ABSTRACT
Although the lower limit of normal (LLN) of FEV1/FVC detects at-risk patients for postoperative outcomes among Japanese chronic obstructive pulmonary disease (COPD) patients with resected lung cancer, there was a lack of a Japanese reference equation to calculate the LLN of FEV1/FVC. Renewed Japanese spirometric reference variables might enable us to verify clinical impact of the LLN of FEV1/FVC among the Japanese population. To evaluate the clinical impact of the LLN of FEV1/FVC by using this renewed reference, data were retrospectively analyzed from 609 newly diagnosed lung cancer patients who had undergone thoracic surgery between 2006 and 2011. The combined assessment of the 0.70 fixed ratio and the LLN of the FEV1/FVC ratio classified the 609 subjects into the COPD (214 subjects), non-COPD (337 subjects), and in-between (58 subjects) groups, respectively. All of the relative odds ratios (ORs) of postoperative outcomes for the comparison between the in-between and non-COPD groups did not show significant confidence intervals (CIs). On the other hand, the adjusted ORs of postoperative outcomes for the COPD group versus the non-COPD group were 2.840 (95% CI: 1.824–4.421) for prolonged oxygen therapy (POT), 1.836 (95% CI: 1.166–2.890) for prolonged postoperative stays, and 1.637 (95% CI: 1.007–2.663) for combined complications. Adjusted comparisons of POT between the in-between and COPD groups also showed a significant relative OR of 2.984 (95% CI: 1.447–6.153). A standardized assessment of the LLN of FEV1/FVC by a renewed Japanese spirometric reference provides risk stratification for postoperative outcomes in the population.
Key Words: chronic obstructive lung disease, lower limit of normal, Japanese, thoracic surgery, lung cancer
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) and lung cancer are projected to continue contributing to an increase in the overall worldwide burden of disease until 2020.1 Mounting evidence suggest a high prevalence of COPD among lung cancer patients.2-4 Postoperative complications among patients with resected lung cancer often need unexpected treatments and consequently result in an enormous economic burden. In comparing patients undergoing thoracic surgery, we found that COPD patients defined by the 0.70 fixed ratio of forced expiration volume in 1 second (FEV1)/forced vital capacity (FVC) had an average postoperative stay that was 61% higher, and a 100% greater need of prolonged oxygen therapy (POT), than patients without COPD.5 Thus, we face an urgent need to establish satisfactory preoperative pulmonary assessments which provide better risk stratification for postoperative outcomes among COPD patients with resected lung cancer.6
Our previous data suggests that airway obstruction defined by the lower fifth percentile of a large healthy reference group-the statistically defined lower limit of normal (LLN)-of FEV1/FVC might be a more important predictor of COPD-related outcomes than that defined by the 0.7 fixed ratio.7-9 Nevertheless, the study has a limitation to utilize a Caucasian reference equation, due to the lack of a Japanese reference equation.8,9 Meanwhile, the spirometric reference variables for the Japanese adult population has recently developed by using the lambda-mu-sigma (LMS) method.10 Whether the renewed Japanese reference could classify the clinical impact of COPD-related outcomes remains elusive.
In the present study, we verified that the LLN-determined airway obstruction might identify at-risk patients on postoperative outcomes by using the new spirometric reference variables for the Japanese population.
PATIENTS AND METHODS
Study population
The medical records of patients with newly diagnosed lung cancer who were sequentially registered and who had undergone thoracic surgery at the Nagoya University Hospital from January 2006 to December 2011 were retrospectively reviewed. The study was approved by the Institutional Review Board of Nagoya University Graduate School of Medicine (Approved date: April 21st 2014; Approved #: 2014-0052). Requirement of written informed consent was waived due to the retrospective design of the study. The medical records of 712 patients with newly diagnosed lung cancer who underwent spirometry and thoracic surgery were reviewed. Twenty-seven patients were excluded from the analysis for the following reasons: preoperative pulmonary assessment by spirometry including FEV1/FVC was not performed (n=8); the findings of emphysema or fibrosis were not evaluated by thin-section CT examination (n=13); combined surgery for lung cancer and other diseases (n=4); emergency thoracic surgery (n=1); and pathologic stage IV disease (n=1). We recently demonstrated that combined pulmonary fibrosis and emphysema (CPFE) and pulmonary fibrosis affect postoperative and survival outcomes among patients with resected lung cancer.11 Therefore, we excluded 76 patients with fibrosis or CPFE from the study population to evaluate the impact of the LLN-determined airway obstruction. Finally, the study population comprised 609 patients (85.5%).
Data collection for patient characteristics and pulmonary function variables by spirometry
Information was obtained retrospectively from the hospital records as previously reported.4,5,8,9,11 Spirometry was performed with a calibrated dry spirometer, (FUDAC-77; Fukuda Denshi Co., Ltd., Tokyo, Japan), according to the American Thoracic Society (ATS) standards applied in our hospital.12 Airflow obstruction was functionally defined by a FEV1/FVC ratio of 0.7 or LLN.10 The LLN of FEV1 and FVC were calculated by using the reference equations of the Japanese healthy non-smokers (J-LLN).10 Subjects were assigned to the COPD, in-between, and non-COPD groups as previously reported (Figure 1).8 Briefly, subjects were assigned to the COPD group if airflow obstruction, determined by the FEV1/FVC ratio, was below 0.70 and below the LLN. If airflow obstruction, according to the FEV1/FVC, was below 0.70 but above the LLN (the below-0.7 subgroup), or if the FEV1/FVC was above 0.70 but below the LLN (the above-0.7 subgroup), subjects were assigned to the in-between group. Subjects that fitted neither criteria were assigned to the non-COPD group. To evaluate the impact of the COPD group on survival outcomes, subjects were assigned to the COPD group, if airflow obstruction determined by the FEV1/FVC ratio was below 0.7 and below the LLN.9 The remaining subjects were also assigned to the other group, including the in-between and non-COPD groups.9
Fig. 1.
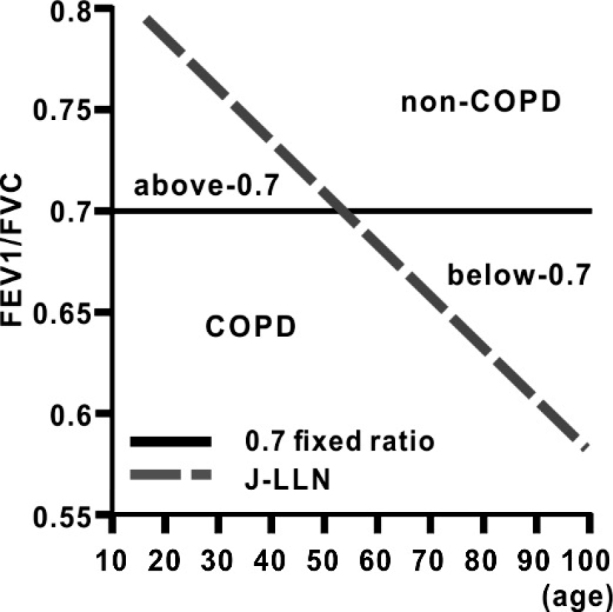
Diagram depicting the 0.7 fixed ratio and the J-LLN of the FEV1/FVC
Diagram depicting the 0.7 fixed ratio of the FEV1/FVC and the decline of the LLN of FEV1/FVC with ageing. The non-COPD indicates the non-COPD group. The COPD indicates the COPD group. The above-0.7 indicates the above-0.7 subgroup among the in-between group. The below-0.7 indicates the below-0.7 subgroup among the in-between group. Solid, the 0.7 fixed ratio of FEV1/FVC; Dash, the J-LLN of FEV1/FVC according to age.
Assessment of emphysema and fibrosis on chest CT
Involvement of emphysema was evaluated by thin-section computed tomography (TSCT) as previously reported.11 After the initial interpretation by at least two radiologists, the evaluation was performed by a chest radiologist (S.I.) who had 20 years of experiences in reading thoracic CT. The severity of emphysema and fibrosis was visually evaluated as previously reported.11 Information on postoperative outcomes and long-term survival was not given to the radiologists during their TSCT evaluation.
Postoperative complications and outcome data collection
In our hospital, all patients received standardized care according to the clinical practices in use at our institution for inpatients. Prolonged oxygen therapy (POT), prolonged postoperative stay (PPS), and postoperative complications were evaluated based on the definition previously reported.5,8 Overall survival (OS) was measured from the date of surgery to the date of death (event) or last date the patient was known to be alive (censored).11,13 Disease-free survival (DFS) was defined as the time from the date of surgery to the first date of recurrence of cancer, or death from any cause.11,13 Date of recurrence was obtained by reviewing the hospital records of all patients.11,13 For the patients who were referred to other hospitals for further support, we contacted the relevant primary physicians to obtain follow-up information.11,13
Statistical analysis
All data were checked for completeness, and the analyzed variables were tested for normality of distribution by the Shapiro-Wilk test. Normally distributed variables were compared among the groups by one-way analysis of variance (ANOVA) and non-normally distributed variables by the Kruskal-Wallis test. Comparisons between the proportions were made using the χ2 test or with Fisher’s exact test. Survival curves were estimated by using the Kaplan-Meier method. Associations between LLN-determined airflow obstruction and survival outcomes were evaluated via the log-rank test. Multivariable logistic regression models were prepared to estimate the risk factors for postoperative outcomes. Inclusion of variables in the models was based on existing knowledge of the risk factors for postoperative outcomes including age, gender, smoking history, and operation time.8 Statistical analyses were performed with PASW Statistics version 24.0 (SPSS Inc., Chicago, IL), and a P value of less than 0.05 was considered statistically significant.
RESULTS
Postoperative outcomes among the study population classified by the J-LLN of FEV1/FVC
The patient characteristics are presented in Table 1. Overall, 387 patients were male and 389 had a history of smoking. The mean age was 67.0 years. Patients with the emphysema determined by TSCT were 36.4% of the study population. The reference equation of the J-LLN of FEV1/FVC was shown in Figure 1. When the J-LLN of FEV1/FVC was utilized to classify the degree of airway obstruction, the study population had 337 patients in the non-COPD group, 58 patients in the in-between group, and 214 patients in the COPD group. The finding of emphysema was observed in 57.4% of all the in-between and COPD groups (156/272 cases; 19 cases in the in-between group and 137 cases in the COPD group, respectively). The mean age in the in-between group was higher than that in the COPD group. The proportion of male patients and a smoking history in the COPD group were higher than those in the in-between group. Especially, the COPD group had almost double the number of patients with more than 40 pack-years, compared with the in-between group. The proportion of grade 2 or 3 emphysema was significantly higher in the COPD group than in the other groups.
Table 1.
Patient characteristics among the study population
| All cases (n=609) |
Non-COPD (n=337) |
In-between (n=58) |
COPD (n=214) |
p value | |
| Age, years* | 67.0 (26–87) | 66.1 (26–87) | 69.3 (31–84)(1) | 67.9 (43–85)(2) | 0.005 |
| Gender, male | 63.5 (387) | 50.7 (171) | 70.7 (41)(1) | 81.8 (175)(3) | 0.001 |
| History of smoking | 63.9 (389) | 47.5 (160) | 70.7 (41)(1) | 87.9(188)(2)(3) | 0.001 |
| Pack-year* | 38.8 (0–174) | 23.6 (0–126) | 40.7 (0–165)(1) | 62.2 (0–174)(2)(3) | 0.001 |
| More than 40 pack-year | 46.8 (285) | 28.8 (97) | 41.4 (24) | 76.6 (164)(2)(3) | 0.001 |
| Spirometric variables | |||||
| VC (%)** | 110.4 (17.5) | 109.2 (17.1) | 110.1 (18.1) | 112.3 (18.2) | 0.311 |
| DLCO (%)** | 113.7 (28.8) | 117.8 (27.7) | 120.0 (29.8) | 105.7 (28.7)(2)(3) | 0.001 |
| %FEV1 predicted** | 104.2 (22.0) | 113.5 (19.0) | 104.6 (16.8)(1) | 89.8 (19.9)(2)(3) | 0.001 |
| Emphysema by TSCT | 0.001 | ||||
| Grade 0 | 63.6 (387) | 80.4 (271) | 67.2 (39) | 36.0 (77) | |
| Grade 1 | 21.8 (133) | 14.8 (50) | 24.1 (14) | 32.2 (69) | |
| Grade 2 | 10.2 (62) | 3.9 (13) | 6.9 (4) | 21.0 (45) | |
| Grade 3 | 4.4 (27) | 0.9 (3) | 1.7 (1) | 10.8 (23) | |
| Pathological stage | 0.131 | ||||
| I | 69.1 (421) | 73.0 (246) | 63.8 (37) | 64.4 (138) | |
| II | 18.1 (110) | 14.5 (49) | 24.1 (14) | 22.0 (47) | |
| III | 12.8 (78) | 12.5 (42) | 12.1 (7) | 13.6 (29) | |
| Histology | 0.001 | ||||
| Adenocarcinoma | 73.4 (447) | 83.1 (280) | 69.0 (40) | 59.3 (127) | |
| Squamous cell carcinoma | 20.2 (123) | 12.8 (43) | 22.4 (13) | 31.3 (67) | |
| Othersa
|
6.4 (39) | 4.1 (14) | 8.6 (5) | 9.4 (20) |
n indicates number. * Data are shown as mean (range). ** Data are shown as mean (standard deviation). All other data are shown as % (number). a Others include large cell carcinoma, non-small cell carcinoma, and carcinoid. (1) indicates a siginificant difference between the non-COPD and in-between groups. (2) indicates a significant difference between the in-between and COPD groups. (3) indicates a significant difference between the non-COPD and COPD groups.
The incidences of POT, PPS, and combined complications among the different groups were evaluated (Table 2). The incidences of all postoperative outcomes in the COPD group were significantly higher than those in the non-COPD group. The incidence of the POT in the COPD group was also more than twice as high as that in the in-between group (42.5% vs 20.7%, respectively). The incidence of PPS and combined complications in the COPD group showed almost 40% and 30% increase compared with those in the in-between, respectively. To determine whether this classification of airflow obstruction determined by the J-LLN of FEV1/FVC could effectively detect the risk of adverse postoperative outcomes, the relative odds ratios (ORs) of postoperative outcomes for both the in-between and the COPD groups were compared with those of the non-COPD group (Table 3A). Although all of the relative ORs for the comparison between the non-COPD and COPD groups showed significant confidence intervals (CIs), there was no significant difference of the relative ORs for the comparison between the non-COPD and in-between groups. When the relative ORs for the comparison between the non-COPD and COPD groups were adjusted by age, gender, smoking history, and operation time (Table 3B), it showed a significant relative OR of 2.840 (95% CI: 1.824–4.421) for POT, 1.836 (95% CI: 1.166–2.890) for PPS, and 1.637 (95% CI: 1.007–2.663) for combined complications (Table 3B). When the relative ORs in POT comparing the in-between group with the COPD group were adjusted, it still showed a significant relative OR of 2.984 (95% CI: 1.447–6.153).
Table 2.
Operative outcomes among the study population
| All cases (n=609) |
Non-COPD (n=337) |
In-between (n=58) |
COPD (n=214) |
p value | |
| Surgical procedure | |||||
| Lobectomy | 78.7 (479) | 79.8 (269) | 82.8 (48) | 75.7 (162) | 0.153 |
| Operation time, minutes* | 232.3 (31–873) | 220.5 (31–604) | 243.6 (111–471)(1) | 248.5 (45–873)(3) | 0.013 |
| Postoperative outcomes | |||||
| Prolonged oxygen treatment | 25.5 (155) | 15.4 (52) | 20.7 (12) | 42.5 (91)(2)(3) | 0.001 |
| Complicationsa | 19.0 (116) | 13.9 (47) | 20.7 (12) | 26.6 (57)(3) | 0.001 |
| Arrythmia | 9.2 (56) | 7.7 (26) | 12.1 (7) | 10.7 (23) | 0.356 |
| Minitracheostomy | 2.8 (17) | 2.1 (7) | 1.7 (1) | 4.2 (9) | 0.293 |
| Prolonged air leakage | 2.8 (17) | 1.8 (6) | 1.7 (1) | 4.7 (10)(3) | 0.116 |
| Prolonged ventilation | 3.1 (19) | 2.1 (7) | 3.4 (2) | 4.7 (10) | 0.230 |
| Pneumonia | 5.3 (32) | 2.1 (7) | 6.9 (4) | 9.8 (21)(3) | 0.001 |
| Prolonged postoperative stay | 26.6 (162) | 19.3 (65) | 27.6 (16) | 37.9 (81)(3) | 0.001 |
| Postoperative stay, days* | 12.0 (3–291) | 9.9 (4–39) | 11.1 (5–35) | 15.5 (3–291)(3) | 0.001 |
| Postoperative deaths | |||||
| 30-day motality | 0.2 (1) | 0.3 (1) | 0 (0) | 0 (0) | 1.000 |
| Hospital motality | 0.2 (1) | 0 (0) | 0 (0) | 0.5 (1) | 0.447 |
n indicates number. * Data are shown as mean (range). All other data are shown as % (number). a Complications include arrhythmia, the need of minitracheostomy, prolonged air leakage, prolonged ventilation, and pneumonia.
(1) indicates a siginificant difference between the non-COPD and in-between groups. (2) indicates a significant difference between the in-between and COPD groups. (3) indicates a significant difference between the non-COPD and COPD groups.
Table 3 (A).
Crude relative Odds Ratio (95% CI) of postoperative outcomes for the non-COPD group with the in-between group and COPD groups with airway obstruction
| Category | Non-COPD | In-between | COPD |
| Prolonged oxygen treatment | 1.0 | 1.430 (0.710–2.881) p =0.317 |
4.055 (2.715–6.005) p =0.001 |
| Prolonged postoperative stay | 1.0 | 1.594 (0.844–3.012) p =0.151 |
2.549 (1.731–3.752) p =0.001 |
| Complications | 1.0 | 1.610 (0.794–3.261) p =0.186 |
2.240 (1.454–3.451) p =0.001 |
Relative Odds Ratio (OR) (95% CI) of postoperative outcomes of the in-between group and the COPD group were compared with those of the non-COPD group were shown.
Table 3 (B).
Adjusted relative Odds Ratio (95% CI) of postoperative outcomes for the non-COPD group with the in-between group and the COPD group
| Category | Non-COPD | In-between | COPD |
| Prolonged oxygen treatment | 1.0 | 0.952 (0.453–2.000) p =0.896 |
2.840 (1.824–4.421) p =0.001 |
| Prolonged postoperative stay | 1.0 | 1.134 (0.565–2.280) p =0.723 |
1.836 (1.166–2.890) p =0.009 |
| Complications | 1.0 | 1.201 (0.570–2.531) p =0.630 |
1.637 (1.007–2.663) p =0.047 |
Adjusted Odds Ratio (OR) (95% CI) of postoperative outcomes was adjusted for age, gender, history of smoking, and operation time. Adjusted OR of postoperative outcomes of the in-between group and the COPD group were compared with those of the non-COPD group.
Patient characteristics and postoperative outcomes in the two populations among the in-between group
The J-LLN-determined in-between group was composed of 14 patients in the above-0.7 subgroup and 44 patients in the below-0.7 subgroup, respectively. We compared patient characteristics and postoperative outcomes in the above-0.7 subgroup with those in the below-0.7 subgroups (Table 4). Overall, the above-0.7 subgroup was more likely to comprise younger female, and to have a less intensive smoking history, as compared with the below-0.7 subgroup. There were no patients with grade 2 and 3 emphysema in the above-0.7 subgroup. The incidences of postoperative outcomes in the above-0.7 subgroup were likely to be similar to those in non-COPD group.
Table 4.
Patient characteristics and outcomes among the In-between group
| Category | In-between Above 0.7 (n=14) |
In-between Below 0.7 (n=44) |
p value |
| Age, years* | 54.9 (31–67) | 73.9 (59–84) | 0.001 |
| Gender, male | 14.3 (2) | 88.6 (39) | 0.001 |
| History of smoking | 28.6 (4) | 84.1 (37) | 0.001 |
| Pack-year* | 8.2 (0–49.5) | 51.0 (0–165) | 0.001 |
| More than 40 pack-year | 7.1 (1) | 52.3 (23) | 0.003 |
| Spirometric variables | |||
| VC (%)** | 119.0 (12.7) | 107.1 (18.7) | 0.031 |
| DLCO (%)** | 113.1 (17.9) | 121.5 (32.7) | 0.228 |
| %FEV1 predicted** | 102.4 (9.9) | 105.0 (18.4) | 0.489 |
| Emphysema by TSCT | 0.470 | ||
| Grade 0 | 85.7 (12) | 61.3 (27) | |
| Grade 1 | 14.3 (2) | 27.3 (12) | |
| Grade 2 | 0 (0) | 9.1 (4) | |
| Grade 3 | 0 (0) | 2.3 (1) | |
| Postoperative outcomes | |||
| Prolonged oxygen treatment | 14.3 (2) | 22.7 (10) | 0.397 |
| Complications | 14.3 (2) | 22.7 (10) | 0.397 |
| Arrythmia | 0 (0) | 15.9 (7) | 0.127 |
| Minitracheostomy | 7.1 (1) | 0 (0) | 0.241 |
| Prolonged air leakage | 0 (0) | 2.3 (1) | 0.759 |
| Prolonged ventilation | 7.1 (1) | 2.3 (1) | 0.428 |
| Pneumonia | 7.1 (1) | 6.8 (3) | 0.680 |
| Prolonged postoperative stay | 28.6 (4) | 27.3 (12) | 0.587 |
| Postoperative stay, days* | 9.5 (5–15) | 11.6 (5–35) | 0.278 |
n indicates number. * Data are shown as mean (range). ** Data are shown as mean (standard deviation). All other data are shown as % (number).
Impact of the J-LLN-determined airflow obstruction on survival in resected lung cancer patients with stage I disease and lobectomy
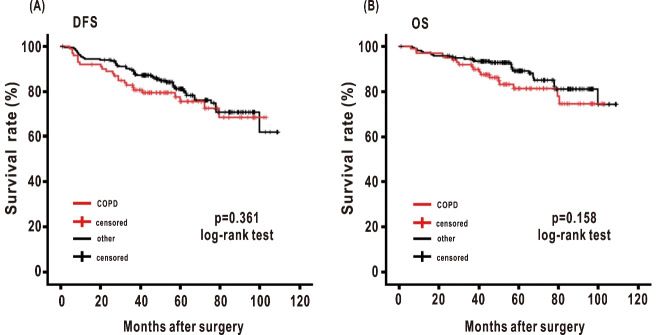
To minimize the influence of pathological stage, the impact of airflow obstruction was evaluated in resected lung cancer patients with stage I disease and lobectomy.9 The study population comprised 317 patients. In the patients with stage I disease and lobectomy, the median follow-up time among subjects who were still alive was 60.2 months (range, 1.0 to 109.1 months). Median DFS and OS were 55.5 months (range, 1.0 to 109.1 months) and 58.2 months (range, 1.0 to 109.1 months), respectively. The 5-year DFS and 5-year OS rates for this population were 79.4% and 86.7%, respectively. There were 70 recurrences and 46 deaths. To evaluate the impact of the airflow obstruction, combined assessment of the 0.70 fixed ratio and the J-LLN of the FEV1/FVC ratio was utilized to classify the population. The COPD group had 100 patients; the other group had 217 patients. Classification by a FEV1/FVC ratio of 0.7 and LLN did not show worse DFS and OS among the groups (p=0.361 in DFS and p=0.158 in OS, respectively) (Figure 2A and 2B).
Fig. 2.
Kaplan-Meier estimates of disease-free and overall survival in resected lung cancer patients with stage I disease and lobectomy by using the 0.7 fixed ratio and the J-LLN of FEV1/FVC
Fig. 2A: Disease-free survival (DFS) in 317 patients with stage I disease and lobectomy classified by the J-LLN of FEV1/FVC (p = 0.361 log rank test).
Fig. 2B: Overall survival (OS) in 317 patients with stage I disease and lobectomy classified by the J-LLN of FEV1/FVC (p = 0.158 log rank test).
Red lines = the COPD group; red hatch marks = COPD censored; black lines = the other group; black hatch marks = other censored.
DISCUSSION
To our knowledge, there has been no evidence to suggest the importance of airflow limitation defined by a renewed Japanese spirometric reference on any clinical settings. This is the first study to show that the LLN of FEV1/FVC by a renewed Japanese spirometric reference provides appropriate risk stratification on postoperative outcomes in Japanese COPD patients with resected lung cancer.
Although older patients with COPD are often known to have a much lower pulmonary function,6 a recent review suggests developing more inclusive considerations for surgical resection with curative intent in lung cancer patients with COPD, as limited surgical resections or nonsurgical therapeutic options might provide inferior survival rates compared with resection.14 Thus, accurate risk stratification on postoperative outcomes in COPD patients with resected lung cancer is warranted.6 In our study, the mean value of %FEV1 predicted in the COPD group was nearly 90%. Indeed, we recently showed that severe airway obstruction below 50% of %FEV1 predicted was one of factors for affecting the proposal of thoracic surgery with curative intent to newly-diagnosed lung cancer patients.4 Thus, the value of %FEV1 predicted did not appear to provide risk stratification on postoperative outcomes in COPD patients with resected lung cancer.
Adult smokers suspected of having COPD were reported to be at no increased risk of respiratory morbidity or all-cause mortality until the ratio falls below the age-corrected LLN (even though it is below 0.7).15 By using the NHANES III reference equation which provides spirometric reference variables for non-Asian population,16 we previously showed that the COPD group defined by combined assessments of the 0.7 fixed ratio and the LLN of FEV1/FVC (C-LLN) showed worse postoperative and survival outcomes among resected lung cancer patients.8,9 This was due to a lack of an appropriate reference equation for the Japanese population to calculate the LLN of FEV1/FVC. The LLN values of FEV1/FVC by two reference equations were slightly different. When the C-LLN of FEV1/FVC was utilized, only one patient was in the above-0.7 subgroup.8,9 When the J-LLN of FEV1/FVC was utilized, the J-LLN determined in-between group involved two subgroups with different patient characteristics. Patient characteristics such as mean age and a smoking history in the below-0.7 subgroup were similar to those in the COPD group. On the other hand, the characteristics in the above-0.7 subgroup were similar to those in the non-COPD group. Two cases with grade 1 emphysema in the above-0.7 subgroup showed history of smoking (One female with 11.3 pack-years and one male with 49.5 pack-years, respectively). The incidences of postoperative outcomes in the above-0.7 subgroup were similar to those in the non-COPD group. Although the factors by which patients in the avobe-0.7 subgroup had airway obstruction below the J-LLN of FEV1/FVC could not be identified from medical records, a recent study suggests that low FEV1 in early adulthood is important in the genesis of COPD.17 Further investigation for the clinical impact of the LLN-determined airflow obstruction among subjects with the FEV1/FVC above 0.7 is warranted. The J-LLN-determined COPD group remains an independent risk factor for postoperative outcomes as compared to the non-COPD and in-between groups. Taken together with previous studies,8,9 the data strengthened that risk stratification as determined by the LLN of FEV1/FVC might detect at-risk patients for postoperative outcomes among COPD patients with resected lung cancer.
Because advanced disease stage was an independent factor associated with decision-making process and survival outcomes on thoracic surgery with curative intent,4,9 we evaluated an impact of the LLN-defined airway obstruction on survival outcomes in resected lung cancer patients with stage I disease and lobectomy. In contrast with the previous study,9 Kaplan-Meier curves and log-rank test did not show the clinical impact of the LLN-determined airway obstruction on DFS and OS.
Our study was based on the retrospective analysis of data from 609 out of a total of 712 cases from a single institution. Nevertheless, data from 85.5% of all patients at a single institution who were sequentially registered and underwent a pulmonary resection from 2006 to 2011 could minimize the possible contribution of the treatment-related prognostic factors and the selection bias. Many studies suggest that a race/ethnic adjustment factor should not be applied to the FEV1/FVC ratio, a finding supported by the recent study comparing Caucasian and Asian-American participants.7,18-1919 Although the Multi-Ethnic Study of Atherosclerosis (MESA) involved only 343 Asian-American participants,19 this renewed Japanese spirometric reference variables were derived from 20,341 Japanese healthy non-smoking participants.10 Thus, this spirometric reference equation for the Japanese population might be more properly utilized to evaluate airway obstruction in the Asian population compared with the NHANES III reference equation for the MESA population.
CONCLUSION
A standardized assessment of the LLN of FEV1/FVC by a renewed Japanese spirometric reference provides risk stratification for postoperative outcomes in COPD patients with resected lung cancer.
ACKNOWLEDGMENTS
This work is partly supported by a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labor and Welfare, Japan [Grants; H26-Nanchi-Ippan-065 and H29-Nanchi-Ippan-023].
AUTHORS’ CONTRIBUTIONS
YO, NH, KY, and YH had full access to all of the data in the study and are responsible for the integrity of the data and the accuracy of the data analysis.
SI: contributed to analysis of the findings of TSCT examination.
SO, KK, TF, FK and TF: contributed to collection of the data.
KW: contributed to the development of the analytic concept, data analyses.
KY and YH: contributed to critical revision of the manuscript.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. [DOI] [PMC free article] [PubMed]
- 2.Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129(5):1305–12. [DOI] [PubMed]
- 3.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. [DOI] [PubMed]
- 4.Hashimoto N, Matsuzaki A, Okada Y, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med. 2014;14:14. [DOI] [PMC free article] [PubMed]
- 5.Matsuo M, Hashimoto N, Usami N, et al. Inspiratory capacity as a preoperative assessment of patients undergoing thoracic surgery. Interact Cardiovasc Thorac Surg. 2012;14(5):560–564. [DOI] [PMC free article] [PubMed]
- 6.Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. 2009;34(1):17–41. [DOI] [PubMed]
- 7.Quanjer PH, Enright PL, Miller MR, et al. The need to change the method for defining mild airway obstruction. Eur Respir J. 2011;37(3):720–722. [DOI] [PubMed]
- 8.Osuka S, Hashimoto N, Sakamoto K, et al. Risk stratification by the lower limit of normal of FEV1/FVC for postoperative outcomes in patients with COPD undergoing thoracic surgery. Respir Investig. 2015;53(3):117–123. [DOI] [PubMed]
- 9.Matsuzaki A, Hashimoto N, Okachi S, et al. Clinical impact of the lower limit of normal of FEV1/FVC on survival in lung cancer patients undergoing thoracic surgery. Respir Investig. 2016;54(3):184–192. [DOI] [PubMed]
- 10.Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig. 2014;52(4):242–250. [DOI] [PubMed]
- 11.Hashimoto N, Iwano S, Kawaguchi K, et al. Impact of thin-section computed tomography-determined combined pulmonary fibrosis and emphysema on outcomes among patients with resected lung cancer. Ann Thorac Surg. 2016;102(2):440–447. [DOI] [PubMed]
- 12.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. [DOI] [PubMed]
- 13.Zhai R, Yu X, Shafer A, Wain JC, Christiani DC. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145(2):346–353. [DOI] [PMC free article] [PubMed]
- 14.Raviv S, Hawkins KA, DeCamp MM, Jr., Kalhan R. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am J Respir Crit Care Med. 2011;183(9):1138–1146. [DOI] [PubMed]
- 15.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of FEV1 to FVC as a basis for establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(5):446–451. [DOI] [PMC free article] [PubMed]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. [DOI] [PubMed]
- 17.Lange P, Celli B, Agusti A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. [DOI] [PubMed]
- 18.Ip MS, Ko FW, Lau AC, et al. Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization. Chest. 2006;129(2):384–392. [DOI] [PubMed]
- 19.Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–145. [DOI] [PMC free article] [PubMed]
- 20.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed]