in our cohort as well. In addition, we found that in KS, AMH secretion is negatively regulated by testosterone, as it is in typically developing boys. As a marker of Sertoli cell function, normal AMH levels suggest some preservation of function through mid-puberty in KS.
In summary, we describe clinical features in a large population of boys with KS that may be used to raise the clinicians’ awareness of the diagnosis of KS during childhood. These include below average sized penis and testes, taller stature than would be expected on the basis of parents’ heights, fifth finger clinodactyly, hypertelorism, hypotonia, and speech and reading delays. These features occur both in boys in whom the diagnosis was made prenatally and in boys in whom the diagnois was made postnatally. In addition, boys with KS have evidence of mild androgen deficiency throughout childhood, as indicated by their decreased penile length and relatively low testosterone levels. The contribution of early androgen deficiency to motor and cognitive functioning and the optimal age for initiation of testosterone replacement are important unanswered questions.
Acknowledgments
Supported by grants from the National Institutes of Health (RO1NS #050597, NS050597) and Institut de Recherche Endocrinienne et Metabolique (Paris, France).
Glossary
- AMH
Antimullerian hormone
- BMI
Body mass index
- DHEAS
Dehydroepiandrosterone sulfate
- FSH
Follicle-stimulating hormone
- KS
Klinefelter syndrome
- LH
Luteinizing hormone
- SDS
Standard deviation score
REFERENCES
- 1.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 2003;88:622–6. [DOI] [PubMed] [Google Scholar]
- 2.Klinefelter H, Reifenstein EC, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis, without A-Leydigism and increased excretion of follicle stimulating hormone. J Clin Endocr Metab 1942;2:615–27. [Google Scholar]
- 3.Bradbury J, Bunge RG, Boccabella RA. Chromatin test in Klinefelter’s syndrome. J Clin Endocrinol Metab 1956;16:689. [DOI] [PubMed] [Google Scholar]
- 4.Stewart DA, Bailey JD, Netley CT, Park E. Growth, development, and behavioral outcome from mid-adolescence to adulthood in subjects with chromosome aneuploidy: the Toronto Study. Birth Defects Orig Artic Ser 1990;26:131–88. [PubMed] [Google Scholar]
- 5.Harvey J, Jacobs PA, Hassold T, Pettay D. The parental origin of 47,XXY males. Birth Defects Orig Artic Ser 1990;26:289–96. [PubMed] [Google Scholar]
- 6.Lahlou N, Fennoy I, Carel JC, Roger M. Inhibin B and anti-Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J Clin Endocrinol Metab 2004;89:1864–8. [DOI] [PubMed] [Google Scholar]
- 7.Zinn AR, Ramos P, Elder FF, Kowal K, Samango-Sprouse C, Ross JL. Androgen receptor CAGn repeat length influences phenotype of 47,XXY (Klinefelter) syndrome. J Clin Endocrinol Metab 2005;90:5041–6. [DOI] [PubMed] [Google Scholar]
- 8.Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder FF, Zinn A. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Horm Res 2005;64:39–45. [DOI] [PubMed] [Google Scholar]
- 9.Robinson A, Bender BG, Linden MG. Summary of clinical findings in children and young adults with sex chromosome anomalies. Birth Defects Orig Artic Ser 1990;26:225–8. [PubMed] [Google Scholar]
- 10.Manning MA, Hoyme HE. Diagnosis and management of the adolescent boy with Klinefelter syndrome. Adolesc Med 2002;13:367–74. [PubMed] [Google Scholar]
- 11.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr 1979;32:607–29. [DOI] [PubMed] [Google Scholar]
- 12.Hall JG, Froster-Iskenius UG, Allanson JE. Handbook of normal physical measurements. Oxford: Oxford University Press; 1995. [Google Scholar]
- 13.Flugel GS. Anthropologischer Atlas Grundlagen und Daten. Frankfurt; 1986. [Google Scholar]
- 14.Greulich WaP SI. Radiographic atlas of skeletal development of the hand and wrist Stanford, Calif: Stanford University Press; 1959. [Google Scholar]
- 15.Soriano-Guillen L, Mitchell V, Carel JC, Barbet P, Roger M, Lahlou N. Activating mutations in the luteinizing hormone receptor gene: a human model of non-follicle-stimulating hormone-dependent inhibin production and germ cell maturation. J Clin Endocrinol Metab 2006;91:3041–7. [DOI] [PubMed] [Google Scholar]
- 16.Simpson JL, De La Cruz F, Swerdloff RS, Samango-Sprouse C, Skakkebaek NE, Graham JM Jr, et al. Klinefelter syndrome: expanding the phenotype and identifying new research directions. Genet Med 2003;5:460–8. [DOI] [PubMed] [Google Scholar]
- 17.Aksglaede L, Wikstrom AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update 2006;12:39–48. [DOI] [PubMed] [Google Scholar]
- 18.Salbenblatt JA, Bender BG, Puck MH, Robinson A, Faiman C, Winter JS. Pituitary-gonadal function in Klinefelter syndrome before and during puberty. Pediatr Res 1985;19:82–6. [DOI] [PubMed] [Google Scholar]
- 19.Gunther DF, Eugster E, Zagar AJ, Bryant CG, Davenport ML, Quigley CA. Ascertainment bias in Turner syndrome: new insights from girls who were diagnosed incidentally in prenatal life. Pediatrics 2004;114:640–4. [DOI] [PubMed] [Google Scholar]
- 20.Ratcliffe SG, Butler GE, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. IV. Birth Defects Orig Artic Ser 1990;26:1–44. [PubMed] [Google Scholar]
- 21.Stewart DA, Netley CT, Park E. Summary of clinical findings of children with 47,XXY, 47,XYY, and 47,XXX karyotypes. Birth Defects Orig Artic Ser 1982;18:1–5. [PubMed] [Google Scholar]
- 22.Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet 1997;16:54–63. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell PD, Smith DW. The XXY (Klinefelter’s) syndrome in childhood: detection and treatment. J Pediatr 1972;80:250–8. [DOI] [PubMed] [Google Scholar]
- 24.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76–9. [DOI] [PubMed] [Google Scholar]
- 25.Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder F, Zinn AR. The phenotype of early androgen deficiency in young boys with 47,XXY Klinefelter syndrome. J Clin Endocrinol Metab 2004;Submitted. [DOI] [PubMed] [Google Scholar]
- 26.Salbenblatt JA, Meyers DC, Bender BG, Linden MG, Robinson A. Gross and fine motor development in 47,XXY and 47,XYY males. Pediatrics 1987;80:240–4. [PubMed] [Google Scholar]
- 27.Samango-Sprouse C Mental development in polysomy X Klinefelter syndrome (47,XXY; 48,XXXY): effects of incomplete X inactivation. Semin Reprod Med 2001;19:193–202. [DOI] [PubMed] [Google Scholar]
- 28.Wikstrom AM, Raivio T, Hadziselimovic F, Wikstrom S, Tuuri T, Dunkel L. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab 2004;89:2263–70. [DOI] [PubMed] [Google Scholar]
- 29.Wikstrom AM, Dunkel L, Wickman S, Norjavaara E, Ankarberg-Lindgren C, Raivio T. Are adolescent boys with Klinefelter syndrome androgen deficient? A longitudinal study of Finnish 47,XXY boys. Pediatr Res 2006;59:854–9. [DOI] [PubMed] [Google Scholar]
- 30.Andersson AM, Skakkebaek NE. Serum inhibin B levels during male childhood and puberty. Mol Cell Endocrinol 2001;180:103–7. [DOI] [PubMed] [Google Scholar]
- 31.Christiansen P, Andersson AM, Skakkebaek NE. Longitudinal studies of inhibin B levels in boys and young adults with Klinefelter syndrome. J Clin Endocrinol Metab 2003;88:888–91. [DOI] [PubMed] [Google Scholar]