Abstract
Purpose
Previous studies observed modestly higher risk of gestational diabetes (GDM) associated with antidepressant use in pregnancy, potentially due to confounding by indication. We assessed the association of antidepressant continuation in pregnancy with GDM, as well as blood glucose levels, after accounting for confounding.
Methods
We conducted a retrospective cohort study of singleton live births from 2001–2014 to women enrolled in Kaiser Permanente Washington, an integrated healthcare delivery system, utilizing electronic health data and linked Washington State birth records. We required that women have ≥1 antidepressant prescription fills ≤6 months before pregnancy. Women with an antidepressant fill during pregnancy were categorized as “continuers” (n=1634); those without a fill were “discontinuers” (n=1211). We calculated relative risks (RRs) for GDM and mean differences in screening blood glucose levels using generalized estimating equations with inverse probability of treatment weighting to account for baseline characteristics, including mental health conditions and indicators of mental health severity.
Results
Compared with discontinuers, antidepressant continuers had comparable risk of GDM (RR: 1.10, 95% confidence interval [CI]: 0.84–1.44) and screening blood glucose levels (mean difference: 2.3 mg/dL, 95% CI: −1.5 to 6.1 mg/dL). We observed generally similar results for specific antidepressants, with the potential exceptions of risk of GDM associated with sertraline (RR: 1.30, 95% CI: 0.90–1.88) and venlafaxine (RR: 1.52, 95% CI: 0.87–2.68), but neither association was statistically significant.
Conclusions
Our study suggests that overall, women who continue antidepressants in pregnancy are not at increased risk for GDM or higher blood glucose, although further study may be warranted for sertraline and venlafaxine.
Keywords: antidepressants, blood glucose levels, gestational diabetes, glucola, oral glucose challenge test, pregnancy
1. INTRODUCTION
Gestational diabetes (GDM) is glucose intolerance in pregnancy that affects approximately 6% of pregnant women, or 280,000 pregnancies, in the US annually.1 The condition occurs when the pancreas is unable to compensate for the natural rise in insulin resistance during the second trimester with greater insulin secretion. In the US, screening for GDM is recommended at 24–28 weeks gestation or earlier in the second trimester for women at high risk.1 Affected women have increased risk of cesarean delivery and of having an infant with macrosomia or birth injuries.2 Over the lifecourse, women with a history of GDM have increased risk of developing type 2 diabetes,3 and their children are more likely to become obese and develop type 2 diabetes.4,5
Antidepressant use may increase type 2 diabetes risk in non-pregnant women,6,7 suggesting that the 7–8% of pregnant women in the US who use antidepressants8 could be at elevated risk of GDM. Antidepressant use has been associated with weight gain, which is known to increase GDM risk.9,10 Additionally, antidepressants may be associated with greater risk of diabetes through antidepressant-induced impairment of mitochondrial function in pancreatic β-cells, which decreases insulin secretion and increases β-cell apoptosis.6,11,12
Two previous studies suggest 1.31 to 1.37-fold increased risk of GDM associated with antidepressant use in pregnancy;13,14 however, both compared women using antidepressants in pregnancy with all women not using antidepressants in pregnancy instead of a group with a more comparable risk profile, such as pregnant women with a mental health diagnosis or pre-pregnancy antidepressant use. These studies also did not adjust for mental health indicators, such as level of mental healthcare utilization or use of psychotropic medications, which could help account for differences in severity of mental health conditions. Another study observed 15% greater risk for users of any antidepressant and 27% greater risk for venlafaxine users,15 and although the investigators accounted for many confounders, they did not include measures capturing severity of mental health conditions.
To address the potential for confounding by indication in prior studies, we investigated the risk of GDM and mean difference in screening blood glucose levels among women who used antidepressants before pregnancy, comparing those who continued versus discontinued antidepressant use during pregnancy. We accounted for differences in maternal characteristics, including mental health conditions and severity, both by restricting to prior users and using inverse probability of treatment weighting (IPTW) in analyses.
2. METHODS
2.1. Overview
We conducted a retrospective cohort study within Kaiser Permanente Washington (KPWA, formerly Group Health Cooperative), an integrated healthcare delivery system in Washington State that maintains extensive data on patient enrollment, demographics, encounters, diagnoses, procedures, and prescription fills. Two-thirds of KPWA members receive comprehensive care through clinics and providers within the KPWA system (the “integrated group practice” (IGP)); for these members we acquired additional data on laboratory values, vital signs, and mental health questionnaires. We used electronic health data from KPWA linked to Washington State birth records.16 The study was approved by the KPWA Institutional Review Board and the Washington State Department of Health Institutional Review Board (both with waivers of consent).
2.2. Study population and design
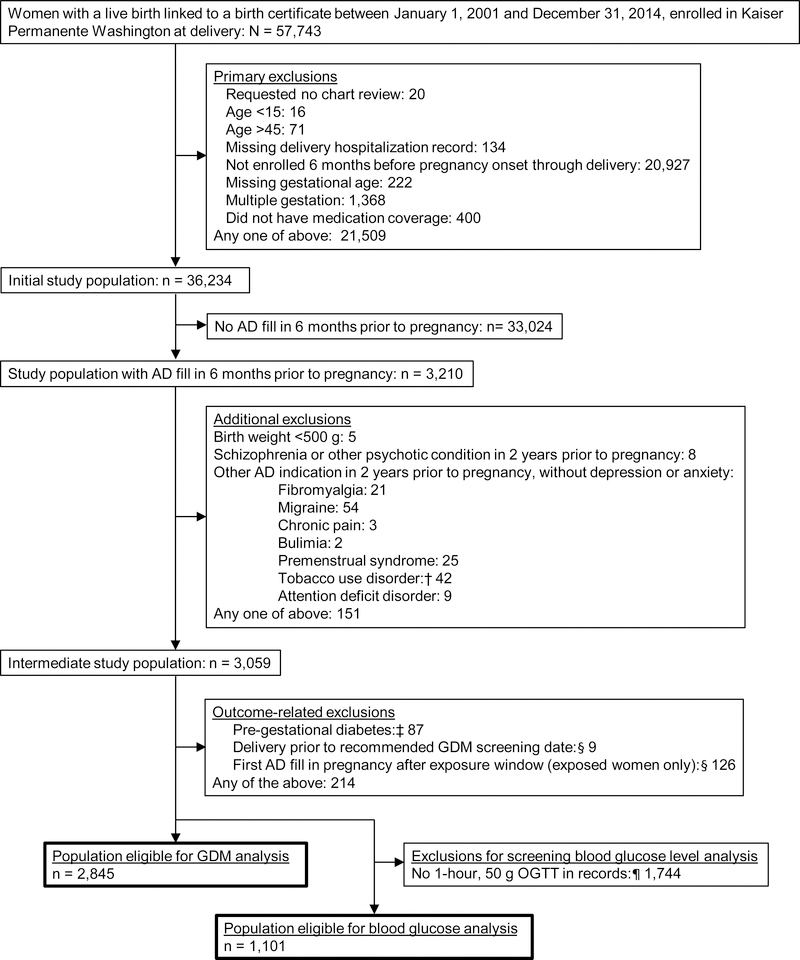
A flow of potentially eligible study subjects through all exclusion criteria is detailed in Figure 1. We identified a cohort of singleton live births that could be linked to a birth certificate from January 1, 2001 through December 31, 2014. These births were to women age 15–45 without pre-existing diabetes (type 1 or 2, Supplementary Table 2) who were enrolled in KPWA from six months before pregnancy through delivery. We required women to have an antidepressant prescription fill during the six months before pregnancy, allowing analyses to: (1) address the clinical decision of whether to continue antidepressants in pregnancy, and (2) limit bias due to confounding by indication. We excluded women with their first antidepressant fill in pregnancy after this exposure window, defined below (Figure 1). Because 7% of women contributed more than one birth to the cohort, our unit of analysis was technically “births” rather than “women”, but we have used the terms interchangeably.
Figure 1. Identification of the study population.
AD: Antidepressant; GDM: Gestational diabetes; OGTT: Oral glucose tolerance test; IGP: Integrated Group Practice
†We only excluded deliveries to women with a tobacco use disorder code in the 2 years prior to pregnancy if they also had a prescription fill for bupropion, the only antidepressant indicated to treat tobacco use disorder.
‡Women with type 1 or type 2 diabetes are, by definition, not at risk for GDM; we excluded women with those diagnostic codes (Supplementary Table 2) recorded between six months prior to pregnancy and 24 weeks gestation.
§The exposure window spans the start of pregnancy through 4 weeks before GDM screening. For the 8% of women missing GDM screening date from a procedure code, we assumed they were screened at 28 weeks, based on screening guidelines. If these women delivered at or before 28 weeks gestation, we excluded them, because they may not have received GDM screening.
¶We expected 1-hour, 50 g OGTTs (part of the 1-step testing strategy) to only be present in our laboratory data for women in the IGP from 2001–2011, after which the IGP switched to the 2-step testing strategy, which used 2-hour, 75-g OGTTs (Methods Appendix 1).
To further decrease confounding by indication, we sought to include only women taking antidepressants for depression or anxiety, as 80–90% of antidepressant use among women is for these indications.17,18 Because women without a healthcare encounter before pregnancy did not have a chance to receive a diagnostic code for depression or anxiety (Supplementary Table 1), we did not require such a code. However, we excluded women without a depression or anxiety code who had a code for another health condition that is an accepted antidepressant indication (Figure 1, Supplementary Table 2).8
Analyses of screening blood glucose levels were restricted to a subset with a 1-hour, 50 g oral glucose challenge test result available, predominantly those receiving care at KPWA-owned clinics prior to 2011 (Figure 1, Methods Appendix 1).
2.3. Exposure
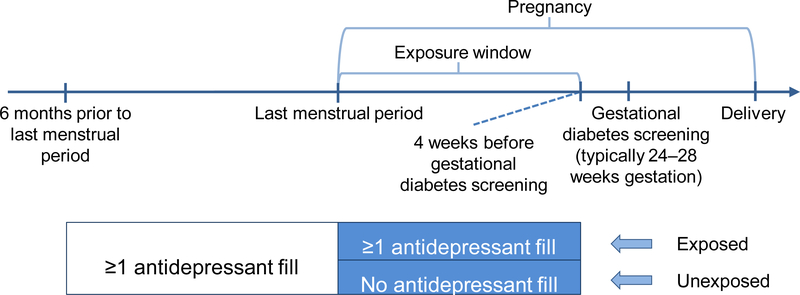
Medication exposure information came from pharmacy dispensing data and included generic antidepressant name, date of prescription fill, and days supplied. We defined the exposure window as the start of pregnancy through four weeks before the date of GDM screening for each woman to allow time for an effect of exposure on outcome (Figure 2). Because this is an insured population and the KPWA guidelines recommend universal GDM screening, we assumed screening occurred at 28 weeks (recommended at 24–28 weeks gestation) for the 8% of women missing a procedure code for GDM screening. We considered women with any antidepressant prescription fill during the pregnancy exposure window to be “continuers” and women without such a fill to be “discontinuers” (Figure 2).
Figure 2.
Study design
Fills for the following medications counted as exposure: selective serotonin reuptake inhibitors (SSRIs; citalopram, escitalopram, fluvoxamine, fluoxetine, paroxetine, sertraline), serotonin-norepinephrine reuptake inhibitors (SNRIs; desvenlafaxine, venlafaxine), and other antidepressants (bupropion, mirtazapine). We did not include trazodone or tricyclic antidepressants in our definition of exposure because they are most commonly used to treat sleep and pain conditions in our population.18 We did not require continuers to fill the same antidepressant during the six months prior to pregnancy and the exposure window. Women with an antidepressant fill that overlapped into pregnancy but without a fill during pregnancy were categorized as discontinuers, an assumption addressed in a sensitivity analysis.
2.4. Outcomes
GDM was ascertained from KPWA diagnostic codes. We required at least two codes of GDM (ICD-9-CM 648.8) from 16 weeks gestation to delivery or one code at the delivery hospitalization. Because there is evidence that even incremental differences in blood glucose levels are associated with adverse outcomes,19,20 we also assessed blood glucose levels (mg/dL) from a 50 g, 1-hour oral glucose challenge from GDM screening, ascertained from KPWA laboratory data.
2.5. Covariates
We ascertained parity, maternal race/ethnicity, maternal education, pre-pregnancy weight, and pre-pregnancy body mass index (BMI) from state birth records because they were not available in the electronic health record for the one-third of members not in the IGP. Primary analyses relied on pre-pregnancy weight because BMI was not available on the birth certificate until 2003.
We obtained the following covariates from KPWA electronic health databases: (1) at the time of delivery: birth year, maternal age, and baby’s sex, and (2) prior to pregnancy: membership in the IGP, Medicaid insurance coverage, smoking and substance use disorder diagnoses, mental health diagnoses, psychotropic and other prescription medication fills, and utilization of psychotherapy, psychiatry, and inpatient psychiatric hospitalization (definitions in Supplementary Tables 1, 9, and 10). We collected covariate data as far back in time as possible given continuous enrollment.21 We also ascertained GDM testing strategy (use of the one-step vs. two-step approach) using procedure codes (Methods Appendix 1).
To further assess depression severity, we extracted the most recent Patient Health Questionnaire-9 (PHQ-9) score in the 2 years prior to pregnancy when available (details about this questionnaire and availability in our population in Methods Appendix 2).22
2.6. Statistical analysis
We described characteristics of eligible women by exposure status and calculated standardized mean differences (SMDs) with and without IPTW. SMDs quantify differences between two groups without being overly affected by sample size.23
Because PHQ-9 scores were available only for a sub-set of women, we described PHQ-9 scores in the two years prior to pregnancy but did not include them in regression models.
To account for correlation among multiple births to the same woman, we used generalized estimating equations with an exchangeable working correlation matrix and robust standard errors for all regression analyses. For the analysis of GDM with antidepressant continuation in pregnancy, we used a Poisson (log) link function to calculate relative risks (RRs) and 95% confidence intervals (CIs). We calculated mean differences and 95% CIs for the association of continuing antidepressant use in pregnancy versus discontinuing use with screening blood glucose levels using an identity link function.
We used IPTW models to include more potential confounders than would be possible in multivariable adjusted models (Methods Appendix 3).23,24 Briefly, IPTW uses propensity scores to weight observations by their likelihood of exposure to improve balance in baseline covariates between exposure groups.
Covariates for IPTW models were chosen a priori based on our knowledge and previous literature, after considering sample size limitations. For the overall analysis of GDM, we weighted the IPTW model by all variables in Variable Set 3 (Supplementary Table 3). For the overall analyses of screening blood glucose levels, the IPTW model included fewer variables due to sample size limitations (Variable Set 2 in Supplementary Table 3). We conducted complete case analyses, excluding observations that were missing covariate values.
We conducted sub-analyses comparing women who continued specific antidepressants with women who discontinued any antidepressant (same group of discontinuers from overall analysis). We used the smaller Variable Set 2 for these sub-analyses due to smaller numbers of exposed women (Supplementary Table 3). For analyses of paroxetine and venlafaxine (which had the fewest users), we chose a parsimonious model based on characteristics with SMDs comparing continuers and discontinuers of ≥0.10 (see Supplementary Table 4 for SMD values), and from these variables, we included only those associated with a >10% change in the RR for GDM when added to the crude model (Variable Set 1 in Supplementary Table 3). For consistency, we used this model for analyses of blood glucose levels for these two antidepressants.
2.7. Sensitivity analyses
We conducted sensitivity analyses to address limitations to our primary analyses. For sensitivity analyses of both GDM and blood glucose levels, we redefined exposure as receiving ≥2 antidepressant fills to address potential exposure misclassification. For GDM only, a multiplicative interaction term was used to assess interaction by type of GDM testing strategy received (1-step and 2-step, Methods Appendix 1). Additional sensitivity analyses are described in Methods Appendix 4.
All statistical analyses were conducted in R version 3.4.2 (R Core Team [https://www.r-project.org]).
3. RESULTS
There were 2,845 births eligible for the GDM analysis, including 1,634 to women who continued antidepressants in pregnancy and 1,211 to women who discontinued. For analysis of blood glucose levels from the 1-hour, 50 g GDM screening, we had 1,101 births (continuers: 613, discontinuers: 488; Figure 1). Eighty-six percent of continuers filled an SSRI in pregnancy; fluoxetine, sertraline, and citalopram were the most commonly used antidepressants (Supplementary Table 5). Ninety percent of women had a fill for one antidepressant medication during pregnancy, 9% had fills for two different antidepressants, and <1% had fills for three or four different antidepressants (Supplementary Table 6). Four percent of women were missing data on a covariate and were excluded from regression analyses.
Women who continued antidepressant medication during pregnancy were similar to discontinuers with respect to baseline factors, with a few exceptions: antidepressant continuers were slightly older, more educated, more likely to have had a prior birth, and more likely to be non-Hispanic white than women who discontinued antidepressants (Table 1). Continuers were also more likely than discontinuers to have had a psychiatry visit or an antidepressant fill more than one year before pregnancy (Supplementary Table 4). The average length of enrollment prior to pregnancy was 4.3 years (standard deviation [SD]: 4.1 years) for continuers and 4.5 years (SD: 4.3 years) for discontinuers. The average exposure window was similar between continuers (164.0 days, SD: 24.5) and discontinuers (164.4 days, SD: 24.6). After weighting, SMDs in baseline characteristics were not meaningfully different (not ≥0.10)23 between continuers and discontinuers (Supplementary Figure 1).
Table 1.
Selected baseline characteristics of women with births eligible for the analysis of gestational diabetes.
| Covariate | No antidepressant fill in pregnancy (n=1211) % | Antidepressant fill in pregnancy (n=1634) % | Unweighted SMD | SMD after IPTW |
|---|---|---|---|---|
| Maternal age at delivery, mean (SD) | 29.6 (5.8) | 31.4 (5.5) | 0.301 | 0.012 |
| Number of prior births (parity) | ||||
| Zero | 45.1 | 37.5 | 0.156 | <0.001 |
| One | 34.4 | 34.9 | 0.010 | 0.006 |
| Two or more | 20.5 | 27.6 | 0.126 | 0.009 |
| Maternal race/ethnicity | ||||
| Hispanic | 6.6 | 5.7 | 0.038 | 0.008 |
| Non-Hispanic Asian | 2.9 | 1.5 | 0.093 | 0.002 |
| Non-Hispanic black | 4.0 | 2.3 | 0.098 | 0.004 |
| Non-Hispanic Native American | 1.1 | 1.5 | 0.040 | 0.003 |
| Non-Hispanic Native Hawaiian/Pacific Islander | 1.7 | 1.3 | 0.037 | <0.001 |
| Non-Hispanic white | 83.6 | 87.6 | 0.114 | 0.004 |
| Maternal education level | ||||
| High school diploma or less | 21.5 | 17.9 | 0.091 | 0.005 |
| Some college | 39.5 | 37.3 | 0.044 | 0.002 |
| Bachelor’s degree or more | 39.0 | 44.8 | 0.117 | 0.002 |
| Medicaid | 3.7 | 3.4 | 0.019 | 0.001 |
| Pre-pregnancy weight in lbs, mean (SD) | 165.8 (45.2) | 168.4 (44.5) | 0.058 | 0.007 |
| 1-step gestational diabetes testing strategy | 17.2 | 16.3 | 0.022 | 0.002 |
| Mental health disorders† | ||||
| Alcohol use disorder | 4.4 | 4.2 | 0.008 | 0.007 |
| Tobacco use | 13.0 | 13.6 | 0.020 | 0.001 |
| Drug use disorder | 4.0 | 3.6 | 0.023 | 0.001 |
| Depressive disorder | 72.4 | 73.4 | 0.023 | <0.001 |
| Anxiety disorder | 38.6 | 41.9 | 0.066 | 0.004 |
| Obsessive compulsive disorder | 1.8 | 3.3 | 0.094 | 0.019 |
| Post-traumatic stress disorder | 3.2 | 3.2 | 0.002 | 0.003 |
| Bipolar affective disorder | 4.7 | 4.3 | 0.017 | 0.007 |
| Medication fills‡ | ||||
| Antipsychotic | 1.8 | 2.3 | 0.036 | 0.003 |
| Benzodiazepine | 22.5 | 24.2 | 0.041 | 0.003 |
| Mood stabilizer medication | 4.9 | 5.3 | 0.021 | 0.014 |
| Medication associated with weight gain | 14.2 | 15.1 | 0.024 | 0.003 |
| Diabetes medication | 2.2 | 2.3 | 0.002 | 0.003 |
| Mental healthcare utilization§ | ||||
| Psychotherapy visit | 18.7 | 17.2 | 0.040 | 0.001 |
| Psychiatry visit | 14.4 | 15.8 | 0.037 | 0.004 |
| Inpatient psychiatric hospitalization | 0.7 | 1.0 | 0.026 | 0.016 |
SD: Standard deviation; SMD: Standardized mean difference; IPTW: Inverse probability of treatment weighting
If the SMD was ≥0.10, a standard cut point,23 we considered the two groups to be substantially different. Weighted SMDs <0.10 indicate that we had achieved good balance in terms of measured baseline characteristics between births to exposed and unexposed women.
This table contains the study population for the analysis of gestational diabetes. The study population for the analysis of blood glucose levels from the 1-hour, 50 g oral glucose tolerance test excluded many births presented in this table (Figure 1).
This table includes a subset of all covariates that were used to create the inverse probability of treatment weights and included in the overall regression models (Variable Set 3 in Supplementary Table 3). The full version of this table (with all included characteristics) is Supplementary Table 4.
Diagnostic codes for conditions are listed in Supplementary Table 1. Specific medications in medication categories are listed in Supplementary Table 9. Definitions of mental healthcare utilization are described in Supplementary Table 10.
At least one diagnostic code in the 2 years prior to pregnancy onset.
At least one medication fill in the year prior to pregnancy onset.
At least one encounter in the year prior to pregnancy onset.
Among antidepressant continuers, we had results from a PHQ-9 screening before pregnancy for 333 women (~59% of women who could have a record, based on when the PHQ-9 was used in KPWA, described in Methods Appendix 2) compared with 285 (~66% of women who could have a record) among discontinuers. The mean pre-pregnancy score was 9.7 for continuers and 10.4 for discontinuers (SDs: 6.6 and 6.7, respectively).
The unadjusted incidence of GDM was 9% in antidepressant continuers and 7% in discontinuers (Table 2). In aggregate, women continuing antidepressants in pregnancy did not have a greater risk of GDM than discontinuers after IPTW (RR 1.10, 95% CI: 0.84–1.44). However, continuation of sertraline and venlafaxine specifically showed some evidence of greater risks of GDM, although findings were non-significant (RR 1.30, 95% CI: 0.90–1.88 and RR 1.52, 95% CI: 0.87–2.68, respectively).
Table 2.
Association of antidepressant continuation in pregnancy with gestational diabetes.
| No. | No. (%) with gestational diabetes | Crude RR (95% CI) | Inverse probability of treatment weighted RR (95% CI) | |
|---|---|---|---|---|
| No antidepressant fill in pregnancy | 1211 | 90 (7%) | ref | ref |
| Any antidepressant fill in pregnancy | 1634 | 149 (9%) | 1.23 (0.95–1.58) | 1.10 (0.84–1.44)† |
| SSRIs | 1403 | 126 (9%) | 1.21 (0.93–1.57) | 1.10 (0.84–1.45)‡ |
| Citalopram | 343 | 28 (8%) | 1.08 (0.71–1.63) | 0.82 (0.51–1.28)‡ |
| Fluoxetine | 474 | 35 (7%) | 1.00 (0.69–1.45) | 1.00 (0.66–1.52)‡ |
| Paroxetine | 147 | 12 (8%) | 1.10 (0.62–1.96) | 0.88 (0.49–1.60)§ |
| Sertraline | 462 | 52 (11%) | 1.40 (1.00–1.97) | 1.30 (0.90–1.88)‡ |
| Bupropion | 217 | 21 (10%) | 1.29 (0.81–2.05) | 1.07 (0.63–1.81)‡ |
| Venlafaxine | 104 | 15 (14%) | 1.95 (1.17–3.26) | 1.52 (0.87–2.68)§ |
RR: relative risk; CI: confidence interval; SSRIs: selective serotonin reuptake inhibitors
Numbers for specific medications may add to >100% due to some women using multiple antidepressants.
Users of escitalopram, fluvoxamine, desvenlafaxine, and mirtazapine were also included in analyses but there were too few births among women exposed to these medications to present them separately.
Weighted by characteristics included in Variable Set 3, listed in Supplementary Table 3.
Weighted by characteristics included in Variable Set 2, listed in Supplementary Table 3.
Weighted by characteristics included in Variable Set 1, listed in Supplementary Table 3.
Overall, there was not a difference in mean blood glucose level between continuers and discontinuers after IPTW (mean difference: 2.3 mg/dL, 95% CI: −1.5 to 6.3 mg/dL), although continuers of sertraline, when assessed separately, had slightly higher blood glucose (mean difference: 5.4 mg/dL, 95% CI: −1.5 to 12.3 mg/dL; Table 3).
Table 3.
Association of antidepressant continuation in pregnancy with blood glucose levels from a screening, 1-hour, 50 g oral glucose tolerance test.
| No. | Blood glucose levels in mg/dL Mean (SD) | Crude mean difference in mg/dL (95% CI) | Inverse probability of treatment weighted mean difference in mg/dL (95% CI) | |
|---|---|---|---|---|
| No antidepressant fill in pregnancy | 488 | 115 (27) | ref | ref |
| Any antidepressant fill in pregnancy | 613 | 117 (30) | 2.1 (−1.4 to 5.5) | 2.3 (−1.5 to 6.1)† |
| SSRIs | 533 | 116 (28) | 1.6 (−1.9 to 5.0) | 1.8 (−2.0 to 5.6)† |
| Citalopram | 102 | 115 (23) | 0.4 (−4.6 to 5.4) | −0.3 (−5.7 to 5.2)† |
| Fluoxetine | 229 | 116 (29) | 0.7 (−4.0 to 5.3) | −0.2 (−5.3 to 4.8)† |
| Paroxetine | 74 | 118 (29) | 3.8 (−3.6 to 11.2) | 2.9 (−4.8 to 10.6)‡ |
| Sertraline | 141 | 118 (27) | 2.7 (−2.4 to 7.9) | 5.4 (−1.5 to 12.3)† |
| Bupropion | 83 | 120 (44) | 5.4 (−4.4 to 15.2) | 4.5 (−6.5 to 15.4)† |
| Venlafaxine | 28 | 117 (29) | 2.2 (−8.3 to 12.7) | 1.9 (−9.2 to 13.1)‡ |
SD: standard deviation; CI: confidence interval; SSRIs: selective serotonin reuptake inhibitors
Numbers for specific medications may add to >100% due to some women having used multiple antidepressants.
Users of escitalopram, fluvoxamine, desvenlafaxine, and mirtazapine were also included in analyses but there were too few births among women exposed to these medications to present them separately.
Weighted by characteristics included in Variable Set 2, listed in Supplementary Table 3.
Weighted by characteristics included in Variable Set 1, listed in Supplementary Table 3.
3.1. Sensitivity analyses
For most sensitivity analyses, we did not find an association between antidepressant continuation in pregnancy and GDM or blood glucose level (Supplementary Tables 7 and 8). There was a suggestion of greater risk of GDM associated with antidepressant continuation in women screened for GDM using the 1-step approach but not in women screened using the 2-step approach (RR: 1.54, 95% CI: 0.89–2.66 and RR: 0.96, 95% CI: 0.69–1.33, respectively); however, the p-value for interaction was not statistically significant (p=0.131; Supplementary Table 7).
4. DISCUSSION
After accounting for maternal characteristics, including mental health diagnoses and indicators of severity, we did not observe greater GDM risk (RR: 1.10, 95% CI: 0.84–1.44) or appreciable differences in screening blood glucose levels for women who continued, as compared with discontinued, antidepressant medication during pregnancy. Findings from blood glucose analyses were generally consistent with those from the GDM analyses. There was a suggestion of greater risk of GDM for women continuing venlafaxine and sertraline, the latter of which was supported by the slightly higher screening blood glucose levels for sertraline. However, none of these findings were statistically significant, and as such, should be viewed only as potential signals to be explored in future research.
In contrast with our study, some previous studies observed greater risk of GDM associated with antidepressant use: a study using Sweden’s national registry data on births from 1995–2007 reported moderately greater risk of GDM associated with use of antidepressants in pregnancy (OR: 1.37, 95% CI 1.18–1.58). The medications studied included SSRI’s, SNRI’s, tricyclic antidepressants, mirtazapine, bupropion, and some rarely used antidepressants such as monoamine oxidase inhibitors.13 A smaller study of births from 1990–2000 in the Canadian province of Saskatchewan also suggested about 30% greater risk with exposure to SSRI’s, although this result was not statistically significant (OR: 1.31, 95% CI: 0.86–2.01).14 Both studies compared pregnant women who used antidepressants with pregnant women who did not use antidepressants, instead of restricting the comparator group to pregnant women with a depression or anxiety diagnosis or pre-pregnancy antidepressant use. Additionally, they did not adjust for indicators of severity of mental health conditions. Although our overall findings were not indicative of elevated risk of GDM, we were not powered to rule out the modest increased risk suggested by these studies, given our upper confidence limit of 1.44. Our findings were more consistent with a sub-analysis from a large, Canadian study conducted within the Quebec Pregnancy Cohort using births from 1998–2015 (presented as an abstract) that restricted to women using antidepressants prior to pregnancy, including SSRI’s, SNRI’s, and tricyclic antidepressants, but did not adjust for indicators of the severity of mental health conditions. The authors reported slightly greater risk of GDM associated with antidepressant use in pregnancy (OR: 1.15, 95% CI: 1.00–1.33).15 In analyses not restricted to women who used antidepressants pre-pregnancy, the authors reported greater risk of GDM specific to venlafaxine users (OR: 1.27, 95% CI: 1.09–1.49) and no greater risk for sertraline users (OR: 1.01, 95% CI: 0.80–1.28). Although our estimates of GDM risk associated with these medications had wide confidence intervals, based on our point estimates, we interpret our findings for venlafaxine (RR: 1.52, 95% CI: 95% CI: 0.87–2.68) as most likely consistent with the aforementioned study and our findings for sertraline (RR: 1.30, 95% CI: 0.90–1.88) as most likely inconsistent. To our knowledge, no previous studies have assessed the association of antidepressant use in pregnancy with blood glucose levels.
There is biological plausibility for a greater risk of GDM associated with sertraline and venlafaxine use. A study in female cynomolgus monkeys found that sertraline treatment was associated with decreased adiponectin levels, which could in turn promote insulin resistance.25 In humans, a study reported that adults using sertraline had about 50% greater risk of developing diabetes than those not using the medication, which was higher than for other antidepressants assessed in our study.26 As an SNRI, venlafaxine affects norepinephrine in addition to serotonin. Evidence in humans indicates that alterations in the gene encoding for the β3 adrenergic receptor (a target for norepinephrine) are associated with increased insulin resistance, lending support to our findings that venlafaxine may increase risk of GDM.27,28 Another study found that risk of developing chronic diabetes in adults using venlafaxine was greater than among other antidepressant users.29 However, the specific pathways through which venlafaxine use in pregnancy may lead to increased risk of GDM are not well understood. Considering that venlafaxine is not typically a first-line treatment for depression and anxiety, venlafaxine users may have differed from other antidepressant users in ways we did not fully capture, leading to confounding.
A major strength of this study is that we restricted to women taking antidepressants prior to pregnancy and used indicators of mental health severity and other demographic and health-related factors in our models to minimize confounding. It is reassuring that for the subgroup with PHQ-9 depression scores available, these scores were very similar between continuers and discontinuers, indicating similar depression severity prior to pregnancy. Other strengths include use of a large, well-defined study population and antidepressant dispensing data, rather than prescriptions alone or maternal self-report.
Our study had several limitations. There were small numbers of women continuing certain antidepressants, which decreased power to detect modest effects for these medications. There also could be residual confounding by factors like mental health condition severity or healthy lifestyle. Additionally, if women with only one antidepressant fill in pregnancy did not actually consume the medication, there could be misclassification of exposure, potentially attenuating our risk estimates. In primary analyses of GDM, we combined women who received the 1-step testing strategy with women who received the 2-step testing strategy; although we adjusted for type of test received, this may not have been appropriate if antidepressant use was differentially associated with GDM based on testing strategy, as suggested by a sensitivity analysis. Also, we studied live births in a largely non-Hispanic white and commercially insured population, which may limit generalizability.
In conclusion, our study suggests that continuation of antidepressant medications during pregnancy is not associated with greater risk of GDM, with the possible exceptions of sertraline and venlafaxine, which need further study. In addition to risk for gestational diabetes, there are many other factors that must be weighed in the decision about whether to continue antidepressants in pregnancy, including other effects on infant health and the woman’s mental health.
Supplementary Material
Figure S1. Unweighted and weighted standardized mean differences (SMDs) comparing characteristics in births to women who continued or discontinued antidepressants in pregnancy in the study population for the gestational diabetes analysis.
Figure S2. Unweighted and weighted standardized mean differences (SMDs) comparing characteristics in births to women who continued or discontinued antidepressants in pregnancy in the study population for the screening blood glucose levels analysis.
Key points.
Antidepressant use has been associated with greater risk of type 2 diabetes.
Previous studies suggest that antidepressant use in pregnancy increases risk of gestational diabetes, but they may have been biased by confounding.
Our study accounts for confounding by maternal characteristics, including presence of mental health conditions and indicators of their severity.
Overall, we did not observe greater risk of gestational diabetes or higher screening blood glucose levels associated with antidepressant use in pregnancy.
We found suggestions of greater risk of gestational diabetes for women who used the antidepressants sertraline or venlafaxine, but these findings may have been due to chance and need confirmation in future research.
Acknowledgements
We thank Sharon Fuller and Eric Baldwin for pulling the information from the Kaiser Permanente Washington database to build our cohort, as well as programming our initial variables. We thank Dr. Greg Simon and Christine Stewart for their advice about how to best extract and operationalize mental health indicators. We also acknowledge the Mental Health Research Network for their resources that we used to define mental health diagnoses, procedures, and medications. We thank James Fraser for his help with Institutional Review Board applications. We thank Dr. Susan Shortreed and Rob Wellman for their advice regarding the statistical analysis.
Funding sources: Dr. Paige Wartko received a graduate student stipend from the Stroum Graduate Fellowship through the University of Washington Diabetes Research Center and the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Reproductive, Perinatal, and Pediatric Epidemiology Training Grant (#T32 HD052462). Funds for data extraction came from a Group Health Foundation Partnership for Innovation grant.
REFERENCES
- 1.ACOG Practice Bulletin No. 190 Summary: Gestational Diabetes Mellitus. Obstetrics and gynecology. 2018;131(2):406–408. [DOI] [PubMed] [Google Scholar]
- 2.Spaight C, Gross J, Horsch A, Puder JJ. Gestational Diabetes Mellitus. Endocr Dev 2016;31:163–178. [DOI] [PubMed] [Google Scholar]
- 3.Chodick G, Elchalal U, Sella T, et al. The risk of overt diabetes mellitus among women with gestational diabetes: a population-based study. Diabetic medicine : a journal of the British Diabetic Association. 2010;27(7):779–785. [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes. 1991;40 Suppl 2:197–201. [DOI] [PubMed] [Google Scholar]
- 5.Gilmore LA, Klempel-Donchenko M, Redman LM. Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Seminars in perinatology. 2015;39(4):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes care. 2013;36(10):3337–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharjee S, Bhattacharya R, Kelley GA, Sambamoorthi U. Antidepressant use and new-onset diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 2013;29(4):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huybrechts KF, Palmsten K, Mogun H, et al. National trends in antidepressant medication treatment among publicly insured pregnant women. General hospital psychiatry. 2013;35(3):265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmacher T. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res 2003;37(3):193–220. [DOI] [PubMed] [Google Scholar]
- 10.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstetrics and gynecology. 2010;115(3):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmorsy E, Al-Ghafari A, Helaly ANM, Hisab AS, Oehrle B, Smith PA. Editor’s Highlight: Therapeutic Concentrations of Antidepressants Inhibit Pancreatic Beta-Cell Function via Mitochondrial Complex Inhibition. Toxicol Sci 2017;158(2):286–301. [DOI] [PubMed] [Google Scholar]
- 12.Isaac R, Boura-Halfon S, Gurevitch D, Shainskaya A, Levkovitz Y, Zick Y. Selective serotonin reuptake inhibitors (SSRIs) inhibit insulin secretion and action in pancreatic beta cells. The Journal of biological chemistry. 2018;293(12):4577–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychological medicine. 2010;40(10):1723–1733. [DOI] [PubMed] [Google Scholar]
- 14.Wen SW, Yang Q, Garner P, et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. American journal of obstetrics and gynecology. 2006;194(4):961–966. [DOI] [PubMed] [Google Scholar]
- 15.Dandjinou M, Sheehy O, Bérard A. Antidepressants Use during Pregnancy and the Risk of Gestational Diabetes Mellitus. Birth Defects Res A Clin Mol Teratol 2018;110(9):775. [Google Scholar]
- 16.Baldwin E, Johnson K, Berthoud H, Dublin S. Linking mothers and infants within electronic health records: a comparison of deterministic and probabilistic algorithms. Pharmacoepidemiology and drug safety. 2015;24(1):45–51. [DOI] [PubMed] [Google Scholar]
- 17.Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. American journal of obstetrics and gynecology. 2012;207(1):49 e41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patten SB, Esposito E, Carter B. Reasons for antidepressant prescriptions in Canada. Pharmacoepidemiology and drug safety. 2007;16(7):746–752. [DOI] [PubMed] [Google Scholar]
- 19.Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes care. 2009;32(9):1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. The New England journal of medicine. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 21.Gilbertson DT, Bradbury BD, Wetmore JB, et al. Controlling confounding of treatment effects in administrative data in the presence of time-varying baseline confounders. Pharmacoepidemiology and drug safety. 2016;25(3):269–277. [DOI] [PubMed] [Google Scholar]
- 22.Manea L, Gilbody S, McMillan D. A diagnostic meta-analysis of the Patient Health Questionnaire-9 (PHQ-9) algorithm scoring method as a screen for depression. General hospital psychiatry. 2015;37(1):67–75. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. American journal of epidemiology. 2006;163(12):1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverstein-Metzler MG, Shively CA, Clarkson TB, et al. Sertraline inhibits increases in body fat and carbohydrate dysregulation in adult female cynomolgus monkeys. Psychoneuroendocrinology. 2016;68:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siafis S, Papazisis G. Detecting a potential safety signal of antidepressants and type 2 diabetes: a pharmacovigilance-pharmacodynamic study. British journal of clinical pharmacology. 2018;84(10):2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widen E, Lehto M, Kanninen T, Walston J, Shuldiner AR, Groop LC. Association of a polymorphism in the beta 3-adrenergic-receptor gene with features of the insulin resistance syndrome in Finns. The New England journal of medicine. 1995;333(6):348–351. [DOI] [PubMed] [Google Scholar]
- 28.Walters JM, Ward GM, Barton J, et al. The effect of norepinephrine on insulin secretion and glucose effectiveness in non-insulin-dependent diabetes. Metabolism. 1997;46(12):1448–1453. [DOI] [PubMed] [Google Scholar]
- 29.Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. The American journal of psychiatry. 2009;166(5):591–598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Unweighted and weighted standardized mean differences (SMDs) comparing characteristics in births to women who continued or discontinued antidepressants in pregnancy in the study population for the gestational diabetes analysis.
Figure S2. Unweighted and weighted standardized mean differences (SMDs) comparing characteristics in births to women who continued or discontinued antidepressants in pregnancy in the study population for the screening blood glucose levels analysis.