Abstract
Children spend over 6 hours a day in schools and have higher asthma morbidity from school environmental exposures. The present study aims to determine indoor and outdoor possible sources affecting indoor PM2.5 in classrooms. Weeklong indoor PM2.5 samples were collected from 32 inner-city schools from a Northeastern U.S. community during three seasons (fall, winter and spring) during the years 2009 to 2013. Concurrently, daily outdoor PM2.5 samples were taken at a central monitoring site located at a median distance of 4,974 m (range 1,065–11,592 m) from the schools. Classroom indoor concentrations of PM2.5 (an average of 5.2 μg/m3) were lower than outdoors (an average of 6.5 μg/m3), and these averages were in the lower range compared to the findings in other schools’ studies. The USEPA PMF model was applied to the PM2.5 components measured simultaneously from classroom indoor and outdoor to estimate the source apportionment. The major sources (contributions) identified across all seasons of indoor PM2.5 were secondary pollution (41%) and motor vehicles (17%), followed by Calcium (Ca)-rich particles (12%), biomass burning (15%), soil dust (6%), and marine aerosols (4%). Likewise, the major sources of outdoor PM2.5 across all seasons were secondary pollution (41%) and motor vehicles (26%), followed by biomass burning (17%), soil dust (7%), road dust (3%), and marine aerosols (1%). Secondary pollution was the greatest contributor to indoor and outdoor PM2.5 over all three seasons, with the highest contribution during spring with 53% to indoor PM2.5 and 45% to outdoor PM2.5. Lower contributions of this source during fall and winter are most likely attributed to less infiltration indoors. In contrast, the indoor contribution of motor vehicles source was highest in the fall (29%) and winter (25%), which was presumably categorized by a local source. From the relationship between indoor-to-outdoor sulfur ratios and each source contribution, we also estimated the local and regional influence on indoor PM2.5 concentration. Overall, the observed differences to indoor PM2.5 are related to seasonality, and the distinct characteristics and behavior of each classroom/school.
Keywords: Source apportionment, Sulfur ratio, Inner-city environment, Classrooms, Indoor air quality, Schools
1. Introduction
Air pollution, including particulate matter with aerodynamic diameter <2.5 μm (PM2.5) has been associated with asthma exacerbation and cognitive impairment (Sunyer et al. 2015; Tétreault et al. 2016) in children but the PM2.5 sources and components most responsible for these health effects are imperfectly understood. PM2.5 sources and components vary by the microenvironments in which children live, learn and play. The school classroom, where children spend a significant portion of their day, has been identified as one of the most important microenvironments where PM2.5 exposure with relevance to health may occur. School proximity to local traffic (Forns et al. 2016), and traffic encountered on the walk to school (Alvarez-Pedrerol et al. 2017), have been implicated in studies of pollution and poorer neurocognitive testing in school children. However, apart from studies on school proximity to traffic (Forns et al. 2016; Green Rochelle et al. 2004), limited data are available on the sources or components of PM2.5 present in the classroom environment that may account for adverse health outcomes like asthma morbidity or neurocognition (Gaffin et al. 2017). One Barcelona study implicated indoor school PM from traffic, but not from other sources, as an influence on child neurocognition (Basagaña et al. 2016).
Key environmental and behavioral factors such as occupancy, ventilation rates, building characteristics and seasonality may influence the school indoor PM2.5 concentration and composition, including the amount of traffic PM2.5 that can come indoors. School buildings characteristics such as age, building materials, and ventilation; and factors such as occupant density per classroom volume, affect the transport and mixing of pollutants, which pose a risk to the health of the occupants, often affecting their performance in school (MacNaughton et al. 2017). Koo, et al. (Koo et al. 1997) found that student learning decreased in natural ventilation as compared to air-conditioned classrooms. Building materials can act as reservoirs of irritants that can contribute to the gas-to-particle processes, affecting the indoor air quality of the classrooms (Smedje and Norbäck 2008). Occupant activities can increase the concentration of pollutants by resuspension of previously deposited particles and by introducing new particles through clothing and shoes (Stranger et al. 2008). Factors that vary throughout the year such as temperature gradients between indoor and outdoor, relative humidity, wind direction and speed have an effect on the penetration rate of outdoor pollutants to indoor settings (Chatzidiakou et al. 2012). Yearlong studies examining seasonal variability of PM levels in schools are scarce and the limited investigations have shown differences in PM concentrations between summer and winter where the temperature difference between indoor and outdoor might affect the infiltration of the pollutants (Fromme et al. 2007; Goyal and Khare 2009).
Inner-city areas tend to have a higher level of asthma morbidity (Gergen and Togias 2015). In particular, higher rates of hospitalizations and asthma morbidity were found in these impoverished areas compared to other urban neighborhoods (Weiss et al. 1992). People living and working in these environments experience high exposure to pollutants such as elemental carbon (EC) given their proximity to highways (Spira-Cohen et al. 2011).
The School Inner City Asthma Study (SICAS) investigates the effect of classroom-based environmental exposures on students with asthma in the northeast United States. In a recent SICAS publication, using the indoor-to-outdoor sulfur ratio as a tracer we showed that the infiltration of outdoor PM2.5 varied by school and that there were indoor contributions to indoor PM2.5 levels (Gaffin et al. 2017). As a part of the SICAS study, using measurements of PM2.5 mass, trace elements and black carbon (BC), we provide detailed characterization of indoor and outdoor sources affecting indoor PM2.5. Our aim is to identify indoor and outdoor PM2.5 sources affecting indoor exposure to children attending inner-city schools to help close the gap in the absence of information about children’s personal exposures in these microenvironments.
2. Materials and Methods
2.1. Studied schools and central outdoor site
Indoor measurements were conducted in classrooms of inner-city schools during occupied and non-occupied periods during the school week but not on weekends. One or two samples per classroom were collected in different seasons (1 per season: either fall, winter, or spring) during the academic years 2009–2013. In total, 139 classrooms across 32 schools were studied. Details of school characteristics and locations are reported in Gaffin et al. (2017). The study was conducted in schools from a single urban metropolitan location. Briefly, the schools in this study varied widely in age (built between 1899 and 2002, with many built before the 1970’s) and in the type of ventilation. Often the buildings within the school were of varying ages with varying types of ventilation. None of the schools had gas cooking in the building and all had recently serviced furnaces. Of the 32 schools studied in this source apportionment analysis, 4 had central HVAC systems, 15 had radiant heat “natural ventilation”, and the remainder had either classroom-based unit vents or a mix of ventilation types. Most of the classrooms (96%) were in the 2nd and ≥ 3rd levels of the building, and the flooring consisted of linoleum/tile (68%) and rugs (80%). For outdoor measurements, a central site was located on the roof of a 6-story building, which is not adjacent to local highways. The median distance between the central site and schools was 4,974 m, ranging from 1,065 to 11,592 m.
2.2. Measurements and Analyses
Indoor samplers were used to collect weeklong indoor PM2.5 sample on Teflon filters at a flow rate of 1.8 L/min (Demokritou et al. 2001). A total of 205 indoor samples were collected during the study period. Concurrently, daily outdoor PM2.5 concentrations were also measured at the central monitoring site. Indoor and outdoor PM2.5 samples collected on Teflon filters including blanks were weighed with an electronic microbalance (MT-5 Mettler Toledo, Columbus, OH) prior to measurement the filters were equilibrated for a period of 48 hours in a room of controlled temperature (22±1.5 °C) and relative humidity (40±5%). Following the weighing, the indoor filters were measured for BC concentrations using a Smokestain Reflectometer (Model EEL M43D, Diffusion Systems Ltd., United Kingdom). X-Ray Fluorescence (XRF) spectroscopy (model Epsilon 5, PANalytical, The Netherlands) was used to determine the elemental composition of indoor and outdoor PM2.5 samples. A total of 48 elements were measured and quality Assurance/Quality Control of XRF analysis is described in Kang et al (2014). At the central site, hourly elemental (EC) and organic carbon (OC) were measured on pre-fired quartz fiber filters based on the thermal optical transmittance (TOT) technique using a semi-continuous OCEC field analyzer (Model 3, Sunset Laboratory Inc., Tigard, OR). Continuous BC concentrations were measured using a single (λ=880 nm) channel aethalometer (model AE-16, Magee Scientific, Berkeley, CA). Hourly and daily data from the central outdoor site were averaged and matched over indoor sampling periods each classroom. All analyses were conducted at the Harvard T.H Chan School of Public Health.
The indoor-to-outdoor sulfur concentration ratios were estimated for every sampling period and classroom. This ratio has been widely used in previous studies to approximate the infiltration fraction of PM2.5 from outdoors (Sarnat et al. 2002; Wallace and Williams 2005). Assuming negligible indoor sources of sulfur, the indoor-to-outdoor sulfur ratios were used as an infiltration factor for each classroom.
2.3. Source apportionment
The US Environmental Protection Agency Positive Matrix Factorization (USEPA PMF 5.0) model was used to determine the possible sources of indoor and outdoor PM2.5. The PMF model has been described in detail elsewhere (Paatero and Tapper 1994). The uncertainty was calculated using the equation below:
where sij is the analytical uncertainty, and DLij is the method detection limit (Polissar et al. 1998; Reff et al. 2007).
Three validation methods were used to assess the stability and variability of the results: (1) bootstrap (BS), which helps to identify the observations that could influence the solution; (2) displacement (DISP), which helps to understand the final solution and its sensitivity to small changes; and (3) BS-DISP analysis, which is a hybrid approach that combines BS and DISP methods. The results from these validation methods are included in Appendices A and B. The goodness of fit for the PMF solutions was examined using the percent Mean Relative Errors (MRE) (Masri et al. 2015):
where, xi is the measured concentration and is the predicted concentration of species i.
Elements with more than 80% of their concentration values below the limit of detection were excluded from further analysis. Exception to this exclusion criteria for indoor analysis were Al which is a tracer of soil; Cu, and Pb, which are tracers of vehicle sources; and V and Ni, which are tracers of oil combustion. The exception for outdoor analysis was Cu. Consequently, 15 elements of 48 elements analyzed were included for further analysis. The PMF model categorizes the species based on their signal-to-noise ratio. Species categorized as ‘bad’ had a signal-to-noise ratio <0.2, ‘weak’ species had 0.2≤ signal-to-noise ratio <2, and ‘strong’ species had a signal-to-noise ratio ≥2. Species categorized as ‘bad’ were excluded from the analysis, ‘strong’ species were included in the analysis and remained unchanged, and ‘weak’ species were also included in the analysis but were down-weighted by tripling their uncertainty values (Paatero and Hopke 2003). PMF was run multiple times using 4 to 8 factors for both indoor and outdoor PM2.5. The results chosen were the ones more interpretable among the different solutions. Sources were assigned according to the abundance of key tracer elements.
3. Results and Discussion
3.1. PM2.5 mass concentrations and chemical compositions
Table 1 represents the summary of weeklong concentrations of indoor and outdoor PM2.5 mass and components. Outdoor concentrations from the central site were averaged over indoor sampling periods at each classroom. Sulfate was calculated stochiometrically from sulfur by multiplying by three, assuming that all sulfur is in the form of sulfate. On average, outdoor concentrations of PM2.5 (6.5 μg/m3) were higher than indoors (5.2 μg/m3). As shown in Table 2, these averages are in the lower range compared to other studies performed in other cities of US (Bozlaker et al. 2017; Hochstetler et al. 2011; Keeler et al. 2002), Europe (Almeida et al. 2011; Amato et al. 2014; Borgini et al. 2011; Canha et al. 2014; Fromme et al. 2008; Janssen et al. 2001; Jovanović et al. 2014; Stranger M et al. 2008; Wichmann et al. 2010; Zwoździak et al. 2013), China (Hou et al. 2015; Xu et al. 2015), and India (Chithra and Nagendra 2012; Habil et al. 2013). In the other US cities, studies of Texas (Bozlaker et al. 2017) and Michigan (Keeler et al. 2002) schools reported that outdoor concentrations were 13.4 and 11.6–20.6 μg/m3, respectively. In Europe, the schools in Spain (29 μg/m3) (Amato et al. 2014), Germany (17.0 μg/m3) (Fromme et al. 2008), Belgium (63.0 μg/m3) (Stranger M et al. 2008), Sweden (9.7 μg/m3) (Wichmann et al. 2010), Italy (77.9 μg/m3) (Borgini et al. 2011) and the Netherlands (24.8 μg/m3) (Janssen et al. 2001) also had on average higher outdoor concentrations than our study. In our study, outdoor daily PM2.5 concentrations did not exceed the daily NAAQS PM2.5 level of 35 μg/m3. Among the 15 elements, Sulfate as S was the most abundant, followed by Na, Cl, Ca, Fe and Si. These elements were also abundant for both indoor and outdoor concentrations. The mean indoor-to-outdoor (I/O) PM2.5 across the schools’ studied was 0.8. The ratio was lower than 1.3 in Belgium (Stranger M et al. 2008) and 0.98 in Serbia (Jovanović et al. 2014) under natural ventilation. Also, these were lower than the schools studies in India where the I/O PM2.5 was 1.4 in the study and had natural ventilation (Chithra and Nagendra 2012). In schools in China, the results were comparable to the ones in our study where the I/O PM2.5 was 0.8 and also had natural ventilation (Xu et al. 2015). For the studies in the other US cities, the results were different: in Texas, the I/O PM2.5 was lower with 0.2, but this school had a mechanical ventilation system; and in Michigan the I/O PM2.5 ranged from 0.5 to 2.8 (Bozlaker et al. 2017; Keeler et al. 2002).
Table 1.
Summary of weekly indoor and outdoor concentrations of PM2.5 mass and its components
| Pollutant | Indoor |
Outdoor |
||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Units: μg/m3 | ||||||
| PM2.5 Mass | 205 | 5.2 | 2.3 | 205 | 6.5 | 2.7 |
| BC | 205 | 0.3 | 0.2 | 205 | 0.6 | 0.3 |
| OC | NA | NA | NA | 195 | 2.9 | 1.2 |
| Sulfate | 205 | 1.1 | 0.5 | 205 | 1.5 | 0.6 |
| Units: ng/m3 | ||||||
| Al | 205 | 6.0 | 11.0 | 205 | 23.0 | 19.0 |
| Ca | 205 | 40.0 | 23.0 | 205 | 21.0 | 7.0 |
| Cl | 205 | 52.0 | 95.0 | 205 | 33.0 | 56.0 |
| Cu | 205 | 3.0 | 2.0 | 205 | 2.0 | 0.9 |
| Fe | 205 | 31.0 | 20.0 | 205 | 46.0 | 13.0 |
| K | 205 | 32.0 | 14.0 | 205 | 38.0 | 16.0 |
| Mg | ND | ND | ND | 205 | 22.0 | 17.0 |
| Na | 205 | 92.0 | 69.0 | 205 | 128.0 | 60.0 |
| Ni | 205 | 0.7 | 0.7 | ND | ND | ND |
| Pb | 205 | 3.0 | 3.0 | 205 | 3.0 | 1.0 |
| Si | 205 | 30.0 | 24.0 | 205 | 44.0 | 29.0 |
| Ti | 205 | 2.0 | 2.0 | 205 | 2.0 | 1.0 |
| V | 205 | 0.7 | 0.9 | ND | ND | ND |
| Zn | 205 | 6.0 | 4.0 | 205 | 8.0 | 4.0 |
NA: not available
ND: below lower detection limit
Table 2.
Indoor and outdoor PM2.5 concentrations and its possible sources from various schools
| Reference | Location | # Schools | Concentration range (μg/m3) |
Ventilation system | Findings | |
|---|---|---|---|---|---|---|
| Indoor | Outdoor | |||||
| This study | Northeast, USA | 32 | 0.8–16.9 | 3.3–16.6 | 17 natural, 3 central HVAC, 12 mixed | I/O PM2.5 = 0.8; Sources: Ca-rich material, road dust, sea salt, vehicles, biomass burning, regional. |
| Hochstetler et al. (2011) | Ohio, USA | 4 | 6.9–28.3 | 3.8–27.6 | 3 schools open windows | I/O PM2.5 = 0.5–2.8 |
| Keeler et al. (2002) | Michigan, USA | 2 | 6.5–16.4 | 11.6–20.6 | Opening windows, no air conditioning | I/O PM2.5 = 0.5–1.4 |
| Bozlaker et al. (2017) | Texas, USA | 1 | 2.3–4.1 | 13.4ⱡ | Mechanical | I/O PM2.5 = 0.2; Sources: soil & road dust, vehicular emissions, petroleum refining, oil combustion, coal combustion, vegetative burning, sea salt, ca-rich material, incineration, steel plant |
| Amato et al. (2014) | Barcelona, Spain | 39 | 7–105 | 1–192 | Natural | Sources: organic/ textile/ chalk, heavy oil, metallurgy, sulfate & organics, nitrate, traffic, road dust, mineral, sea salt |
| Jovanović et al. (2014) | Serbia | 1 | 26.9–63.9 | -- | Natural | I/O PM2.5 = 1.0 |
| Fromme et al. (2008) | Munich, Germany | 1 | 19.3–105.9 | 5.1–67.4 | Natural | Indoor PM consists mainly of crustal materials, detrition of the building materials and chalk |
| Canha et al. (2014) | Rural Portugal | 1 | 100ⱡ | -- | Natural | Sources: bakery industry, wood burning process/ soil re-suspension/ chalk use, crustal, and marine contributions |
| Almeida et al. (2011) | Lisbon, Portugal | 3 | 10ⱡ | 3 – 10 | Natural | Elemental composition suggested sources: crustal materials, detritions of the building materials and chalk |
| Zwoździak et al. (2013) | Wroclaw, Poland | 1 | 13.5–59.8 | 9.1–15.6 | Natural | I/O PM2.5 = 2.0 for winter, 4.1 for summer |
| Stranger et al. (2008) | Antwerp, Belgium | 27 | 26–129 | 12–148 | Opening windows | I/O PM2.5 = 1.3 |
| Borgini et al. (2011) | Milan, Italy | 3 | 79.4ⱡ | 77.9ⱡ | Natural | I/O PM2.5 = 1.0 |
| Janssen et al. (2001) | Netherlands | 24 | 7.7–52.8 | 5.2–60.8 | -- | I/O PM2.5 = 0.9 |
| Wichmann et al. (2010) | Stockholm, Sweden | 6 | 2.8–13.9 | 5.2–24.2 | Mechanical | I/O PM2.5 = 0.9 |
| Hou et al. (2015) | Beijing, China | 2 | 11 – 79 | 17 – 87 | Natural | I/O PM2.5 = 0.6 (closed), 0.9 (open) windows and doors |
| Xu et al. (2015) | Xi’an, China | 1 | 141.8ⱡ | 167.8ⱡ | Natural | I/O PM2.5 = 0.8; sources: coal combustion, motor vehicle exhaust, other primary sources, and secondary formation |
| Habil et al. (2013) | Agra, India | 10 | 71.3–90.1 | -- | Natural | I/O PM2.5 = 1.0 for winter, 1.2 for summer, 1.1 for monsoon; Sources: vehicle, soil, metal processes, wind-blown dust, industrial |
| Chithra and Nagendra (2012) | Chennai, India | 1 | 32–61 | -- | Natural | I/O PM2.5 = 1.4 ± 0.7 |
Mean concentration
3.2. Indoor and outdoor sources contributing indoor PM2.5
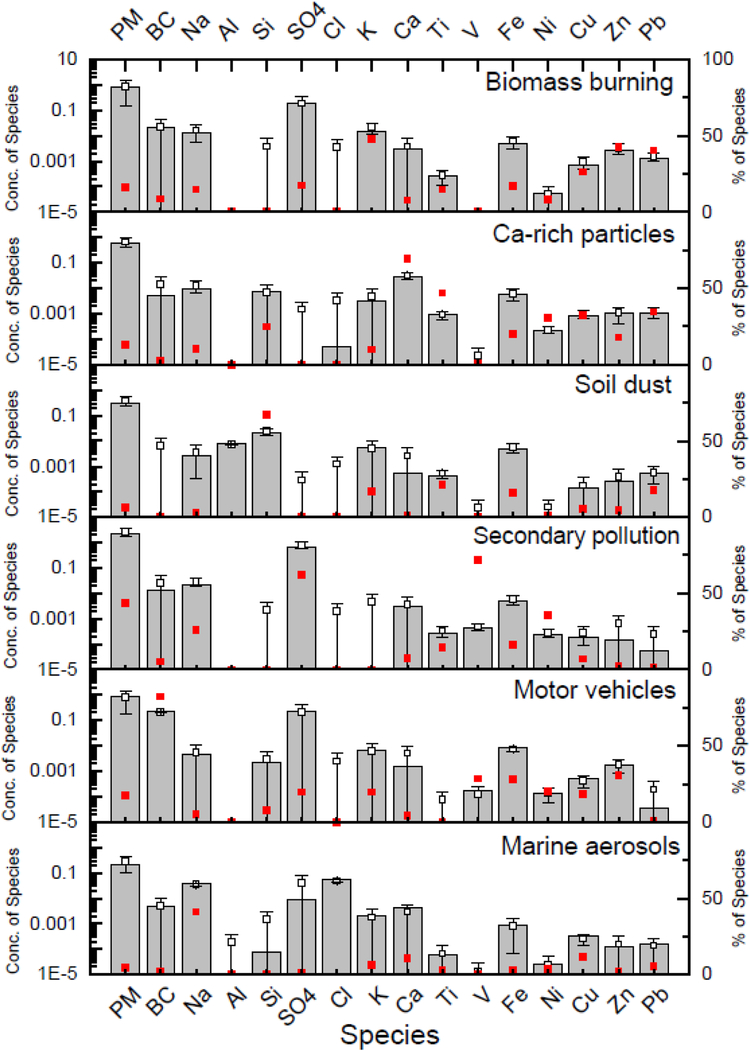
The PMF receptor model identified six source factors that contributed to indoor PM2.5 concentrations as shown in Table 3 and Figure 1. Percent mean relative errors (%MRE) were used to assess the goodness of fit for the PMF model solution. The results showed a good prediction of indoor PM2.5 mass and elemental concentrations with low MREs (<15%), except for Al (27.6%), which had concentration values below the limit of detection.
Table 3.
Source contributions to indoor PM2.5 mass and elemental concentration (ng/m3)
| Ca-rich particles | Motor vehicles | Secondary pollution | Soil dust | Marine aerosol | Biomass burning | Estimate | Measured | %MRE | |
|---|---|---|---|---|---|---|---|---|---|
| Mass | 619.1 | 856.9 | 2,142.1 | 302.4 | 207.3 | 787.3 | 4,915.1 | 5,247.9 | 6.3 |
| BC | 5.0 | 212.7 | 13.8 | 0.0 | 4.9 | 21.9 | 258.3 | 260.0 | 0.7 |
| Sulfate | 0.0 | 211.9 | 668.6 | 0.0 | 8.6 | 184.8 | 1,073.9 | 1,074.6 | 0.1 |
| Al | 0.0 | 0.0 | 0.0 | 7.4 | 0.0 | 0.0 | 7.4 | 5.8 | 27.6 |
| Ca | 27.6 | 1.5 | 3.0 | 0.5 | 4.1 | 3.1 | 39.8 | 39.9 | 0.3 |
| Cl | 0.0 | 0.0 | 0.0 | 0.0 | 51.7 | 0.0 | 51.7 | 51.8 | 0.2 |
| Cu | 0.9 | 0.5 | 0.2 | 0.1 | 0.3 | 0.7 | 2.7 | 2.8 | 3.6 |
| Fe | 5.9 | 8.2 | 4.7 | 4.6 | 0.9 | 5.0 | 29.3 | 30.2 | 3.0 |
| K | 3.1 | 6.5 | 0.0 | 5.4 | 2.0 | 15.7 | 32.7 | 32.7 | 0.0 |
| Na | 9.2 | 4.5 | 23.1 | 2.7 | 36.8 | 13.0 | 89.3 | 92.7 | 3.7 |
| Ni | 0.2 | 0.1 | 0.2 | 0.0 | 0.0 | 0.1 | 0.6 | 0.7 | 14.3 |
| Pb | 1.1 | 0.0 | 0.1 | 0.5 | 0.2 | 1.3 | 3.2 | 3.5 | 8.6 |
| Si | 7.5 | 2.3 | 0.0 | 20.5 | 0.1 | 0.0 | 30.4 | 30.4 | 0.0 |
| Ti | 0.9 | 0.0 | 0.3 | 0.4 | 0.1 | 0.3 | 2.0 | 1.9 | 5.3 |
| V | 0.0 | 0.2 | 0.4 | 0.0 | 0.0 | 0.0 | 0.6 | 0.7 | 14.3 |
| Zn | 1.1 | 1.8 | 0.1 | 0.3 | 0.1 | 2.6 | 6.0 | 6.0 | 0.0 |
Figure 1.
Source profiles contributing to indoor PM2.5. The grey filled-in rectangle represents the estimated average concentration of each species by source factor estimated using PMF. The small closed (red) square represents the percentage of a specific species ascribed to the specific source (factor) by PMF. For example, using PMF, the mean concentration of indoor SO4 ascribed to secondary pollution is 0.7 μg/m3, which is 62% of the total sulfate concentration (See also Table 3). The small open square represents the estimated average concentration of each species by source factor estimated using DISP with the error bars indicating the confidence intervals.
Ca-rich particles, motor vehicles, secondary pollution, soil dust, biomass burning and marine aerosols were identified as sources affecting PM2.5 in school classrooms, which account for 12, 17, 41, 6, 15, and 4% of the total mass, respectively. The first factor, Ca-rich particles was characterized by the high contributions of Ca, Fe, and Ti, which suggests the contribution of road-dust from outdoors, or possibly the wear-out of cement and dry-wall indoors. The second factor, soil dust, was characterized by the contributions of terrestrial elements Al and Si. Overall, we observed sulfate mass concentration in every factor, although its relative contribution to indoor sources was, as expected, low. Other studies (Song et al. 2001) in this region have shown the contribution of sulfate to their source profiles due to the contribution of coal-fired power plants located in the Midwest USA. For the Ca-rich particles and soil dust, the crustal particles can enter classrooms through windows or be released from the children’s shoes and clothes, especially after playing outside. These two may be related to children’s activities as has been reported by other school studies in Texas, Germany, Spain and Portugal (Almeida et al. 2011; Bozlaker et al. 2017; Canha et al. 2014; Fromme et al. 2008). The study of Bozlaker et al. (2017) in Texas found contributions of 2.1 and 10.2% from Ca-rich particles and soil/road dust, respectively. Canha et al. (2014) reported a 27% contribution from soil and a contribution of 53% from chalk/resuspension in a rural area of Portugal. In Barcelona, Amato et al. (2014) estimated contributions of 17% for a mineral source and 45% for textile/chalk source. It is worth noting that in this study’s school classrooms chalk is not used, the possible sources of Ca-rich particles might be due to cement or drywall wear-out processes. The third factor was identified as secondary pollution because it was associated with sulfate, which is a tracer of regional and long-range transport of particulate air pollution (Ling-Da et al. 2013; Viana et al. 2008). Nitrate and ammonium markers have been used in source apportionment studies to identify a secondary nitrate factor, but the nitrate concentration is low in the Northeast USA, with mean concentration of 0.79 μg/m3, representing a PM2.5 mass fraction of 9% during the study period. This factor was also associated with V and Ni, which are tracers of oil combustion. However, since oil combustion was not significant, it might not be identified as an individual factor. The fourth factor was identified as the motor vehicles with a high contribution of BC, which is a clear tracer of vehicular emissions (Viana et al. 2008). The fifth factor was related to marine aerosols because of its association with Na and Cl, which are major constituents of sea water (Mazzei et al. 2008; Viana et al. 2008). The sixth factor was identified as biomass burning because was characterized by contributions from K and Zn, which are tracers of biomass burning (Echalar et al. 1998).
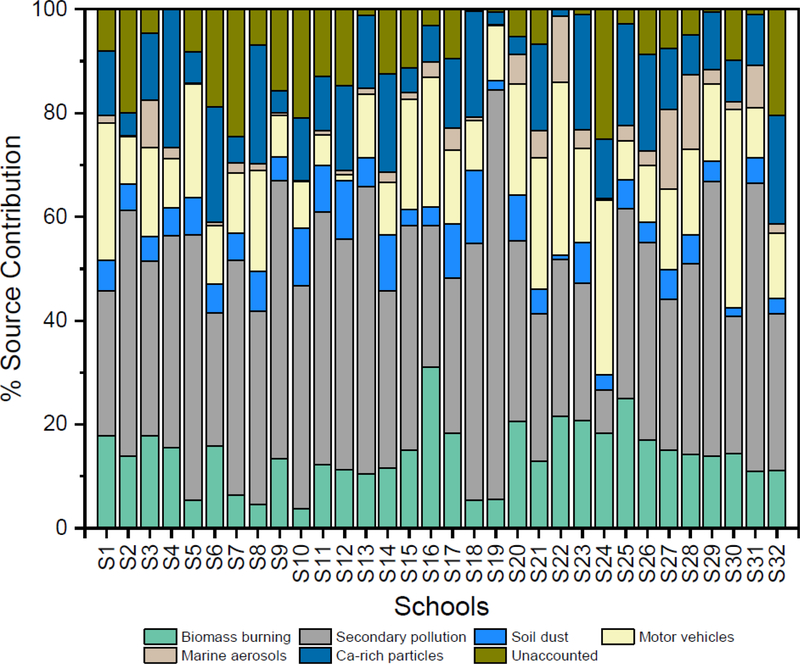
In Figure D.1. in Appendix D, we show the percentage contribution of these sources to indoor PM2.5 in each school. The secondary pollution factor was the dominant source among all the schools followed by motor vehicles factor and biomass burning. Low contribution from the marine aerosols factor was also observed. The unaccounted fraction was defined as: total measured mass – total modeled mass. This mass fraction could be contribution from secondary nitrate or other secondary organic aerosols.
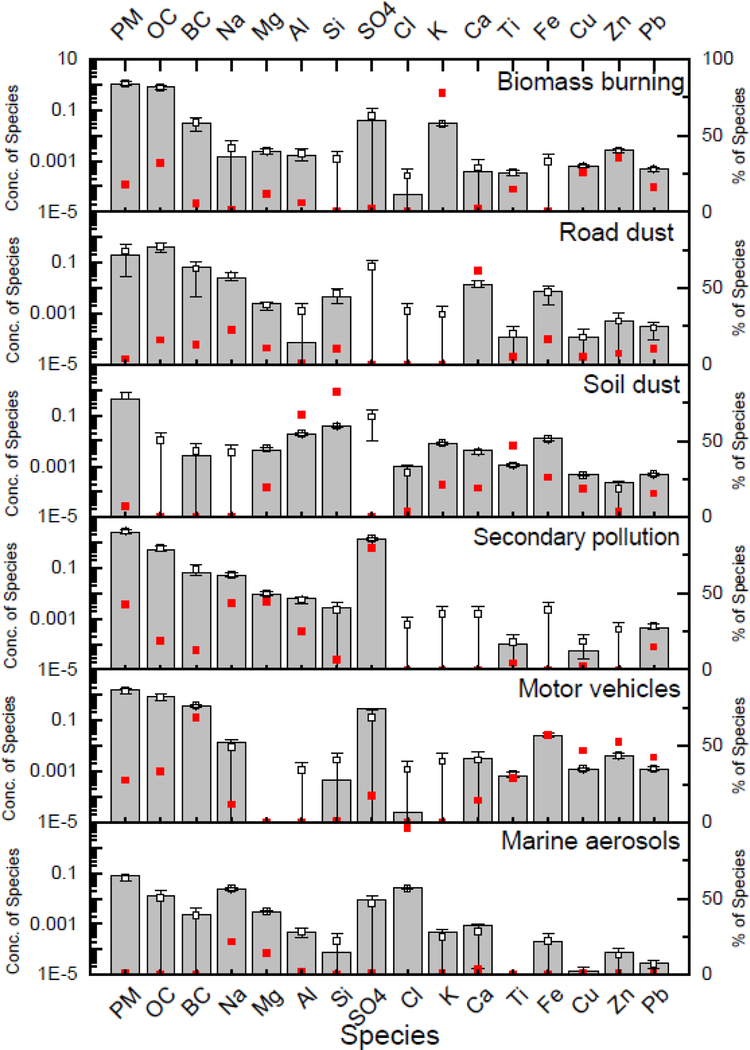
The source apportionment analysis of outdoor PM2.5 concentrations identified six source factors shown in Table 4 and Figure 2. Goodness of fit showed a good prediction of outdoor PM2.5 mass and elemental concentrations with low (<15%) of MREs, except for Cl (18%). The factors included: biomass burning (17%), road dust (3%), soil dust (7%), secondary pollution (41%), motor vehicles (26%), and marine aerosols (1%). The first factor, biomass burning, was associated with K, which is a tracer of biomass burning. The second factor was identified as road dust because of the high contributions of Ca and Fe. Soil dust, the third factor, was characterized by the high contributions of Al and Si. The fourth factor was identified as secondary pollution due to high contributions of Sulfate and Na. The fifth factor was identified as the motor vehicles source for having high contributions from BC, OC, Cu, and Zn. The last factor was marine aerosols and identified with high contribution of Cl. Contrary to indoor environment, the ambient molar ratio of Na to Cl could not be estimated because a considerable amount of particle Cl could be converted to gas-phase HCl by the reactions with HNO3 and H2SO4 in urban atmosphere (Seinfeld and Pandis 2016). For this reason, we used Na and Cl as a different source tracer for outdoor PM2.5.
Table 4.
Source contributions to outdoor PM2.5 mass and elemental concentration (ng/m3)
| Road dust | Motor vehicles | Secondary pollution | Soil dust | Marine aerosols | Biomass burning | Estimate | Measured | %MRE | |
|---|---|---|---|---|---|---|---|---|---|
| Mass | 209.2 | 1,698.7 | 2,630.9 | 434.8 | 75.0 | 1,107.2 | 6,155.8 | 6,497.3 | 5.3 |
| BC | 68.0 | 365.0 | 66.1 | 2.8 | 2.5 | 29.0 | 533.4 | 628.0 | 15.1 |
| OC | 400.3 | 846.7 | 485.8 | 0.0 | 13.3 | 811.3 | 2,557.4 | 2,868.6 | 10.8 |
| Sulfate | 0.0 | 287.3 | 1,314.8 | 0.0 | 9.4 | 40.0 | 1,651.5 | 1,508.4 | 9.5 |
| Al | 0.1 | 0.0 | 6.6 | 17.9 | 0.5 | 1.6 | 26.7 | 23.4 | 14.1 |
| Ca | 13.1 | 3.1 | 0.0 | 4.1 | 0.8 | 0.4 | 21.5 | 21.0 | 2.4 |
| Cl | 0.0 | 0.0 | 0.0 | 0.9 | 25.9 | 0.0 | 26.8 | 32.7 | 18.0 |
| Cu | 0.1 | 1.1 | 0.1 | 0.5 | 0.0 | 0.6 | 2.4 | 2.4 | 0.0 |
| Fe | 7.5 | 26.3 | 0.0 | 12.0 | 0.2 | 0.0 | 46.0 | 46.1 | 0.2 |
| K | 0.0 | 0.0 | 0.0 | 8.2 | 0.5 | 30.5 | 39.2 | 37.9 | 3.4 |
| Mg | 2.3 | 0.0 | 9.7 | 4.2 | 3.1 | 2.5 | 21.8 | 21.9 | 0.5 |
| Na | 25.5 | 13.2 | 49.6 | 0.0 | 24.7 | 1.4 | 114.4 | 128.7 | 11.1 |
| Pb | 0.3 | 1.3 | 0.4 | 0.5 | 0.0 | 0.5 | 3.0 | 2.9 | 3.4 |
| Si | 4.5 | 0.4 | 2.9 | 36.1 | 0.1 | 0.0 | 44.0 | 44.4 | 0.9 |
| Ti | 0.1 | 0.7 | 0.1 | 1.1 | 0.0 | 0.3 | 2.3 | 2.2 | 4.5 |
| Zn | 0.5 | 3.8 | 0.0 | 0.2 | 0.1 | 2.6 | 7.2 | 8.0 | 10.0 |
Figure 2.
Source profiles contributing to outdoor PM2.5. The grey filled-in rectangle represents the estimated average concentration of each species by source factor estimated using PMF. The small closed (red) square represents the percentage of a specific species ascribed to the specific source (factor) by PMF. For example, using PMF, the mean concentration of indoor SO4 ascribed to secondary pollution is 1.3 μg/m3, which is 80% of the total sulfate concentration (See also Table 4). The small open square represents the estimated average concentration of each species by source factor estimated using DISP with the error bars indicating the confidence intervals.
3.3. Indoor-to-outdoor (I/O) ratios
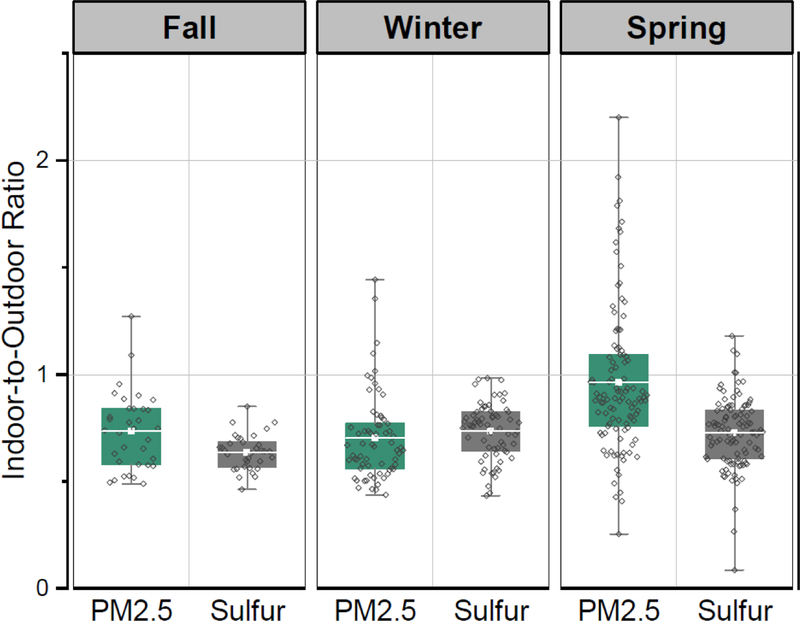
Indoor-to-outdoor sulfur ratio is used widely to estimate the infiltration of PM2.5 from outdoor to indoor assuming that there is non-significant indoor source of sulfur (Sarnat et al. 2002; Wallace and Williams 2005). For this study, four schools out of 32 schools had a sulfur ratio greater than 1. A ratio above 1 suggests that there might be indoor sulfur sources or possibly outdoor sulfur sources near the schools which do not impact the central site.
Figure 3 shows the I/O ratios for sulfur and PM2.5 by season. The highest I/O sulfur ratios were observed during the spring (0.73). The highest I/O PM2.5 ratios were observed during the spring (0.96). The opening of windows during the spring season might be the reason for the higher I/O ratios indicating a high infiltration. Higher I/O ratios were observed in schools in Agra, India (Habil et al. 2013): 1.0 for winter, 1.2 for summer, and 1.1 for the monsoon period. In Beijing, Hou et al. (2015) reported an I/O PM2.5 ratio of 0.6 when the windows and doors were closed. This is comparable to those observed during the fall and winter seasons in Boston. In addition, the climatic conditions in Boston and Beijing are similar, where winter seasons are very cold. Furthermore, the I/O PM2.5 was 0.9 in Hou et al. (2015) study when the windows and doors were open, which is similar to that we observed in Boston during the spring when windows and doors might more frequently open as compared to the other seasons.
Figure 3.
Box plots of indoor-to-outdoor PM2.5 and sulfur ratios by season. Green boxes represent indoor-to-outdoor PM2.5 ratios and gray boxes represent indoor-to-outdoor sulfur ratios. The box plot shows the mean, 25th and 75th percentiles, while the end of the whiskers indicates the minimum and maximum of the data.
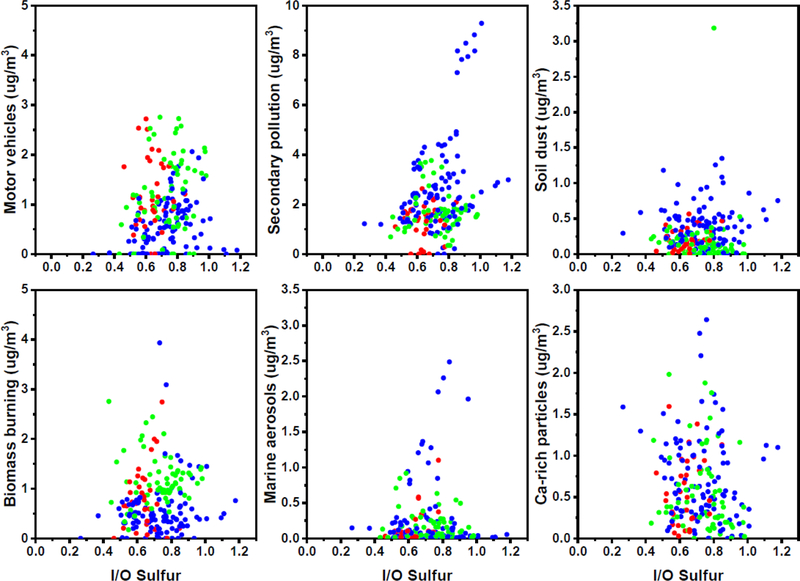
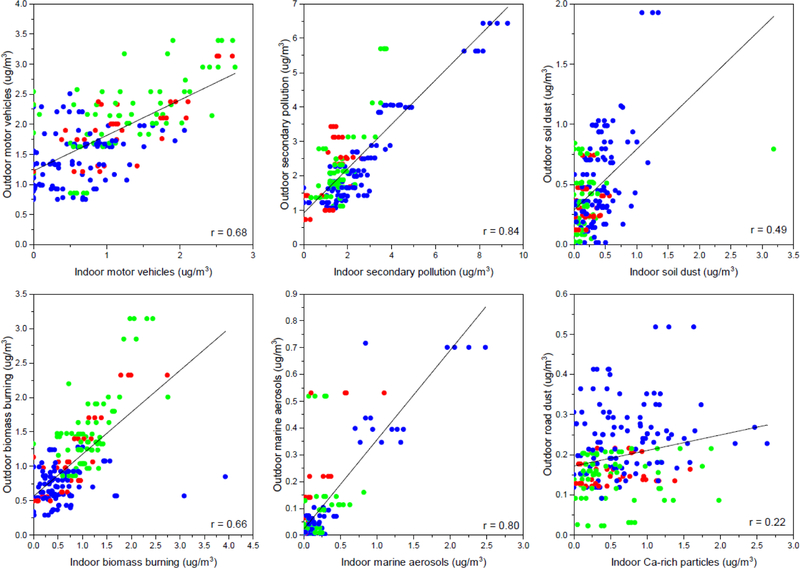
Soil dust and Ca-rich particles sources can be categorized as indoor origins as described above, whereas motor vehicles, secondary pollution, biomass burning, and marine aerosols are categorized as outdoor origins. Seasonal relationships between source contributions and I/O sulfur ratios are shown in Figure 4. As it can be seen, the relationships of indoor origin differ from those observed for the outdoor ones. Indoor sources exhibit high variability between schools because the contributions are not related with infiltration (I/O sulfur ratio). Furthermore, motor vehicles and biomass burning sources have a different variation from the other two outdoor-origin sources (i.e., marine aerosols and secondary pollution) that have similar seasonal patterns, as also shown in Table 5 and Figure C.1 in Appendix C. Higher contributions of motor vehicles in the winter and fall is more likely derived from local sources in the vicinity of the schools. Activities in the school environment during winter such as using motor equipment/vehicles to clean the snow and spreading salt, and during the fall such as cleaning the leaves may have driven these differences to the other sources. In addition, higher contribution of biomass burning in winter is more likely associated with wood burning at the neighborhood of schools. Consequently, each source would have different distance scale: soil dust and Ca-rich particles are in the schools; motor vehicles and biomass burning are in the vicinity of schools; secondary and marine aerosols have a regional scale.
Figure 4.
Relationship between classroom indoor PM2.5 source contributions and I/O sulfur ratio by seasons: Blue dots (spring); Red dots (fall); and Green dots (winter).
Table 5.
Sources affecting PM2.5 by season
| Sources | Fall (N = 34) | Winter (N = 66) | Spring (N = 103) | |||
|---|---|---|---|---|---|---|
| μg/m3 | %* | μg/m3 | % | μg/m3 | % | |
| Indoor | ||||||
| Observed PM2.5 | 4.38 | - | 4.94 | - | 5.73 | - |
| Estimated PM2.5 | 4.23 | 97 | 4.88 | 99 | 5.23 | 91 |
| Motor vehicles | 1.23 | 29 | 1.20 | 25 | 0.55 | 11 |
| Secondary pollution | 1.28 | 30 | 1.66 | 34 | 2.75 | 53 |
| Marine aerosols | 0.13 | 3 | 0.15 | 3 | 0.27 | 5 |
| Soil dust | 0.21 | 5 | 0.18 | 4 | 0.41 | 8 |
| Ca-rich particles | 0.56 | 13 | 0.51 | 10 | 0.71 | 14 |
| Biomass burning | 0.82 | 19 | 1.16 | 24 | 0.54 | 10 |
| Outdoor | ||||||
| Observed PM2.5 | 6.17 | - | 7.50 | - | 5.97 | - |
| Estimated PM2.5 | 5.80 | 94 | 6.39 | 85 | 5.48 | 92 |
| Motor vehicles | 2.00 | 34 | 2.11 | 33 | 1.42 | 26 |
| Secondary pollution | 2.06 | 36 | 2.21 | 35 | 2.46 | 45 |
| Marine aerosols | 0.12 | 2 | 0.08 | 1 | 0.09 | 2 |
| Soil dust | 0.35 | 6 | 0.32 | 5 | 0.55 | 10 |
| Road dust | 0.16 | 3 | 0.14 | 2 | 0.24 | 4 |
| Biomass burning | 1.12 | 19 | 1.53 | 24 | 0.72 | 13 |
Percentage of observed PM2.5 mass
4. Conclusions
Possible sources contributing to indoor PM2.5 and seasonal variability were investigated at inner-city schools’s environment. Indoor PM2.5 concentrations were comparable to those observed outdoors, demonstrating penetration of outdoor pollution to indoors, but the concentrations were in the lower range compared to other school studies. Six source factors (Ca-rich particles, soil dust, motor vehicles, marine aerosols, secondary pollution and biomass burning) were identified to affect indoor PM2.5 concentration. Ca-rich particles and soil factors were likely associated with child-related activities that transport materials from playgrounds. Concurrent outdoor PM2.5 concentrations were estimated to be contributed by six source factors (motor vehicles, secondary pollution, marine aerosols, soil dust, road dust, biomass burning), which were similar to the previous studies of the studied area. From the relationship between I/O sulfur ratio and each source contribution, seasonal patterns of motor vehicles and biomass burning were different with a lower in spring and a higher in winter from those of other outdoor source factors (i.e., secondary pollution and marine aerosols) with a higher in spring with a higher infiltration, which indicates difference in distance scales. Contrary to regional scale of secondary pollution and marine aerosols, motor vehicles and biomass burning have local scale in the vicinity of the schools.
Our study has some limitations. First, the use of a single outdoor measurement might somewhat misclassify the outdoor-to-indoor comparison of PM2.5. However, we have previously demonstrated that ambient measurement at the ambient monitoring site reasonably represented the outdoor levels in the geographic area of this study. Also, the difference in distance to the ground between the schools and the 6-story building might affect the precision of the outdoor-to-indoor comparison. In addition, we did not measure the air exchange rate in each of the classrooms, and, given the variability of the classroom ventilation within as well as between schools, source apportionment by classroom or school ventilation type becomes difficult. Second, we did not measure secondary organic aerosols and nitrate indoor and outdoor, which might cause bias of our results. However, note that nitrate concentration are low in the northeast USA, with mean concentration of 0.79 μg/m3 accounting for a 9% of the PM2.5 mass during the study period.
Schools are complex microenvironments with different factors that can contribute to the transport, mixing and generation of indoor particles. Further investigations should incorporate indoor child behavior and activities to better assess exposure and the relevance of PM2.5 in inner-city school indoor environments. If school-level exposures and their sources are assessed along with their relative importance in influencing child health, then targeted interventions may improve children’s health and well-being.
Highlights.
Relative source contributions of indoor and outdoor PM2.5 were determined for inner-city school classrooms;
Four outdoor sources and two indoor sources were identified as contributors to indoor PM2.5 concentrations, and;
Regional sources were the greatest contributor to indoor and outdoor PM2.5 in all seasons.
Acknowledgements
This publication was made possible by the National Institutes of Health (NIH) grants (R01 AI 073964, K23AI106945, U01 AI 110397, K24 AI 106822, R01HL137192), Coway grant (ISRA-2017) and US EPA grant (RD-835872). Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the granters. Further, the granters do not endorse the purchase of any commercial products or services mentioned in the publication. We acknowledge the efforts of Jack M. Wolfson, all other laboratory and field staffs for laboratory and field measurement support.
Abbreviations
- PM2.5
Particulate Matter less than 2.5 microns in diameter
- Ca
Calcium
- USEPA
US Environmental Protection Agency
- PMF
Positive Matrix Factorization
- EC
Elemental carbon
- BC
Black carbon
- SICAS
School Inner City Asthma Study
- OC
Organic carbon
- TOT
Thermal optical transmittance
- XRF
X-Ray Fluorescence
- MRE
Mean Relative Errors
- NAAQS
US National Ambient Air Quality Standards
- V
Vanadium
- Cr
Chromium
- Ni
Nickel
- Cu
Copper
- Br
Bromine
- Pb
Lead
- Al
Aluminum
- Na
Sodium
- Cl
Chlorine
- Fe
Iron
- Si
Silicon
- K
Potassium
- Mg
Magnesium
- Ti
Titanium
- Zn
Zinc
- I/O
Indoor-to-outdoor
- S
Sulfur
Appendix A. PMF input and diagnostics summary
A.1. Indoor PM2.5
**** Input Data Statistics ****
| Species | Category | S/N |
|---|---|---|
| Mass | Weak | 9 |
| BC | Strong | 5.6 |
| Na | Weak | 1.3 |
| Mg | Bad | 0 |
| Al | Weak | 0.1 |
| Si | Strong | 4.8 |
| Sulfate | Strong | 10 |
| Cl | Strong | 7.4 |
| K | Strong | 9.9 |
| Ca | Strong | 9.7 |
| Ti | Weak | 0.9 |
| V | Weak | 0.6 |
| Cr | Bad | 0 |
| Mn | Bad | 0 |
| Fe | Weak | 6.5 |
| Ni | Weak | 0.1 |
| Cu | Weak | 0.1 |
| Zn | Weak | 2.5 |
| Br | Bad | 0.1 |
| Pb | Weak | 0.3 |
**** Constrained Run Summary ****
| Base model run number: | 5 |
| Base user-selected seed: | 67 |
| Number of factors: | 6 |
| Extra modeling uncertainty (%): | 0 |
**** Constraints ****
| Factor | Element | Type | Value | dQ | % dQ |
|---|---|---|---|---|---|
| Factor 2 | Sulfate | Pull Down Maximally | NA | 3.48 | 1 |
| Factor 5 | Sulfate | Pull Down Maximally | NA | 3.48 | 1 |
**** Constrained Run Summary Table ****
| Constrained # | dQ(Robust) | Q(Robust) | Q(Aux) | Q(True) | Converged | # Steps |
|---|---|---|---|---|---|---|
| 1 | 1.6 | 349.7 | 1.7 | 348 | Yes | 1,079 |
**** Constrained Run Statistics ****
| Species | Intercept | Slope | SE | r2 | KS test stat | KS test p value |
|---|---|---|---|---|---|---|
| Mass | 1.17 | 0.71 | 0.92 | 0.76 | 0.06 | 0.42 |
| BC | 0 | 0.99 | 0.01 | 1 | 0.19 | 0 |
| Na | 0.01 | 0.88 | 0.03 | 0.84 | 0.04 | 0.95 |
| Al | 0 | 0.56 | 0 | 0.67 | 0.15 | 0 |
| Si | 0 | 1 | 0 | 1 | 0.05 | 0.6 |
| Sulfate | 0 | 1 | 0 | 1 | 0.07 | 0.3 |
| Cl | 0 | 1 | 0 | 1 | 0.12 | 0.01 |
| K | 0 | 1 | 0 | 1 | 0.05 | 0.7 |
| Ca | 0 | 1 | 0 | 1 | 0.03 | 0.97 |
| Ti | 0 | 0.41 | 0 | 0.4 | 0.13 | 0 |
| V | 0 | 0.15 | 0 | 0.15 | 0.18 | 0 |
| Fe | 0.01 | 0.61 | 0.01 | 0.51 | 0.09 | 0.08 |
| Ni | 0 | 0.16 | 0 | 0.17 | 0.10 | 0.02 |
| Cu | 0 | 0.15 | 0 | 0.12 | 0.12 | 0 |
| Zn | 0 | 0.55 | 0 | 0.50 | 0.08 | 0.13 |
| Pb | 0 | 0.1 | 0 | 0.04 | 0.06 | 0.47 |
A.2. Outdoor PM2.5
**** Input Data Statistics ****
| Species | Category | S/N |
|---|---|---|
| Mass | Weak | 9 |
| OC | Strong | 4.5 |
| EC | Bad | 0.9 |
| BC | Strong | 7.2 |
| UVC | Bad | 7.2 |
| DC | Bad | 3.5 |
| Na | Strong | 3.1 |
| Mg | Weak | 0.5 |
| Al | Strong | 2.5 |
| Si | Strong | 7.1 |
| Sulfate | Strong | 10 |
| Cl | Strong | 5.1 |
| K | Strong | 10 |
| Ca | Strong | 9.7 |
| Ti | Strong | 1.9 |
| V | Bad | 1.9 |
| Cr | Bad | 0.1 |
| Mn | Bad | 0.1 |
| Fe | Strong | 9.2 |
| Ni | Bad | 0.4 |
| Cu | Weak | 0.1 |
| Zn | Strong | 4.2 |
| Br | Bad | 1 |
| Ba | Bad | 0.3 |
| Pb | Weak | 0.9 |
**** Constrained Run Summary ****
| Base model run number: | 15 |
| Base user-selected seed: | 86 |
| Number of factors: | 6 |
| Extra modeling uncertainty (%): | 0 |
**** Constraints ****
| Factor | Element | Type | Value | dQ | % dQ |
|---|---|---|---|---|---|
| Factor 1 | Sulfate | Pull Down Maximally | NA | 139.87 | 0.5 |
| Factor 6 | Sulfate | Pull Down Maximally | NA | 139.87 | 0.5 |
**** Constrained Run Summary Table ****
| Constrained # | dQ(Robust) | Q(Robust) | Q(Aux) | Q(True) | Converged | # Steps |
|---|---|---|---|---|---|---|
| 1 | 71.1 | 28044.2 | 70 | 63321.1 | Yes | 2,028 |
**** Constrained Run Statistics ****
| Species | Intercept | Slope | SE | r2 | KS test stat | KS test p value |
|---|---|---|---|---|---|---|
| Mass | 1.15 | 0.74 | 1.6 | 0.81 | 0.05 | 0.01 |
| OC | 0.84 | 0.58 | 1.22 | 0.33 | 0.06 | 0 |
| BC | 0.15 | 0.65 | 0.17 | 0.64 | 0.05 | 0.01 |
| Na | 0.02 | 0.78 | 0.07 | 0.65 | 0.06 | 0 |
| Mg | 0.01 | 0.49 | 0.01 | 0.44 | 0.06 | 0 |
| Al | 0 | 0.88 | 0.01 | 0.88 | 0.03 | 0.26 |
| Si | 0 | 1 | 0 | 0.99 | 0.06 | 0 |
| Sulfate | 0 | 1 | 0 | 1 | 0.08 | 0 |
| Cl | 0 | 1 | 0 | 1 | 0.13 | 0 |
| K | 0 | 1 | 0 | 1 | 0.07 | 0 |
| Ca | 0 | 0.99 | 0 | 1 | 0.02 | 0.52 |
| Ti | 0 | 0.64 | 0 | 0.69 | 0.1 | 0 |
| Fe | 0 | 0.96 | 0 | 0.98 | 0.04 | 0.02 |
| Cu | 0 | 0.47 | 0 | 0.43 | 0.05 | 0 |
| Zn | 0.01 | 0.09 | 0 | 0.08 | 0.23 | 0 |
| Pb | 0 | 0.27 | 0 | 0.18 | 0.03 | 0.14 |
Appendix B. PMF base error estimation summary
B.1. Indoor PM2.5
**** Constrained BS-DISP Diagnostics ****
| # of Cases Accepted: | 101 | |||||
| %dQ: | −2.63126 | |||||
| Swaps by Factor: | 0 | 0 | 0 | 0 | 0 | 0 |
**** Constrained DISP Diagnostics ****
| Error Code: | 0 | |||||
| %dQ: | −0.39317 | |||||
| Swaps by Factor: | 0 | 0 | 0 | 0 | 0 | 0 |
**** Constrained BS Mapping ****
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Unmapped | |
|---|---|---|---|---|---|---|---|
| Boot Factor 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Boot Factor 2 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Boot Factor 3 | 0 | 0 | 100 | 0 | 0 | 0 | 0 |
| Boot Factor 4 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Boot Factor 5 | 0 | 0 | 0 | 0 | 100 | 0 | 0 |
| Boot Factor 6 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
B.2. Outdoor PM2.5
**** Constrained BS-DISP Diagnostics ****
| # of Cases Accepted: | 90 | |||||
| %dQ: | −0.07431 | |||||
| Swaps by Factor: | 0 | 0 | 0 | 0 | 0 | 0 |
**** Constrained DISP Diagnostics ****
| Error Code: | 0 | |||||
| %dQ: | −0.0398 | |||||
| Swaps by Factor: | 0 | 0 | 0 | 0 | 0 | 0 |
**** Constrained BS Mapping ****
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Unmapped | |
|---|---|---|---|---|---|---|---|
| Boot Factor 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Boot Factor 2 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Boot Factor 3 | 0 | 0 | 100 | 0 | 0 | 0 | 0 |
| Boot Factor 4 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Boot Factor 5 | 0 | 0 | 0 | 0 | 100 | 0 | 0 |
| Boot Factor 6 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
Appendix C. I/O Correlation
Figure C.1.
Correlation between sources contributing to indoor and outdoor PM2.5 by season. Blue dots represent samples collected during the spring season. Red dots represent samples collected during fall season. Green dots represent samples collected during winter season.
Appendix D. Source contribution by school
Figure D.1.
Source contribution by schools.
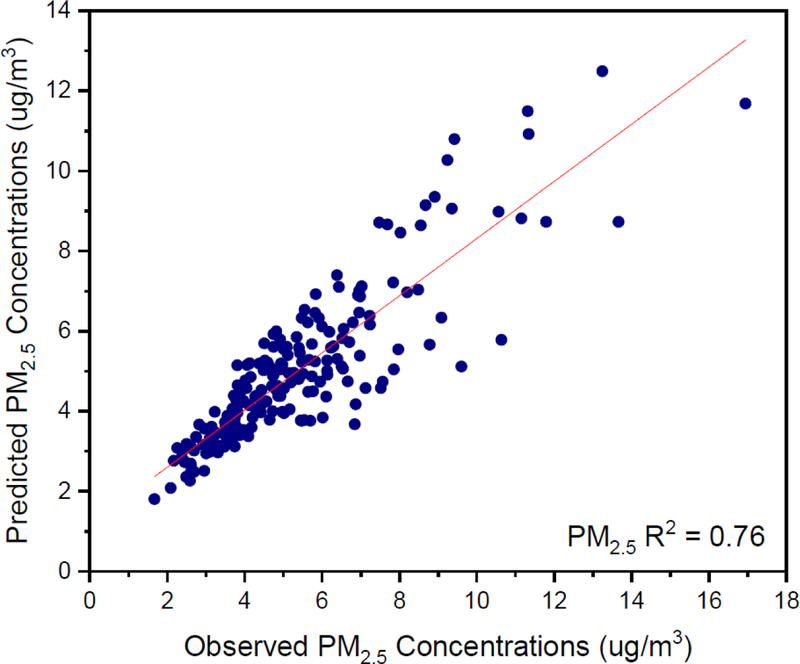
Appendix E. Observed vs Predicted Concentrations
Figure E.1.
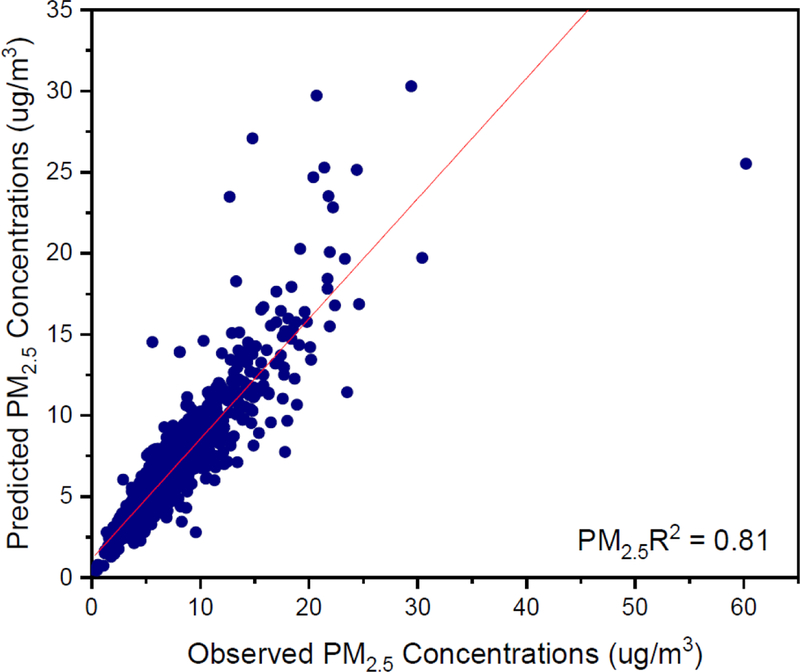
Observed vs Predicted Concentrations of indoor PM2.5 mass.
Figure E.2.
Observed vs Predicted Concentrations of outdoor PM2.5 mass.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Almeida SM, Canha N, Silva A, do Carmo Freitas M, Pegas P, Alves C, et al. 2011. Children exposure to atmospheric particles in indoor of lisbon primary schools. Atmospheric Environment 45:7594–7599. [Google Scholar]
- Alvarez-Pedrerol M, Rivas I, López-Vicente M, Suades-González E, Donaire-Gonzalez D, Cirach M, et al. 2017. Impact of commuting exposure to traffic-related air pollution on cognitive development in children walking to school. Environmental Pollution 231:837–844. [DOI] [PubMed] [Google Scholar]
- Amato F, Rivas I, Viana M, Moreno T, Bouso L, Reche C, et al. 2014. Sources of indoor and outdoor pm2. 5 concentrations in primary schools. Science of the Total Environment 490:757–765. [DOI] [PubMed] [Google Scholar]
- Basagaña X, Esnaola M, Rivas I, Amato F, Alvarez-Pedrerol M, Forns J, et al. 2016. Neurodevelopmental deceleration by urban fine particles from different emission sources: A longitudinal observational study. Environmental Health Perspectives 124:1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgini A, Tittarelli A, Ricci C, Bertoldi M, De Saeger E, Crosignani P. 2011. Personal exposure to pm2. 5 among high-school students in milan and background measurements: The eurolifenet study. Atmospheric environment 45:4147–4151. [Google Scholar]
- Bozlaker A, Peccia J, Chellam S. 2017. Indoor/outdoor relationships and anthropogenic elemental signatures in airborne pm2. 5 at a high school: Impacts of petroleum refining emissions on lanthanoid enrichment. Environmental science & technology 51:4851–4859. [DOI] [PubMed] [Google Scholar]
- Canha N, Almeida SM, do Carmo Freitas M, Wolterbeek HT, Cardoso J, Pio C, et al. 2014. Impact of wood burning on indoor pm2. 5 in a primary school in rural portugal. Atmospheric environment 94:663–670. [Google Scholar]
- Chatzidiakou L, Mumovic D, Summerfield AJ. 2012. What do we know about indoor air quality in school classrooms? A critical review of the literature. Intelligent Buildings International 4:228–259. [Google Scholar]
- Chithra V, Nagendra SS. 2012. Indoor air quality investigations in a naturally ventilated school building located close to an urban roadway in chennai, india. Building and Environment 54:159–167. [Google Scholar]
- Demokritou P, Kavouras IG, Ferguson ST, Koutrakis P. 2001. Development and laboratory performance evaluation of a personal multipollutant sampler for simultaneous measurements of particulate and gaseous pollutants. Aerosol Science & Technology 35:741–752. [Google Scholar]
- Echalar F, Artaxo P, Martins JV, Yamasoe M, Gerab F, Maenhaut W, et al. 1998. Long-term monitoring of atmospheric aerosols in the amazon basin: Source identification and apportionment. Journal of Geophysical Research: Atmospheres 103:31849–31864. [Google Scholar]
- Forns J, Dadvand P, Foraster M, Alvarez-Pedrerol M, Rivas I, López-Vicente M, et al. 2016. Traffic-related air pollution, noise at school, and behavioral problems in barcelona schoolchildren: A cross-sectional study. Environmental Health Perspectives 124:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Twardella D, Dietrich S, Heitmann D, Schierl R, Liebl B, et al. 2007. Particulate matter in the indoor air of classrooms—exploratory results from munich and surrounding area. Atmospheric Environment 41:854–866. [Google Scholar]
- Fromme H, Diemer J, Dietrich S, Cyrys J, Heinrich J, Lang W, et al. 2008. Chemical and morphological properties of particulate matter (pm10, pm2. 5) in school classrooms and outdoor air. Atmospheric Environment 42:6597–6605. [Google Scholar]
- Gaffin JM, Petty CR, Hauptman M, Kang C-M, Wolfson JM, Awad YA, et al. 2017. Modeling indoor particulate exposures in inner city school classrooms. Journal of exposure science & environmental epidemiology 27:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen PJ, Togias A. 2015. Inner city asthma. Immunology and Allergy Clinics 35:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R, Khare M. 2009. Indoor–outdoor concentrations of rspm in classroom of a naturally ventilated school building near an urban traffic roadway. Atmospheric Environment 43:6026–6038. [Google Scholar]
- Green Rochelle S, Smorodinsky S, Kim Janice J, McLaughlin R, Ostro B. 2004. Proximity of california public schools to busy roads. Environmental Health Perspectives 112:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habil M, Massey DD, Taneja A. 2013. Exposure of children studying in schools of india to pm levels and metal contamination: Sources and their identification. Air Quality, Atmosphere & Health 6:575–587. [Google Scholar]
- Hochstetler HA, Yermakov M, Reponen T, Ryan PH, Grinshpun SA. 2011. Aerosol particles generated by diesel-powered school buses at urban schools as a source of children’s exposure. Atmospheric environment 45:1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Liu J, Li J. 2015. Investigation of indoor air quality in primary school classrooms. Procedia Engineering 121:830–837. [Google Scholar]
- Janssen NA, van Vliet PH, Aarts F, Harssema H, Brunekreef B. 2001. Assessment of exposure to traffic related air pollution of children attending schools near motorways. Atmospheric environment 35:3875–3884. [Google Scholar]
- Jovanović M, Vučićević B, Turanjanin V, Živković M, Spasojević V. 2014. Investigation of indoor and outdoor air quality of the classrooms at a school in serbia. Energy 77:42–48. [Google Scholar]
- Keeler GJ, Dvonch T, Yip FY, Parker EA, Isreal BA, Marsik FJ, et al. 2002. Assessment of personal and community-level exposures to particulate matter among children with asthma in detroit, michigan, as part of community action against asthma (caaa). Environmental Health Perspectives 110:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo L, Luk M, Mok M, Yuen J, Yuen T. 1997. Health effects from air conditioning: Epidemiologic studies on schools and offices in hong kong. Proceedings of indoor and built environment problems in Asia at Kuala Lumpur, Malaysia 45. [Google Scholar]
- Ling-Da Y, Guang-Fu W, Ren-Jian Z, Lei-Ming Z, Yu S, Bing-Bing W, et al. 2013. Characterization and source apportionment of pm2.5 in an urban environment in beijing. Aerosol and Air Quality Research 13:574–583. [Google Scholar]
- MacNaughton P, Eitland E, Kloog I, Schwartz J, Allen J. 2017. Impact of particulate matter exposure and surrounding “greenness” on chronic absenteeism in massachusetts public schools. International journal of environmental research and public health 14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S, Kang C-M, Koutrakis P. 2015. Composition and sources of fine and coarse particles collected during 2002–2010 in boston, ma. Journal of the Air & Waste Management Association 65:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei F, D’Alessandro A, Lucarelli F, Nava S, Prati P, Valli G, et al. 2008. Characterization of particulate matter sources in an urban environment. Science of The Total Environment 401:81–89. [DOI] [PubMed] [Google Scholar]
- Paatero P, Tapper U. 1994. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5:111–126. [Google Scholar]
- Paatero P, Hopke PK. 2003. Discarding or downweighting high-noise variables in factor analytic models. Analytica Chimica Acta 490:277–289. [Google Scholar]
- Polissar AV, Hopke PK, Paatero P, Malm WC, Sisler JF. 1998. Atmospheric aerosol over alaska: 2. Elemental composition and sources. Journal of Geophysical Research: Atmospheres 103:19045–19057. [Google Scholar]
- Reff A, Eberly SI, Bhave PV. 2007. Receptor modeling of ambient particulate matter data using positive matrix factorization: Review of existing methods. Journal of the Air & Waste Management Association 57:146–154. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Long CM, Koutrakis P, Coull BA, Schwartz J, Suh HH. 2002. Using sulfur as a tracer of outdoor fine particulate matter. Environmental Science & Technology 36:5305–5314. [DOI] [PubMed] [Google Scholar]
- Seinfeld JH, Pandis SN. 2016. Atmospheric chemistry and physics: From air pollution to climate change:John Wiley & Sons. [Google Scholar]
- Smedje G, Norbäck D. 2008. Irritants and allergens at school in relation to furnishings and cleaning. Indoor Air 11:127–133. [DOI] [PubMed] [Google Scholar]
- Song X-H, Polissar AV, Hopke PK. 2001. Sources of fine particle composition in the northeastern us. Atmospheric Environment 35:5277–5286. [Google Scholar]
- Spira-Cohen A, Chen LC, Kendall M, Lall R, Thurston GD. 2011. Personal exposures to traffic-related air pollution and acute respiratory health among bronx schoolchildren with asthma. Environmental Health Perspectives 119:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger M, Potgieter-Vermaak SS, Van Grieken R. 2008. Characterization of indoor air quality in primary schools in antwerp, belgium. Indoor Air 18:454–463. [DOI] [PubMed] [Google Scholar]
- Stranger M, Potgieter-Vermaak SS, R. VG. 2008. Characterization of indoor air quality in primary schools in antwerp, belgium. Indoor Air 18:454–463. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Esnaola M, Alvarez-Pedrerol M, Forns J, Rivas I, López-Vicente M, et al. 2015. Association between traffic-related air pollution in schools and cognitive development in primary school children: A prospective cohort study. PLoS Medicine 12:e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétreault L-F, Doucet M, Gamache P, Fournier M, Brand A, Kosatsky T, et al. 2016. Severe and moderate asthma exacerbations in asthmatic children and exposure to ambient air pollutants. International Journal of Environmental Research and Public Health 13:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana M, Kuhlbusch TAJ, Querol X, Alastuey A, Harrison RM, Hopke PK, et al. 2008. Source apportionment of particulate matter in europe: A review of methods and results. Journal of Aerosol Science 39:827–849. [Google Scholar]
- Wallace L, Williams R. 2005. Use of personal-indoor-outdoor sulfur concentrations to estimate the infiltration factor and outdoor exposure factor for individual homes and persons. Environmental science & technology 39:1707–1714. [DOI] [PubMed] [Google Scholar]
- Weiss KB, Gergen PJ, Crain EF. 1992. Inner-city asthma: The epidemiology of an emerging us public health concern. Chest 101:362S–367S. [DOI] [PubMed] [Google Scholar]
- Wichmann J, Lind T, Nilsson M-M, Bellander T. 2010. Pm2. 5, soot and no2 indoor–outdoor relationships at homes, pre-schools and schools in stockholm, sweden. Atmospheric Environment 44:4536–4544. [Google Scholar]
- Xu H, Guinot B, Shen Z, Ho KF, Niu X, Xiao S, et al. 2015. Characteristics of organic and elemental carbon in pm2. 5 and pm0. 25 in indoor and outdoor environments of a middle school: Secondary formation of organic carbon and sources identification. Atmosphere 6:361–379. [Google Scholar]
- Zwoździak A, Sówka I, Krupińska B, Zwoździak J, Nych A. 2013. Infiltration or indoor sources as determinants of the elemental composition of particulate matter inside a school in wrocław, poland? Building and Environment 66:173–180. [Google Scholar]