Abstract
Although Alzheimer’s disease (AD) is an international health research priority for our aging population, little therapeutic progress has been made. This lack of progress may be partially attributable to disease heterogeneity. Previous studies have identified an inverse association of cancer and AD, suggesting that cancer history may be one source of AD heterogeneity. These findings are particularly interesting in light of the number of common risk factors and two-hit models hypothesized to commonly drive both diseases. We reviewed the ten hallmark biological alterations of cancer cells to investigate overlap with the AD literature and identified overlap of all ten hallmarks in AD, including: 1) potentially common underlying risk factors, such as increased inflammation, deregulated cellular energetics, and genome instability, 2) inversely regulated mechanisms, including cell death and evading growth suppressors, and 3) functions with more complex, pleiotropic mechanisms, some of which may be stage-dependent in AD, such as cell adhesion/contact inhibition and angiogenesis. Additionally, we discuss the recent observation of a biological link between cancer and AD neuropathology. Finally, we address the therapeutic implications of this topic. The significant overlap of functional pathways and molecules between these diseases, some similarly and some oppositely regulated or functioning in each disease, supports the need for more research to elucidate cancer-related AD genetic and functional heterogeneity, with the aims of better understanding AD risk mediators, as well as further exploring the potential for some types of drug repurposing towards AD therapeutic development.
Keywords: Alzheimer Disease, Cancer, Risk Factors, Genetic Heterogeneity, Systems Biology
Introduction
Alzheimer’s disease (AD) imposes a terrible cost on millions of patients as well as their caretakers and is a national health priority. Unfortunately, to date, there has been little progress in developing disease modifying treatments for this devastating disease. One reason that treatment attempts have been largely unsuccessful may be a result of considering late onset AD (LOAD) as a ‘one size fits all’ disease. For decades, researchers have been suggesting that, much like cancer, LOAD is actually a heterogeneous compilation of risk and causative factors converging towards similar symptoms and disease processes. While there are no doubt important commonalities, such as the biological mechanisms leading to build-up of amyloid plaques and tau neurofibrillary tangles, the two major neuropathological characteristics of AD [1], there are also likely to be important differences in subgroups of LOAD patients with differing genetic risk, environmental exposures, and medical histories. While this viewpoint is supported by research, therapeutic research in AD has been largely focused on addressing the two core neuropathologies, with little accommodation for the heterogeneous nature of disease risk, etiology, and progression.
Cancer history/comorbidity is an aspect of a patient’s medical history that may have an important impact on LOAD development and progression. Cancer has been inversely associated with LOAD in numerous epidemiological studies, with cancer shown to reduce the risk of developing LOAD and vice versa [2–7]. These studies accounted for risk factors such as smoking history, sex, and the presence of the apolipoprotein E (APOE) ε4 allele, the major known genetic risk factor for LOAD. Additionally, many of these studies have provided evidence that survival bias is not driving this disease association, including a study by Roe et al. (2010) showing that cancer and LOAD were inversely associated, while cancer and vascular dementia were not [6]. A recent study of 3,499,378 mostly male US veterans further supports these findings, showing that survivors of numerous cancer types had lower AD risk, though reduced risk was not observed for other age-related conditions, and that chemotherapy was independently associated with lower AD risk [8]. Our previous study showed that cancer history was associated with later onset of LOAD, suggesting that cancer and/or cancer treatment may delay LOAD onset [4]. Importantly, we also found that non-melanoma skin cancer, which is generally benign and only treated with surgical removal, also showed later age of LOAD onset, supporting the idea that beyond cancer treatment effects, the genetic background or environmental exposures predisposing to cancer may be protective towards LOAD.
Although cancer and LOAD have been shown to be inversely associated, there are a surprising number of parallels that can be drawn between these two diseases. Risk for both diseases increases with age and comorbid conditions such as diabetes and metabolic syndrome [9, 10], and appears to be affected by behavioral and environmental factors such as vitamin levels and nutrition, sunlight exposure, smoking, diet and exercise, and heavy metal exposure [11–24]. Genetic factors including microRNAs show evidence of differential expression and activity related to both diseases [25]. Preliminary evidence supports the hypothesis that microRNAs may act as central regulators of both oncogenesis and neurodegeneration [26]. A transcriptomic study using multiple cancer and neurodegenerative disease data sets found significant overlap of pathways both inversely and commonly expressed in cancer and LOAD [27]. Another parallel involves the two-hit model of cancer progression, wherein multiple DNA mutations are necessary for oncogenesis [28]. A similar model has been recently adapted to explain the risk for and progression of AD as well. The two-hit model, when applied to AD, suggests that early life harmful exposures and/or recessive genetic variants are latent risk factors until later life insults are incurred, at which time additive genetic and epigenetic changes initiate a neuropathological cascade culminating in dementia and death [29–33].
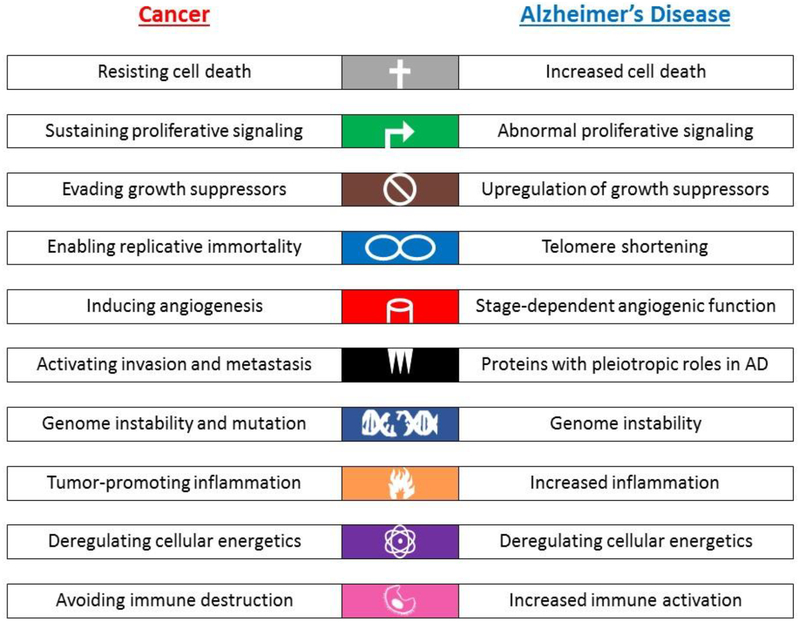
Another interesting parallel between the cancer and LOAD disease processes involves the ten hallmarks of cancer, as updated by Hanahan and Weinberg in 2011 from their original publication in 2000 [34, 35]. All of these hallmarks show some evidence for involvement of key molecules, pathways, or mechanisms in the risk, onset, or progression of AD (Figure 1), with some showing evidence of an inverse relationship between cancer and LOAD, while others appear to have parallel or more complicated relationships.
Figure 1. Hallmarks of Cancer in AD.
The ten hallmarks of cancer (Hanahan et al., 2011) are listed in the left column (Cancer), while in the right column (Alzheimer’s disease, AD), known and potential roles of the hallmark pathways or genes/proteins in the hallmark pathways are listed. As shown, all ten hallmarks share functional overlap with known or postulated AD-related biological mechanisms.
Hallmarks of Cancer in LOAD
The first hallmark of cancer is resistance to cell death. Cell death occurs either by necrosis, in which cell injury results in death by unregulated autolysis, or by apoptosis, programmed cell death [35]. Cancer cells must be able to escape apoptosis, and a common mechanism involves mutations in the pro-apoptotic tumor protein 53 (TP53) gene. Somatic inactivating TP53 mutations have been found in numerous cancer types, while germline mutations in this gene result in Li-Fraumeni syndrome, an autosomal dominant hereditary cancer pre-disposition syndrome [28, 36–38]. Another example of this mechanism is downregulation of tumor suppressive regulators such as DAPK1; downregulation of this molecule suppresses apoptosis in cancer cells [39].
In contrast, increased cell death is a hallmark of AD; however, there is ongoing debate regarding the precise mechanisms driving this process [40, 41]. It has been suggested that there are two main types of cell death in AD, apoptosis and neurofibrillary formation. In neurofibrillary formation, cells re-enter the cell cycle, similar to apoptotic initiation. However, there is evidence to suggest that in neurofibrillary formation cells escape classical apoptosis and instead progress farther through the cell cycle before experiencing neurofibrillary degeneration [42]. There is increasing evidence for involvement of both these types of cell death in AD, supporting the idea of an inverse tendency towards apoptosis in cancer compared to AD. A study of transcriptome data from three types of cancer and neurodegenerative diseases showed that genes in the apoptotic pathway were upregulated in AD [27]. Upregulation of apoptotic proteins has also been observed in the platelets of individuals with Mild Cognitive Impairment (MCI) and AD [43], and studies of genes mutated in familial AD have highlighted the potential involvement of neurodegenerative apoptotic mechanisms [44, 45]. Additionally, it has been shown that amyloid-beta induces neuronal death in vitro, and evidence has implicated the pro-apoptotic p53 signaling pathway in this effect [46, 47]. A caspase-independent mechanism involving apoptosis-inducing factor (AIF) has also been identified in AD. Translocation of this protein to the nucleus, which leads to caspase-independent apoptosis, has been observed in AD and was colocalized with neurofibrillary tangles [48]. Upregulation of tumor suppressive regulators such as DAPK1 have been shown in AD brains and have been associated with neuronal apoptotic activity in response to variety of stimuli, further supporting the potential involvement of cancer-related apoptotic processes influencing neuronal cell death in AD [49]. Thus, though there is some debate about cellular outcomes (apoptosis vs. neurofibrillary degeneration), it is clear that while apoptotic processes are commonly downregulated or inactivated in cancer, these processes are commonly upregulated or activated in AD. It is possible that a genetic background predisposing towards either upregulation or downregulation of apoptotic processes may contribute towards the inverse association of cancer and AD observed in epidemiological studies.
The second hallmark of cancer is sustaining proliferative signaling [35]. This can occur through a variety of pathways, including producing more growth factor ligands, signaling to neighboring cells to produce more growth factors, increasing receptor proteins, mutations activating ligand-independent receptor firing, or constitutive activation of downstream growth factor signaling molecules [34, 35]. Somatic mutations for genes within these signaling pathways have been commonly identified in various cancers. For example, activating mutations in the B-Raf proto-oncogene (BRAF) are common in melanoma. The mutated protein activates the mitogen-activated protein kinase (MAPK) pathway, inducing proliferation, differentiation, and cell survival [35, 50]. An alternate pathway to MAPK activation common across various cancer types involves activating mutations of small GTPase RAS family oncogenes [51, 52]. Interestingly, it has been shown that while upregulation of oncoproteins involved in tumor growth is a hallmark of cancer, excessive upregulation of these proteins can actually lead to cellular senescence, suggesting that precise regulatory changes are necessary to achieve carcinogenesis [35, 53].
Dysregulated protein kinases important for proliferative signaling have also been identified in AD patients, which is unsurprising given that hyperphosphorylation of neurofibrillary protein tau by protein kinases is one of the hallmarks of AD. The following examples highlight a few results in this complex area, and demonstrate the importance of proliferative signaling genes in AD. MAPK/ERK-p has been measured in neurons and glial cells in patients with early stage tauopathies, and MAPK/ERK-p and phosphorylated protein kinase of 38 kDa (p38) co-localize in neurons and glial cells with phosphorylated tau deposits [54]. Cavallini et al. (2013) performed a network analysis that suggested involvement of Ras family GTPases in tau phosphorylation pathways [55]. The small G-protein p21ras has been associated with neuritic plaques and tangles in AD [56, 57]. Studies of gene expression in AD have identified the MAPK/ERK pathway as downregulated in AD, as well as brain-region-specific altered expression of genes in the PI3K/AKT growth signaling pathway [58–60]. There have been various regional and phosphorylation (activation-state) dependent differences in expression changes observed in kinase signaling pathways for some AD studies, suggesting that altered expression may be associated with an increase in the proportion of activated kinases; this makes sense in the context of increased phosphorylation of tau associated with advancing disease state [61–63]. The MAPK/ERK pathway is also known to be involved in synaptic plasticity and learning, linking downregulation of genes in this pathway to the cognitive dysfunction observed in AD [58]. These results support the involvement of proliferative signaling genes and pathways in both cancer and AD, and also show the different effects resulting from differential regulation and activity of the same genes in different tissues. Activation/upregulation in somatic tissue is associated with proliferation as one of the hallmarks of cancer, while activation in neurons and glial cells may be one of the initiating steps of tau hyperphosphorylation leading to neurofibrillary tangle formation and cell death, and subsequent downregulation may contribute to decreased synaptic plasticity and cognitive dysfunction in AD.
The third hallmark of cancer is evading growth suppressors. A number of growth suppressor genes, also known as ‘tumor suppressors’, have been identified as commonly containing inactivating mutations in different cancer types, including the retinoblastoma transcriptional corepressor 1 (RB1), TP53, and the phosphatase and tensin homolog PTEN [35, 64]. Both pRb and p53 proteins regulate cell cycle progression; thus, inactivation of these tumor suppressors permits cell growth and division [65]. PTEN negatively regulates the proliferative AKT/PKB signaling pathway [66]. Cells must also be capable of avoiding terminal differentiation, in which cells enter an irreversible post-mitotic state. An oncoprotein commonly over-expressed in cancer, c-Myc, is an example of this process. Upregulation of c-Myc enables it to outcompete transcription factors responsible for initiating differentiation and prevent cells from entering the post-mitotic state [35, 67].
Similarly, evading growth suppressors is a known occurrence in AD. Aberrant neural cell cycle progression is a hallmark of AD, and has been linked to neuropathology [68, 69]. Cell cycle studies in AD model organisms suggest that cell cycle re-entry is accompanied by or followed by increased neuropathological processes and apoptosis, while inhibiting cell cycle progression may reduce neurodegeneration [70–72]. Numerous tumor suppressors appear to play important roles in AD neuropathological development [73–77]. For example, p27, an important negative cell cycle regulator, shows enhanced degradation in AD patients compared to controls. Phosphorylated p27, which is marked for degradation, overlaps with neurofibrillary pathology [78, 79]. A number of cell cycle proteins are upregulated in the peripheral lymphocytes of AD patients as well [80–82], suggesting systemic cell cycle dysregulation may be a hallmark of both cancer and AD.
The fourth hallmark of cancer is enabling replicative immortality. All normal cultured cell types have a finite replicative potential, termed the ‘Hayflick limit’. Once they reach this limit, normal cells stop growing and enter senescence [83]. While cultured cells can circumvent this limit by disabling the pRb and p53 tumor suppressor proteins, the precise mechanisms of cell senescence are still unclear [35]. If cells are able to circumvent the Hayflick limit and continue to divide, they will eventually reach a second limit called the ‘crisis’ state, in which massive genomic instability typically results in cell death [35]. This genomic instability is attributed to critically shortened telomeres, the sequences of DNA that normally protect the ends of each chromosome. To survive this crisis state, the cell must activate telomere maintenance machinery. Typically, this involves upregulation of the telomerase enzyme, which is responsible for elongating telomere sequences in dividing cells and is normally absent or significantly downregulated in somatic cells [35, 84–86]. Activation of telomerase is a critical step in the development of most cancer cells [87].
In contrast, there has recently been accumulating evidence that AD patients have shorter telomeres, and that shorter telomere length is a risk factor for AD [88, 89]. Shorter telomere length could potentially compromise genomic stability, leading to increased amounts of cells undergoing apoptosis in the AD brain. Shorter telomeres would theoretically first impact neurogenesis, as neural stem cells would have shorter telomeres than differentiated neurons due to cell division. There is growing evidence supporting the downregulation of neurogenesis in neurodegenerative diseases, and in particular the importance of neurogenesis in AD [73, 90]. There is also some preliminary evidence from studies of depression supporting the possibility that stress or disease-mediated telomere shortening or reduced telomerase activity could have a deleterious impact on neurogenesis and hippocampal volume [91–93]. The activity of the catalytic subunit of telomerase, TERT, has been shown to have neuroprotective effects in cell and animal models following injury, as well as neuroprotective functions in neurons; due to this, there is increasing interest in telomerase as a therapeutic target in neurological diseases [94].
The fifth hallmark of cancer is inducing angiogenesis, or blood vessel formation. All cells in a tissue must reside within 100 μm of a capillary blood vessel to obtain the nutrients and oxygen necessary for survival [34]. In order to grow new cancerous tissue, blood vessel growth is also required [35, 95]. Angiogenesis is carefully regulated in normal tissue, requiring cancer cells to develop the ability to initiate additional angiogenic signaling and sustain angiogenesis [35, 95]. Based on observations of tumor development, it is postulated that angiogenic induction is an early to middle cancer stage event, which is required for clonal expansion to form a macroscopic tumor [35, 95]. This process is typically achieved by altering the balance of angiogenic inducers (such as vascular endothelial growth factor, VEGF) and inhibitors (such as β-interferon) [35, 96].
Angiogenesis is also a topic of great interest in LOAD research. Cerebral blood flow has been suggested as a biomarker of LOAD, as measurable differences can be detected years before clinical disease presentation via neuroimaging, and can differentiate between cognitively normal individuals, those at risk for LOAD (assessed by family history or brain amyloid), and individuals diagnosed with LOAD [97–99]. There is some evidence that cerebral blood flow may be initially increased, though hypoperfusion is characteristic of later disease stages. Research on cerebral blood flow is complicated by the relationship of cerebral blood flow and brain metabolism, as if metabolism decreases, blood flow will also decrease [100]. Another complication is that it appears that cerebral blood flow may become uncoupled under pathological conditions [101]. Vascular and LOAD pathology commonly co-occur, and the presence of cerebral infarcts has been shown to increase the odds of dementia, specifically impairing memory function [102–104]. Capillary cerebral amyloid angiopathy, the pathological deposition of amyloid-beta within cerebral vessels, has been associated with LOAD [105, 106]. All of these findings support a significant role of vascular dysfunction in LOAD. It has been hypothesized that increases in brain regional blood flow observed years prior to LOAD symptoms may represent upregulated angiogenesis as a compensatory mechanism, which then eventually is overtaken by the significant, widespread decrease in blood flow observed in later stage LOAD [97]. A key mechanism driving this stage-dependent vascular functional change may be the different effects of upregulation of hypoxia-inducible factor-1α (HIF-1α), known to be upregulated by hypoxia. HIF-1α upregulates angiogenic factors such as vascular endothelial growth factor (VEGF), which can upregulate angiogenesis, attenuate the pathogenic impact of hypoxia, and potentially delay the onset of LOAD symptoms [107]. However, there is also evidence that HIF-1α upregulation could exacerbate LOAD neuropathological processes and the activation of the cellular stress response and impairment of autophagy [108]. Thus, while HIF-1α and angiogenic upregulation may be beneficial in early disease stages to compensate for vascular dysfunction, it may also promote LOAD pathological processes. Furthermore, LOAD neuropathology is associated with reduced capillary expression of VEGF and nitric oxide, two markers of angiogenesis [109], suggesting that late stage LOAD brain blood flow decrease may be the result of a vicious neuropathological feedback loop which may also inhibit brain angiogenic repair and maintenance pathways. Thus, angiogenesis regulation may be LOAD-stage-dependent, and may be an important factor in disease progression and related cognitive dysfunction.
The sixth hallmark of cancer is activating invasion and metastasis [35]. While a complete overview of this extremely complex area of study is beyond the scope of this review, we present a few key points for consideration. Advanced stage cancer cells downregulate cell adhesion molecules promoting contact inhibition, such as E-cadherin and its upstream regulator Reelin, and upregulate adhesion molecules associated with cell migration, such as N-cadherin [110, 111]. Transcription factors associated with migratory processes during embryogenesis are upregulated, possibly in response to stimuli in the tumor microenvironment, resulting in numerous cell structure changes and facilitating the various steps involved in invasion and metastasis [35]. Reelin is a key signaling molecule that has been shown to mediate RAS/PI3K signaling promoting cell motility and tumor metastasis [110].
Cell adhesion and contact inhibition molecules have also been shown to play a role in LOAD. Ibanez et al. (2014) found both cell adhesion and extracellular matrix receptor molecules to be downregulated in cancer, but upregulated in LOAD [27]. Cell adhesion was one of the pathways showing significant enrichment of single nucleotide polymorphisms when tested for association with a composite memory score in an LOAD cohort [112], and was also significantly associated with LOAD in two other independent cohorts [113]. Neural cell adhesion molecules are very important for proper synaptic functioning, playing roles in the regulation of synaptic vesicle recycling, stabilization of synaptic membrane interactions, and recruitment of scaffolding proteins and neurotransmitter receptors to the synapse [114]. Interestingly, while Reelin has been shown to mediate cell motility and tumor invasion in cancer, it has also been shown to regulate nervous system development and modulate synaptic plasticity in the adult brain [115]. Reelin also appears to play a more direct role in AD neuropathology, with evidence from mice and humans supporting involvement of Reelin in amyloid plaque formation and tau hyperphosphorylation, and may also be subject to feedback regulation or aggregation by amyloid beta [115–119]. Thus, it appears that in addition to a more straightforward influence on disease risk/progression, cell adhesion pathway genes may also play important pleiotropic roles in LOAD.
The seventh hallmark of cancer is genome instability and mutation. This concept was introduced more recently in Hanahan et al., 2011, as one of two enabling characteristics of cancer. Acquisition of genomic instability generates random mutations, some of which result in the required hallmarks of cancer [35]. It is postulated the genomic instability in cancer cells commonly occurs due to deactivation of genomic maintenance and/or genomic integrity surveillance though such proteins as p53 and BRCA1 [120], as well as telomere erosion accompanied by chromosomal breaks and fusions, resulting in deletion and amplification of numerous segments of DNA [121]. Another piece of evidence for the importance of genomic instability in cancer is the observation of increased aneuploidy, particularly loss of the Y chromosome in men, which has been associated with various cancer types as well as with variants in cancer-related genes [122–125].
Genomic instability is also a topic that has long been of interest in LOAD. Inspired by the knowledge that patients with Down syndrome are more likely to have early onset AD, linked to an extra copy of the chromosome 21 APP gene, researchers have tried to determine whether neurons in LOAD patients experience copy number gain linked to pathology or cell death. This question is also linked to the theory that neurons re-enter the cell cycle as part of a pathological process leading to cell death [126]. Direct studies of aneuploidy in LOAD have shown mixed results, with some studies supporting copy number gains in select chromosomes measured by fluorescent in situ hybridization (FISH), potentially related to increased neuronal cell death [127–129], while others suggest that aneuploidy may not play an important role in disease initiation/progression [130, 131]. Given the small sample sizes and diverse methods of these studies, as well as the lack of knowledge regarding correlation of aneuploidy with biomarkers and pathology of LOAD, at present it is not possible to confidently conclude whether autosomal copy number or genome amplification plays an important role in LOAD pathological processes. However, there is increasing literature to suggest that systemic sex chromosome loss may play an important role in aging and LOAD [132–134]. Micronuclei arising from chromosome mis-segregation have been observed at greater frequency in older individuals, those at increased risk for LOAD, and LOAD patients [135], further supporting the importance of genomic instability in LOAD. Finally, shorter telomeres, altered telomere architecture, and telomere shortening over time have been associated with LOAD [88, 89, 136, 137], though there is some inconsistency in this literature as well [138–141], likely partially due to differences in telomere length by cell/tissue type, as well as the very large number of medical, genetic, behavioral, and environmental factors capable of influencing telomere length. Chromosome gain and loss and shorter telomeres may all be important factors in LOAD risk/progression but more research is needed to clarify the roles of these mechanisms.
The eighth hallmark of cancer is tumor-promoting inflammation. This concept is the second enabling characteristic of cancer more recently posited by Hanahan et al., 2011 [35]. Tumors have been compared to chronic non-healing wounds, involving chronic inflammation caused by the persistent presence of immune inflammatory cells [142]. These innate immune cells, normally involved in wound healing, are also associated with tissue pathologies including fibrosis, aberrant angiogenesis, and neoplasia [35, 143]. Macrophage subtypes, mast cells, neutrophils, and T and B lymphocytes have all been identified as potentially tumor-promoting [143–147]. To survive, tumors must shift the balance from the subclasses of lymphocytes and innate immune cells attacking the tumor towards the types of immune cells promoting inflammation and tumor growth [35, 147]. It has been posited that inflammation might promote the development of the earliest stages of neoplastic progression, and that a state of chronic systemic inflammation is oncogenic.
The topic of inflammation has long been of interest in AD research [148, 149]. Amyloid plaques and tangles promote a chronic inflammatory feedback loop by inducing the expression and release of pro-inflammatory cytokines by activated microglia, which in turn enhance amyloidogenic processing [150]. ‘Gliosis’, or the inflammation of microglia and astrocytes, is a hallmark of LOAD; amyloid plaques are typically surrounded by activated microglial cells in early and late phases of disease [151]. While there is evidence that the inflammatory negative feedback loop is likely a toxic contributor to neuropathology, there is also evidence that normal microglial function includes the clearance of amyloid beta, though it is unclear how effective microglia are at this task [151, 152]. A review of select microRNAs in cancer and LOAD literature identified common functional overlap in innate immunity and inflammation and oxidative stress [25]. On an epidemiological level, type 2 diabetes increases the risk of LOAD, and it has been postulated that one of the molecular mechanisms driving this relationship is central and peripheral inflammation [153]. It has been strongly established that systemic inflammation increases with age, and that chronic inflammation is more common in elderly individuals [154]. Inflammation may be one of the common risk factors tying together age-related diseases including cancer and LOAD.
The ninth ‘emerging’ hallmark of cancer is deregulating cellular energetics. Cancer cells have been shown to modify or reprogram cellular metabolism to support proliferation. Cancer cells are known to switch from aerobic oxidative phosphorylation by mitochondria to primarily glycolysis (the Warburg effect [155]) even under aerobic conditions, which better enables hypoxic cells to survive. This switch is accompanied by upregulated glucose import, which helps to compensate for the difference in glycolytic energy production [35]. In each cell there are numerous complex, interconnected signaling networks that regulate cellular energetics; in cancer cells genetic instability promotes the acquisition of genetic mutations, including some that can orchestrate the hallmark functions of cancer and alter cellular metabolism [35]. The hubs of these pleiotropic networks include many genes discussed above, including RAS oncogenes, MYC, PTEN, and HIF-1α, raising the question of whether this is an independent hallmark or a phenomenon tied inextricably to other hallmarks, including proliferation and angiogenesis. One of the regulators of glycogen synthesis, glycogen synthase kinase-3 (GSK3), is regulated by insulin and by the PI3K/Akt signaling pathway, suggesting another point of connection between cancer and diabetes / metabolic syndrome [156]. It should be noted that this emerging hallmark is under debate, however, as other researchers have proposed a ‘reverse Warburg’ hypothesis in which cancer cell metabolism is more complicated [156]. In the reverse Warburg model, cancer cells induce oxidative stress in cancer-associated fibroblasts, causing them to initiate autophagy and undergo glycolysis. Glycolytic byproducts lactate and pyruvate are transferred to epithelial cancer cells undergoing oxidative phosphorylation, essentially allowing cancer cells to feed off the surrounding tissue [157–159]. Notably, stromal cells exhibiting what appears to be a key biomarker of this effect, caveolin-1, show transcriptional similarities to the AD brain transcriptome [157, 158].
Metabolic dysregulation has been studied for decades in LOAD research [160], and LOAD has even been referred to as ‘type 3 diabetes’ [161]. Brain insulin and insulin-like growth factor signaling disturbances and hypometabolism are characteristic of LOAD, including some studies of early disease stages, and hypometabolism has been shown to be correlated with neuropsychological performance deficits [160–168]. This known decrease in glucose metabolism was deemed so significant that [18(F)] fluorodeoxyglucose (FDG) positron emission tomography (PET), an imaging method that can measure glucose metabolism in the brain, has been proposed as a sensitive measure of LOAD progression and a feasible treatment outcome measure [169]. Based on these studies and the hypothesized biology driving metabolic differences in LOAD, a new area of therapeutic interventions is targeting upregulating ketone body metabolism to compensate for dysregulated glucose metabolism in the brain [170–174]. Metabolism was also one of the key functional categories identified as inversely associated in cancer and LOAD by Ibanez et al. (2014). While various metabolic pathways were up and downregulated in cancer, metabolic pathways were only downregulated in neurodegenerative diseases, including LOAD, and there was a concentration of overlapping metabolic pathways upregulated in cancer and downregulated in LOAD, including oxidative phosphorylation and the Krebs (citric acid) cycle [27]. Interestingly, one of the key proteins identified in cancer metabolic research, GSK3, has also been identified as playing important roles in LOAD neuropathology, with overexpression linked to increases in tau hyperphosphorylation and tangles as well as differences in amyloid precursor protein processing leading to increased amyloid-beta [175]. However, the emerging picture of metabolic differences in LOAD is complex and likely stage- and sex-specific, highlighting this as a topic requiring further investigation.
The tenth ‘emerging’ hallmark of cancer is avoiding immune destruction (immunoevasion) [35]. Currently, while there is some evidence to support the importance of immune function in suppressing cancer, the importance and efficacy of this function require further research [176, 177]. There is limited evidence supporting the involvement of both the adaptive and innate immune systems in immune surveillance and tumor eradication. Theoretically, cancer cells are selected for those that are weakly immunogenic, not strongly targeted by the immune system. Tumors have also been shown to subvert progenitor immune cells, which then function to suppress normal immune function [35]. Cancer cells could also theoretically disable immune cells by secreting immune-suppressive factors [178]. Though much work remains to be done in this area, immunoevasion likely plays a role in at least some types of cancer.
While some types of cancer may be characterized by immune resistance, it appears that peripheral as well as central immune function may be a risk factor for LOAD. Peripheral immune cells have been identified in the brain, and it has been suggested that these cells may play a role in exacerbating LOAD processes, though there is currently debate on this topic [179–181]. The top 20 genes identified by the International Genomics of Alzheimer’s Project (IGAP) showed significant enrichment for the immune response pathway [182]. Furthermore, a recent study identified a SNP in the interleukin-1 receptor accessory protein (IL1RAP) gene as significantly associated with brain amyloid deposition, implicating microglial activation as an important pathway in LOAD [183]. IL1RAP has also been identified as highly upregulated in myeloid leukemia [184], highlighting the potential importance of differential regulation of immune functions in cancer and LOAD. The complement and coagulation cascades and cytokine-cytokine pathways were identified by Ibanez et al. (2014) as downregulated in cancer and upregulated in LOAD, suggesting inverse regulation of immune pathways may play different roles in cancer and LOAD progression. However, more work remains to investigate the roles of the immune system in LOAD risk and progression.
Cancer and LOAD Neuropathology
While some hallmarks of cancer show an inverse association with known function in LOAD, and some appear to have common or complex pleiotropic effects, it is difficult to interpret these findings without a direct link to the two neuropathological hallmarks of LOAD, amyloid and tau pathology. Recent research has started to elucidate this link to LOAD neuropathology. As discussed above, IL1RAP was identified as an immune protein that is upregulated in cancer, but also has been recently shown to potentially mediate amyloid deposition [183]. Work by Jane Driver and colleagues has identified the protein Pin1 as having key roles in both oncogenesis and LOAD neuropathology [185]. Pin1, which can change the conformation of numerous substrates, has been shown to modulate the amplitude and duration of cellular responses or processes [186]. Pin1 coordinates cell division and is upregulated in many cancer types; however, in LOAD, Pin1 is downregulated or inactivated, and in mouse models, Pin1 absence has been shown to impair tau function and amyloid precursor protein processing, resulting in accumulation of tau tangles and amyloid plaques [185]. Pin1 has neuroprotective functions, as it has been shown to act on both APP and tau to switch their conformations from dysfunction to a functional shape, preventing toxic pathological processes [186]. A study of microRNA roles in cancer and AD by Holohan et al. (2013) showed that of the eight reviewed microRNAs, while all had cancer-specific functions, all but one also had functions identified in LOAD studies related to amyloid precursor protein processing or amyloid-beta plaque accumulation, or to tau neurofibrillary tangles [25]. Finally, in two large cohorts of deceased older men and women with and without dementia, while there was no difference in neuritic plaque count by cancer history, individuals with cancer history were shown to have lower odds of LOAD proximate to death and significantly fewer neurofibrillary tau tangles at autopsy [187]. While the biological mechanism(s) driving this association remain to be elucidated, there are a number of plausible explanations for the observation of a reduced load of neurofibrillary tau tangles in individuals with cancer history, including cancer survivor selection for improved immune function, survivor behavioral changes leading to healthier brain aging, the influence of chemotherapeutic agents on tau pathology, and/or underlying molecular mechanisms influencing LOAD/cancer risk as well as LOAD neuropathological processes. All of these mechanisms require further investigation to identify potential novel therapeutic directions for cancer and LOAD.
Therapeutic Implications
Cancer drug repurposing has gained significant attention recently as a novel source of potential LOAD therapeutic agents, and was recently reviewed by Heather Snyder and colleagues (2017) [7]. The significant overlap of all ten hallmarks of cancer with functions or molecules linked to AD, albeit some with opposite regulation or functions, highlights the importance of this area of study. This is a complicated subject, clearly, with some hallmarks likely to be more suitable for exploration than others. Some molecules may potentially be directly repurposed (i.e. immune checkpoint inhibitors or microtubule stabilizers), while others may need further study (activators vs. inhibitors targeting pathways that appear to be differentially regulated in cancer and AD, such as proliferative and apoptotic pathways). A recent study by Frain et al. (2017) showed that cancer survivors who had received chemotherapy had a lower risk of AD than those who had not received chemotherapy, further supporting the idea of drug repurposing towards AD therapeutics [8]. Efforts towards cancer drug repurposing in AD are already underway. Based on studies of tau conformational changes related to Pin1 function, it may soon be possible to use an antibody or vaccine to specifically target abnormal protein tau in AD and other tauopathies [186, 188], providing novel treatment options that may be more effective than past efforts aimed at ameliorating amyloid pathology. Another interesting drug repurposing study involves the myeloid cell-surface receptor CD33. A SNP in this gene is associated with AD risk [189, 190], while other studies have identified another SNP in linkage disequilibrium that is associated with treatment response in acute myeloid leukemia [191, 192]. Both SNPs were shown to decrease full-length CD33 expression in tissues of interest, which was associated with decreased AD odds ratio, suggesting that antibodies developed for acute myeloid leukemia such as lintuzumab could be effectively repurposed as AD treatments [193]. Drugs blocking immune checkpoints have been shown to mobilize the immune system, resulting in anti-tumor activity [194]. Recent research suggests that these drugs may be repurposed towards AD, as repeated treatment in mouse models has shown reduction of cerebral amyloid-beta plaques as well as improved cognitive performance [195]. NSAIDs, which reduce peripheral inflammatory factors, have shown mixed evidence for reduced risk of both cancer and AD [196, 197]. Research on this topic has been complicated by the effects of study duration and design; further research on this type of medication in both diseases seems merited to reach any conclusions. Another interesting target for cancer drug repurposing that has been rather extensively studied is bexarotene, a retinoid X receptor that in cancer functions to suppress proliferation and inhibit inflammatory cell activation [198]. There is some evidence that this drug may also stimulate expression of APOE and increase beta amyloid clearance [199]. The effects of treatment on beta amyloid reduction may be dependent on APOE genotype, and there may be significant cardiovascular risk associated with use [200]; however, this class of drugs represents an interesting new direction leveraging the pleiotropic effects of molecules associated with cancer and AD. While the drugs discussed here are largely involved in immune/inflammatory pathways, given the significant functional overlap of pathways in cancer and AD it is possible that there are cancer drugs targeting a few of the other hallmarks that may also prove efficacious in AD, or that may suggest novel targets in these pathways. This represents a rich and largely unexplored opportunity for novel AD therapeutic development.
Conclusions
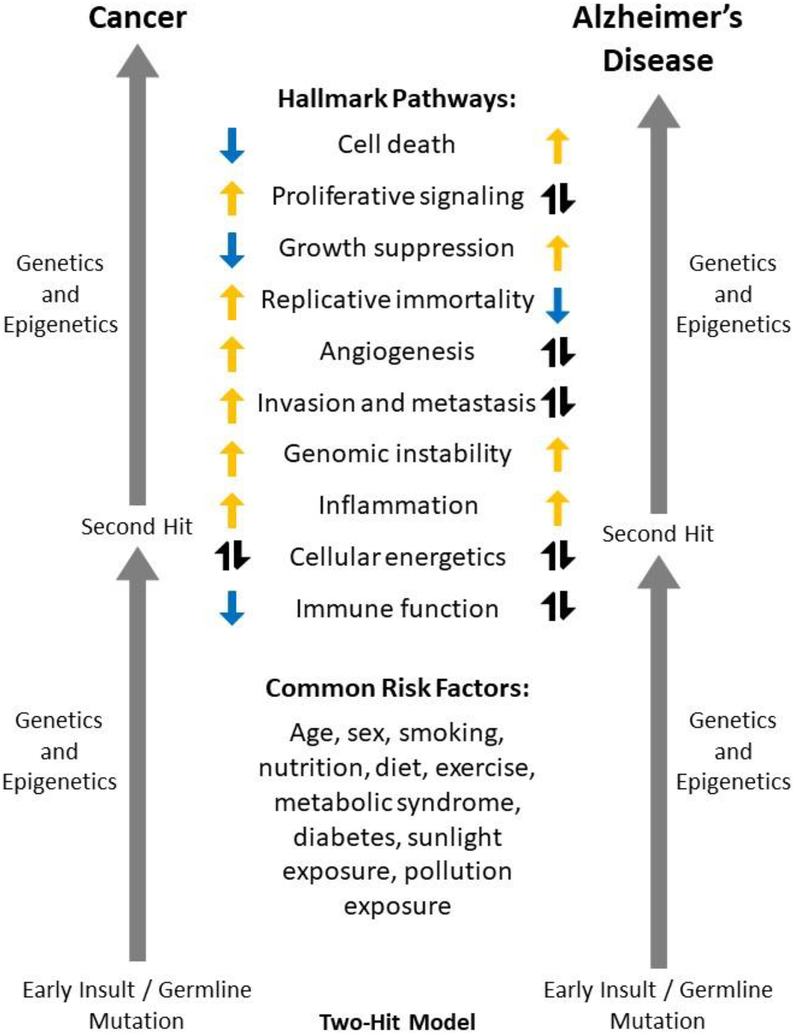
There appears to be significant overlap in disease risk factors and the hallmarks of cancer cells and LOAD, some with opposite, similar, or pleiotropic functions in each disease, as well as a potential direct connection between cancer history and LOAD neuropathology, supporting the idea that cancer history may be an important factor influencing LOAD heterogeneity, risk, and progression (Figure 2). Some pathways, such as resisting cell death and upregulating growth suppressors, appear to be inversely regulated between these diseases and may help to explain the inverse association of cancer and LOAD observed in epidemiological studies. Other pathways exhibit more complicated pleiotropic roles, including potential effects on LOAD neuropathological pathways. More research is needed to elucidate the molecular mechanisms driving the inverse association of LOAD and specific cancer types, particularly focusing on the mechanism(s) mediating AD neuropathological processes. Additionally, future studies are needed to investigate genetic and molecular factors as biomarkers of risk for both diseases, as well as to continue to explore existing cancer agents in some hallmarks for repurposing towards AD treatments.
Figure 2. Cancer and AD Model.
This model shows the two-hit hypothesis widely accepted as a common mechanism of oncogenesis and more recently proposed to underlie Alzheimer’s disease (AD) onset, risk factors including many common to both cancer and AD, and the potential contributions of the Hallmarks of Cancer to cancer (left) and AD (right). Orange arrows pointing up indicate a posited or observed increase in this hallmark for each disease; blue arrows pointing down indicate a posited or observed decrease in this hallmark for each disease. Black arrows pointing up and down indicate that this hallmark (or molecules involved in this hallmark) show bi-directional, stage-specific, or pleiotropic effects for the disease. It is important to note that many of these hallmarks could be important both before and after the second hit in both diseases, or could be acting at different stages of disease. ‘Genetics and Epigenetics’ are listed for both diseases before and after the second hit, as genetic background has been posited to contribute to risk and progression for both diseases (beyond the two key mutations cited in the two-hit model), and also may play a role in differential risk for cancer vs. AD.
Acknowledgements
This work was supported by funding from the National Institutes of Health and National Institute on Aging (R01 AG042437, AG051086, AG029672, AG019771, CA129769, R35 CA197289, P30 AG010133, and U01 AG24904).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest/Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1.Perl DP, Neuropathology of Alzheimer’s disease. Mt Sinai J Med, 2010. 77(1): p. 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driver JA, et al. , Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ, 2012. 344: p. e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musicco M, et al. , Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology, 2013. 81(4): p. 322–8. [DOI] [PubMed] [Google Scholar]
- 4.Nudelman KN, et al. , Association of cancer history with Alzheimer’s disease onset and structural brain changes. Front Physiol, 2014. 5: p. 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roe CM, et al. , Alzheimer disease and cancer. Neurology, 2005. 64(5): p. 895–8. [DOI] [PubMed] [Google Scholar]
- 6.Roe CM, et al. , Cancer linked to Alzheimer disease but not vascular dementia. Neurology, 2010. 74(2): p. 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder HM, et al. , Exploring the nexus of Alzheimer’s disease and related dementias with cancer and cancer therapies: A convening of the Alzheimer’s Association & Alzheimer’s Drug Discovery Foundation. Alzheimers Dement, 2017. 13(3): p. 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frain L, et al. , Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimers Dement, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JQ, et al. , Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry, 2016. 87(5): p. 476–84. [DOI] [PubMed] [Google Scholar]
- 10.Vanhanen M, et al. , Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology, 2006. 67(5): p. 843–7. [DOI] [PubMed] [Google Scholar]
- 11.Roskies M, et al. , Vitamin D deficiency as a potentially modifiable risk factor for thyroid cancer. J Otolaryngol Head Neck Surg, 2012. 41(3): p. 160–3. [PubMed] [Google Scholar]
- 12.Malekshah AF, et al. , Vitamin deficiency in Golestan Province, northern Iran: a high-risk area for esophageal cancer. Arch Iran Med, 2010. 13(5): p. 391–4. [PubMed] [Google Scholar]
- 13.Baena Ruiz R and Salinas Hernandez P, Diet and cancer: risk factors and epidemiological evidence. Maturitas, 2014. 77(3): p. 202–8. [DOI] [PubMed] [Google Scholar]
- 14.Palmer S, Diet, nutrition, and cancer. Prog Food Nutr Sci, 1985. 9(3–4): p. 283–341. [PubMed] [Google Scholar]
- 15.Mosconi L and McHugh PF, Let Food Be Thy Medicine: Diet, Nutrition, and Biomarkers’ Risk of Alzheimer’s Disease. Curr Nutr Rep, 2015. 4(2): p. 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoel DG, et al. , The risks and benefits of sun exposure 2016. Dermatoendocrinol, 2016. 8(1): p. e1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayeux R and Stern Y, Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med, 2012. 2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong G, et al. , Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One, 2015. 10(3): p. e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ordonez-Mena JM, et al. , Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med, 2016. 14: p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fair AM and Montgomery K, Energy balance, physical activity, and cancer risk. Methods Mol Biol, 2009. 472: p. 57–88. [DOI] [PubMed] [Google Scholar]
- 21.Barnard RJ and Aronson WJ, Preclinical models relevant to diet, exercise, and cancer risk. Recent Results Cancer Res, 2005. 166: p. 47–61. [DOI] [PubMed] [Google Scholar]
- 22.Mates JM, et al. , Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med, 2010. 49(9): p. 1328–41. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, et al. , Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci, 2008. 28(1): p. 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunez O, et al. , Association between heavy metal and metalloid levels in topsoil and cancer mortality in Spain. Environ Sci Pollut Res Int, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holohan KN, et al. , Functional microRNAs in Alzheimer’s disease and cancer: differential regulation of common mechanisms and pathways. Front Genet, 2012. 3: p. 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh J, Molecular network analysis of human microRNA targetome: from cancers to Alzheimer’s disease. BioData Min, 2012. 5(1): p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibanez K, et al. , Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet, 2014. 10(2): p. e1004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knudson AG Jr., Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A, 1971. 68(4): p. 820–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahiri DK and Maloney B, The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp Gerontol, 2010. 45(4): p. 291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahiri DK, et al. , How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res, 2007. 4(2): p. 219–28. [DOI] [PubMed] [Google Scholar]
- 31.Lahiri DK, Maloney B, and Zawia NH, The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry, 2009. 14(11): p. 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lahiri DK, et al. , Early-life events may trigger biochemical pathways for Alzheimer’s disease: the “LEARn” model. Biogerontology, 2008. 9(6): p. 375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maloney B, et al. , Applying epigenetics to Alzheimer’s disease via the latent early-life associated regulation (LEARn) model. Curr Alzheimer Res, 2012. 9(5): p. 589–99. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D and Weinberg RA, The hallmarks of cancer. Cell, 2000. 100(1): p. 57–70. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D and Weinberg RA, Hallmarks of cancer: the next generation. Cell, 2011. 144(5): p. 646–74. [DOI] [PubMed] [Google Scholar]
- 36.Leroy B, et al. , The TP53 website: an integrative resource centre for the TP53 mutation database and TP53 mutant analysis. Nucleic Acids Res, 2013. 41(Database issue): p. D962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petitjean A, et al. , Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat, 2007. 28(6): p. 622–9. [DOI] [PubMed] [Google Scholar]
- 38.Olivier M, et al. , Li-Fraumeni and related syndromes: correlation between tumor type, family structure, and TP53 genotype. Cancer Res, 2003. 63(20): p. 6643–50. [PubMed] [Google Scholar]
- 39.Tur MK, et al. , Restoration of DAP Kinase Tumor Suppressor Function: A Therapeutic Strategy to Selectively Induce Apoptosis in Cancer Cells Using Immunokinase Fusion Proteins. Biomedicines, 2017. 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeBlanc AC, The role of apoptotic pathways in Alzheimer’s disease neurodegeneration and cell death. Curr Alzheimer Res, 2005. 2(4): p. 389–402. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, et al. , Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res, 2006. 3(4): p. 393–6. [DOI] [PubMed] [Google Scholar]
- 42.Hamdane M, et al. , Neurofibrillary degeneration of the Alzheimer-type: an alternate pathway to neuronal apoptosis? Biochem Pharmacol, 2003. 66(8): p. 1619–25. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, et al. , Increased apoptosis in the platelets of patients with Alzheimer’s disease and amnestic mild cognitive impairment. Clin Neurol Neurosurg, 2016. 143: p. 46–50. [DOI] [PubMed] [Google Scholar]
- 44.Czech C, Tremp G, and Pradier L, Presenilins and Alzheimer’s disease: biological functions and pathogenic mechanisms. Prog Neurobiol, 2000. 60(4): p. 363–84. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs DM, et al. , Staurosporine-induced activation of caspase-3 is potentiated by presenilin 1 familial Alzheimer’s disease mutations in human neuroglioma cells. J Neurochem, 1999. 73(6): p. 2278–85. [DOI] [PubMed] [Google Scholar]
- 46.Akhter R, Sanphui P, and Biswas SC, The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in beta-amyloid-induced neuron death. J Biol Chem, 2014. 289(15): p. 10812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhter R, et al. , The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death. J Neurochem, 2015. 134(6): p. 1091–103. [DOI] [PubMed] [Google Scholar]
- 48.Yu W, et al. , Evidence for the involvement of apoptosis-inducing factor-mediated caspase-independent neuronal death in Alzheimer disease. Am J Pathol, 2010. 176(5): p. 2209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.You MH, et al. , Death-associated protein kinase 1 phosphorylates NDRG2 and induces neuronal cell death. Cell Death Differ, 2017. 24(2): p. 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantwell-Dorris ER, O’Leary JJ, and Sheils OM, BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther, 2011. 10(3): p. 385–94. [DOI] [PubMed] [Google Scholar]
- 51.Bos JL, ras oncogenes in human cancer: a review. Cancer Res, 1989. 49(17): p. 4682–9. [PubMed] [Google Scholar]
- 52.Santarpia L, Lippman SM, and El-Naggar AK, Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets, 2012. 16(1): p. 103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson DM, et al. , A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age (Dordr), 2014. 36(3): p. 9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrer I, et al. , Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm (Vienna), 2001. 108(12): p. 1397–415. [DOI] [PubMed] [Google Scholar]
- 55.Cavallini A, et al. , An unbiased approach to identifying tau kinases that phosphorylate tau at sites associated with Alzheimer disease. J Biol Chem, 2013. 288(32): p. 23331–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gartner U, et al. , Induction of p21ras in Alzheimer pathology. Neuroreport, 1995. 6(10): p. 1441–4. [DOI] [PubMed] [Google Scholar]
- 57.Gartner U, Holzer M, and Arendt T, Elevated expression of p21ras is an early event in Alzheimer’s disease and precedes neurofibrillary degeneration. Neuroscience, 1999. 91(1): p. 1–5. [DOI] [PubMed] [Google Scholar]
- 58.Hallock P and Thomas MA, Integrating the Alzheimer’s disease proteome and transcriptome: a comprehensive network model of a complex disease. OMICS, 2012. 16(1–2): p. 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T, et al. , Transcriptional signaling pathways inversely regulated in Alzheimer’s disease and glioblastoma multiform. Sci Rep, 2013. 3: p. 3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang WS, et al. , Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol Genomics, 2008. 33(2): p. 240–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrer I, et al. , Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res, 2005. 2(1): p. 3–18. [DOI] [PubMed] [Google Scholar]
- 62.Zhu X, et al. , Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem, 2001. 76(2): p. 435–41. [DOI] [PubMed] [Google Scholar]
- 63.Griffin RJ, et al. , Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem, 2005. 93(1): p. 105–17. [DOI] [PubMed] [Google Scholar]
- 64.Robinson DR, et al. , Integrative clinical genomics of metastatic cancer. Nature, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer M, et al. , Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F target gene analyses identifies cell cycle gene regulatory networks. Nucleic Acids Res, 2016. 44(13): p. 6070–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim HJ, Crowe P, and Yang JL, Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol, 2015. 141(4): p. 671–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wall M, et al. , Translational control of c-MYC by rapamycin promotes terminal myeloid differentiation. Blood, 2008. 112(6): p. 2305–17. [DOI] [PubMed] [Google Scholar]
- 68.Keeney JT, et al. , Cell cycle proteins in brain in mild cognitive impairment: insights into progression to Alzheimer disease. Neurotox Res, 2012. 22(3): p. 220–30. [DOI] [PubMed] [Google Scholar]
- 69.Bonda DJ, et al. , Pathological implications of cell cycle re-entry in Alzheimer disease. Expert Rev Mol Med, 2010. 12: p. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khurana V, et al. , TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol, 2006. 16(3): p. 230–41. [DOI] [PubMed] [Google Scholar]
- 71.Absalon S, et al. , MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J Neurosci, 2013. 33(37): p. 14645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HG, et al. , Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochem Int, 2009. 54(2): p. 84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blalock EM, et al. , Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A, 2004. 101(7): p. 2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sonoda Y, et al. , Accumulation of tumor-suppressor PTEN in Alzheimer neurofibrillary tangles. Neurosci Lett, 2010. 471(1): p. 20–4. [DOI] [PubMed] [Google Scholar]
- 75.Wilson C, et al. , The p53 homologue p73 accumulates in the nucleus and localizes to neurites and neurofibrillary tangles in Alzheimer disease brain. Neuropathol Appl Neurobiol, 2004. 30(1): p. 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arendt T, et al. , Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. Neuroreport, 1996. 7(18): p. 3047–9. [DOI] [PubMed] [Google Scholar]
- 77.Lovell MA, et al. , Wilms’ tumor suppressor (WT1) is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer’s disease. Brain Res, 2003. 983(1–2): p. 84–96. [DOI] [PubMed] [Google Scholar]
- 78.Munoz U, et al. , Enhanced proteasome-dependent degradation of the CDK inhibitor p27(kip1) in immortalized lymphocytes from Alzheimer’s dementia patients. Neurobiol Aging, 2008. 29(10): p. 1474–84. [DOI] [PubMed] [Google Scholar]
- 79.Ogawa O, et al. , Increased p27, an essential component of cell cycle control, in Alzheimer’s disease. Aging Cell, 2003. 2(2): p. 105–10. [DOI] [PubMed] [Google Scholar]
- 80.Kim H, et al. , Overexpression of Cell Cycle Proteins of Peripheral Lymphocytes in Patients with Alzheimer’s Disease. Psychiatry Investig, 2016. 13(1): p. 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song J, et al. , G1/S checkpoint proteins in peripheral blood lymphocytes are potentially diagnostic biomarkers for Alzheimer’s disease. Neurosci Lett, 2012. 526(2): p. 144–9. [DOI] [PubMed] [Google Scholar]
- 82.Tan M, et al. , Combination of p53(ser15) and p21/p21(thr145) in peripheral blood lymphocytes as potential Alzheimer’s disease biomarkers. Neurosci Lett, 2012. 516(2): p. 226–31. [DOI] [PubMed] [Google Scholar]
- 83.Hayflick L, Mortality and immortality at the cellular level. A review. Biochemistry (Mosc), 1997. 62(11): p. 1180–90. [PubMed] [Google Scholar]
- 84.Bryan TM and Cech TR, Telomerase and the maintenance of chromosome ends. Curr Opin Cell Biol, 1999. 11(3): p. 318–24. [DOI] [PubMed] [Google Scholar]
- 85.Counter CM, et al. , Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J, 1992. 11(5): p. 1921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Counter CM, et al. , Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci U S A, 1998. 95(25): p. 14723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akincilar SC, Unal B, and Tergaonkar V, Reactivation of telomerase in cancer. Cell Mol Life Sci, 2016. 73(8): p. 1659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forero DA, et al. , Meta-analysis of Telomere Length in Alzheimer’s Disease. J Gerontol A Biol Sci Med Sci, 2016. 71(8): p. 1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhan Y, et al. , Telomere Length Shortening and Alzheimer Disease--A Mendelian Randomization Study. JAMA Neurol, 2015. 72(10): p. 1202–3. [DOI] [PubMed] [Google Scholar]
- 90.Horgusluoglu E, et al. , Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am J Med Genet B Neuropsychiatr Genet, 2017. 174(1): p. 93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mamdani F, et al. , Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl Psychiatry, 2015. 5: p. e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolkowitz OM, et al. , PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res, 2015. 232(1): p. 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yun S, et al. , Stress-Induced Anxiety- and Depressive-Like Phenotype Associated with Transient Reduction in Neurogenesis in Adult Nestin-CreERT2/Diphtheria Toxin Fragment A Transgenic Mice. PLoS One, 2016. 11(1): p. e0147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez-Giraldo Y, et al. , Neuroprotective effects of the catalytic subunit of telomerase: A potential therapeutic target in the central nervous system. Ageing Res Rev, 2016. 28: p. 37–45. [DOI] [PubMed] [Google Scholar]
- 95.Folkman J, Tumor angiogenesis: therapeutic implications. N Engl J Med, 1971. 285(21): p. 1182–6. [DOI] [PubMed] [Google Scholar]
- 96.Otrock ZK, et al. , Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis, 2007. 39(2): p. 212–20. [DOI] [PubMed] [Google Scholar]
- 97.Wierenga CE, Hays CC, and Zlatar ZZ, Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis, 2014. 42 Suppl 4: p. S411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tosun D, et al. , Discriminative Power of Arterial Spin Labeling Magnetic Resonance Imaging and 18F-Fluorodeoxyglucose Positron Emission Tomography Changes for Amyloid-beta-Positive Subjects in the Alzheimer’s Disease Continuum. Neurodegener Dis, 2016. 16(1–2): p. 87–94. [DOI] [PubMed] [Google Scholar]
- 99.Okonkwo OC, et al. , Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cereb Cortex, 2014. 24(4): p. 978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Franceschi M, et al. , Correlations between cognitive impairment, middle cerebral artery flow velocity and cortical glucose metabolism in the early phase of Alzheimer’s disease. Dementia, 1995. 6(1): p. 32–8. [DOI] [PubMed] [Google Scholar]
- 101.Tohgi H, et al. , Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology, 1998. 40(3): p. 131–7. [DOI] [PubMed] [Google Scholar]
- 102.Schneider JA, et al. , Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology, 2004. 62(7): p. 1148–55. [DOI] [PubMed] [Google Scholar]
- 103.Schneider JA, et al. , Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol, 2007. 62(1): p. 59–66. [DOI] [PubMed] [Google Scholar]
- 104.Schneider JA, et al. , The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol, 2009. 66(2): p. 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thal DR, et al. , Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol, 2008. 115(6): p. 599–609. [DOI] [PubMed] [Google Scholar]
- 106.Jeynes B and Provias J, The possible role of capillary cerebral amyloid angiopathy in Alzheimer lesion development: a regional comparison. Acta Neuropathol, 2006. 112(4): p. 417–27. [DOI] [PubMed] [Google Scholar]
- 107.Ashok BS, Ajith TA, and Sivanesan S, Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin Exp Pharmacol Physiol, 2017. 44(3): p. 327–334. [DOI] [PubMed] [Google Scholar]
- 108.Salminen A, Kauppinen A, and Kaarniranta K, Hypoxia/ischemia activate processing of Amyloid Precursor Protein: impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J Neurochem, 2017. 140(4): p. 536–549. [DOI] [PubMed] [Google Scholar]
- 109.Provias J and Jeynes B, Neurofibrillary tangles and senile plaques in Alzheimer’s brains are associated with reduced capillary expression of vascular endothelial growth factor and endothelial nitric oxide synthase. Curr Neurovasc Res, 2008. 5(3): p. 199–205. [DOI] [PubMed] [Google Scholar]
- 110.Castellano E, et al. , RAS signalling through PI3-Kinase controls cell migration via modulation of Reelin expression. Nat Commun, 2016. 7: p. 11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Angelucci C, et al. , Epithelial-stromal interactions in human breast cancer: effects on adhesion, plasma membrane fluidity and migration speed and directness. PLoS One, 2012. 7(12): p. e50804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramanan VK, et al. , Genome-wide pathway analysis of memory impairment in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort implicates gene candidates, canonical pathways, and networks. Brain Imaging Behav, 2012. 6(4): p. 634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu G, et al. , Cell adhesion molecules contribute to Alzheimer’s disease: multiple pathway analyses of two genome-wide association studies. J Neurochem, 2012. 120(1): p. 190–8. [DOI] [PubMed] [Google Scholar]
- 114.Leshchyns’ka I and Sytnyk V, Synaptic Cell Adhesion Molecules in Alzheimer’s Disease. Neural Plast, 2016. 2016: p. 6427537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chin J, et al. , Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci, 2007. 27(11): p. 2727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kocherhans S, et al. , Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer’s disease mice. J Neurosci, 2010. 30(27): p. 9228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deutsch SI, Rosse RB, and Deutsch LH, Faulty regulation of tau phosphorylation by the reelin signal transduction pathway is a potential mechanism of pathogenesis and therapeutic target in Alzheimer’s disease. Eur Neuropsychopharmacol, 2006. 16(8): p. 547–51. [DOI] [PubMed] [Google Scholar]
- 118.Knuesel I, et al. , Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol Aging, 2009. 30(5): p. 697–716. [DOI] [PubMed] [Google Scholar]
- 119.Cuchillo-Ibanez I, et al. , The beta-amyloid peptide compromises Reelin signaling in Alzheimer’s disease. Sci Rep, 2016. 6: p. 31646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Konishi H, et al. , Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc Natl Acad Sci U S A, 2011. 108(43): p. 17773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Desmaze C, et al. , Telomere-driven genomic instability in cancer cells. Cancer Lett, 2003. 194(2): p. 173–82. [DOI] [PubMed] [Google Scholar]
- 122.Forsberg LA, et al. , Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet, 2014. 46(6): p. 624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Noveski P, et al. , Loss of Y Chromosome in Peripheral Blood of Colorectal and Prostate Cancer Patients. PLoS One, 2016. 11(1): p. e0146264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wright DJ, et al. , Genetic variants associated with mosaic Y chromosome loss highlight cell cycle genes and overlap with cancer susceptibility. Nat Genet, 2017. 49(5): p. 674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Minner S, et al. , Y chromosome loss is a frequent early event in urothelial bladder cancer. Pathology, 2010. 42(4): p. 356–9. [DOI] [PubMed] [Google Scholar]
- 126.Yurov YB, Vorsanova SG, and Iourov IY, The DNA replication stress hypothesis of Alzheimer’s disease. ScientificWorldJournal, 2011. 11: p. 2602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arendt T, et al. , Selective cell death of hyperploid neurons in Alzheimer’s disease. Am J Pathol, 2010. 177(1): p. 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arendt T, Cell cycle activation and aneuploid neurons in Alzheimer’s disease. Mol Neurobiol, 2012. 46(1): p. 125–35. [DOI] [PubMed] [Google Scholar]
- 129.Yang Y, Geldmacher DS, and Herrup K, DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci, 2001. 21(8): p. 2661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mosch B, et al. , Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci, 2007. 27(26): p. 6859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van den Bos H, et al. , Single-cell whole genome sequencing reveals no evidence for common aneuploidy in normal and Alzheimer’s disease neurons. Genome Biol, 2016. 17(1): p. 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spremo-Potparevic B, et al. , Alterations of the X Chromosome in Lymphocytes of Alzheimer’s Disease Patients. Curr Alzheimer Res, 2015. 12(10): p. 990–6. [DOI] [PubMed] [Google Scholar]
- 133.Dumanski JP, et al. , Mosaic Loss of Chromosome Y in Blood Is Associated with Alzheimer Disease. Am J Hum Genet, 2016. 98(6): p. 1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spremo-Potparevic B, et al. , Premature centromere division of the X chromosome in neurons in Alzheimer’s disease. J Neurochem, 2008. 106(5): p. 2218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Migliore L, et al. , Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis, 2011. 26(1): p. 85–92. [DOI] [PubMed] [Google Scholar]
- 136.Liu M, et al. , Telomere Shortening in Alzheimer’s Disease Patients. Ann Clin Lab Sci, 2016. 46(3): p. 260–5. [PubMed] [Google Scholar]
- 137.Mathur S, et al. , Three-dimensional quantitative imaging of telomeres in buccal cells identifies mild, moderate, and severe Alzheimer’s disease patients. J Alzheimers Dis, 2014. 39(1): p. 35–48. [DOI] [PubMed] [Google Scholar]
- 138.Lukens JN, et al. , Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimers Dement, 2009. 5(6): p. 463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thomas P, NJ OC, and Fenech M, Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev, 2008. 129(4): p. 183–90. [DOI] [PubMed] [Google Scholar]
- 140.Guan JZ, et al. , Analysis of telomere length and subtelomeric methylation of circulating leukocytes in women with Alzheimer’s disease. Aging Clin Exp Res, 2013. 25(1): p. 17–23. [DOI] [PubMed] [Google Scholar]
- 141.Roberts RO, et al. , Short and long telomeres increase risk of amnestic mild cognitive impairment. Mech Ageing Dev, 2014. 141–142: p. 64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Singh K and Singh K, Carcinogenesis and diabetic wound healing: evidences of parallelism. Curr Diabetes Rev, 2015. 11(1): p. 32–45. [DOI] [PubMed] [Google Scholar]
- 143.Goswami KK, et al. , Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol, 2017. 316: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 144.van de Nieuwenhof HP, et al. , Specific intraepithelial localization of mast cells in differentiated vulvar intraepithelial neoplasia and its possible contribution to vulvar squamous cell carcinoma development. Histopathology, 2010. 57(3): p. 351–62. [DOI] [PubMed] [Google Scholar]
- 145.Tang X, et al. , Preoperative High Neutrophil-to-Lymphocyte Ratio Is Associated with High-grade Bladder Cancer. Anticancer Res, 2017. 37(8): p. 4659–4663. [DOI] [PubMed] [Google Scholar]
- 146.Lieto E, et al. , Preoperative Neutrophil to Lymphocyte Ratio and Lymphocyte to Monocyte Ratio are Prognostic Factors in Gastric Cancers Undergoing Surgery. J Gastrointest Surg, 2017. [DOI] [PubMed] [Google Scholar]
- 147.DeNardo DG, Andreu P, and Coussens LM, Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev, 2010. 29(2): p. 309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McGeer PL and McGeer EG, Glial cell reactions in neurodegenerative diseases: pathophysiology and therapeutic interventions. Alzheimer Dis Assoc Disord, 1998. 12 Suppl 2: p. S1–6. [PubMed] [Google Scholar]
- 149.McGeer PL and McGeer EG, The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev, 1995. 21(2): p. 195–218. [DOI] [PubMed] [Google Scholar]
- 150.Serpente M, et al. , Innate immune system and inflammation in Alzheimer’s disease: from pathogenesis to treatment. Neuroimmunomodulation, 2014. 21(2–3): p. 79–87. [DOI] [PubMed] [Google Scholar]
- 151.Guillot-Sestier MV and Town T, Innate immunity in Alzheimer’s disease: a complex affair. CNS Neurol Disord Drug Targets, 2013. 12(5): p. 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heneka MT, Golenbock DT, and Latz E, Innate immunity in Alzheimer’s disease. Nat Immunol, 2015. 16(3): p. 229–36. [DOI] [PubMed] [Google Scholar]
- 153.De Felice FG and Ferreira ST, Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes, 2014. 63(7): p. 2262–72. [DOI] [PubMed] [Google Scholar]
- 154.De la Fuente M and Miquel J, An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des, 2009. 15(26): p. 3003–26. [DOI] [PubMed] [Google Scholar]
- 155.Warburg O, The Metabolism of Carcinoma Cells. Journal of Cancer Research, 1925. 9(1): p. 148–163. [Google Scholar]
- 156.Xu XD, et al. , Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat, 2015. 38(3): p. 117–22. [DOI] [PubMed] [Google Scholar]
- 157.Pavlides S, et al. , Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: a transcriptional informatics analysis with validation. Cell Cycle, 2010. 9(11): p. 2201–19. [DOI] [PubMed] [Google Scholar]
- 158.Pavlides S, et al. , Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer’s disease, and “Neuron-Glia Metabolic Coupling”. Aging (Albany NY), 2010. 2(4): p. 185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pavlides S, et al. , The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle, 2009. 8(23): p. 3984–4001. [DOI] [PubMed] [Google Scholar]
- 160.de Leon MJ, et al. , Regional correlation of PET and CT in senile dementia of the Alzheimer type. AJNR Am J Neuroradiol, 1983. 4(3): p. 553–6. [PMC free article] [PubMed] [Google Scholar]
- 161.de la Monte SM and Wands JR, Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol, 2008. 2(6): p. 1101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Steen E, et al. , Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis, 2005. 7(1): p. 63–80. [DOI] [PubMed] [Google Scholar]
- 163.de Leon MJ, et al. , Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am J Neuroradiol, 1983. 4(3): p. 568–71. [PMC free article] [PubMed] [Google Scholar]
- 164.Cutler NR, Cerebral metabolism as measured with positron emission tomography (PET) and [18F] 2-deoxy-D-glucose: healthy aging, Alzheimer’s disease and Down syndrome. Prog Neuropsychopharmacol Biol Psychiatry, 1986. 10(3–5): p. 309–21. [DOI] [PubMed] [Google Scholar]
- 165.McGeer PL, et al. , Positron emission tomography and the possible origins of cytopathology in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry, 1986. 10(3–5): p. 501–18. [DOI] [PubMed] [Google Scholar]
- 166.Tamminga CA, et al. , Alzheimer’s disease: low cerebral somatostatin levels correlate with impaired cognitive function and cortical metabolism. Neurology, 1987. 37(1): p. 161–5. [DOI] [PubMed] [Google Scholar]
- 167.Mann UM, et al. , Heterogeneity in Alzheimer’s disease: progression rate segregated by distinct neuropsychological and cerebral metabolic profiles. J Neurol Neurosurg Psychiatry, 1992. 55(10): p. 956–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Silverman DH, et al. , Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA, 2001. 286(17): p. 2120–7. [DOI] [PubMed] [Google Scholar]
- 169.Alexander GE, et al. , Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am J Psychiatry, 2002. 159(5): p. 738–45. [DOI] [PubMed] [Google Scholar]
- 170.Ding F, et al. , Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One, 2013. 8(11): p. e79977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Henderson ST, Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics, 2008. 5(3): p. 470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hertz L, Chen Y, and Waagepetersen HS, Effects of ketone bodies in Alzheimer’s disease in relation to neural hypometabolism, beta-amyloid toxicity, and astrocyte function. J Neurochem, 2015. 134(1): p. 7–20. [DOI] [PubMed] [Google Scholar]
- 173.VanItallie TB, Biomarkers, ketone bodies, and the prevention of Alzheimer’s disease. Metabolism, 2015. 64(3 Suppl 1): p. S51–7. [DOI] [PubMed] [Google Scholar]
- 174.Cunnane SC, et al. , Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer’s Disease. Front Mol Neurosci, 2016. 9: p. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Small SA and Duff K, Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron, 2008. 60(4): p. 534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Osborn JL and Greer SF, Metastatic melanoma cells evade immune detection by silencing STAT1. Int J Mol Sci, 2015. 16(2): p. 4343–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pandey JP, Immunoglobulin GM Genes, Cytomegalovirus Immunoevasion, and the Risk of Glioma, Neuroblastoma, and Breast Cancer. Front Oncol, 2014. 4: p. 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kawasaki BT and Farrar WL, Cancer stem cells, CD200 and immunoevasion. Trends Immunol, 2008. 29(10): p. 464–8. [DOI] [PubMed] [Google Scholar]
- 179.McManus RM, Mills KH, and Lynch MA, T Cells-Protective or Pathogenic in Alzheimer’s Disease? J Neuroimmune Pharmacol, 2015. 10(4): p. 547–60. [DOI] [PubMed] [Google Scholar]
- 180.McManus RM and Heneka MT, Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther, 2017. 9(1): p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mietelska-Porowska A and Wojda U, T Lymphocytes and Inflammatory Mediators in the Interplay between Brain and Blood in Alzheimer’s Disease: Potential Pools of New Biomarkers. J Immunol Res, 2017. 2017: p. 4626540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.International Genomics of Alzheimer’s Disease, C., Convergent genetic and expression data implicate immunity in Alzheimer’s disease. Alzheimers Dement, 2015. 11(6): p. 658–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ramanan VK, et al. , GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer’s disease implicates microglial activation gene IL1RAP. Brain, 2015. 138(Pt 10): p. 3076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Askmyr M, et al. , Selective killing of candidate AML stem cells by antibody targeting of IL1RAP. Blood, 2013. 121(18): p. 3709–13. [DOI] [PubMed] [Google Scholar]
- 185.Driver JA, Zhou XZ, and Lu KP, Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer’s disease. Biochim Biophys Acta, 2015. 1850(10): p. 2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Driver JA, Zhou XZ, and Lu KP, Regulation of protein conformation by Pin1 offers novel disease mechanisms and therapeutic approaches in Alzheimer’s disease. Discov Med, 2014. 17(92): p. 93–9. [PMC free article] [PubMed] [Google Scholar]
- 187.Yarchoan M, et al. , Association of Cancer History with Alzheimer’s Disease Dementia and Neuropathology. J Alzheimers Dis, 2017. 56(2): p. 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lu KP, et al. , Potential of the Antibody Against cis-Phosphorylated Tau in the Early Diagnosis, Treatment, and Prevention of Alzheimer Disease and Brain Injury. JAMA Neurol, 2016. 73(11): p. 1356–1362. [DOI] [PubMed] [Google Scholar]
- 189.Lambert JC, et al. , Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet, 2013. 45(12): p. 1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Naj AC, et al. , Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet, 2011. 43(5): p. 436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lamba JK, et al. , Coding polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML: a pilot study. Leukemia, 2009. 23(2): p. 402–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mortland L, et al. , Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clin Cancer Res, 2013. 19(6): p. 1620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Malik M, et al. , Genetics of CD33 in Alzheimer’s disease and acute myeloid leukemia. Hum Mol Genet, 2015. 24(12): p. 3557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peng W, et al. , PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res, 2012. 72(20): p. 5209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Baruch K, et al. , PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med, 2016. 22(2): p. 135–7. [DOI] [PubMed] [Google Scholar]
- 196.Sauer CM, et al. , Effect of long term aspirin use on the incidence of prostate cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol, 2018. 132: p. 66–75. [DOI] [PubMed] [Google Scholar]
- 197.Wang J, et al. , Anti-inflammatory drugs and risk of Alzheimer’s disease: an updated systematic review and meta-analysis. J Alzheimers Dis, 2015. 44(2): p. 385–96. [DOI] [PubMed] [Google Scholar]
- 198.Liby KT and Sporn MB, Rexinoids for prevention and treatment of cancer: opportunities and challenges. Curr Top Med Chem, 2016. [PubMed] [Google Scholar]
- 199.Cramer PE, et al. , ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science, 2012. 335(6075): p. 1503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cummings JL, et al. , Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer’s disease. Alzheimers Res Ther, 2016. 8: p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]