Abstract
Background:
Whole-body and thoracic ionizing radiation exposure are both associated with the development of renal dysfunction. However, whether low-level environmental radiation from air pollution affects renal function remains unknown.
Objectives:
We investigated the association of particle radioactivity (PR) with renal function defined by the estimated glomerular filtration rate (eGFR) and chronic kidney disease (CKD) in the Normative Aging Study.
Methods:
This longitudinal analysis included 2,491 medical visits from 809 white males enrolled between 1999 and 2013. The eGFR was calculated using the CKD-EPI and MDRD equations, and CKD cases were identified as those with an eGFR <60 mL/min/1.73 m2 Gross β activity measured by five monitors of the U.S. Environmental Protection Agency’s RadNet monitoring network was utilized to represent PR.
Results:
Ambient PR levels from 1 to 28 days prior to clinical visit demonstrated robust negative associations with both forms of eGFR, but not with the increased odds of CKD. An interquartile range higher 28-day average ambient PR level was significantly associated with 0.83-mL/min/1.73 m2 lower eGFR estimated by the CKD-EPI equation (95% confidence interval: −1.46, −0.20, p-value=0.01). Controlling for PM2.5 or black carbon in the model slightly attenuated the PR effects on eGFR. However, in individuals with the highest levels (3rd tertile) of C-reactive protein (CRP) or fibrinogen, we observed robust associations of PR with eGFR and CKD, suggesting that systemic inflammation may modify the PR-eGFR and PR-CKD relationships.
Conclusions:
Our study reveals adverse health effects of short-term low-level ambient PR on the renal function, providing evidence to guide further study of the interplay between PR, inflammation, and renal health.
Keywords: air pollution, particle radioactivity, renal function, eGFR, CKD
1. Introduction
Ambient air pollution, especially fine particulate matter <2.5 μm in aerodynamic diameter (PM2.5), has been associated with reduced life expectancy and increased risks of cardiovascular disease (CVD), stroke, and mortality (Brook et al. 2010). As previous studies suggested that CVD is highly related to impaired renal function (Gansevoort et al. 2013), several epidemiologic studies have further found that ambient PM levels, including PM10 and PM2.5, are associated with declined estimated glomerular filtration rate (eGFR) and increased risk of incident chronic kidney disease (CKD) (Bowe et al. 2017, 2018; Chan et al. 2018; Mehta et al. 2016). Indeed, three recent studies indicated that long-term exposure to PM2.5 could significantly elevate the risk of CKD among 1,164,057 U.S. Medicare population, 2,482,737 U.S. veterans, and 100,629 Asians (Bowe et al. 2018; Bragg-Gresham et al. 2018; Chan et al. 2018). Further, a monotonic increasing association of PM10, NO2, and CO concentrations with the risk of adverse kidney outcomes was reported in the U.S. veterans (Bowe et al. 2017).
Air pollution also releases low-level radiation, an attribute of the exposure that has been largely neglected by studies of health effects of air pollution. Radiation may cause damage to macromolecules (Kempner 2011) and thereby increase the risk of aging-related diseases (Richardson and Schadt 2014). Exposures to ionizing radiation from an atomic bomb or radiotherapy, i.e., high-level radiation, induce renal dysfunction and may increase CVD risk (Adams et al. 2012; Valkema et al. 2005). However, whether low-level radiation from air pollution affects renal function remains unknown. Particle radioactivity (PR) is a critical component of PM2.5. A study on PR suggested that inhaled particulates could act as vectors for radionuclides, which may emit radiation after inhalation and deposition in the respiratory tract and promote an acute rise in blood pressure by changing the vascular structure or function via inflammation (Nyhan et al. 2018). And a recent study further showed the robust associations of PR with biomarkers of oxidative stress and inflammation (Li et al. 2018). Because hypertension is a critical risk factor for eGFR decline and CKD progression, and inflammation appears to increase kidney vulnerability and accelerate eGFR decline in older individuals (Goicoechea et al. 2008; Gupta et al. 2012; Kugler et al. 2015), we hypothesized that the level of PR from air pollution might also be related to renal function decline and risk of CKD.
We investigated whether short-term (≤28 days) low-level ambient PR is associated with an acute decline in eGFR and increased odds of CKD in an ongoing prospective cohort study of older men living in the Boston metropolitan area. We further sought to clarify the role of inflammation in the relationship between PR and renal function by exploring whether the associations of PR with eGFR and CKD were stronger in participants with higher levels of C-reactive protein (CRP) and fibrinogen, two plasma biomarkers of systemic inflammation.
2. Methods
2.1. Study design and population
The Normative Aging Study (NAS) is an ongoing longitudinal study on aging established by the U.S. Department of Veterans Affairs in 1963. Details of the study are published elsewhere (Mehta et al. 2016). Briefly, the NAS is a closed cohort of 2,280 male veterans living in the Greater Boston area. Participants were enrolled after an initial health screening to determine whether they were free of known chronic medical conditions. Most participants were examined up to four times between 1999 and 2015, and have been reevaluated every three to five years on a continuous rolling basis using detailed on-site physical examinations and questionnaires. Participants eligible for this study had continued participation as of 1999 when the data on renal function, PR, air pollution, and inflammation biomarkers started being collected, and kept living in the Greater Boston area. Because only a very small number of non-white participants were enrolled in the NAS cohort (89 visits from 22 participants) and the heterogeneity of race, a total of 2,491 medical visits from 809 white participants were used in the analysis. The NAS was approved by the Department of Veterans Affairs Boston Healthcare System and written informed consent was obtained from each participant.
2.2. Data collection
As previously described (Gao et al. 2019a; Mehta et al. 2016), participants were asked to provide detailed information about their lifestyles, dietary habits, activity levels, and demographic factors at each visit. Height and weight were used to calculate body mass index (BMI, in kg/m2). Blood samples were collected at each visit after overnight fasting to assess clinical biomarkers, such as total cholesterol (mg/dL), serum triglyceride (mg/dL), and high-density lipoprotein (HDL, mg/dL). Serum creatinine concentration (mg/dL) for estimating renal function was determined using Hitachi 747 analyzer (Boehringer-Mannheim Corp, Indianapolis, IN). Two inflammatory markers, C-reactive protein (CRP, mg/L) and fibrinogen (mg/dL), both highly related to the decline of eGFR and the development of CKD (Goicoechea et al. 2008; Gupta et al. 2012; Kugler et al. 2015), were measured. We determined plasma CRP concentrations with an immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics-Indianapolis, IN), and plasma fibrinogen was measured using MDA Fibriquick method (Bind et al. 2012). Systolic and diastolic blood pressures (SBP & DBP) were measured once in each arm while the subject was seated, using a standard cuff. Major diseases were assessed based on participants’ medical history and prior diagnoses (Nyhan et al. 2018).
2.3. Assessments of particle radioactivity, air particulate pollution levels, and weather variants
Short-term particle radioactivity, PM2.5, black carbon (BC), temperature, and relative humidity were measured on the day of the visit, and mean values were computed up to 28 days before the medical visit for each blood draw.
Methods for measurement of PR are detailed elsewhere (Nyhan et al. 2018). Gross β activity (unit: becquerel; Bq/m3), a measurement of all particle-bound ambient β activity, was used as a surrogate for PR. Gross β activity data were acquired from the U.S. Environmental Protection Agency’s (USEPA) RadNet monitoring network (https://www.epa.gov/radnet). The network comprises 139 stationary air monitor stations. Each air monitoring station is equipped with a total suspended particle high-volume air sampler collecting particles on filters (10-cm-diameter synthetic fiber). Filters are collected every 3-5 days and sent to the National Analytical Radiation Environmental Laboratory (NAREL) for measurement of gross β activity. The time between collection and measurement is approximately seven days, which allows time for the decay of short-lived radon progenies (e.g., 214Pb and 214Bi) that may be attached to the particles. Despite the decay of most of the short-lived radionuclides, there is still radioactivity from the decay of relatively long-lived thoron progenies (e.g., 210Pb and 210Bi). In the absence of real-time β activity, we used these data as a qualitative indicator of radiation activity of particles collected on the filter. All days within a sampling period were assigned the β activity measured from the respective sample. Gross β activity were measured at five monitors in the Greater Boston area (Boston, MA, Concord NH, Hartford CT, Providence RI and Worcester MA), which were highly correlated with each other (Table S1). We calculated a regional beta concentration for the study area by taking a standardized mean of these five monitors. This method prevents missing days at a monitor from adding false variability to the calculated mean daily value. In brief, we first calculated daily deviations from each monitor’s overall mean, and standardized these daily deviations by the monitor’s standard deviation. We then calculated the regional daily standardized deviation as the average of all monitor’s standardized deviations. Finally, we transformed this daily regional standardized deviation to daily β values by multiplying by the mean deviation of all monitors and adding back the average of all monitors. Due to the log-normal nature of the β measurements, all calculations were done on the log-transformed β values before being exponentiated to attain the final daily values. The final daily regional beta concentration was highly correlated with that from all five monitors in the Greater Boston area (Pearson R >0.8). Our final daily β values represent the daily PR exposure for our study population residing in the eastern Massachusetts area.
As previously described (Gao et al. 2019b; Mehta et al. 2015), concentrations of PM2.5 (μg/m3) and BC (μg/m3) were measured at the Harvard University supersite located near downtown Boston and approximately 1 km from the examination center. Since study participants live in the Greater Boston area within a median distance of 20 km, we assumed that these measurements could serve as surrogates of their exposure levels. Daily integrated PM2.5 concentrations were collected by the Harvard Impactor (Koutrakis et al. 1993), and BC was measured continuously using an Aethalometer (Magee Scientific Co., Model AE-16). We also obtained temperature and relative humidity data from the National Weather Service Station at Logan Airport (Boston, MA), located approximately 12 km from the examination site. Since study participants lived throughout the metropolitan area, we assumed that these temperature and humidity measurements could serve as surrogates of their exposures.
2.4. Renal function assessment
As previously described, serum creatinine (Scr) concentration was determined at each visit. We calculated eGFR (mL/min/1.73 m2) at each visit using the equation of Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [eGFR = 141 × min (Scr/0.9, 1)−0.411 × max (Scr/0.9, 1)−1.209 × 0.993Age] (Levey et al. 2009), and the equation of Modification of Diet in Renal Disease (MDRD) Study [eGFR = 175 × (Scr)−1.154 × (Age)−0.203] (Levey et al. 1999). The Pearson correlation coefficient between both estimates in our study was 0.96 and the intraclass Pearson correlation coefficients were ~0.7 for both forms of eGFR indicating a high degree of stability over time. CKD cases were identified by either form of eGFR of less than 60 mL/min/1.73 m2
2.5. Statistical analysis
Descriptive statistics were used to summarize socio-demographics and lifestyle factors for the first visit and the total visits of the participants. We examined whether the same-day and up to 28-day (7, 14, and 28 days before the study visit) average PR levels (as the exposures) were associated with both forms of eGFR (as the outcomes). We used time-varying linear mixed-effects regression models with random participant-specific intercepts (via PROC MIXED), accounting for the correlation of repeated measures. We applied three models increasingly adjusting for potential covariates. Model 1 adjusted for age (years), total cholesterol, triglycerides, HDL, SBP, DBP, season of medical visits (spring/ summer/ fall/ winter), corresponding temperature, and relative humidity. Model 2 additionally adjusted for BMI (underweight or normal weight/ overweight/ obese), smoking status (current/ former/ never smoker), alcohol intake (<2 drinks per day versus 2+ drinks per day). Model 3 additionally adjusted for hypertension, stroke, coronary heart disease (CHD), and diabetes diagnosed by physicians (yes/no). Effect estimate was reported as the mean difference in eGFR per interquartile range (IQR) increase in exposure, which was calculated separately for each exposure window studied. The IQR reflects the distribution (25th–75th percentile) in the observed data, while also enabling a comparison of the effects of different exposure types measured with different units. Similar analyses on the association of PR with the levels of CRP and fibrinogen were also performed using the same analysis models. Moreover, we estimated the effects of PR on the odds of CKD occurring using logistic regression models with generalized estimating equations adjusting for all potential covariates and the random participant-specific intercepts accounting for the correlation of repeated measures.
We further explored how the independent PR-eGFR associations changed with and without adjusting for other air pollutants. We included PM2.5 or BC in the fully-adjusted regression models for the PR-eGFR relationship and compared the coefficients of PR with those from the main analysis models. We also examined whether the potential interactions of PR with PM2.5 or BC levels could affect the change of eGFR by adding interaction terms. Additionally, to understand whether systemic inflammation modified the associations of PR with eGFR and the odds of CKD, we performed subgroup analyses by stratifying the PR-eGFR and PR-CKD associations based on the tertiles of CRP and fibrinogen levels.
2.6. Sensitivity analysis
Healthier study participants may be more likely to participate in subsequent clinical examinations over time (Seaman and White 2013). To evaluate the validity of missing at random assumption and assess the impact of potential selection bias caused by non-random unavailability for follow-up, we used inverse probability weighting (IPW) to correct for this potential survival bias as a sensitivity analysis. We estimated the inverse probability of coming to a subsequent clinical visit using logistic regressions given all relevant covariates at the previous visit among eligible participants: age, BMI, smoking status and pack-years, cholesterol level, hypertension, and medication use (diuretics and beta blockers). Rather than a constant of ‘1’, the inverse probability of being included in the analysis was assigned to the baseline visit. A weighted model simultaneously adjusted for the inverse probability and the aforementioned potential covariates of the main analysis. Furthermore, we re-performed all main analyses in the subset of participants who were free of CKD at the initial visit, to remove possible confounding effects from the risk of CKD at the baseline (Levey et al. 2009).
SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used to perform data cleaning and all analyses. A two-sided p-value <0.05 was considered to be statistically significant.
3. Results
3.1. Characteristics of participants and distributions of environmental exposures
Table 1 describes the characteristics of the 809 study participants. At the first visit included in the analysis, participants were on average 72 years old with an average CRP of 3.2 mg/L and fibrinogen of 342 mg/dL. More than 60% of participants were former smokers and less than 5% were current smokers. Most participants were overweight or obese and consumed less than two drinks per day. Participants were healthier at the first visit than at the subsequent visits in terms of the prevalence of major diseases. Most visits were conducted between March and November. At the first visit, 178/809 participants (~22%) had CKD, and 746/2491 (~30%) of the total visits involved participants with CKD. Average values for ambient PR and air particulate pollution levels over the course of follow-up remained stable across 1 - 28 days (Table 2). The 28-day average level was 2.90±1.08 ×10−4 Bq/m3 (IQR=1.43) for PR, 9.67±2.75 μg/m3 (IQR=3.55) for PM2.5, and 5.39±2.71 ×10−1 μg/m3 (IQR=3.17) for BC. All mutual correlations between the above-listed air pollutants were statistically significant (Table 3, p-value<0.05).
Table 1.
Characteristics of participants reported upon first clinical examination and averaged across all examinations a
| Characteristics | First visit (N=809) | All visits (N=2491) |
|---|---|---|
| Age (years) | 72.32 (6.65) | 75.71 (7.07) |
| Total cholesterol (mg/dL) | 199.93 (38.04) | 183.90 (38.22) |
| Serum triglyceride (mg/dL) | 144.54 (93.60) | 128.49 (76.18) |
| HDL cholesterol (mg/dL) | 49.29 (12.87) | 48.96 (13.02) |
| Systolic blood pressure (mm Hg) | 133.50 (16.88) | 129.13 (17.53) |
| Diastolic blood pressure (mm Hg) | 78.25 (9.21) | 71.51 (10.65) |
| C-reactive protein (CRP, mg/L) | 3.16 (5.90) | 3.09 (7.74) |
| Fibrinogen (mg/dL) b | 341.89 (87.65) | 339.75 (80.96) |
| Smoking status c | ||
| Current smoker | 36 (4.5%) | 100 (4.0%) |
| Former smoker | 527 (65.2%) | 1596 (64.2%) |
| Never smoker | 245 (30.3%) | 792 (31.8%) |
| Body mass index (BMI) | ||
| Underweight or normal weight (<25) | 157 (19.4%) | 576 (23.1%) |
| Overweight (≥25 to <30) | 430 (53.2%) | 1268 (50.9%) |
| Obese (≥30) | 222 (27.4%) | 647 (26.0%) |
| Alcohol consumption (2+ drinks per day) | 158 (19.5%) | 464 (18.6%) |
| Major diseases | ||
| Hypertension | 574 (71.0%) | 1896 (76.1%) |
| Stroke | 58 (7.2%) | 204 (8.2%) |
| Coronary heart disease (CHD) | 236 (29.2%) | 866 (34.8%) |
| Diabetes | 110 (13.6%) | 392 (15.7%) |
| Season of visit | ||
| Spring | 194 (24.0%) | 591 (23.7%) |
| Summer | 218 (26.9%) | 707 (28.4%) |
| Fall | 243 (30.0%) | 773 (31.0%) |
| Winter | 154 (19.1%) | 420 (16.9%) |
| Chronic kidney disease (CKD) | 178 (22.0%) | 746 (29.9%) |
Mean values (standard deviation) for continuous variables and n (%) for categorical variables;
Data missing for 460 visits;
Data missing for 64 visits
Table 2.
Distributions of particle radioactivity, PM2.5, and black carbon for all visits from NAS
| Exposure (Unit) | Exposure window (days) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
7 |
14 |
21 |
28 |
||||||
| Mean (SD) | IQR | Mean (SD) | IQR | Mean (SD) | IQR | Mean (SD) | IQR | Mean (SD) | IQR | |
| Particle radioactivity (Bq/m3, × 10−4) | 2.90 (1.08) | 1.43 | 2.84 (0.88) | 1.19 | 2.83 (0.74) | 1.03 | 2.84 (0.67) | 0.93 | 2.84 (0.62) | 0.84 |
| PM2.5 (μg/3) | 9.75 (5.94) | 6.25 | 9.55 (3.34) | 4.15 | 9.58 (2.93) | 3.79 | 9.62 (2.78) | 3.61 | 9.67 (2.75) | 3.55 |
| Black carbon (μg/m3, ×10−1) | 5.93 (2.81) | 4.26 | 5.37 (2.81) | 3.37 | 5.36 (2.73) | 3.24 | 5.37 (2.74) | 3.16 | 5.39 (2.71) | 3.17 |
SD = standard deviation; IQR = interquartile range
Table 3.
Correlation matrix of 28-day average levels of particle radioactivity, PM2.5, and black carbon (Spearman Coefficient)
| Exposure | Particle radioactivity | PM2.5 | Black carbon |
|---|---|---|---|
| Particle radioactivity | 1 | ||
| PM2.5 | 0.426 | 1 | |
| Black carbon | 0.303 | 0.325 | 1 |
Bolded coefficients have a p-value <0.05
3.2. Associations of particle radioactivity with renal function and chronic kidney disease
PR demonstrated robust negative associations with both forms of eGFR even after adjusting for all potential covariates (Table 4). Estimated magnitudes of the associations were the strongest on the same day of visit and slightly attenuated in the next 28 days. An IQR higher PR level on the same day was significantly associated with 0.84-mL/min/1.73 m2 lower eGFR estimated by the CKD-EPI method [95% confidence interval (CI): −1.41, −0.27] and 1.03-mL/min/1.73 m2 lower eGFR estimated by the MDRD method (95% CI: −1.68, −0.37). An IQR higher 28-day average PR level was significantly associated with 0.83-mL/min/1.73 m2 lower eGFR estimated by the CKD-EPI method (95% CI: −1.46, −0.20) and 0.93-mL/min/1.73 m2 lower eGFR estimated by the MDRD method (95% CI: −1.66, −0.20). The 14-day average PR level was marginally associated with both determinations of eGFR with the same decreasing pattern. However, PR was not associated with the odds of CKD (Table S2). The 28-day average PR level was marginally associated with the odds of CKD [Odds ratio (OR) = 1.15; 95% CI: 0.99, 1.34; p-value = 0.06]. A fluctuation in PR impact on eGFR was also observed, the coefficients of PR for both eGFR were the strongest for the same-day PR level and were then slightly attenuated.
Table 4.
Associations between 28-day average particle radioactivity level and renal function (eGFR)
| Exposures | Model 1 a |
Model 2 b |
Model 3 c |
||||
|---|---|---|---|---|---|---|---|
| Change per IQR (SE) | p-value | Coefficients (SE) | p-value | Coefficients (SE) | p-value | ||
| eGFR (CKD-EPI) | |||||||
| Exposure window (days) | 1 | −0.770 (0.291) | 0.008 | −0.777 (0.290) | 0.007 | −0.840 (0.289) | 0.004 |
| 7 | −0.879 (0.310) | 0.005 | −0.896 (0.309) | 0.004 | −0.905 (0.308) | 0.003 | |
| 14 | −0.605 (0.324) | 0.062 | −0.617 (0.323) | 0.056 | −0.644 (0.322) | 0.045 | |
| 21 | −0.699 (0.332) | 0.035 | −0.720 (0.331) | 0.030 | −0.728 (0.330) | 0.028 | |
| 28 | −0.808 (0.325) | 0.013 | −0.823 (0.325) | 0.011 | −0.830 (0.323) | 0.010 | |
| eGFR (MDRD) | |||||||
| Exposure window (days) | 1 | −0.955 (0.335) | 0.004 | −0.964 (0.334) | 0.004 | −1.027 (0.333) | 0.002 |
| 7 | −0.993 (0.357) | 0.006 | −1.011 (0.356) | 0.005 | −1.019 (0.355) | 0.004 | |
| 14 | −0.606 (0.372) | 0.104 | −0.620 (0.372) | 0.095 | −0.647 (0.371) | 0.081 | |
| 21 | −0.743 (0.381) | 0.052 | −0.768 (0.381) | 0.044 | −0.774 (0.380) | 0.042 | |
| 28 | −0.910 (0.374) | 0.015 | −0.929 (0.373) | 0.013 | −0.932 (0.372) | 0.012 | |
Model adjusted for age (years), total cholesterol, triglycerides, HDL, SBP, DBP, season of medical visits (spring/ summer/ fall/ winter), temperature (°C), and relative humidity (%);
Model additionally adjusted for BMI (underweight or normal weight/ overweight/ obese), smoking status (current/ former/ never smoker), and alcohol intake (<2 drinks per day versus 2+ drinks per day;
Model additionally adjusted for hypertension, stroke, coronary heart disease (CHD), and diabetes diagnosed by physicians (yes/no)
Figure 1 and Table S3 illustrate the coefficients of PR in the PR-eGFR associations with and without mutually adjusting for PM2.5 or BC. Estimates of PR were slightly attenuated after adjusting for other air pollutants, except for the 7-day average PR level for eGFR (CKD-EPI). Although estimates of PR were slightly reduced for >7 days of ambient PR levels, their magnitudes were in the same direction. Similarly, both PM2.5 and BC showed negative correlations with eGFR in the 28-day exposure window separately, while the estimates were attenuated after adjusting for PR (Figure S1). We further tested whether the interactions of PR with PM2.5 or BC in the models with two exposure variables, but did not observe any statistically significant results (p-value <0.05).
Figure 1.
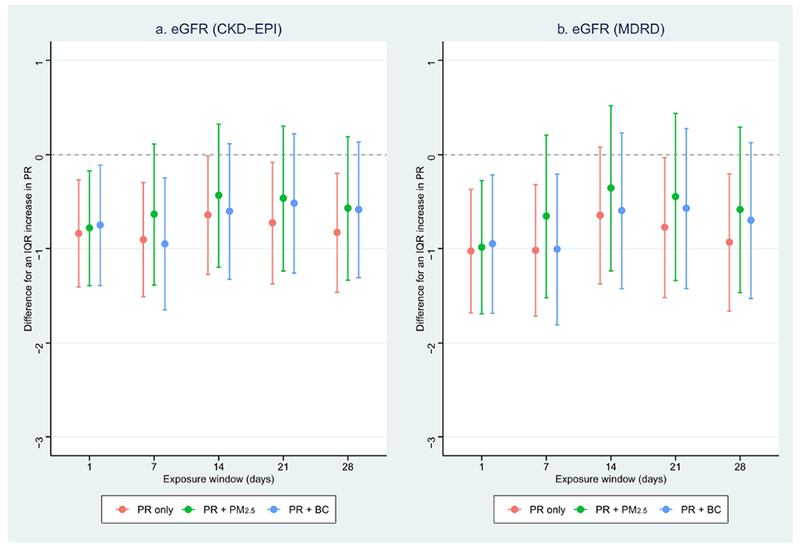
Associations between particle radioactivity and kidney function in the exposure window of 28 days with and without controlling for air pollution
PR: particle radioactivity; BC: black carbon; eGFR: estimated glomerular filtration rate
Dots: Point estimates; Error bars: Confidence intervals;
Red: Model with particle radioactivity only;
Green: Model with particle radioactivity and PM2.5;
Blue: Model with particle radioactivity and black carbon;
PR was negatively correlated with levels of CRP and fibrinogen, though not statistically significant (data not shown). However, after stratifying the observations based on the tertiles of CRP and fibrinogen levels (Table 5), the PR-eGFR and PR-CKD risk associations showed monotonic decreasing and increasing patterns, respectively, with the tertiles of both markers. An IQR higher 28-day PR level was significantly associated with ~1.9-mL/min/1.73 m2 lower eGFR (CKD-EPI) and ~2.3-mL/min/1.73 m2 lower eGFR (MDRD) among individuals with the highest levels of CRP and fibrinogen (3rd tertile), respectively. Further, an IQR higher 28-day average PR level was positively associated with the odds of CKD by ~34% among individuals with the highest levels of CRP (OR = 1.34, 95% CI: 1.04, 1.72; p-value = 0.011) and fibrinogen (OR = 1.34, 95% CI: 0.98, 1.83; p-value = 0.067). Similar decreasing/increasing patterns were observed for same-day, 7-day, 14-day, and 21-day average PR levels (Table S4).
Table 5.
Association of 28-day average particle radioactivity levels with renal function (eGFR) and the relative odds of chronic kidney diseases (CKD) stratified by CRP and fibrinogen levels a
| Exposures | eGFR (CKD-EPI) |
eGFR (MDRD) |
Odds of CKD |
|||||
|---|---|---|---|---|---|---|---|---|
| Change per IQR (SE) | p-value | Coefficients (SE) | p-value | Ncase/NTotal | Odds ratio (95% CI) | p-value | ||
| CRP (mg/L) b | ||||||||
| Tertile | 1 (<0.91) | 0.021 (0.542) | 0.970 | −0.343 (0.649) | 0.598 | 189/810 | 1.01 (0.76 – 1.34) | 0.949 |
| 2 (0.91 ~ <2.25) | −1.175 (0.621) | 0.060 | −1.092 (0.737) | 0.140 | 247/809 | 1.21 (0.95 – 1.55) | 0.273 | |
| 3 (≥2.25) | −1.951 (0.697) | 0.006 | −2.379 (0.763) | 0.002 | 298/808 | 1.34 (1.04 – 1.72) | 0.011 | |
| Fibrinogen (mg/dL) c | ||||||||
| Tertile | 1 (<302) | −0.380 (0.604) | 0.529 | −0.535 (0.720) | 0.459 | 179/679 | 1.16 (0.89 – 1.51) | 0.265 |
| 2 (302 ~ <362) | −0.989 (0.634) | 0.121 | −1.173 (0.756) | 0.123 | 203/675 | 1.18 (0.88 – 1.50) | 0.283 | |
| 3(≥362) | −1.881 (0.787) | 0.018 | −2.331 (0.861) | 0.007 | 258/677 | 1.34 (0.98 – 1.83) | 0.067 | |
Model adjusted for age (years), total cholesterol, triglycerides, HDL, SBP, DBP, season of medical visits (spring/ summer/ fall/ winter), temperature (°C), and relative humidity (%), BMI (underweight or normal weight/ overweight/ obese), smoking status (current/ former/ never smoker), and alcohol intake (<2 drinks per day versus 2+ drinks per day), hypertension, stroke, coronary heart disease (CHD), and diabetes (yes/no);
Data missing for 64 visits;
Data missing for 460 visits
3.3. Sensitivity analyses
Sensitivity analysis controlling for IPW yielded essentially unchanged estimates of PR at each time point from those at base case analysis, suggesting that the results are not biased by loss to follow-up. Another sensitivity analysis among participants without CKD at the first visit also showed similar results to the main analyses, indicating that confounding effect from the lack of normal renal function at initial visit on the identified PR-eGFR relationship is limited (data not shown).
4. Discussion
We investigated the effect of short-term ambient PR level on renal function by assessing eGFR using longitudinal data with repeated measurements among 809 older males. After fully adjusting for potential covariates, PR level (gross β activity) demonstrated strong associations with two forms of eGFR, but not with the odds of CKD. Even though controlling for PM2.5 or BC in the analysis model attenuated the effects of PR, the overall magnitudes and directions of PR effects remained substantially unchanged. CRP and fibrinogen modified the PR-eGFR and PR-CKD relationships with much stronger associations at the highest levels of CRP and fibrinogen (3rd tertile). Our findings indicate that short-term PR levels are associated with renal function decline, which may be modified by systemic inflammation.
Previous studies observed renal dysfunction caused by high-level radiation exposure among patients who received radiation therapies or who survived whole-body irradiation (Adams et al. 2012; Valkema et al. 2005). Our study expands the understanding of radiation by providing evidence that low-level exposure to PR in daily activity could also significantly affect renal function in the short-term. We further observed a fluctuation of PR impact on eGFR; the coefficients of PR for both eGFR estimations were strongest for the same-day PR level and later became slightly attenuated. This might be explained by the short half-life (<1 day) of some radon progenies, such as 214Pb and 214Bi. Additionally, controlling for PM2.5 and BC attenuated the coefficients of PR effects, especially for the >7 days average PR levels, indicating that not only the PR, but also other PM components may contribute to the decline of renal function. More exposure error of PR than that of PM2.5 and BC might also contribute to the changing coefficients after the mutual adjustment (Nyhan et al. 2018). As the PR effects were higher in participants exposed to higher levels of PM or BC, Since low eGFR has also been associated with the risk of CVD (Gansevoort et al. 2013), the identified PR-eGFR relationship suggests a possible linkage of PR with the risk of CVD and indicates that low-level exposure to environmental radiation is likely to be a neglected, yet crucial environmental risk factor for CVD.
Since ambient PR levels were recently linked to elevated blood pressure (Nyhan et al. 2018), a risk factor for renal dysfunction and CKD (Gansevoort et al. 2013), we adjusted for blood pressure and hypertension in our analysis models to exclude any effect of blood pressure on the PR-eGFR/CDK relationship. Our significant findings suggest that PR could lead to renal function decline via mechanisms other than increasing blood pressure. Accordingly, we did not observe an increasingly intensified negative association between PR and eGFR in response to the increased exposure window. Previous studies have revealed associations between air pollution and vascular/endothelial dysfunction, and the latter has been reported to be associated with eGFR decline (Krishnan et al. 2012). We therefore hypothesize that the pathways related to vascular/endothelial dysfunction may mediate the association between ambient PR and eGFR decline. Nevertheless, we note that emerging evidence also suggests that exposure to air pollutants including PR can cause oxidative stress, disorders of metabolic traits, such as glucose intolerance, decreased insulin sensitivity, higher blood lipid concentrations, weight gain, and increased risk of diabetes mellitus (Chen et al. 2016; Li et al. 2018; Wei et al. 2016; Wolf et al. 2016). Such mechanistic pathways might also play roles in the observed PR-eGFR relationship (Gansevoort et al. 2013). Thus, the pathophysiological mechanisms linking PR to renal function decline and CKD remain incompletely understood, necessitating further exploration of the mechanistic underpinnings.
Mounting evidence points to systemic inflammation as a mechanism through which air pollution could trigger vascular/endothelial dysfunction and promote renal dysfunction and other cardiovascular outcomes (Bind et al. 2012; Delfino et al. 2008). But we observed a non-significant negative associations between PR and the two biomarkers of systemic inflammation, which is consistent with the findings of Li et al. in Framingham Heart Study (Li et al. 2018). Given the direct associations of the two biomarkers with CKD described in previous reports (Goicoechea et al. 2008; Gupta et al. 2012; Kugler et al. 2015) and their modification of the PR-eGFR and PR-CKD relationships identified in our study, we suggest that higher levels of systemic inflammation could make renal function more vulnerable to PR. However, since Li et al. also reported robust associations between PR and other inflammation biomarkers such as interleukin-6, 8-epi-prostaglandin F2α, and ICAM-1 (Li et al. 2018), this suggests that the adverse effects of low-dose radiation exposure might be mediated by the inflammation-related pathways other than CRP and fibrinogen. Further studies with larger population n are warranted to explore the role of inflammation in the PR-renal function relationship.
Strengths of this study include the longitudinal study design, repeated measurements of serum creatinine-derived eGFR and environmental exposures, and adjustment for multiple potential confounders and individual-level risk factors. Several limitations should be noted, however. First, both equations for determining eGFR did not account for non-GFR determinants that could affect eGFR, such as muscle mass and diet, which may cause overestimation of serum creatinine, particularly for participants with preserved renal function. Future studies should consider using other methods to determine eGFR. Furthermore, there may be unmeasured confounding from other factors associated with eGFR that may be correlated with PR, such as the environmental noise from traffic, aircraft, and railway, which is associated with markers of CVD (Munzel et al. 2014). Whether environmental noise is associated with renal function is unknown. Additionally, we used PR measurements acquired from the RadNet monitoring network. As per the U.S. EPA’s protocol, integrated filter samples were collected over several days (typically from 5 to 7 days). Since gross β activity is measured several days after the end of the multi-day sampling period, a certain fraction of short-lived radionuclides may have partially decayed and their contribution to PR may not be fully captured by the β value. Finally, participants in this study were older white males, limiting the generalizability of our results to other racial/ethnic groups and women.
5. Conclusions
In conclusion, our study demonstrates that low-level environmental radioactivity received from air pollution is significantly associated with a decline in renal function and could be modified by systemic inflammation. Due to the worldwide exposure to low levels of ionizing radiation, our findings may be widely implemented into the evaluation and prevention of CKD and other circulatory diseases in response to radiation exposure. To date, research on the adverse effects of air-pollution PR is quite limited. Understanding the roles of chemical, biological or physical particle properties may open new avenues to future preventive and regulatory efforts. Future multidisciplinary studies are required to validate our findings in larger cohorts and to determine the biological connections between PR and renal function.
Supplementary Material
Highlights.
Particle radioactivity has been largely neglected by studies of health effects.
Few studies examined association between particle radioactivity and renal function.
Short-term particle radioactivity is associated with the decline of renal function.
Systemic inflammation may modify this adverse effect of particle radioactivity.
Acknowledgments:
The authors would like to thank all Normative Aging Study participants and Dr. Sarah Rasmussen for sharing the codes adapted for inverse probability weighting.
Sources of funding: This work was supported by the National Institute of Environmental Health Sciences (grants P30ES009089, R01ES021733, R01ES025225, and R01ES027747). The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center in Boston, Massachusetts. This work was also supported by U.S. EPA grant (RD-834798 and RD-835872) through the Harvard University U.S. EPA sponsored Air, Climate & Environment (ACE) center. Contents of this study are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, the U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Abbreviations
- PM2.5
fine particulate matter with diameter <2.5 μm
- PR
particle radioactivity
- eGFR
estimated glomerular filtration rate
- CKD
chronic kidney disease
- CRP
C-reactive protein
- BMI
body mass index
- HDL
high-density lipoprotein
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- IPW
inverse probability weighting
- NAS
Normative Aging Study
- USEPA
U.S. Environmental Protection Agency
- BC
black carbon
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- MDRD
Modification of Diet in Renal Disease
- IQR
interquartile range
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflict of interest to disclose.
References
- Adams MJ, Grant EJ, Kodama K, Shimizu Y, Kasagi F, Suyama A, et al. 2012. Radiation dose associated with renal failure mortality: a potential pathway to partially explain increased cardiovascular disease mortality observed after whole-body irradiation. Radiat Res 177:220–228. PMID:22149958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, et al. 2012. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology 23:332–340. PMID:22237295. doi: 10.1097/EDE.0b013e31824523f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. 2017. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: a cohort study. Lancet Planet Health 1:e267–e276. PMID:29851625. doi: 10.1016/S2542-5196(17)30117-1 [DOI] [PubMed] [Google Scholar]
- Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. 2018. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 29:218–230. PMID:28935655. doi: 10.1681/ASN.2017030253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg-Gresham J, Morgenstern H, McClellan W, Saydah S, Pavkov M, Williams D, et al. 2018. County-level air quality and the prevalence of diagnosed chronic kidney disease in the US Medicare population. PLoS One 13:e0200612 PMID:30063741. doi: 10.1371/journal.pone.0200612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121:2331–2378. PMID:20458016. doi: 10.1161/CIR.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- Chan TC, Zhang Z, Lin BC, Lin C, Deng HB, Chuang YC, et al. 2018. Long-term exposure to ambient fine particulate matter and chronic kidney disease: a cohort study. Environ Health Perspect 126:107002 PMID:30392394. doi: 10.1289/EHP3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, et al. 2016. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 39:547–554. PMID:26868440. doi: 10.2337/dc15-1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, et al. 2008. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect 116:898–906. PMID:18629312. doi: 10.1289/ehp.11189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. 2013. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382:339–352. PMID:23727170. doi: 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- Gao X, Colicino E, Shen J, Just AC, Nwanaji-Enwerem JC, Wang C, et al. 2019a. Comparative validation of an epigenetic mortality risk score with three aging biomarkers for predicting mortality risks among older adult males. Int J Epidemiol. PMID:31038702. doi: 10.1093/ije/dyz082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Colicino E, Shen J, Kioumourtzoglou MA, Just AC, Nwanaji-Enwerem JC, et al. 2019b. Impacts of air pollution, temperature, and relative humidity on leukocyte distribution: An epigenetic perspective. Environ Int 126:395–405. PMID:30826618. doi: 10.1016/j.envint.2019.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea M, de Vinuesa SG, Gomez-Campdera F, Aragoncillo I, Verdalles U, Mosse A, et al. 2008. Serum fibrinogen levels are an independent predictor of mortality in patients with chronic kidney disease (CKD) stages 3 and 4. Kidney Int Suppl:S67–70. PMID:19034331. doi: 10.1038/ki.2008.519 [DOI] [PubMed] [Google Scholar]
- Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, et al. 2012. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7:1938–1946. PMID:23024164. doi: 10.2215/CJN.03500412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempner ES. 2011. Direct effects of ionizing radiation on macromolecules. J Polym Sci B Polym Phys 49:827–831. PMID:21643521. doi: 10.1002/polb.22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutrakis P, Sioutas C, Ferguson ST, Wolfson JM, Mulik JD, Burton RM. 1993. Development and evaluation of a glass honeycomb denuder filter pack system to collect atmospheric gases and particles. Environmental Science & Technology 27:2497–2501. PMID:WOS:A1993ME57800043. doi:DOI 10.1021/es00048a029 [DOI] [Google Scholar]
- Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, et al. 2012. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution). J Am Coll Cardiol 60:2158–2166. PMID:23103035. doi: 10.1016/j.jacc.2012.08.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler E, Cohen E, Goldberg E, Nardi Y, Levi A, Krause I, et al. 2015. C reactive protein and long-term risk for chronic kidney disease: a historical prospective study. J Nephrol 28:321–327. PMID:24981713. doi: 10.1007/s40620-014-0116-6 [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470. PMID:10075613. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. PMID:19414839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Nyhan MM, Wilker EH, Vieira CLZ, Lin H, Schwartz JD, et al. 2018. Recent exposure to particle radioactivity and biomarkers of oxidative stress and inflammation: The Framingham Heart Study. Environ Int 121:1210–1216. PMID:30376999. doi: 10.1016/j.envint.2018.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Kubzansky LD, Coull BA, Kloog I, Koutrakis P, Sparrow D, et al. 2015. Associations between air pollution and perceived stress: the Veterans Administration Normative Aging Study. Environ Health 14:10 PMID:25627872. doi: 10.1186/1476-069X-14-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, et al. 2016. Long-term exposure to ambient fine particulate matter and renal function in older men: The Veterans Administration Normative Aging Study. Environ Health Perspect 124:1353–1360. PMID:26955062. doi: 10.1289/ehp.1510269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Gori T, Babisch W, Basner M. 2014. Cardiovascular effects of environmental noise exposure. Eur Heart J 35:829–836. PMID:24616334. doi: 10.1093/eurheartj/ehu030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhan MM, Coull BA, Blomberg AJ, Vieira CLZ, Garshick E, Aba A, et al. 2018. Associations between ambient particle radioactivity and blood pressure: The NAS (Normative Aging Study). J Am Heart Assoc 7 PMID:29545261. doi: 10.1161/JAHA.117.008245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Schadt EE. 2014. The role of macromolecular damage in aging and age-related disease. J Gerontol A Biol Sci Med Sci 69 Suppl 1:S28–32. PMID:24833583. doi: 10.1093/gerona/glu056 [DOI] [PubMed] [Google Scholar]
- Seaman SR, White IR. 2013. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 22:278–295. PMID:21220355. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. 2005. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med 46 Suppl 1:83S–91S. PMID:15653656. [PubMed] [Google Scholar]
- Wei Y, Zhang JJ, Li Z, Gow A, Chung KF, Hu M, et al. 2016. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB J 30:2115–2122. PMID:26891735. doi: 10.1096/fj.201500142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, et al. 2016. Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes 65:3314–3326. PMID:27605624. doi: 10.2337/db15-1567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


