Abstract
Background:
Prenatal exposures to certain per- and polyfluoroalkyl substances (PFAS) have been linked to lower weight and adiposity at birth but greater weight and adiposity in childhood. We hypothesized that faster growth in early infancy may be associated with maternal PFAS concentrations.
Methods:
Among 415 mother-infant pairs in a longitudinal cohort study, we estimated associations between maternal pregnancy serum concentrations of six PFAS and offspring weight and adiposity at ~5 months of age, and growth in early infancy. Linear and logistic regression models were adjusted for potential confounders including maternal pre-pregnancy body mass index. Effect modification by infant sex was evaluated. We evaluated potential confounding by correlated exposures via multipollutant linear regression and elastic net penalized regression.
Results:
Associations between maternal PFAS concentrations and infant weight and adiposity differed by offspring sex. In male infants, maternal perfluorooctanoate and perfluorononanoate were positively associated with adiposity, with percent fat mass increases of 1.5-1.7% per ln-ng/mL increase in PFAS (median adiposity at ~5 months: 24.6%). Maternal perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS) were associated with lower weight-for-age z-score among female infants only (−0.26 SD per ln-ng/mL PFOS, 95% CI −0.43, −0.10; −0.17 SD per ln-ng/mL PFHxS, 95% CI −0.33, −0.01). In analyses pooled by sex, 2-(N-methyl-perfluorooctane sulfonamido) acetate above vs. below the limit of detection was associated with greater odds of rapid growth in weight-for-age (odds ratio [OR] 2.2, 95% CI 1.1, 4.3) and weight-for-length (OR 3.3, 95% CI 1.8, 6.2). Multipollutant models generally confirmed the results and strengthened some associations.
Discussion:
We observed sex- and chemical-specific associations between maternal serum PFAS concentrations and infant weight and adiposity. Multipollutant models suggested confounding by correlated PFAS with opposing effects. Although maternal PFAS concentrations are inversely associated with infant weight and adiposity at birth, rapid gain may occur in infancy, particularly in fat mass.
Keywords: Perfluoroalkyl substances, polyfluoroalkyl substances, pregnancy, infancy, adiposity, rapid growth, weight-for-age, weight-for-length
Introduction1
Per- and polyfluoroalkyl substances (PFAS) are persistent environmental chemicals used widely in a variety of consumer and industrial products. Despite the phase-out of production of certain PFAS in the USA, many chemicals within this class continue to be detected in groundwater (Anderson et al. 2016; Barzen-Hanson et al. 2017), drinking water (Hu et al. 2016), and in human blood in the United States (Centers for Disease Control and Prevention 2019), including in women of reproductive age. It is therefore important to identify and quantify any adverse health effects that may result from PFAS exposure experienced by pregnant women and their infants.
Prenatal exposure to PFAS may have lasting effects on offspring metabolic health. Several epidemiologic studies have reported that maternal concentrations of PFAS during pregnancy are associated with greater offspring weight and/or adiposity in childhood or early adulthood (Braun et al. 2016; Halldorsson et al. 2012; Høyer et al. 2015; Karlsen et al. 2016; Lauritzen et al. 2018; Mora et al. 2017), although other studies have reported null (Andersen et al. 2013; Hartman et al. 2017; Manzano-Salgado et al. 2017) or inverse associations (Wang et al. 2016). A recent meta-analysis of 10 prospective studies found an overall positive association between prenatal perfluorooctanoate (PFOA) and child overweight or body mass index (BMI) (Liu et al. 2018).
In mouse models, in utero exposure to low doses of PFOA produced higher body weight in midlife (Hines et al. 2009), while in utero exposure to perfluorooctane sulfonate (PFOS) led to insulin resistance in adult offspring (Wan et al. 2014). Potential biological mechanisms by which prenatal PFAS exposure may affect adiposity and related metabolic disruption include effects on peroxisome proliferator-activated receptors (regulators of lipid metabolism and adipogenesis), as well as estrogen receptor-mediated activity (Bjerregaard-Olesen et al. 2019; Heindel et al. 2017; Rosen et al. 2017). Maternal PFOA concentrations during pregnancy have consistently been inversely associated with infant weight at birth (Bach et al. 2015; Johnson et al. 2014), and we recently reported in the Healthy Start study that women with higher concentrations of certain PFAS during pregnancy also delivered infants with lower adiposity (percent fat mass) at birth (Starling et al. 2017).
The different directions of association between prenatal PFAS and offspring adiposity at different ages may be reconciled if there is a period of rapid growth during which the smaller, more highly exposed infants begin to exceed the weight and adiposity of their less-exposed peers. However, previous findings on the association between prenatal PFAS exposure and weight, adiposity, or growth in infancy have been inconsistent across studies (Alkhalawi et al. 2016; Andersen et al. 2010; Chen et al. 2017; de Cock et al. 2014; Gyllenhammar et al. 2018; Maisonet et al. 2012; Manzano-Salgado et al. 2017; Shoaff et al. 2018).
Rapid infant growth is associated with greater risk of obesity later in life, and systematic reviews have identified birth to 4-6 months of age as a critical period (Monteiro and Victora 2005; Young et al. 2012). We therefore estimated associations between maternal PFAS concentrations during pregnancy and the body weight, adiposity, and growth of infants during the first ~5 months of life, in a Colorado cohort with background exposure to PFAS. We hypothesized that prenatal exposure to PFAS would be positively associated with weight and adiposity as well as rapid growth in early infancy.
Materials and Methods
Study participants and design
The Healthy Start study is an ongoing, longitudinal cohort study that enrolled 1,410 women from outpatient obstetrics clinics at the University of Colorado Hospital from 2009-2014. To be eligible for enrollment in Healthy Start, women were aged 16 or older, pregnant with a single fetus, with no history of stillbirth or extremely preterm birth and no serious chronic illness including diabetes, asthma treated with steroids, cancer or medication-dependent psychiatric illness, and had completed fewer than 24 weeks of gestation at enrollment. Participants provided written informed consent prior to enrollment in the study, and protocols were approved by the Colorado Multiple Institutional Review Board. The analysis of blinded specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research. Of the original 1,410 women enrolled, 19 experienced fetal demise and an additional 9 withdrew from the study prior to delivery.
Study participants attended two research visits during pregnancy and one visit shortly after the birth of their child, at which questionnaires were administered, samples collected and anthropometric data recorded. An additional postnatal follow-up visit at approximately 5 months of age was added to the study protocol in November 2010. An ancillary study was conducted to quantify PFAS in maternal serum, and 652 participants were selected for this sub-study based on availability of maternal mid- to late-pregnancy serum and umbilical cord blood, which was collected when delivery conditions allowed. To be eligible for the present analysis, mother-infant pairs enrolled in Healthy Start were required to have serum PFAS measurements during pregnancy and to have infant weight and body composition measurements at the postnatal follow-up visit. Complete data on essential covariates were also required. Of the 652 mother-infant pairs with prenatal PFAS measurements, 433 had 5-month body composition measurements and 18 were excluded due to missing information on breastfeeding during the first 5 months of life, resulting in a sample size of 415 mother-infant pairs. For analysis of growth in weight-for-length z-score only, the sample size was reduced to 402 following the exclusion of 13 participants with missing length measurements at birth.
Exposure assessment
Maternal fasting blood samples were collected during mid- to late-pregnancy (median 27 weeks of gestation, range 20-34 weeks) and immediately separated and frozen at −80°C. Frozen serum samples were shipped on dry ice to the CDC’s National Center for Environmental Health, Division of Laboratory Sciences, where 11 PFAS were quantified using a previously described approach (Kato et al. 2011): perfluorooctane sulfonamide (FOSA; also known as PFOSA), 2-(N-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA; also known as Et-PFOSA-AcOH), 2-(N-methyl perfluorooctane sulfonamido) acetate (MeFOSAA; also known as Me-PFOSA-AcOH), perfluorohexane sulfonate (PFHxS), linear PFOA (n-PFOA), sum of branched isomers of PFOA (Sb-PFOA), perfluorodecanoate (PFDA; also known as PFDeA), linear PFOS (n-PFOS), sum of perfluoromethylheptane sulfonate isomers (Sm-PFOS), sum of perfluorodimethylhexane sulfonate isomers (Sm2-PFOS), and perfluorononanoate (PFNA). The limit of detection (LOD) for all analytes was 0.1 ng/mL. The sums of all branched and linear isomers of PFOA and PFOS, respectively, were calculated to obtain concentrations of total PFOS and total PFOA. We restricted our analysis to those six PFAS with detectable concentrations in greater than 50% of participants: PFOA, PFOS, PFNA, PFDA, PFHxS, and MeFOSAA.
Outcome assessment
Infant length, weight, and adiposity were measured by the staff of the Pediatric Clinical and Translational Research Center at the Children’s Hospital of Colorado. Weight (to the nearest gram) and adiposity (fat mass as a percentage of total body mass) were evaluated in infants wearing only a spandex cap to cover their hair, using the PEAPOD device (COSMED, Rome, Italy). Length was measured in a recumbent position using an O’Leary Length Board, and recorded to the nearest millimeter. The PEAPOD uses air displacement plethysmography (ADP) to determine body volume and calculates whole-body density, which is then used to estimate fat mass and fat-free mass using infant-specific equations (Urlando et al. 2003). Adiposity was assessed at least twice in all infants; a third measurement was conducted if the first two measurements differed by >2%, and the mean of the two closest measurements was used. We calculated weight-for-age z-score (WAZ) and weight-for-length z-score (WLZ) from the World Health Organization growth charts for children under 2 years of age, using freely available software (WHO Multicentre Growth Reference Study Group 2006). We calculated change in WAZ and WLZ by subtracting the z-score at birth from the z-score at approximately 5 months. Rapid growth was defined as increase in WAZ or WLZ >0.67 standard deviation units from birth to the postnatal follow-up visit (Monteiro and Victora 2005).
Other variables
Information on maternal age, race/ethnicity, education, income, smoking status, and history of previous pregnancies was obtained via questionnaires administered to the mother during pregnancy. Maternal height was measured by research staff using a stadiometer at the first study visit during pregnancy. Pre-pregnancy weight was obtained by maternal self-report or from the medical record, and BMI was calculated as height in meters divided by weight in kilograms squared. All maternal weights recorded during pregnancy were abstracted from the prenatal medical record by research staff, and gestational weight gain was calculated as the difference between the last recorded weight and the pre-pregnancy weight. To remove the correlation with gestational age, we converted gestational weight gain to a z-score based on the gestational week of last measurement and the BMI of the mother prior to pregnancy, following Hutcheon et al. (Hutcheon et al. 2013). Research staff abstracted infant sex and birthweight from the medical record.
At the 5-month follow-up visit, mothers were asked a series of questions regarding their child’s health, feeding, and development. A binary variable for exclusive breastfeeding to follow-up was created based on the mother’s answers to the following questions: (1) “Have you ever breastfed your baby?”, (1a) “Are you now feeding your baby any breastmilk?”, and (2) “Have you ever fed your baby any formula?” If mothers reported currently feeding breastmilk and never having fed formula, the infants were classified as “exclusively breastfed.” This classification refers only to breastmilk vs. formula and does not consider the timing of introduction to solid foods, which was evaluated separately. Mothers were asked to report whether or not their child had yet consumed any items on a regular basis (defined as 2 or more consecutive days) from nine separate categories of food and beverages, including cow’s milk and juice as well as typical weaning foods such as cereals, fruits, vegetables, and pureed meats.
Statistical analysis
PFAS concentrations were natural log transformed to reduce the influence of outliers. Instrumental readings were used for values reported as below the LOD, unless the output value was zero, in which case the LOD/2 was substituted. We calculated Spearman rank correlations between each natural-log transformed pair of PFAS, including only detectable (i.e., above the LOD) concentrations.
We fit separate multiple linear regression models to estimate the associations between each PFAS and each of the continuous outcomes: adiposity at follow-up, WAZ at follow-up, WLZ at follow-up, change in WAZ and change in WLZ from birth to follow-up. We fit logistic regression models to estimate the associations between each PFAS and the odds of rapid growth in WAZ or WLZ (change in z-score >0.67 between birth and follow-up). To evaluate the linearity of the dose-response relationships, we fit generalized additive models using spline transformations of natural log-transformed PFAS with 4 knots for each PFAS-outcome pair. If a significant departure from linearity was detected (p<0.05), we entered PFAS into regression models only as categorical variables. We categorized PFOA, PFOS, and PFHxS into tertiles, while we divided PFDA and PFNA into only two categories, above vs. at or below the median, because of relatively low detectability and limited variability in concentrations. The number of women in each tertile was unequal for some PFAS because of limited concentration variability. Otherwise, we entered the five PFAS with detectable concentrations in >90% of participants into models as continuous, natural log-transformed variables, and we entered MeFOSAA (57% above the LOD) as a binary variable (detected vs. not detected) only.
We selected covariates using directed acyclic graphs representing hypothesized causal effects and associations reported in published literature. We adjusted for the following potential confounders in all models: maternal age, race/ethnicity (non-Hispanic white, Hispanic, non-Hispanic African American, and all others combined), pre-pregnancy BMI (kg/m2), gravidity (any previous pregnancies versus none), smoking during pregnancy (any versus none), maternal education (less than 12th grade, high school or GED, some college or associate’s degree, four year college degree, or graduate degree), gestational weight gain z-score, infant sex, exclusive breastfeeding until the follow-up visit (yes/no), and age at postnatal follow-up visit (days). We hypothesized that birthweight and gestational age at birth were causal intermediates and we therefore did not include them as covariates in the models (Schisterman et al. 2009). We evaluated effect modification by infant sex in all adjusted models by including an interaction term between sex and the PFAS of interest. We considered effect modification by sex to be present if the interaction term had a p-value less than 0.20 (Selvin 2004). For consistency, if any significant PFAS interactions with sex were present for an outcome, we presented all results for that outcome stratified by infant sex.
We conducted a sensitivity analysis to evaluate the possible confounding role of infant introduction to solid foods prior to the follow-up visit, by adjusting all models for a binary variable indicating regular consumption of any foods or beverages other than breastmilk or formula. We conducted additional sensitivity analyses to evaluate the potential role of breastfeeding as a mediator of observed associations. We fit models for adiposity, WAZ, and WLZ without adjustment for exclusive breastfeeding to include any potential indirect effect via breastfeeding in the total estimated effect of prenatal PFAS on infant weight and growth. Finally, we fit models for change in WAZ, change in WLZ, and rapid growth in WAZ and WLZ additionally adjusted for the baseline measure (WAZ or WLZ at birth) in a sensitivity analysis.
We examined the potential for confounding in each single-PFAS model by other, correlated PFAS concentrations using two approaches, for the following three outcomes: diposity at follow-up, WAZ at follow-up, and WLZ at follow-up. The first approach was a simple multipollutant model for which we entered all six PFAS as predictors in linear regression models, in addition to the covariates listed above. In the presence of highly correlated groups of exposures, these models are often characterized by variance inflation and may fail to identify independent effects. The second approach was elastic net penalized regression models. Elastic net regression selects variables that significantly contribute to the prediction of an outcome, even in the presence of groups of correlated variables (Zou and Hastie 2005), however resulting estimates are biased by design and therefore may be better used to identify rather than quantify associations. All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
The average age of the child at the follow-up visit was 5.1 months (range 2.8 to 9.4 months). Self-reported race and ethnicity were generally representative of the Denver metropolitan area, with 59% non-Hispanic white women, 22% Hispanic women, 13% non-Hispanic African-American women, and 5% of all other racial/ethnic groups combined (Table 1). The characteristics of participants in this analysis are similar to those of the larger Healthy Start study population (Supplemental Table 1).
Table 1.
Characteristics of 415 mother-infant Healthy Start pairs eligible for this analysis.
| Maternal and infant characteristics | Mean ± SD or N (%) |
|---|---|
| Maternal age (years) | 28.4 ± 6.1 |
| Race/ethnicity | |
| Non-Hispanic white | 246 (59) |
| Hispanic | 93 (22) |
| Non-Hispanic African American | 55 (13) |
| All others | 21 (5) |
| Pre-pregnancy BMI (kg/m2) | 25.8 ± 6.6 |
| Highest education level completed | |
| Less than 12th grade | 54 (13) |
| High school degree or GED | 59 (14) |
| Some college or associate’s degree | 99 (24) |
| Four year college degree | 92 (22) |
| Graduate degree | 111 (27) |
| Household income in the past year | |
| $20,000 or less | 46 (11) |
| $20,001-$40,000 | 60 (14) |
| $40,001-$70,000 | 85 (20) |
| $70,001 or more | 152 (37) |
| Don’t know | 72 (17) |
| Any previous pregnancies | 264 (64) |
| Any smoking during pregnancy | 27 (7) |
| Gestational weight gain (kg) | 13.7 ± 6.3 |
| Weight gain for gestational age z-score | −0.07 ± 1.06 |
| Gestational age at blood sample collection (days) | 191 ± 18 |
| Infant gestational age at birth (days) | 277 ± 9 |
| Preterm (<37 completed weeks of gestation) | 10 (2) |
| Birth weight (g) | 3254 ± 438 |
| Adiposity at birth (%)a | 9.0 ± 3.7 |
| Infant sex: male | 213 (51) |
| Exclusively breastfed to postnatal follow-up | 161 (39) |
| Regular solid foods prior to postnatal follow-up b | 168 (41) |
| Age at postnatal follow-up (months) | 5.1 ± 1.2 |
| Adiposity at postnatal follow-up (%) | 24.6 ± 5.4 |
| Weight-for-age z-score (WAZ) at postnatal follow-up | −0.46 ± 0.94 |
| Difference in WAZ from birth to postnatal follow-up | −0.34 ±0.99 |
| Rapid growth in WAZ to postnatal follow-up | 63 (15) |
| Weight-for-length z-score (WLZ) at postnatal follow-up | −0.22 ± 1.07 |
| Difference in WLZ from birth to postnatal follow-up a | −0.34 ± 1.17 |
| Rapid growth in WLZ to postnatal follow-up a | 74 (18) |
N=402
N=412
Median PFAS concentrations were somewhat lower than those reported among females in the general U.S. population during the period 2009-2014 (Centers for Disease Control and Prevention 2019) (Table 2; Supplemental Table 2). We observed moderate to high pairwise Spearman correlations among most PFAS concentrations, with the strongest correlation between PFOA and PFNA (ρ=0.77). MeFOSAA was only weakly correlated with the other PFAS (ρ=0.02-0.18) (Table 2).
Table 2.
Serum concentrations of per- and polyfluoroalkyl substances (PFAS, in ng/mL) and pairwise Spearman correlations among 415 eligible participants in Healthy Start, 2009-2014.
| PFAS | Abbreviation | % above limit of detection |
Percentiles | Correlations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | PFOA | PFOS | PFNA | PFDA | PFHxS | MeFOSAA | |||
| Perfluorooctanoate | PFOA | 100 | 0.3 | 0.7 | 1.0 | 1.6 | 2.7 | 1 | 0.69 | 0.77 | 0.52 | 0.61 | 0.06 |
| Perfluorooctane sulfonate | PFOS | 99.5 | 0.6 | 1.4 | 2.2 | 3.4 | 6.3 | 1 | 0.60 | 0.39 | 0.68 | 0.18 | |
| Perfluorononanoate | PFNA | 99.5 | 0.2 | 0.2 | 0.4 | 0.6 | 1.2 | 1 | 0.57 | 0.44 | 0.02 | ||
| Perfluorodecanoate | PFDA | 90.6 | <LOD | 0.1 | 0.1 | 0.2 | 0.4 | 1 | 0.25 | 0.04 | |||
| Perfluorohexane sulfonate |
PFHxS | 99.3 | 0.2 | 0.5 | 0.7 | 1.2 | 2.5 | 1 | 0.10 | ||||
| 2-(N-methyl perfluorooctane sulfonamido) acetate |
MeFOSAA | 57.1 | <LOD | <LOD | 0.1 | 0.1 | 0.3 | 1 | |||||
Note: Correlations among values above the limit of detection (LOD) only (PFOA, n=415; PFOS, n=413; PFNA, n=413; PFDA, n=376; PFHxS, n=412; MeFOSAA, n=237). The LOD for all PFAS was 0.1 ng/mL.
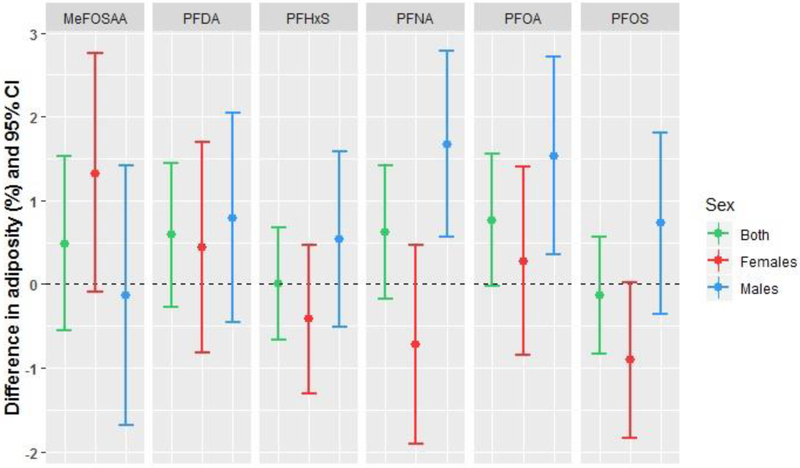
Adiposity at 5 months of age
In models for adiposity at follow-up, we noted interactions (p<0.20) between PFAS concentrations and infant sex for PFOS and PFNA (Table 3). In male infants, maternal serum concentrations of PFOA and PFNA during pregnancy were positively associated with adiposity, showing percent fat mass increases of 1.5-1.7% per ln-ng/mL increase in PFAS (Table 3; Figure 1). Women in the highest vs lowest tertile of PFOA had male infants with 2.8% greater adiposity (95% CI 0.8, 4.8%) (Supplemental Table S3). In female infants, maternal PFOS was associated with lower adiposity, although not statistically significant. Female infants of women in the highest tertile vs lowest tertile of PFOS had adiposity that was 2.1% lower (95% CI −3.8, −0.3%).
Table 3.
Maternal serum per- and polyfluoroalkyl substances (PFAS, in ng/mL) and adjusted differences in infant adiposity at 5-month follow-up among 415 mother-infant pairs in the Healthy Start study, 2009-2014.
| Difference and 95% confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| PFAS | N | Both sexes combined | p-value for interaction |
N | Males only | N | Females only |
| PFOA | 415 | 0.76 (−0.03, 1.55) | 0.44 | 213 | 1.53 (0.35, 2.71) | 202 | 0.27 (−0.85, 1.40) |
| PFOS | 415 | −0.13 (−0.83, 0.57) | 0.05 | 213 | 0.73 (−0.36, 1.81) | 202 | −0.91 (−1.84, 0.02) |
| PFNA | 415 | 0.62 (−0.17, 1.41) | 0.04 | 213 | 1.67 (0.56, 2.78) | 202 | −0.72 (−1.91, 0.47) |
| PFDA | 415 | 0.59 (−0.27, 1.44) | 0.94 | 213 | 0.79 (−0.46, 2.04) | 202 | 0.44 (−0.82, 1.69) |
| PFHxS | 415 | 0.01 (−0.67, 0.68) | 0.31 | 213 | 0.54 (−0.51, 1.58) | 202 | −0.42 (−1.31, 0.47) |
| MeFOSAA | |||||||
| <LOD | 178 | Ref | 0.53 | 98 | Ref | 80 | Ref |
| 0.1-2.1 ng/mL | 237 | 0.48 (−0.56, 1.53) | 115 | −0.13 (−1.68, 1.41) | 122 | 1.32 (−0.10, 2.75) | |
Note: Adjusted for maternal age, race/ethnicity, pre-pregnancy BMI, any previous pregnancies, any smoking during pregnancy, education, gestational weight gain z-score, infant sex, exclusive breastfeeding to follow-up visit, and age in days at follow-up visit. The limit of detection for all PFAS was 0.1 ng/mL. Differences are presented per 1 ln-unit change in each per- or polyfluoroalkyl substance, with the exception of MeFOSAA, which is presented in categories only due to low detection frequency. Abbreviations: LOD, limit of detection; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFNA, perfluorononanoate; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonate; MeFOSAA, 2-(N-methyl perfluorooctane sulfonamido) acetate; Ref, Reference level for categorical variables.
Figure 1.
Adjusted associations between maternal PFAS concentrations and infant adiposity at 5-month follow-up among 415 mother-infant pairs in the Healthy Start study. Differences are presented per 1 ln-unit change in each per- or polyfluoroalkyl substance, with the exception of MeFOSAA, which is presented as above versus below the limit of detection (0.1 ng/mL).
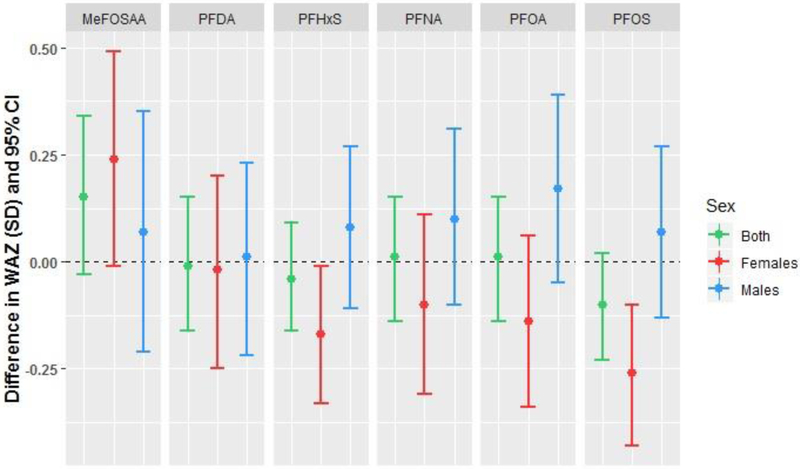
Weight-for-age z-score at 5 months
We observed a significant interaction (p<0.20) between PFOS and infant sex in models for WAZ at follow-up (Table 4). Maternal serum concentrations of PFOS and PFHxS were associated with lower WAZ among female infants only (−0.26 SD per ln-ng/mL PFOS, 95% CI −0.43, −0.10; −0.17 SD per ln-ng/mL PFHxS, 95% CI −0.33, −0.01; Table 4; Figure 2). Female infants of women in the highest vs. lowest tertiles of PFOS and PFHxS had significantly lower WAZ (Supplemental Table S4). Maternal concentrations of MeFOSAA above vs. below the LOD were associated with higher WAZ in both sexes combined and in females, although confidence intervals included the null (Table 4).
Table 4.
Maternal serum per- and polyfluoroalkyl substances (PFAS, in ng/mL) and adjusted differences in infant weight-for-age z-score at 5-month follow-up among 415 mother-infant pairs in the Healthy Start study, 2009-2014.
| Difference and 95% confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| PFAS | N | Both sexes combined | p-value for interaction |
N | Males only | N | Females only |
| PFOA per ln-(ng/mL) | 415 | 0.01 (−0.14, 0.15) | 0.45 | 213 | 0.17 (−0.05, 0.39) | 202 | −0.14 (−0.34, 0.06) |
| PFOS per ln-(ng/mL) | 415 | −0.10 (−0.23, 0.02) | 0.10 | 213 | 0.07 (−0.13, 0.27) | 202 | −0.26 (−0.43, −0.10) |
| PFNA per ln-(ng/mL) | 415 | 0.01 (−0.14, 0.15) | 0.64 | 213 | 0.10 (−0.10, 0.31) | 202 | −0.10 (−0.31, 0.11) |
| PFDA per ln-(ng/mL) | 415 | −0.01 (−0.16, 0.15) | 0.69 | 213 | 0.01 (−0.22, 0.23) | 202 | −0.02 (−0.25, 0.20) |
| PFHxS per ln-(ng/mL) | 415 | −0.04 (−0.16, 0.09) | 0.25 | 213 | 0.08 (−0.11, 0.27) | 202 | −0.17 (−0.33, −0.01) |
| MeFOSAA | |||||||
| <LOD | 178 | Ref | 0.80 | 98 | Ref | 80 | Ref |
| 0.1-2.1 ng/mL | 237 | 0.15 (−0.03, 0.34) | 115 | 0.07 (−0.21, 0.35) | 122 | 0.24 (−0.01, 0.49) | |
Note: Adjusted for maternal age, race/ethnicity, pre-pregnancy BMI, any previous pregnancies, any smoking during pregnancy, education, gestational weight gain z-score, infant sex, exclusive breastfeeding to follow-up visit, and age in days at follow-up visit. The limit of detection (LOD) for all PFAS was 0.1 ng/mL. Differences are presented per 1 ln-unit change in each per- or polyfluoroalkyl substance, with the exception of MeFOSAA, which is presented in categories only due to low detection frequency. Abbreviations: PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFNA, perfluorononanoate; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonate; MeFOSAA, 2-(N-methyl perfluorooctane sulfonamido) acetate; Ref, Reference level for categorical variables.
Figure 2.
Adjusted associations between maternal PFAS concentrations and infant weight-for-age z-score (WAZ) at 5-month follow-up among 415 mother-infant pairs in the Healthy Start study. Differences are presented per 1 ln-unit change in each per- or polyfluoroalkyl substance, with the exception of MeFOSAA, which is presented as above versus below the limit of detection (0.1 ng/mL).
We observed no PFAS-by-sex interactions in the models for growth in weight-for-age z-score from birth to 5 months; therefore, we present results for both sexes combined only. Infants of mothers with concentrations of MeFOSAA above the LOD had twice the odds of rapid growth in early infancy vs those below the LOD (odds ratio [OR] 2.2, 95% CI 1.1, 4.3; Supplemental Table S5). There were no significant associations between other PFAS and growth in WAZ.
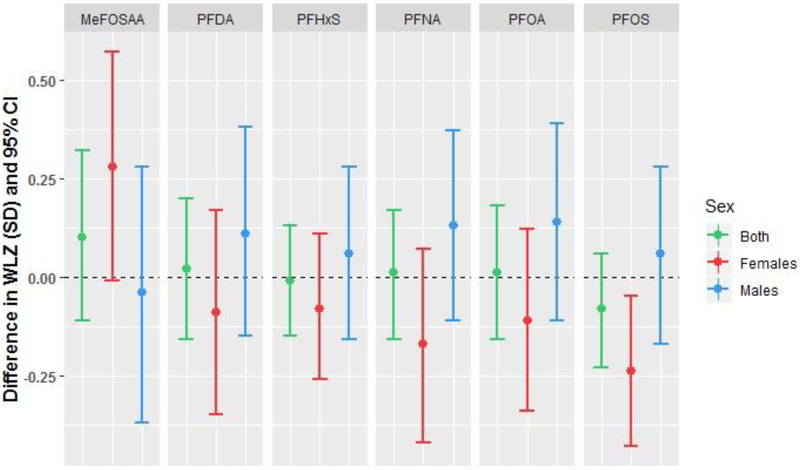
Weight-for-length z-score at 5 months
As in the models for WAZ, we observed a significant interaction (p<0.20) between PFOS and infant sex in models for WLZ at follow-up (Table 5). Maternal PFOS was associated with lower WLZ among female infants only (−0.24 SD per ln-ng/mL PFOS, 95% CI −0.43, −0.05; Table 5; Figure 3). Additionally, female infants of women in the highest vs. lowest tertiles of PFOS had lower WLZ (Supplemental Table S6). There were no associations between PFAS and WLZ in males.
Table 5.
Maternal serum per- and polyfluoroalkyl substances (PFAS, in ng/mL) and adjusted differences in infant weight-for-length z-score at 5-month follow-up among 415 mother-infant pairs in the Healthy Start study, 2009-2014.
| Difference and 95% confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| PFAS | N | Both sexes combined | p-value for interaction |
N | Males only | N | Females only |
| PFOA | 415 | 0.01 (−0.16, 0.18) | 0.64 | 213 | 0.14 (−0.11, 0.39) | 202 | −0.11 (−0.34, 0.12) |
| PFOS | 415 | −0.08 (−0.23, 0.06) | 0.17 | 213 | 0.06 (−0.17, 0.28) | 202 | −0.24 (−0.43, −0.05) |
| PFNA | 415 | 0.01 (−0.16, 0.17) | 0.37 | 213 | 0.13 (−0.11, 0.37) | 202 | −0.17 (−0.42, 0.07) |
| PFDA | 415 | 0.02 (−0.16, 0.20) | 0.63 | 213 | 0.11 (−0.15, 0.38) | 202 | −0.09 (−0.35, 0.17) |
| PFHxS | 415 | −0.01 (−0.15, 0.13) | 0.67 | 213 | 0.06 (−0.16, 0.28) | 202 | −0.08 (−0.26, 0.11) |
| MeFOSAA | |||||||
| <LOD | 178 | Ref | 0.43 | 98 | Ref | 80 | Ref |
| 0.1-2.1 ng/mL | 237 | 0.10 (−0.11, 0.32) | 115 | −0.04 (−0.37, 0.28) | 122 | 0.28 (−0.01, 0.57) | |
Note: Adjusted for maternal age, race/ethnicity, pre-pregnancy BMI, any previous pregnancies, any smoking during pregnancy, education, gestational weight gain z-score, infant sex, exclusive breastfeeding to follow-up visit, and age in days at follow-up visit. The limit of detection (LOD) for all PFAS was 0.1 ng/mL. Differences are presented per 1 ln-unit change in each per- or polyfluoroalkyl substance, with the exception of MeFOSAA, which is presented in categories only due to low detection frequency. Abbreviations: PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFNA, perfluorononanoate; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonate; MeFOSAA, 2-(N-methyl perfluorooctane sulfonamido) acetate; Ref, Reference level for categorical variables.
Figure 3.
Adjusted associations between maternal PFAS concentrations and infant weight-for-length z-score (WLZ) at 5-month follow-up among 415 mother-infant pairs in the Healthy Start study. Differences are presented per 1 ln-unit change in each per- or polyfluoroalkyl substance, with the exception of MeFOSAA, which is presented as above versus below the limit of detection (0.1 ng/mL).
There were no PFAS-by-sex interactions in the models for growth in weight-for-length z-score from birth to 5 months therefore we present results for both sexes combined only. Maternal PFHxS and MeFOSAA were associated with greater odds of rapid growth in WLZ in early infancy (PFHxS OR 1.5 per ln-ng/mL, 95% CI 1.0, 2.2; MeFOSAA OR 3.3 for above vs. below LOD, 95% CI 1.8, 6.2; Supplemental Table S7).
Sensitivity analyses
Additional adjustment for solid food introduction prior to the follow-up visit resulted in no meaningful changes to effect estimates (data not shown). Models without adjustment for exclusive breastfeeding produced somewhat attenuated or unchanged effect estimates (Supplemental Tables S8, S9, and S10). Additional adjustment for baseline measures in models for change in WAZ, change in WLZ, and rapid growth in WAZ or WLZ produced only minimal changes to effect estimates (data not shown).
Multipollutant models generally confirmed the results of the single pollutant models, but revealed the potential for mutual confounding effects, particularly by PFOS and PFOA (rs=0.69). In the multipollutant linear regression models for adiposity at follow-up, both the positive association with PFOA and the negative association with PFOS were generally strengthened, suggesting that the positive correlation between PFOA and PFOS may have led to confounding bias that attenuated both effects in single pollutant models (Supplemental Table S11). However, the positive association between PFOA and adiposity in males was not significant in the elastic net penalized regression models. PFNA associations were prominent and in opposite directions for males and females.
Multipollutant models for WAZ at follow-up confirmed the negative association with PFOS in females and strengthened the positive association with MeFOSAA (Supplemental Table S12). Notably, the negative association between PFHxS and WAZ in females was attenuated to the null, suggesting that the association may have been due to confounding by PFOS (rs=0.68) in the single pollutant models. Similar patterns were observed in multipollutant models for WLZ at follow-up (Supplemental Table S13).
Discussion
In this sample of mother-infant pairs from a Colorado cohort, maternal serum concentrations of select PFAS during pregnancy were associated with sex-specific and chemical-specific differences in infant weight and adiposity to ~5 months of age. In male infants, maternal PFOA and PFNA were associated with greater adiposity, while in female infants, PFOS and PFHxS were associated with lower weight-for-age, and PFOS was associated with lower weight-for-length. Only MeFOSAA, a PFAS less frequently measured in previous studies, was associated with rapid growth in WAZ, and both MeFOSAA and PFHxS were associated with rapid growth in WLZ, although other PFAS showed elevated but not statistically significant odds ratios.
Previously in the same cohort, we observed that prenatal PFOA and PFNA concentrations were associated with lower weight and adiposity at birth in both male and female infants, and no significant interaction with sex was observed at birth (Starling et al. 2017). The current results lend limited support to the theory that accelerated growth in infancy may follow PFAS-associated growth restriction in utero. Specifically, male infants born to women with higher prenatal concentrations of PFOA and PFNA had greater adiposity already by ~5 months of age, despite their lower adiposity at birth. However, maternal PFOA and PFNA were not associated with “rapid” growth by the definition used here (change in z-score >0.67 SD units) in this period, and no significant PFAS-by-sex interactions were observed for growth in WAZ or WLZ. In female infants, we observed evidence of persistent suppression of growth and adiposity to ~5 months, particularly among those whose mothers had greater prenatal PFOS concentrations.
Rapid growth in early infancy is believed to be an important predictor of later obesity (Bjerregaard et al. 2014; Monteiro and Victora 2005; Young et al. 2012). Infants exposed prenatally to PFAS who experience accelerated growth during infancy or in early childhood may demonstrate greater adiposity and obesity risk in childhood (Braun et al. 2016; Høyer et al. 2015; Karlsen et al. 2016; Lauritzen et al. 2018; Liu et al. 2018; Mora et al. 2017) and young adulthood (Halldorsson et al. 2012). However, in our study, only prenatal MeFOSAA was associated with greater odds of rapid growth in WAZ and WLZ in the first ~5 months of life. We are unaware of previous studies associating prenatal MeFOSAA with childhood obesity or adiposity.
Several previous studies have examined associations between prenatal PFAS exposure and offspring weight and growth in infancy (Alkhalawi et al. 2016; Andersen et al. 2010; Chen et al. 2017; de Cock et al. 2014; Gyllenhammar et al. 2018; Maisonet et al. 2012; Manzano-Salgado et al. 2017; Shoaff et al. 2018). In all of these previous studies, adiposity was not measured via ADP or other highly accurate methods, but rather approximated using BMI, WLZ, or ponderal index. ADP is a non-invasive and rapid method for measuring body mass and volume to calculate adiposity, and has been shown to be reliable and valid relative to hydrostatic weighing, dual-energy X-ray absorptiometry (DXA), and a 4-compartment reference model (Ellis et al. 2007; Fields et al. 2002). Specifically, estimates of percent fat mass in 6-month old infants from the PEAPOD were shown to be highly correlated with those from DXA (r=0.925), although PEAPOD estimates were lower (Fields et al. 2012). In a previous investigation in the Healthy Start study, we found WLZ, BMI, and sum of skinfold thicknesses to be adequate proxies for ADP-measured adiposity at 5 months of age, while WAZ was equally associated with fat mass and lean mass (Perng et al. 2017). In the present study, we found different associations with prenatal PFAS for WAZ, WLZ, and ADP-measured adiposity at ~5 months of age, confirming that these measures evaluate somewhat different components of body weight and growth.
The results of previous studies have been inconsistent regarding the direction of association between maternal PFAS and weight/adiposity in infancy, and regarding the specific PFAS with significant associations. A study including both male and female infants of Danish women (Andersen et al. 2010) reported that prenatal plasma PFOA was associated with lower weight and BMI at 5 months in boys only, and PFOS was associated with lower weight and BMI at 12 months in both sexes combined. By contrast, our results show higher adiposity at 5 months in boys born to women with higher pregnancy PFOA concentrations, while only girls born to women with higher PFOS have lower WAZ and adiposity. Inconsistencies may be partially explained by differing PFAS concentrations, which were notably higher among the Danish participants enrolled in 1996-2002 (median PFOA 5.3 ng/mL; median PFOS 33.4 ng/mL) (Andersen et al. 2010) than among U.S. participants in our study (enrolled in 2009-2014). It is possible that greater prenatal exposure to PFAS is associated with more enduring suppression of growth, as observed in some animal exposure studies (Lau et al. 2006).
Some previous studies have noted that PFAS-exposed infants with lower birth weight “catch up” in weight at some point during infancy or childhood. A Spanish study reported that prenatal PFOA (but not PFOS, PFNA, or PFHxS) was associated with greater growth in WAZ in the first 6 months of life, in boys only (Manzano-Salgado et al. 2017). A study of female offspring of British women reported lower birth weight associated with prenatal serum PFOS, PFOA, and PFHxS, but found that these differences were eliminated by 9 months of age, and girls whose mothers had greater prenatal PFOS concentrations had greater WAZ by 20 months of age (Maisonet et al. 2012). Therefore, it is possible that growth recovery in PFOS-exposed girls may only occur later in infancy or early childhood. This theory is supported by the results of another study in a Taiwanese cohort that examined associations between umbilical cord blood PFAS and WAZ and BMI in different periods of early life (Chen et al. 2017). In that study, PFOS was associated with lower weight and BMI at birth, but greater BMI among girls only at 5-9 years of age.
Other studies did not observe recovery of weight or adiposity throughout infancy following reduced fetal growth associated with PFAS. A German study (Alkhalawi et al. 2016), using a combination of maternal plasma and umbilical cord blood samples for exposure assessment, reported inverse associations of PFOS, PFOA and PFHxS with birth weight, but no linear trends in weight across concentration quartiles at any other time point in infancy (1, 4, 6, 12 months). That study did, however, report a positive association between prenatal PFHxS and ponderal index across infancy; sex-specific effects were not examined. A U.S. study of pregnant women with serum PFAS concentrations (PFOA median 5.5 ng/mL; PFOS median 14 ng/mL) higher than in the present study reported inverse longitudinal associations of prenatal PFOA and PFOS with offspring BMI, WAZ, and WLZ, and no evidence of rapid growth from age 4 weeks to 2 years of age (Shoaff et al. 2018).
Finally, some previous studies reported null findings during infancy. A Dutch study with repeated measures of weight and length throughout infancy found no significant associations between cord blood PFOS and PFOA with weight or BMI up to 11 months (de Cock et al. 2014). Similarly, a Swedish study reported that maternal post-partum concentrations of six PFAS were not significantly associated with offspring weight-for-age z-score in infancy, although they did observe positive associations of PFOA, PFOS, PFNA, and PFHxS with child BMI at age 4 years (Gyllenhammar et al. 2018). Differences among study results may be partially explained by varying ranges of PFAS concentrations during pregnancy, as well as by geographic variation in the composition of PFAS mixtures.
The reasons for sex-specific effects of prenatal PFAS on offspring weight and adiposity are unknown; however, certain PFAS have demonstrated endocrine-disrupting activity. For example, PFAS extracted from serum of pregnant women were found to have estrogenic activity in vitro (Bjerregaard-Olesen et al. 2016), and maternal serum PFAS concentrations were associated with decreased anogenital distance (a hormone-sensitive developmental outcome) in female infants only (Lind et al. 2017). PFAS have been found to interact with peroxisome proliferator-activated receptors to influence the expression of genes involved in glucose and lipid metabolism (Abbott et al. 2012; Vanden Heuvel et al. 2006; Wolf et al. 2008; Wolf et al. 2012), and this may partially explain PFAS’ ability to enhance adipocyte differentiation in vitro (Ma et al. 2018; Watkins et al. 2014; Xu et al. 2016). However, the action of PFAS in the body may be more complex, as recent studies have reported PFAS activity with other receptors, including the constitutive androstane receptor (Abe et al. 2017), the pregnane-X receptor (Zhang et al. 2017), and estrogen receptor β (Xu et al. 2017) and with the phosphatidylinositol 3-kinase-serine/threonine protein kinase pathway (Li et al. 2017). The potential for endocrine-disrupting effects of PFAS in children is highlighted by two studies reporting associations between PFOA and later age at menarche (Kristensen et al. 2013; Lopez-Espinosa et al. 2011), although an earlier investigation reported null results (Christensen et al. 2011). It should be noted that males and females have different growth trajectories in utero and during infancy (Broere-Brown et al. 2016), and therefore potential effects of PFAS on growth may be best understood in sex-stratified models. Animal literature also provides evidence of early environmental exposures producing sexually dimorphic effects (Sundrani et al. 2017).
Strengths of this study include the examination of a large, ethnically diverse U.S. population, and the measurement of adiposity using the highly accurate and reproducible method of ADP. Given the moderate to high degree of correlation among PFAS concentrations, we examined the potential for confounding by including all six PFAS studied into the same model, and checked for robustness of these findings using a penalized elastic net regression (Zou and Hastie 2005). We considered several important potential confounders of the prenatal PFAS-growth association, including maternal pre-pregnancy BMI and gestational weight gain, and smoking during pregnancy.
We also adjusted for exclusive breastfeeding to the follow-up visit, a strong predictor of adiposity in infancy in this and other studies (Gale et al. 2012). Previous studies reported that prenatal PFAS concentrations are associated with reduced (Fei et al. 2010; Romano et al. 2016; Timmermann et al. 2016) or increased (Rosen et al. 2018) duration of breastfeeding, suggesting that breastfeeding may act as a causal intermediate on a pathway linking prenatal PFAS to infant growth and adiposity. In our sensitivity analyses, we found only minor changes in effect estimates after removing breastfeeding from the models, and these changes were generally toward the null. This suggests that the potential for mediation is low, and that exclusive breastfeeding status may have more influence as a negative confounder in these data. However, we note that breastfeeding may have a complex role as both a source of postnatal PFAS exposure (Mogensen et al. 2015; Mondal et al. 2014) and as a potential effect modifier or mediator of the associations between maternal PFAS and infant adiposity, and merits investigation in future studies.
We were unable to adjust for maternal plasma volume expansion during pregnancy or glomerular filtration rate, which have been previously considered as potential confounders of the association between prenatal PFAS concentrations and birthweight (Verner et al. 2015). While some studies which have adjusted for markers of these factors have reported no meaningful change in the associations between PFAS and fetal growth (Gyllenhammar et al. 2018; Sagiv et al. 2017), maternal hemodynamics remains a potential source of unmeasured confounding for studies of maternal PFAS measured late in pregnancy (Steenland et al. 2018).
An additional limitation of this study is the relatively low concentrations of PFAS in this population, with median concentrations approximately 20-70% lower than those of females in the general U.S. population. This led to limited variability and limited ability to analyze the results for some PFAS because of the low percentage of samples with detectable concentrations. However, we identified some chemical-specific and sex-specific associations, suggesting the potential for prenatal PFAS exposures even at such low concentrations to influence infant growth and body composition. Finally, many statistical comparisons were made in this analysis and we cannot rule out the possibility that some results were significant due to chance.
Conclusions
These findings suggest that maternal serum concentrations of certain PFAS during pregnancy may influence infant growth and adiposity in a sex-specific manner. While prenatal PFAS exposure is inversely associated with weight and adiposity at birth, certain PFAS are associated with more rapid growth or greater weight and adiposity in the first half of infancy, and this may lead to greater weight and adiposity in early childhood. Continued follow-up of the participants in Healthy Start and similar birth cohort studies will allow the examination of longitudinal patterns of growth resulting from in utero PFAS exposure.
Supplementary Material
Highlights.
Associations between maternal PFAS and infant weight and adiposity differed by sex.
Prenatal PFOA and PFNA were associated with greater 5-month adiposity in males.
Prenatal PFOS and PFHxS were associated with lower weight-for-age in females.
MeFOSAA was associated with greater odds of rapid growth from 0 to 5 months of age.
Acknowledgements
We acknowledge the technical assistance of K. Kato, J. Ma, A. Kalathil, T. Jia, and the late Xiaoyun Ye (CDC, Atlanta, GA) in measuring the serum concentrations of PFAS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Funding sources
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK076648), the National Institute of Environmental Health Sciences of the National Institutes of Health (R01ES022934), and the Office of the Director of the National Institutes of Health (UH3OD023248). Dr. Starling was supported by funding from the National Institute of Environmental Health Sciences of the National Institutes of Health (R00ES025817).
Footnotes
Competing Financial Interests
The authors declare they have no actual or potential competing financial interests.
Abbreviations: CDC, Centers for Disease Control and Prevention; LOD, limit of detection; MeFOSAA, 2-(N-methyl perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; WAZ, weight-for-age z-score; WLZ, weight-for-length z-score.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkhalawi E, Kasper-Sonnenberg M, Wilhelm M, Völkel W, Wittsiepe J. 2016. Perfluoroalkyl acids (PFAAs) and anthropometric measures in the first year of life: Results from the Duisburg birth cohort. J Toxicol Environ Health A 79:1041–1049. [DOI] [PubMed] [Google Scholar]
- Andersen CS, Fei C, Gamborg M, Nohr EA, Sorensen TI, Olsen J. 2010. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am J Epidemiol 172:1230–1237. [DOI] [PubMed] [Google Scholar]
- Andersen CS, Fei C, Gamborg M, Nohr EA, Sørensen TI, Olsen J. 2013. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol 178:921–927. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Long GC, Porter RC, Anderson JK. 2016. Occurrence of select perfluoroalkyl substances at U.S. Air force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 150:678–685. [DOI] [PubMed] [Google Scholar]
- Barzen-Hanson KA, Roberts SC, Choyke S, Oetjen K, McAlees A, Riddell N, et al. 2017. Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ Sci Technol 51:2047–2057. [DOI] [PubMed] [Google Scholar]
- Bjerregaard LG, Rasmussen KM, Michaelsen KF, Skytthe A, Mortensen EL, Baker JL, et al. 2014. Effects of body size and change in body size from infancy through childhood on body mass index in adulthood. Int J Obes (Lond) 38:1305–1311. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Wielsøe M, Bech BH, Henriksen TB, et al. 2019. Associations of fetal growth outcomes with measures of the combined xenoestrogenic activity of maternal serum perfluorinated alkyl acids in Danish pregnant women. Environ Health Perspect 127:17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 24:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere-Brown ZA, Baan E, Schalekamp-Timmermans S, Verburg BO, Jaddoe VW, Steegers EA. 2016. Sex-specific differences in fetal and infant growth patterns: A prospective population-based cohort study. Biol Sex Differ 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. Fourth national report on human exposure to environmental chemicals, updated tables, January 2019. Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Chen MH, Ng S, Hsieh CJ, Lin CC, Hsieh WS, Chen PC. 2017. The impact of prenatal perfluoroalkyl substances exposure on neonatal and child growth. Science of the Total Environment 607:669–675. [DOI] [PubMed] [Google Scholar]
- Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, et al. 2011. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ Int 37:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. 2014. First year growth in relation to prenatal exposure to endocrine disruptors - a Dutch prospective cohort study. Int J Environ Res Public Health 11:7001–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. 2007. Body-composition assessment in infancy: Air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr 85:90–95. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. 2010. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand J Work Environ Health 36:413–421. [DOI] [PubMed] [Google Scholar]
- Fields DA, Goran MI, McCrory MA. 2002. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am J Clin Nutr 75:453–467. [DOI] [PubMed] [Google Scholar]
- Fields DA, Demerath EW, Pietrobelli A, Chandler-Laney PC. 2012. Body composition at 6 months of life: Comparison of air displacement plethysmography and dual-energy x-ray absorptiometry. Obesity (Silver Spring) 20:2302–2306. [DOI] [PubMed] [Google Scholar]
- Gale C, Logan KM, Santhakumaran S, Parkinson JR, Hyde MJ, Modi N. 2012. Effect of breastfeeding compared with formula feeding on infant body composition: A systematic review and meta-analysis. Am J Clin Nutr 95:656–669. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Diderholm B, Gustafsson J, Berger U, Ridefelt P, Benskin JP, et al. 2018. Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environment International 111:191–199. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: A prospective cohort study. Environ Health Perspect 120:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TJ, Calafat AM, Holmes AK, Marcus M, Northstone K, Flanders WD, et al. 2017. Prenatal exposure to perfluoroalkyl substances and body fatness in girls. Child Obes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. 2017. Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology 68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female cd-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 304:97–105. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. 2013. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr 97:1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer BB, Ramlau-Hansen CH, Vrijheid M, Valvi D, Pedersen HS, Zviezdai V, et al. 2015. Anthropometry in 5- to 9-year-old Greenlandic and Ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ Health Perspect 123:841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen M, Grandjean P, Weihe P, Steuerwald U, Oulhote Y, Valvi D. 2016. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol 68:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218:2133–2137. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, et al. 2013. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod 28:3337–3348. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90:510–518. [DOI] [PubMed] [Google Scholar]
- Lauritzen HB, Larose TL, Øien T, Sandanger TM, Odland J, van de Bor M, et al. 2018. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: A prospective cohort study. Environ Health 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Yang F, Wang Y, Yuan Z. 2018. Perfluorooctanoic acid (PFOA) exposure in early life increases risk of childhood adiposity: A meta-analysis of prospective cohort studies. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, et al. 2011. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol 45:8160–8166. [DOI] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, et al. 2012. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect 120:1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iñiguez C, Martinez D, et al. 2017. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA birth cohort study. Environ Health Perspect 125:097018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol 49:10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, et al. 2014. Breastfeeding: A potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect 122:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro PO, Victora CG. 2005. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev 6:143–154. [DOI] [PubMed] [Google Scholar]
- Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, et al. 2017. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect 125:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng W, Ringham BM, Glueck DH, Sauder KA, Starling AP, Belfort MB, et al. 2017. An observational cohort study of weight- and length-derived anthropometric indicators with body composition at birth and 5 mo: The Healthy Start study. Am J Clin Nutr 106:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, et al. 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen EM, Brantsæter AL, Carroll R, Haug L, Singer AB, Zhao S, et al. 2018. Maternal plasma concentrations of per- and polyfluoroalkyl substances and breastfeeding duration in the Norwegian Mother and Child Cohort. Environ Epidemiol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Das KP, Rooney J, Abbott B, Lau C, Corton JC. 2017. PPAR±-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 387:95–107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Fleisch AF, Webster TF, Calafat AM, Ye X, et al. 2017. Early-pregnancy perfluoroalkyl substance plasma concentrations and birth outcomes in Project Viva: Confounded by pregnancy hemodynamics? Am J Epidemiol 187:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaff J, Papandonatos GD, Calafat AM, Chen A, Lanphear BP, Ehrlich S, et al. 2018. Prenatal exposure to perfluoroalkyl substances: Infant birth weight and early life growth. Environ Epidemiol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Ye X, et al. 2017. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: Examining mediation by maternal fasting glucose in the Healthy Start study. Environ Health Perspect 125:067016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Barry V, Savitz D. 2018. Serum perfluorooctanoic acid (PFOA) and birthweight: An updated meta-analysis with bias analysis. Epidemiology 29:765–776 . [DOI] [PubMed] [Google Scholar]
- Sundrani DP, Roy SS, Jadhav AT, Joshi SR. 2017. Sex-specific differences and developmental programming for diseases in later life. Reproduction Fertility and Development 29:2085–2099. [DOI] [PubMed] [Google Scholar]
- Timmermann CA, Budtz-Jørgensen E, Petersen MS, Weihe P, Steuerwald U, Nielsen F, et al. 2016. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol 68:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlando A, Dempster P, Aitkens S. 2003. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res 53:486–492. [DOI] [PubMed] [Google Scholar]
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, McDougall R, et al. 2015. Associations of perfluoroalkyl substances (PFASs) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ Health Perspect 123:1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Leung PY, Wong CKC. 2014. Perinatal exposure to perfluorooctane sulfonate affects glucose metabolism in adult offspring. Plos One 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Adgent M, Su PH, Chen HY, Chen PC, Hsiung CA, et al. 2016. Prenatal exposure to perfluorocarboxylic acids (PFCAs) and fetal and postnatal growth in the Taiwan Maternal and Infant Cohort Study. Environ Health Perspect 124:1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group. 2006. WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Available: http://www.who.int/childgrowth/publications/en/.
- Young BE, Johnson SL, Krebs NF. 2012. Biological determinants linking infant weight gain and child obesity: Current knowledge and future directions. Adv Nutr 3:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T. 2005. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society, Series B 67:301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.