ABSTRACT
Stereotactic radiosurgery for large brain metastases (BM) not amenable to surgical resection is associated with limited local control and neurotoxicity, while hypofractionated stereotactic radiotherapy (HFSRT) has emerged as a promising option. We retrospectively evaluated 61 patients with BM larger than 2 cm in the maximum diameter, who were treated with HFSRT (mainly 35 Gy/5 fractions) in our center between 2006–2016, focusing on the effect of BM size on outcomes. Eligible patients were divided according to the maximum BM diameter (group A [23 patients]: ≥3 cm, group B [22 patients]: <3 cm) to assess the relationship between tumor size and prognosis or safety. The primary outcome was the local control rate (LCR), and secondary outcomes were the response rate (RR), brain progression-free survival (BPFS), median survival time (MST), and radionecrosis (RN). Univariate and multivariate analyses for LCR were conducted using Cox’s proportional hazards model. In the 45 eligible patients (58 lesions) enrolled in this study, the RR was 86.4% with an overall LCR of 64.7% at 12 months (67.1% for group A and 61.5% for group B [p = 0.45]). The median BPFS and MST were 11.6 and 14.2 months, respectively. Univariate analyses revealed that female patients and gynecological cancer patients had poorer LCR, but they were not significantly independent prognostic factors (p = 0.06, 0.09, respectively). Two patients (4.4%) experienced RN that was detected more than 4 years after HFSRT. We conclude that HFSRT is safe for large BM but further studies are needed to determine optimal doses and fractions.
Key Words: helical tomotherapy, stereotactic radiotherapy, radiosurgery, brain metastases, radiation necrosis
INTRODUCTION
Stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) are useful options for treating patients with brain metastases, and several randomized controlled trials have confirmed their efficacy.1-7 However, most of those trials included tumors with a maximum diameter of 3 cm. The RTOG 90-05 trial revealed that larger tumors were associated with limited local control and a higher risk of neurotoxicity.8 In general, patients with a single large metastasis and good expected survival are recommended for surgical treatment and postoperative radiotherapy.2,7,9,10 However, the optimal treatment is unclear if the case is considered inoperable, with available options including whole-brain radiotherapy (WBRT), SRS/SRT, and/or chemotherapy. Some recent retrospective studies have demonstrated that hypofractionated SRT (HFSRT) is a reasonable option for unresectable large tumors, relative to single-fraction SRS,11-23 although those studies included variable doses and fractions. Nevertheless, HFSRT may cause less brain radionecrosis (RN) than single-fraction SRS, while still maintaining local tumor control.12,14,16
In 2006, our center began providing HFSRT using helical tomotherapy (HT; TomoTherapy Hi-Art system, Accuray Inc., Sunnyvale, CA) for brain metastases, and we have previously reported our protocol with early clinical results.24 This retrospective study aimed to evaluate the efficacy and safety of HFSRT for large brain metastases.
MATERIALS AND METHODS
Patient selection
Between July 2006 and September 2016, 61 patients with one or two brain metastases with at least one lesion larger than 2 cm in the maximum diameter underwent HFSRT at our institution. To assess the presence of extracranial lesions and the control situation before treatment, all patients provided a blood sample for laboratory testing, and underwent computed tomography (CT) from their head to their abdomen, as well as enhanced brain magnetic resonance imaging (MRI). The exclusion criteria were: (1) no available head MRI and/or CT results after HFSRT, (2) prior brain radiotherapy or surgery, (3) combination with WBRT, and (4) brainstem metastases. The present study’s retrospective protocol was approved by our institutional review board (2017-1-058), and all patients provided written informed consent before their treatment.
Radiation therapy
Patients were immobilized in the supine position with a head shell before undergoing enhanced CT. A slice thickness of 2.5 mm was used before March 2015, although this was subsequently changed to 2.0 mm. The target volumes and organs at risk were contoured using Pinnacle3 workstation (Philips Medical Systems, Andover, MA) and MIM Maestro (MIM Software Inc., Beachwood, OH). The clinical target volume was considered the gross tumor volume (GTV), which was defined based on enhanced lesions that were detected during MRI. The planning target volume (PTV) was set as the clinical target volume plus a 2-mm margin in all dimensions. The prescription dose was 35 Gy in 5 fractions, and routinely assigned to the 90% isodose surface used for PTV coverage of 95%, normalized to 100% of the dose at the maximum dose point (Dmax). The dose calculation grid size was 2.0 mm. All patients were treated using HT. The treatment isocenter was around the geometrical center of the cranium, not related to the target lesions. In cases where patients who had two lesions, we planned to meet the 90% isodose surface for each PTV coverage of 95%, and confirmed this with DVH analyses. All patients started treatment after setup verification using daily megavoltage CT acquisitions.24 Their setup positions were all corrected by the initially calculated error value. After each irradiation finished, the actual setup error value was calculated to ensure it was within the setup margin of 2.0 mm.
Assessment
Treatment response was evaluated using enhanced MRI and judged based on the revised Response Evaluation Criteria in Solid Tumors. The MRI evaluations were scheduled for before treatment, 2–3 months after the treatment, and then every 3–4 months until treatment failure or death. Complete response (CR) was defined as the disappearance of all target lesions. Partial response (PR) was defined as ≥30% decrease in the sum of the target lesions’ diameters, and progressive disease (PD) was defined as ≥20% increase in those diameters compared to the smallest diameters from treatment. Stable disease (SD) was defined as insufficient changes to qualify as PR or PD.25 Non-PD (CR, PR, and SD) response was defined as locally controlled. For patients with 2 lesions, they were not considered to be locally controlled unless both lesions were controlled. We also evaluated the actual target volume during the enhanced MRI follow-ups. Adverse events were classified according to version 4.0 of the Common Terminology Criteria for Adverse Events.
Statistical analysis
To assess the efficacy of HFSRT for large lesions, we divided the patients according to the maximum diameter of their largest lesions (group A: ≥3 cm vs. group B: <3 cm). The primary outcome was local control rate (LCR). The secondary outcomes were median survival time (MST), response rate (RR), distant brain metastases (DBM), and intracranial recurrence. The Kaplan-Meier method with the log-rank test was used to compare LCR and survival time. The RR was calculated as the sum of patients who achieved CR, PR, and SD divided by the total number of patients. Intracranial recurrence included local recurrence and DBM. In a univariate analysis, factors of sex (male vs. female), age (≥65 years vs. <65 years), ECOG performance status (PS; 0 vs. 1–2), Karnofsky performance status (KPS; ≥70 vs. <70), maximum tumor length (≥3 cm vs. <3 cm), controlled primary tumor (yes vs. no), systemic metastases (yes vs. no), number of brain metastases (single vs. multiple), and histological status were evaluated. Variables with p values less than 0.15 were further assessed using Cox’s proportional hazards model in a multivariate analysis. Statistical significance was defined as p <0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R software (version 2.13.0; The R Foundation for Statistical Computing, Vienna, Austria).26
RESULTS
Patient and treatment characteristics
All patients completed the planned HFSRT schedule without delay or discontinuation. Imaging follow-up was performed in 45 patients, with a total of 58 lesions. The remaining 16 patients did not undergo follow-up imaging due to early death within 2 months (n=9) or were lost to follow-up imaging (n=7). At the last follow-up, 7 patients (15.6%) were alive, 34 patients (75.6%) had died, and 4 patients (8.9%) were lost to follow-up. The median follow-up period for all patients was 11.3 months (range: 1.7–93.9 months), and the median follow-up period among patients who had not died was 15.2 months (range: 6.9–93.9 months). The eligible patients underwent a median number of three MRI evaluations (range: 1–37 times). Table 1 shows the patients’ baseline characteristics. The median GTV for all lesions was 7.2 mL (range: 0.1–115.4 mL), and large lesions had a median GTV of 19.3 mL (range: 6.21–115.4 mL). Twenty-three patients (51.1%) had large lesions (≥3 cm) and 8 patients (17.8%) had lesions with a diameter of ≥4 cm. A single metastasis was detected in 32 patients (71.1%) and 15 patients had multiple lesions. The only significant inter-group difference was maximum tumor length (p < 0.001). Thirty-one patients (68.9%) were treated using a dose of 35 Gy in 5 fractions. Two larger tumors (≥45 mm) were treated using a dose of 35–38.5 Gy in 7 fractions, and 12 lesions near the brainstem were treated using a dose of 30–33 Gy in 5 fractions.
Table 1.
Patient characteristics according to tumor size
| All
n = 45 |
Group A
(≥3 cm) n = 23 |
Group B
(<3 cm) n = 22 |
p | ||
| Sex, n | Male | 19 | 11 | 8 | 0.44 |
| Female | 26 | 12 | 14 | ||
| Age in years | Median (range) | 61 (23–81) | 65 (42–81) | 59 (23–74) | 0.19 |
| ≥65 years old | 20 | 13 | 7 | ||
| Eastern Cooperative Oncology Group performance status | 0 | 6 | 2 | 4 | 0.55 |
| 1 | 31 | 16 | 15 | ||
| 2 | 4 | 2 | 2 | ||
| 3 | 4 | 3 | 1 | ||
| 4 | 0 | 0 | 0 | ||
| Karnofsky performance scale score | ≥70 | 41 | 20 | 21 | 0.32 |
| <70 | 4 | 3 | 1 | ||
| Controlled primary tumor | Yes | 32 | 14 | 18 | 0.12 |
| No | 13 | 9 | 4 | ||
| Systemic metastases | Yes | 39 | 19 | 20 | 0.41 |
| No | 6 | 4 | 2 | ||
| Recursive partitioning analysis | 1 | 4 | 2 | 2 | 0.92 |
| 2 | 37 | 18 | 19 | ||
| 3 | 4 | 3 | 1 | ||
| Primary tumor site | Breast | 10 | 6 | 4 | 0.52 |
| NSCLC | 9 | 6 | 3 | ||
| SCLC | 2 | 2 | 0 | ||
| Renal cell | 2 | 1 | 1 | ||
| Gastrointestinal | 5 | 3 | 2 | ||
| Head and neck | 6 | 0 | 6 | ||
| Gynecology | 6 | 3 | 3 | ||
| Other | 5 | 2 | 3 | ||
| Number of brain metastases | 1 | 32 | 18 | 14 | 0.30 |
| 2 | 11 | 5 | 8 | ||
| Maximum tumor length (cm) | Median (range) | 3.0 (2.0–7.1)
IQR: 3.0–4.0 |
IQR: 2.5–3.7
2.5 (2.0–2.9) |
3.8 (3.0–7.1)
IQR: 2.0–2.7 |
<0.001 |
NSCLC: non-small cell lung cancer, SCLC: small cell lung cancer, IQR: interquartile range.
Treatment outcomes
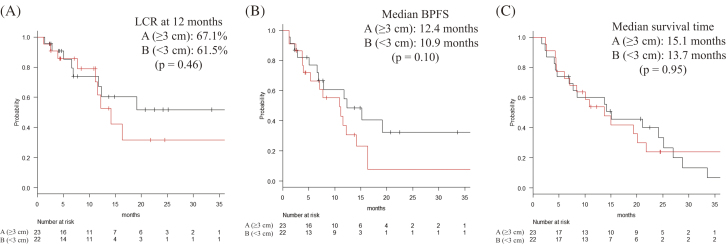
The first radiological evaluation revealed a CR rate of 2.2%, a PR rate of 73.3%, an SD rate of 11.1%, and a PD rate of 13.3%. The RRs were 86.7% overall, 87.0% in group A, and 86.4% in group B (p = 0.97). The LCR values at 6/12 months were 85.4%/64.7% among all patients, 85.2%/67.1% in group A, and 85.6%/61.5% in group B (Fig. 1A., p = 0.46). The BPFS values at 12 months were 54.6% in group A, and 36.9% in group B (Fig. 1B, p = 0.10). The MST was 15.0 months in group A (95% CI: 7.0–25.2 months) and 13.7 months in group B (95% CI: 6.0–21.8 months) (Fig. 1C, p = 0.95).
Fig. 1.
Local control and survival analyses
(A) Local control rate (LCR) curves, (B) Brain progression-free survival (BPFS) curves, and (C) Overall survival curves for each of the 2-group of patients in our study.
Univariate and multivariate analyses
Table 2 shows the result of univariate analysis for local control. The variables of sex (male vs. female) and histological status (gynecological vs. others) had a p value of <0.15, and were further assessed in a multivariate analysis. Using multivariate analyses, female patients and gynecological cancer patients had relatively poorer LCR, but the difference was not significant (male vs. female: p = 0.06, gynecological cancer vs. others: p = 0.09).
Table 2.
The results of univariate and multivariate analysis for local control rate
| Variables | Univariate
p value |
Multivariate
p value |
HR | 95% CI | |
| Sex (male vs. female) | 0.012 | 0.06 | 0.23 | 0.051–1.06 | |
| Age (≥65 years vs. <65 years) | 0.72 | N/A | N/A | N/A | |
| PS (0 vs. 1–2) | 0.74 | N/A | N/A | N/A | |
| KPS (≥70 vs. <70) | 0.49 | N/A | N/A | N/A | |
| Tumor length (≥3 cm vs. <3 cm) | 0.46 | N/A | N/A | N/A | |
| Controlled primary tumor (yes vs. no) | 0.25 | N/A | N/A | N/A | |
| Systemic metastases (yes vs. no) | 0.20 | N/A | N/A | N/A | |
| Number of brain metastases (single vs. multiple) | 0.66 | N/A | N/A | N/A | |
| Histological status | NSCLC | 0.49 | N/A | N/A | N/A |
| SCLC | 0.82 | N/A | N/A | N/A | |
| Gynecology | 0.0066 | 0.09 | 2.48 | 0.84–7.31 | |
| Renal cell | 0.86 | N/A | N/A | N/A | |
| Head and neck | 0.96 | N/A | N/A | N/A | |
| Gastrointestinal tract | 0.95 | N/A | N/A | N/A | |
| Breast | 0.56 | N/A | N/A | N/A | |
| Others | 0.43 | N/A | N/A | N/A | |
PS: eastern cooperative oncology group performance status, KPS: Karnofsky performance status, NSCLC: non-small cell lung cancer, SCLC: small cell lung cancer, HR: hazard ratio, CI: confidence interval
Recurrence and salvage treatment
Intracranial recurrence was detected for 29 patients (64.4%), including 6 patients with PD who were treated using salvage surgery followed by SRT (1 patient), WBRT (1 patient), or other supportive care (4 patients). Fifteen patients developed local recurrence (A: 8 patients, B: 7 patients), 15 patients developed DBM (A: 6 patients, B: 9 patients), and 7 patients experienced both forms of recurrence (A: 3 patients, B: 4 patients). The cumulative local recurrence rates among all patients were 13.5% at 6 months and 27.2% at 12 months. The local recurrence rates at 12 months were 27.2% in group A and 27.3% in group B (p = 0.72). The DBM rates at 12 months were 13.3% in group A and 27.3% in group B (p = 0.32).
Table 3 shows the sites of recurrence and salvage treatments. Six patients, including 1 patient with PD, underwent surgery and 15 patients underwent reirradiation (11 patients underwent SRS/SRT and 4 patients underwent WBRT). The second SRS/SRT was performed for 3 local recurrences and 8 distant metastases. All patients who underwent surgery achieved successful salvage, and the MST from salvage surgery was 16.3 months (range: 10.3–77.3 months). Some patients who underwent reirradiation achieved PR (2 patients) or SD (1 patient), although 12 patients developed PD. The MST after reirradiation was 5.7 months (range: 0.7–36.9 months).
Table 3.
Recurrence sites and salvage treatments
| Local | Distant brain | Local and distant | |
| Surgery | 2 | 1 | 1 |
| SRS/SRT | 5 | 9 | 4 |
| Surgery + SRS/SRT | 0 | 0 | 0 |
| WBRT | 2 | 3 | 2 |
| Chemotherapy | 2 | 0 | 0 |
| BSC | 3 | 2 | 0 |
| Unknown | 1 | 0 | 0 |
| All | 15 | 15 | 7 |
SRS: stereotactic radiosurgery, SRT: stereotactic radiotherapy, WBRT: whole-brain radiotherapy, BSC: best supportive care.
Adverse events
There were no patients who had grade 3 or higher acute toxicities. As for late toxicities, 2 patients were suspected of local recurrence or RN based on their symptoms, MRI, and methionine positron emission tomography (PET). One patient underwent resection and RN diagnosis 6 years after the HFSRT. Her primary lesion was breast cancer, and she had 2 brain metastases (GTV = 7.56 mL and 1.99 mL), which were irradiated with 32.5 Gy in 5 fractions. The volume receiving >20 Gy (V20Gy) was 40.8 mL. The other patient was diagnosed with RN 5 years after the HFSRT (30 Gy in 5 fractions). Her primary lesion was breast cancer, and she had 2 brain metastases (GTV = 20.2 mL and 0.53 mL). V20Gy was 112.3 mL. Their symptoms subsequently improved after resection and they remain alive. There were no other RN or severe late toxicities observed in this study.
DISCUSSION
As modern systemic therapies (including chemotherapy, molecular targeted therapy, or immunotherapy) improve the control of extracranial lesions and survival, SRS/SRT for brain metastases requires local control and safety more than before. Although there is good evidence supporting the use of SRT for small brain tumors, few trials have evaluated SRT for large lesions.9
Several researchers have indicated that safe and effective local control can be achieved using HFSRT for large tumors,11-13,15-18,23 although the optimal doses and fractionation remain unclear, as they are heavily influenced by institutional practice and patient preference (Table 4). Most recent studies have used 3 cm as the diameter cut-off value for defining large tumors.11,16-20,22,23
Table 4.
Studies of hypofractionated stereotactic radiotherapy for large brain metastases
| First author
(reference) |
Year | Patients | Tumor size | Dose/fractions | MTV
(mL) |
MST (months) | LCR at 1 year (%) | RN (%) |
| Ernst-Stecken
(11) |
2006 | 51 | ≥3 mL | 30–35 Gy/5 fr | 6.0 | 11.0 | 76.0 | 39.2 |
| Marginal (90% IDS) | ||||||||
| Kim (12) | 2011 | 98 | ≥5 mL | 36 Gy/6 fr | 5.0 | 7.0 | 71.0 | 0 |
| Marginal (91% IDS) | ||||||||
| Higuchi (13) | 2009 | 43 | ≥10 mL | 30 Gy/3 fr | 17.6 | 8.8 | 75.9 | N/A |
| Marginal (50% IDS) | (mean) | |||||||
| Minniti (14) | 2016 | 289 | ≥2 cm | 27 Gy/3 fr | 12.5 | 13.4 | 73.0 | 8.0 |
| Marginal (80–90% IDS) | ||||||||
| Murai (15) | 2014 | 54 | ≥2.5 cm | 18–30 Gy/3 fr | N/A | 6.0 | 69.0 | 7.4 |
| 21–35 Gy/5 fr | ||||||||
| Fokas (16) | 2012 | 260 | ≥3 cm | 35 Gy/5 fr | N/A | 9.0 | 71–75 | 4.1 |
| 40 Gy/4 fr | ||||||||
| Wegner (17) | 2015 | 36 | ≥3 cm | 24 Gy/3 fr | 15.6 | N/A | 63.0 | 0 |
| Jeong (18) | 2015 | 37 | ≥3 cm | 30 Gy/3 fr | 17.6 | 16.0 | 87.0 | 15.8 |
| 35 Gy/5 fr | ||||||||
| Marginal (80% IDS) | ||||||||
| Current study | 2018 | 45 | ≥2 cm | 35 Gy/5 fr | 19.3 | 14.2 | 64.7 | 4.4 |
| Marginal (90% IDS) |
Fr: fractions, MTV: median target volume, MST: median survival time, LCR: local control rate, RN: radionecrosis, IDS: isodose surface
Ernst-Stecken et al treated 51 patients (72 lesions that were >3 mL) using HFSRT (30–35 Gy in 5 fractions), and achieved good RR and LCR results.11 Although their phase 2 trial included 29 patients who underwent prior WBRT, the severe toxicity rate was not high. Interestingly, the median volume receiving >4 Gy per fraction (V4Gy) was significantly correlated with RN, as only 14% of patients with a V4Gy of <23 cc developed RN, whereas 70% of patients with a V4Gy of >23 cc had MRI-detectable RN.11 Kim et al retrospectively compared SRS versus HFSRT for tumors >5 mL, and reported that the HFSRT group (36 Gy in 6 fractions) exhibited a similar LCR with a lower risk of toxicity than the SRS group (20 Gy in a single fraction), despite the HFSRT being used for larger lesions.12 Minniti et al also retrospectively compared SRS versus HFSRT for tumors >2 cm, and reported that HFSRT (27 Gy in 3 fractions) was used for larger tumors rather than SRS (15–18 Gy), and that the HFSRT group had significantly better LCR and RN rates.14
Kocher et al demonstrated that adjuvant WBRT after SRS reduced the rates of intracranial relapse and neurological death.2 However, SRS plus WBRT did not improve overall survival and was associated with a higher risk of significant decline in neurocognitive function than SRS alone.5,6 The present study compared the intracranial control rate for HFSRT alone, among patients with large lesions (group A) or small lesions (group B), but failed to detect significant differences in the 1-year BPFS or MST. In this study, none of the 8 patients who underwent salvage SRT for DBM developed severe toxicities, suggesting that cases of DBM after HFSRT could be safely managed using salvage SRT.
Previous studies have indicated that HFSRT for large brain tumors provided RN rates of 0–39.2%.11,12,14-21 In addition, two retrospective studies have indicated that HFSRT has a lower rate of RN than SRS, with Minniti et al reporting rates of 8% for HFSRT and 20% for SRS14 and Kim et al reporting rates of 0% for HFSRT (36 Gy in 6 fractions) and 6.9% for SRS.12 In the present study, only 2 patients (4.4%) developed RN years after HFSRT. Hence, long-term survivors, such as breast cancer patients, should be carefully followed with monitoring of cranial symptoms and imaging studies.
The present study has three important limitations. The first limitation is its retrospective single-center design, with variable frequency of post-treatment MRI evaluations (median: 3 times, range: 1–33 times). The second limitation is that we did not conduct a competing risk analysis in our study. This could bias the Kaplan-Meyer estimate. The third limitation is that our HFSRT plans adopted higher % isodose surface for target coverage (90% isodose for PTV coverage) than that of regular SRS/SRT plans (70–80% isodose for target coverage). This was related to the lower center dose of PTV and might have resulted in relatively lower LCRs than those of regular SRS/SRT plans for large BM. Further studies are needed to determine the optimal doses, fractions, and dose distribution (target dose heterogeneity).
CONCLUSION
In conclusion, HFSRT can be considered a safe treatment for brain metastases larger than 2 cm given the low rate of RN associated with this method.
ACKNOWLEDGEMENTS
We gratefully acknowledge the work of past and present members of our department.
CONFLICTS OF INTEREST STATEMENT
The authors have no conflicts of interest.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article.
Abbreviations
- SRS
stereotactic radiosurgery
- SRT
stereotactic radiotherapy
- WBRT
whole-brain radiotherapy
- HFSRT
hypofractionated stereotactic radiotherapy
- RN
radionecrosis
- HT
helical tomotherapy
- ECOG
eastern cooperative oncology group
- PS
performance status
- KPS
Karnofsky performance status
- CT
computed tomography
- MRI
magnetic resonance imaging
- GTV
gross tumor volume
- CTV
clinical tumor volume
- PTV
planning target volume
- CR
complete response
- PR
partial response
- SD
stable disease
- PD
progressive disease
- LCR
local control rate
- MST
median survival time
- RR
response rate
- DBM
distant brain metastases
- BPRS
brain progression-free survival
- PET
positron emission tomography
REFERENCES
- 1.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. [DOI] [PubMed]
- 2.Kocher M, Soffietti R, Abacioglu U, et al. EORTC 22952-26001: Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed]
- 3.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed]
- 4.Kondziolka D, Patel A, Dade Lunsford L, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427–434. [DOI] [PubMed]
- 5.Chang EL, Rey J, Wefel S, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed]
- 6.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed]
- 7.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed]
- 8.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. [DOI] [PubMed]
- 9.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. [DOI] [PMC free article] [PubMed]
- 10.Prabhu RS, Press RH, Patel KR, et al. Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99(2):459–467. [DOI] [PubMed]
- 11.Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2006;81(1):18–24. [DOI] [PubMed]
- 12.Kim YJ, Cho KH, Kim JY, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 2011;81(2):483–489. [DOI] [PubMed]
- 13.Higuchi Y, Serizawa T, Nagano O, et al. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009;74(5):1543–1548. [DOI] [PubMed]
- 14.Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3 × 9 gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142–1148. [DOI] [PubMed]
- 15.Murai T, Ogino H, Manabe Y, et al. Fractionated stereotactic radiotherapy using cyberknife for the treatment of large brain metastases: a dose escalation study. Clin Oncol. 2014;26(3):151–158. [DOI] [PubMed]
- 16.Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol. 2012;109(1):91–98. [DOI] [PubMed]
- 17.Wegner RE, Leeman JE, Kabolizadeh P, et al. Fractionated stereotactic radiosurgery for large brain metastases. Am J Clin Oncol. 2015;38(2):135–139. [DOI] [PubMed]
- 18.Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH. Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc. 2015;58(3):217–224. [DOI] [PMC free article] [PubMed]
- 19.Lee CC, Yen CP, Xu Z, Schlesinger D, Sheehan J. Large intracranial metastatic tumors treated by Gamma Knife surgery: outcomes and prognostic factors. J Neurosurg. 2014;120(1):52–59. [DOI] [PubMed]
- 20.Ebner D, Rava P, Gorovets D, Cielo D, Hepel JT. Stereotactic radiosurgery for large brain metastases. J Clin Neurosci. 2015;22(10):1650–1654. [DOI] [PubMed]
- 21.Han JH, Kim DG, Kim CY, Chung HT, Jung HW. Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys. 2012;83(1):113–120. [DOI] [PubMed]
- 22.Yang H, Kano H, Lunsford LD, Niranjan A, Flickinger JC, Kondziolka D. What factors predict the response of larger brain metastases to radiosurgery? Neurosurgery. 2011;68(3):682–690. [DOI] [PubMed]
- 23.Jiang X, Xiao J, Zhang Y, et al. Hypofractionated stereotactic radiotherapy for brain metastases larger than three centimeters. Radiat Oncol. 2012;7:36. [DOI] [PMC free article] [PubMed]
- 24.Tomita N, Kodaira T, Tachibana H, et al. Helical tomotherapy for brain metastases: dosimetric evaluation of treatment plans and early clinical results. Technol Cancer Res Treat. 2008;7(6):417–424. [DOI] [PubMed]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed]
- 26.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed]