Abstract
Collapsin response mediator proteins (CRMPs) are a family of ubiquitously expressed, homologous phosphoproteins best known for coordinating cytoskeletal formation and regulating cellular division, migration, polarity, and synaptic connection. CRMP2, the most studied of the five family members, is best known for its affinity for tubulin heterodimers and function in regulating the microtubule network. These functions are tightly regulated by post-translational modifications including phosphorylation, SUMOylation, oxidation and O-GlcNAcylation. While CRMP2’s physiological functions rely mostly on its non-phosphorylated state, dysregulation of CRMP2 phosphorylation and SUMOylation has been reported to be involved in the pathophysiology of multiple diseases including cancer, chronic pain, spinal cord injury, neurofibromatosis type 1 and others. Here, we provide a consolidated update on what is known about CRMP2 signaling and function, first focusing on axonal growth and neuronal polarity, then illustrating the link between dysregulated CRMP2 post-translational modifications and diseases. We additionally discuss the roles of CRMP2 in non-neuronal cells, both in the CNS and regions of the periphery. Finally, we offer thoughts on the therapeutic implications of modulating CRMP2 function in a variety of diseases.
Keywords: CRMP2, interactome, neurite outgrowth, post-translational modifications, human disease, Alzheimer’s disease, Multiple sclerosis, Chronic pain, Cancer, Stroke, therapeutics, non-neuronal cells
1. INTRODUCTION
First identified as a regulator of growth cone collapse during brain development [1], the members of the Collapsin Response Mediator Protein (CRMP) family are now well known for their role in cytoskeletal formation, cellular division, migration, neuronal polarity and synapse dynamics [2–8]. Starting with Alzheimer’s disease [9], CRMP dysregulation has now been described in numerous diseases such as pain [10–13], neurodegenerative disorders [14–19], psychiatric syndromes [20–22], and cancer [23,2,24–28], making them of high interest for therapeutic drug targeting across these diverse human conditions [29]. There are five designated CRMPs (CRMP1–5), with CRMP1, 3 and 5 contributing to dendrite morphogenesis while CRMP2 and 4 have roles in axon guidance, elongation and specification [30,3,4,29,2,31,32,8,33]. Here, we focus on the known mechanisms of CRMP2, the most studied member of the CRMP family. We provide a consolidated overview on post-translational modifications of CRMP2, tying these alterations into functional consequences in the central nervous system (CNS). We also discuss CRMP2’s potential role(s) in non-neuronal CNS cells and cells outside the nervous system. Finally, we touch on the therapeutic implications of these basic scientific studies, with the aim of providing a platform for future drug discovery endeavors.
2. The DPYSL2 (CRMP2) gene
The CRMP2 homolog from C. elegans, unc-33, was first described in 1985 in a screen for gene mutations effecting axon growth [34]. Mutations in unc-33 likely resulted in axon guidance and growth defects due to a super-abundance of microtubules, whereby mutant microtubules of a larger diameter formed doublets or triplets [34]. These early reports on unc-33 represents the first findings that fueled research on CRMP2 functions for the following 20 years. After the sequencing of unc-33 in 1992 [35], CRMP2 (alternatively named CRMP-62, TOAD-64, DRP-2, Ulip) was identified in chicken [36], rat [37], human [38] and mouse [39].
The coding region for DPYSL2 (gene coding for CRMP2) is found on chromosome 8p21 in humans. Multiple protein isoforms can rise from the alternative splicing of the first exon of CRMP2 mRNA [40,22] (Figure 1). The extensive genome and transcriptome sequencing performed in the last 20 years allowed us to mine experimental evidence of transcripts for any gene. In the Ensembl (EMBL) and Nucleotide (NIH) databases alternative transcripts leading to a long form of CRMP2 are referenced for humans with published evidence at the protein level [22]. We found that pigs express several splice variants for CRMP2 corresponding to the CRMP2-A (long, Ensembl: ENSSSCT00000031898.2, Nucleotide: XM_001927801.6, Uniprot: I3LJE2) and CRMP2-B (short, Ensembl: ENSSSCT00000052840.1, Nucleotide: XM_021073575.1, Uniprot: A0A286ZKK0) forms. These splice variants are not present in mice (Ensembl: ENSMUSG00000022048, Nucleotide: NM_009955.3, Uniprot: O08553) or rats (Ensembl: ENSRNOG00000009625, Nucleotide: NM_001105717.2, Uniprot: P47942) but were originally described in the chick embryo (CRMP2-A, Ensembl: ENSGALT00000031500.2, Nucleotide: XM_015297296.2, Uniprot: Q71SG2 and CRMP2-B, Ensembl: ENSGALT00000000305.6, Nucleotide: XM_015297298.2, Uniprot: Q90635)[40]. Although CRMP2-A and CRMP2-B isoforms have been reportedly observed in rodents, these are inferred from higher molecular weight bands in western blots, which does not necessarily allow conclusions on whether they are a result of alternative splicing of the mRNA. Thus, the differential detection of multiple molecular weight bands for CRMP2 in western blots may not necessarily represent CRMP2 isoforms but is likely a result of cleavage and/or post-translational modifications. One publication claimed mass spectrometry evidence of CRMP2-A isoform in an immunoprecipitate from mouse brain, but this interpretation remains controversial because the identified peptides can be mapped on the common sequence between CRMP2-A and CRMP2-B but not on the alternative exon 1 belonging exclusively to CRMP2-A (long isoform) (see supplementary figure 1 in [41]). Further, the authors had to use a heterologous system (human HEK293 cells) expressing recombinant human CRMP2-A to confirm their CRMP2-A interaction of interest (Prolyl Isomerase Pin1/CRMP2-A interaction) [41]. Finally, mining unbiased RNA-seq data from multiple brain cell populations showed no significant reads on the region from the mouse chromosome 14 corresponding to the putative alternatively spliced CRMP2-A variant (see notes in [42]). However, an antibody raised against the exon 1 of CRMP2-A (a protein blast indicated that this epitope is not predicted to cross react with other proteins on the mouse proteome) was able to detect a higher molecular weight band in western blot on adult mouse brain and primary cortical neurons [43]. We conclude that expression of alternatively spliced CRMP2 isoforms in rodents is controversial from lack of evidence at the transcript and protein levels. Further studies need to be carried to definitively confirm or exclude whether CRMP2-A is expressed in rodents or if higher molecular weight bands are evidence of a post-translational modification increasing CRMP2 molecular weight by ~10 kDa, which could be a SUMOylation event [44,12,45–47].
Figure 1. Schematic representation of DPYSL2 gene across species.
The intron/exon DPYSL2 gene organization is shown for the indicated species. Each alternative splicing product is identified by its Ensembl ID and corresponding Uniprot ID. Protein length was extracted from Uniprot. The red boxes indicate the alternative Exon 1 specific to each mRNA isoform. The amino-acid sequence shows the positions of known CRMP2 post-translational modifications, color-coded to indicate the exon carrying the modified amino-acid. When known, the enzyme responsible for each modification is indicated.
Not much is known about the distinct role of the two CRMP2 isoforms. CRMP2-A protein product represents the longer form at ~75 kDa, while CRMP2-B is ~62 kDa [40,29,43]. CRMP2-A expression had no effect on primary axon elongation but was able to counteract CRMP2-B-mediated inhibition of axonal growth [40]. It is important to note that the main protein isoform found in all species (572 amino-acids) is conserved with 96.9% identity and 99.1% similarity (amino-acids with similar side chains). All known phosphorylation, SUMOylation, oxidation and O-GlcNAcylation sites are fully conserved from chicken to humans. This suggests that CRMP2 functions are likely conserved between species and that using rodents or pigs to study CRMP2 therapeutic targeting has high translational potential.
Crystal structures of CRMPs have shown them to be homologous to the catalytic dihydropyrimidinase (DHPase) family, though CRMPs do not share the catalytic activity of DHPase [48,45]. What would have been the catalytic site on CRMP2 is maintained as a corresponding pocket that may allow for ligand binding when opened [48,49]. Rather than being catalytic, CRMP2 is modified by a series of post-translational modifications, including redox regulation, O-GlcNAcylation, SUMOylation, and phosphorylation at multiple sites [48,50–53].
3. CRMP2 expression pattern the central nervous system and other cells:
a. CRMP2 expression in neurons
CRMP2 is expressed mainly in the nervous system as inferred from positive immunoblotting signals from brain and DRG homogenates while no expression was detected in intestine, lung, liver, kidney, heart, muscle or any other tissues from chicken [36]. CRMP2 expression pattern in the mouse and cat brain is very high during embryonic development and decreases significantly after birth [43,54,55]. In the adult brain, CRMP2 is expressed in the soma, axons, and dendrites of neurons from the primary motor and somatosensory cortex but also in the hippocampus and the cerebellum [43]. CRMP2 expression was found in progenitors of oligodendrocytes thus showing that CRMP2 expression is not restricted to neurons in the CNS [56]. RNA-seq and machine learning mapping of all cell types of the mouse nervous system allowed for a more detailed analysis of CRMP2 expression pattern in different neuronal and non-neuronal cell populations (http://mousebrain.org/genesearch.html [57]). This revealed high CRMP2 expression in the sensory neuron afferents to the cranial nerves III to XII, the adrenergic/noradrenergic neurons of the medulla, the serotonergic neurons from the nucleus raphe pons, excitatory neurons of the hindbrain, cholinergic enteric neurons, and in immature oligodendrocytes from the pons. Thus, CRMP2 expression in the CNS is mostly contained in neurons or precursor cells.
b. CRMP2 expression in non-neuronal cells
i. Oligodendrocytes
Early evidence for CRMP2 expression in oligodendrocytes (OLs) came from in situ hybridization studies, where it was revealed to be the most highly expressed of the five CRMP isoforms in the adult brain, with mRNA present in the majority of immature OLs [56]. CRMP2 immunolabeling has also been observed in primary non-myelinating OL cultures [43]. More specifically, CRMP2 immunolabeling has been observed in the white matter of the cerebellum, as well as in other areas containing white matter, including the myelinated tracts in the brain and spinal cord [56,58]. Consistent with RNA-seq data, CRMP2 immunolabeling in vivo and in vitro was weak or absent present in astrocytes, microglia, ependymal cells and pericytes (http://mousebrain.org/genesearch.html [56,57]). CRMP2 has been involved in OL process retraction and oligodendrocyte progenitor cell (OPC) migration and directionality [59]. Both OL and OPCs express the Semaphorin3A (Sema3A) receptor Neuropilin-1 [60–62,58]. Sema3A can inhibit oligodendrocyte process extension in vitro [58]. Blocking CRMP2 by adding an anti-CRMP2 antibody to the culture medium prevented the Sema3A induced inhibition of oligodendrocyte branching.
ii. Astrocytes
Although CRMP2 mRNA has been detected in highly purified adult mouse astrocytes (Figure 2 and see notes [63]), there are only two published studies demonstrating CRMP2 protein expression in these cells by either immunostaining or immunoblotting. An immunolabeling study based on anti-bovine CRMP2 N-terminal residues 1–17 found a positive signal in mouse astrocytes (as well as in neurons and OLs), however the epitope used is not fully conserved in rodents (K7N mutation) and is identical between CRMP2 and CRMP4 [64]. In contrast, immunostaining using an antibody directed against CRMP2 residues 217–232 failed to detect CRMP2 in astrocytes [56]. With the consideration of the recent RNA-seq data, and the inconsistent data obtained using antibodies and immunostaining, whether CRMP2 is expressed in astrocytes remains undetermined.
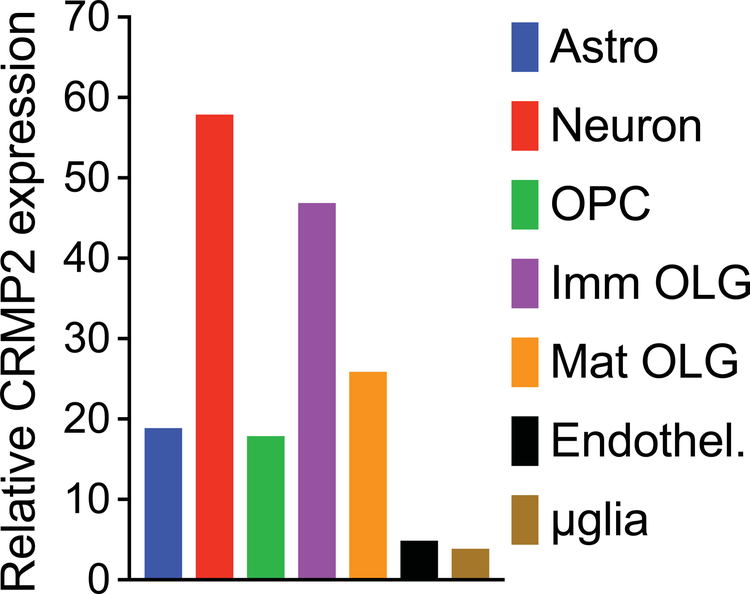
Figure 2. Relative CRMP isoform mRNA expression in neural cell types determined by RNA-seq analysis.
RNA-seq was carried out using highly enriched cell populations isolated from healthy adult mouse brain [42]. The expression level is plotted relative to the level of CRMP2 in neurons which is set to 100%. After neurons, the 2nd most abundant cell source are immature oligodendrocytes (Imm OLGs) at about 80% of that in neurons, followed by mature myelinating OLGs (Mat OLG, about 45% of that in neurons). Astrocyte and OLG progenitor cell (OPC) expression is about 30% that of neurons, while expression in endothelial cells and microglia is the lowest, roughly 10% that of neurons. The level of CRMP2 measured in neurons was 59.9 FPKM (Fragments Per Kilobase of transcript per Million mapped reads). For comparison, the FPKM for several housekeeping genes were 1,733 for α-tubulin, 334 for β3-tubulin, and 323 for β-actin.
iii. Microglia
Similar to astrocytes, RNAseq analysis identified low levels of CRMP2 in microglia of adult mice [42,57]. Subsequent analysis across developmental stages showed CRMP2 microglial expression at E17 (132 reads per kilobase million (RPKM) through post-natal day 60 (90 RPKM) (Supplementary dataset 1 in [65]). Microglial CRMP2 mRNA is also influenced by inflammatory activation as evidenced by treatment with bacterial endotoxin lipopolysaccharide (LPS). CRMP2 expression level in microglia (392 RPKM) was reduced by 2-fold following treatment with LPS, suggesting a regulation of CRMP2 transcript levels during microglial activation (see supplementary table 1 in [66]). Aside from RNAseq analyses, there is no direct evidence documenting CRMP2 expression in microglia. Indirect support for microglial CRMP2 comes from studies showing the inflammatory responses of mouse EOC-20 microglial cells and primary rat microglia are reduced by treatment with lanthionine ketimine ester (LKE), a naturally occurring metabolite shown to interact with CRMP2 [67]. Similarly, providing LKE to mice with spinal cord injury (SCI) suppresses microglia activation, as indicated by reduced numbers and more ramified appearance (quiescent) of ionized calcium-binding adapter molecule 1 (Iba1) – a microglia/macrophage-specific calcium-binding protein – positive cells in the spinal cord [68]. LKE treatment also reduced pathology in a mouse model of Alzheimer’s disease (AD), in which it reduced amyloid deposition, phospho-tau accumulation, and the density of Iba1 stained microglial cells [69]. However, whether LKE’s actions are mediated through CRMP2 or through an alternate target protein (LKE also binds to LanCl1, complement H factor, heat shock protein-70, myelin basic protein (MBP), myelin proteolipid protein, syntaxin binding protein-1 (STXBP1/Munc-18)[67]) have not been addressed. The mechanism of action of LKE on CRMP2 remains unclear. For instance, LKE decreases CRMP2 interaction with tubulin but increases neurite outgrowth and axon formation [68,67]. It is still unknown whether LKE changes CRMP2 expression level or targets CRMP2’s post-translational modifications. Since CRMP2 has been shown to be expressed in peripheral macrophages [70], it is also not clear if the effects of LKE in the above neurological models were due to suppression of parenchymal microglia or of blood derived macrophages, both of which express the Iba1 antigen. Taken together, possible roles for CRMP2 in these cells remain to be fully explored, though it is possible that CRMP2 exerts similar roles in regulating microtubule dynamics in these cell types. As the majority of studies have been carried out in rodents, it remains to be determined if localization of CRMP2 expression (and function) in human cells is similar or distinct from rodents. In this respect, RNA-seq analysis of different cell types isolated from adult human brain shows significant differences compared to mouse, with mature astrocytes showing higher CRMP2 expression than either neurons or OLs (http://brainrnaseq.org [63]). As such, more work remains to be done in the field of CRMP2 dynamics in non-neuronal CNS cell populations.
4. CRMP2 functions and regulation in neuronal polarity establishment:
CRMP2 plays an important role in neuronal polarity establishment and in axonal guidance. A role for CRMP2 in axon guidance was identified in an oocyte screen of proteins participating in the Collapsin (former name for Semaphorin 3A –Sema3A) response pathway [36]. Using a complementary DNA library from E7 chicken dorsal root ganglia (DRG), the CRMP2 coding sequence allowed for an inward current response to the bath application of Sema3A [36]. CRMP2’s role in axonal guidance is due to the CRMP2-dependent collapse of the growth cone of axons in response to Sema3A [36]. Neuronal polarization is the developmental stage where small processes from a neuron will differentiate into an axon and dendrites [71]. Increasing CRMP2 expression level was able to change the polarization of hippocampal neurons and induced multiple axons in 57% of neurons [72]. CRMP2 functions in axonal growth and guidance are tightly regulated through different post-translational modifications.
a. Mechanism of CRMP2-dependent tubulin polymerization
CRMP2 is a cytosolic phosphoprotein regulating the microtubule network, playing an important role in in cell division, neuronal polarity establishment, and axonal guidance in the developing cerebral cortex [72,73,36]. As basal levels, CRMP2 can form hetero-tetramers with the other members of the CRMP family [74]. In the presence of tubulin α/β heterodimers, CRMP2 homotetramers are dissociated into monomers to re-associate as hetero-trimers with α/β tubulin, promoting their transport to the PLUS end of the growing microtubule with the aid of the motor protein kinesin-1 tubulin [75,5,76]. Several regions of CRMP2 have been identified as key for this mechanism. First, the domain corresponding to the aa 323–381 is sufficient to induce microtubule assembly in vitro [5]. However, the aa 466–490 domain of CRMP2 is required to form GTP-state (class I, usually found at the tip of the growing axon [77]) growing microtubules, due to CRMP2 GAP (GTPase activating protein) activity located within its C-terminal domain aa 480–510 [75,78]. Second, CRMP2 interaction with tubulin heterodimers engages the aa T246-N247 and K483-R485-R487, and X-ray crystallography has shown helix H19 of CRMP2’s N-terminal globular domain interacting tightly with β-tubulin [75,76]. Upon interaction, CRMP2 enhances the GTPase activity of the β-tubulin subunit [78] to promote the polymerization of tubulin heterodimers on the curved sheets (class I) of the microtubule ends [75]. Interestingly, this function of CRMP2 can be pharmacologically inhibited by two enantiomers ((2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide; Vimpat®) (R- and S-) of lacosamide, the former enantiomer a clinically available anti-epileptic [79]. Lacosamide binds to CRMP2 on the pocket formed by the residues E360-S363-K418-I420-P443, a binding domain located away from helix H19 [80,81]. Thus, it appears that this region could have an allosteric function in regulating CRMP2 dependent polymerization while maintaining CRMP2’s affinity for tubulin.
Finally, the interaction of CRMP2 with tubulin also engages the unresolved C-terminus of CRMP2 with the E-hook of tubulin, allowing CRMP2 to additionally tether into the existing microtubule network, providing crucial stabilization and allowing CRMP2 to act as a cargo adaptor [82,75]. This region of tubulin is rich in acidic (negatively charged) residues while the C-terminus of CRMP2 is rich in positively charged lysine residues (aa K511-K520-K525). Importantly, this domain of CRMP2 is enriched in phosphorylation sites (aa S522-T509-T514-S518) which add multiple negative charges upon phosphorylation, thereby dissociating CRMP2 tetramers via novel negative charges, and adding repulsive forces between the CRMP2 C-terminus and the E-hook of tubulin [82]. Thus, the phosphorylation of CRMP2 inhibits its interaction with tubulin heterodimers, modulating CRMP2’s role in multiple processes throughout neuronal development.
b. CRMP2 phosphorylation events regulate neurite outgrowth, polarity, and division
The regulation of CRMP2 by phosphorylation (Table 1) was first identified in 1996 in the now classic semaphorin-Cdk5-GSK3β pathway [39]. The semaphorins are prominent extracellular chemorepulsant cues that bind to either neuropilin 1 (NP1) or plexin A1 receptors [83]. Semaphorins have been implicated in coupling with various plexinA signaling pathways to modulate CRMP2, complicating the understanding of precise mechanisms of each of these ligand-receptor pathways [84,85]. Classically, Semaphorin 3A (Sema3A) binds to the ligand NP1, which forms a complex with Plexin A1, promoting an intracellular signaling cascade leading to the activation of Cdk5 (Figure 3A) [86]. Cdk5 phosphorylates CRMP2 at S522, priming CRMP2 for subsequent phosphorylation by GSK3β at residues T509-T514-S518 [87,73,86,88]. Importantly, Cdk5 phosphorylation is resistant to dephosphorylation, thus making Cdk5 a permanent regulatory switch of CRMP2 functions, including tubulin polymerization, cellular proliferation [27], and ion channel trafficking [46,89,90]. Alternatively, Sema cascades may activate members of specific tyrosine kinases (Fes/Fps, Fer, Fyn), leading to phosphorylation of PlexinA1 and subsequently CRMP2 (Figure 3A). These kinases can phosphorylate CRMP2 [91–93] on residues Y32 (Fyn, Fes/Fps [91], not tested for Fer), Y479 (Yes [94], Fer [92], putatively Fyn, Fes [91]) and Y499 (Fer [92], not tested for Fyn, Fes/Fps). Interestingly, Fyn can also activate Cdk5 through phosphorylation at residue Y15 [95], suggesting a coordination between all CRMP2 kinases during Sema3A induced growth cone collapse.
Table 1.
Summary of CRMP2 post-translational modifications and functional consequences.
| Enzyme | Function | Effect of loss of function mutations | Diseases | Targeting peptides and compounds | Reference(s) | |
|---|---|---|---|---|---|---|
| Y32 (phosphorylation) | Fyn, Fes/Fps | Inhibition of Neurite outgrowth, Decreased CRMP2 SUMOylation and NaV1.7 membrane localization | Promotes Neurite outgrowth, Protects NaV1.7 from endocytosis | Decreased at pre- synaptic sites in neuropathic pain | Unknown | [91,95,46,13] |
| Y479 (phosphorylation) | Yes, Fer, Fyn, Fes/Fps | Cytoskeletal reorganization in T-cell migration, loss of affinity for microtubules, disruption of CRMP2 tetramers | Reduced cell polarization and motility | HTLV infections | Unknown | [94,189] |
| Y499 (phosphorylation) | Fer | loss of affinity for microtubules, disruption of CRMP2 tetramers | Unknown | Ovarian cancer | Unknown | [92] |
| T509/T514/S518 (phosphorylation) | GSK3β | loss of affinity for tubulin, cellular proliferation, disruption of CRMP2 tetramers | Unknown | AD, neuropathic pain, bipolar disorder | Naringenin, (S)- lacosamide, lanthionine ketimine ester, edonerpic maleate, | [27,46,89,90,109,137,22,82,67,188] |
| S522 (phosphorylation) | Cdk5 | loss of affinity for tubulin, cancer cell proliferation, promotes CaV2.2 and NaV1.7 membrane localization, disruption of CRMP2 tetramers | Promotes neurite outgrowth, decreases CaV2.2 And NaV1.7 trafficking | AD, neuropathic pain, NF1, bipolar disorder, cancer | (S)- lacosamide, lanthionine ketimine ester, edonerpic maleate | [131–133,9,134,27,46,89,90,166,11,165,10,169,22,180,181,23,182,82,67,188] |
| T555 (phosphorylation) | RhoK, CaM KII | Decreased affinity for tubulin, decreased neurite outgrowth | Promotes neurite outgrowth, decreased Ca2+ influx | AD, MS | Unknown | [19,110,105,111,112] |
| K374 (SUMOylation) | Ubc9 | NaV1.7 trafficking | Decreases NaV1.7 surface expression | neuropathic pain | CRMP2 SUMOylation motif peptide | [12,13,44,161,124,47,45,46,160] |
| S517 (O- GlcNAcylation) | Unknown | Unknown | Unknown | Unknown | Unknown | [52,53] |
| C504 (oxidation) | Unknown | CRMP2 dimerization, microtubule regulation, growth cone collapse | Unknown | Unknown | Unknown | [117,116,118] |
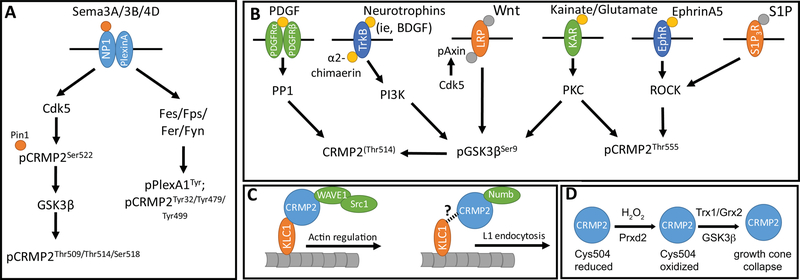
Figure 3. Summary of signaling cascades regulating CRMP2 microtubule dynamics.
(A) Description of Semaphorin mediated signaling, resulting in 1) CDK5 and GSK3β mediated pCRMP2 (stabilized by Pin1) and/or 2) Fes/Fps/Fer/Fyn mediated pCRMP2 at tyrosine sites. (B) Description of various signaling pathways that result in 1) the dephosphorylation of CRMP2, 2) the phosphorylation of GSK3β, and/or 3) pCRMP2 at Thr555. (C) CRMP2’s association with Src-1/WAVE1 and kinesin light chain 1 (KLC1) modulates actin remodeling. Numb mediated endocytosis of the adhesion molecule L1 may be shuttled using similar processes. (D) Description of redox regulation of CRMP2, modifying its affinity to thioredoxin (TRX).
As such, Sema3A and other similar Semaphorin cascades are negative regulators of microtubule dynamics. Indeed, this has been reported to be crucial for proper positioning of caudal primary motor neurons of the zebrafish spinal cord, the proper bifurcation of apical dendrites in hippocampal CA1 pyramidal neurons, axon elongation, and cell division [96–98]. Specifically, in cell division, extracellular Sema3B released from cerebrospinal fluid modulates CRMP2 at T514, thereby regulating the cell division cleavage plane of neuroepithelial cells in the developing mouse spinal cord [85]. CRMP2 has been additionally implicated in the proper formation and localization of the mitotic spindle in anaphase and metaphase using residues 488–572 of its C-terminal tail [99,100], as well as the proper centrosome movement and positioning of Drosophila sensory organ precursor cells [101] through CRMP2’s interaction with Numb, a crucial component of endocytosis [102,103,75]. Specifically, CRMP2-Numb mediated endocytosis promotes the recycling of L1, a neuronal cell adhesion molecule whose incorporation into the leading plasma membrane is necessary for forming new adhesions and promoting axon outgrowth (Figure 3C). A reasonable hypothesis is that CRMP2 tethers Numb-mediated endocytic complexes to motor proteins, such as kinesin and dynein, but the specific role of CRMP2-binding to motor proteins has not been investigated in the context of endocytosis.
A number of other signaling pathways have been found to modulate CRMP2 phosphorylation, and as a consequence, microtubule outgrowth and stabilization. Neurotropins, such as brain derived neurotrophic factor (BDNF) and neurotrophin-3; kainates, such as glutamate; and Axin, a scaffolding protein important in Wnt signaling; can all induce phosphorylation of GSK3β at residue S9, thereby increasing CRMP2’s affinity for tubulin (Figure 3B) [104–106,73,87]. Neurotrophins in particular achieve this through the activation of phosphoinositide 3-kinase (PI3K), which in turn phosphorylates Akt at residue S473, thereby phosphorylating GSK3β at S9. Interestingly, upon Sema3A binding, phosphatase and tensin homolog (PTEN) accumulates in the growth cone [107] and inactivates PI3K [108]. This, in turn, decreases Akt activity to allow GSK3β to inactivate CRMP2 by phosphorylation after Sema3A stimulation [108].
CRMP2’s affinity for tubulin can also be modulated by platelet derived growth factor (PDGF), whose stimulation leads to the activation of protein phosphatase 1, thereby dephosphorylating CRMP2 at T514 and directing cell migration[109]. Conversely, kainates, ephrins, lysophosphatidic acid (LPA), and lipids such as sphingosine-1-phosphate (S1P) can simulate CRMP2 phosphorylation at T555 via their association with their respective ligands. This leads to the activation of either protein kinase C (PKC) or rho associated kinases (ROCK), thereby promoting dissociation with Numb and tubulin (Figure 3B) [110,105,111,112]. As such, these pathways have been implicated in the regulation of sensory neuron and peripheral neuron outgrowth [104,111]. However, inhibiting T555 phosphorylation had only moderate effects on reducing the growth cone collapse induced by LPA [110] or ephrin-A5 [112]. Thus, CRMP2 phosphorylation at T555 plays a role in regulating CRMP2 affinity for tubulin heterodimers but is probably accompanied by additional phosphorylations of CRMP2 (likely by Cdk5 and GSK3β) to induce growth cone collapse.
CRMP2 also functions as a cargo adaptor to the molecular motors kinesin-1 [76] (for anterograde transport) and dynein [113] (for retrograde transport). Direct interaction between CRMP2 and kinesin light chain (KLC) was confirmed by immunoprecipitation and in vitro binding assays, whereby CRMP2’s C-terminus domain at residues aa 440–572 was found to bind to the six-tetratricopeptide repeat domain of KLC1 to transport various cargoes to the distal tip of the axon [76,114]. The same domain of CRMP2 can bind to dynein and inhibit its retrograde transport activity [113], thus controlling the balance between anterograde and retrograde transport [113]. As such, CRMP2 binds tubulin dimers/oligomers and protein complexes such as the Sra-1/WAVE1 complex to KLC-1, thereby regulating axon specification and elongation through Sra-1/WAVE1’s regulation of actin (Figure 3C) [76,114]. Additionally, other proteins such as dynactin 1, a critical component of dynein function, and Numb may be tethered to kinesin-1 via CRMP2 as well to regulate microtubule dynamics, though Numb remains to be confirmed [76,114,115,102].
Taken together, these studies identified numerous phosphorylation sites on CRMP2 that can regulate the function of the protein. CRMP2 phosphorylation: (i) dissociates CRMP2 tetramers, (ii) inhibits CRMP2 interaction with tubulin, (iii) inhibits CRMP2 interaction with endocytic proteins, and (iv) appears to be irreversible at T555 via Cdk5. Thus, while CRMP2 expression levels remain stable during neuronal differentiation, CRMP2 phosphorylation is a dynamic event acting to remove CRMP2 from its function in cytoskeleton remodeling and force it into other cellular functions. As such, post translational modifications of CRMP2 have the ability to regulate a number of dynamic processes throughout neuronal development and maintenance, including cell division, migration, polarity, outgrowth, and synaptic output.
c. The regulation of CRMP2 by other means: oxidation, protein-protein interactions, SUMOylation, and O-GlcNAcylation
CRMP2-mediated neurite outgrowth can be reportedly regulated by a variety of other means. For example, oxidation or reduction by either peroxiredoxin 2 (Prdx2) and glutaredoxin 2 (Grx2) or thioredoxin 1 (Trx1) respectively, aid in the regulation of CRMP2 dimerization and phosphorylation [116–118]. When oxidized at Cys504 via a hydrogen peroxide (H2O2) dependent process following Sema3A stimulation, a disulfide-linked CRMP2 homodimer forms a transient complex with Trx1, enabling the activation of GSK3β, stimulation of p514-CRMP2, and growth cone collapse (Figure 3D) [117]. CRMP2 was identified as a Trx1 protein substrate in vivo [119], highlighting the physiological relevance of the CRMP2 redox switch for regulation of microtubule dynamics during redox perturbations [120].
CRMP2’s association with the ion and metabolite channel Pannexin 1 (Panx1) [121] and recently discovered glycation at approximately K406–483 were predicted [121,122]. Conversely, CRMP2’s association with CRMP4 leads to stabilization of both tubulin and actin fibers simultaneously [32]. Lastly, while CRMP2 is SUMOylated at Lys374, there has been only one report stating this modification regulates microtubule dynamics [123,124,47,45]. As such, further study on this, and all these mechanisms of action, should be pursued. Taken together, the vast diversity of CRMP2 post-translational modifications underscore the dazzling variety of functions this protein performs in neuronal development.
Another post-translational modification that occurs on CRMP2 is the addition of β-linked N-acetylglucosamine (O-GlcNAcylation). While this has been known since 2001 [53], the role of this modification is still unclear. Proteomic analyses revealed that CRMP2 O-GlcNAcylation is located on the residue S517 and co-exists with CRMP2 phosphorylation on the neighboring residue S518 [125]. Phosphorylation of the Cdk5 site (S522) alone did not change the amount of O-GlcNAcylation on the residue S517 [52]. However, adding a phosphorylation of the GSK3β sites (T509-T514-S518) inhibited the subsequent O-GlcNAcylation of S517 [52]. Similarly, CRMP2 S517 O-GlcNAcylation did not impact subsequent phosphorylation by Cdk5 (at S522) but interfered with GSK3β phosphorylation of CRMP2[52]. Thus, this O-GlcNAcylation site on CRMP2 could act as a regulatory point preventing the subsequent phosphorylation by GSK3β even when the priming phosphorylation event has occurred. Interestingly, CRMP2 cleavage by calpain occurs between the amino-acids S517-S518 (US patent: US20120196307A1). Thus, CRMP2 O-GlcNAcylation could also regulate CRMP2 cleavage by Calpain. The exact function of this post-translational modification remains to be elucidated.
5. CRMP2 post-translational modifications in pathology:
a. Multiple sclerosis
Multiple sclerosis is a degenerative disease occurring in the brain and spinal cord and characterized by inflammation, axonal damage and demyelination. In an animal model of multiple sclerosis (myelin oligodendrocyte glycoprotein peptide 25–35 (MOG35–55) induced model of experimental autoimmune encephalomyelitis (EAE)), CRMP2 phosphorylation on T555 was increased in the axons located around inflammatory lesions in the spinal cord during the onset and the peak of EAE [19]. In the CNS, CRMP2 pT555 was detected in all active MS lesions while it was absent from non-diseased samples. Additionally, MS degenerating axons and motor neurons became immune-positive for CRMP2 pT555 as well [19]. Importantly, the CD3+ T-cells infiltrating the spinal cord from MS patients were not positive for CRMP2 pT555. In the EAE model, inhibiting CRMP2 phosphorylation on T555 prevented axonal degeneration, while an antibody to Nogo-A, a neurite outgrowth inhibitor, was able to prevent both the development of EAE and increase of CRMP2 pT555. Whether an anti-Nogo-A antibody was able to prevent both the development of EAE and the increase of CRMP2 pT555. Whether Nogo signaling activation directly increases CRMP2 T555 phosphorylation level remains unknown but, in this example, inhibiting the pathological phosphorylation of CRMP2 was beneficial to prevent some features of this chronic disease [19].
b. Alzheimer’s disease
As the therapeutic implications of CRMP2 modulation in AD have been extensively reviewed previously, we will briefly summarize what has already been discussed while expanding on new insights [3,14,126–129,29,130]. In AD, hyperphosphorylated CRMP2 (on S522, T509-T514-S518, T555) aggregates in amyloid plaques and neurofibrillary tangles prior to the onset of disease pathology as shown in various AD mouse models and human samples. Aβ accumulation increases CRMP2 phosphorylation by Cdk5 at S522 [131–133,9,134], removing the pool of CRMP2 from the microtubule network, thereby dysregulating many basic neuronal processes. Genetically interfering with CRMP2 phosphorylation on S522 prevented Aβ-mediated impairment of long-term potentiation (LTP) [133]. A number of Cdk5, GSK3β, and CRMP2 phosphorylation blocking compounds have been used with varying success in AD models, resulting in fewer and smaller aggregates, improved memory and learning, and improved synaptic signaling [133,135–137]. Further studies are needed to fully characterize the therapeutic potential of CRMP2 phosphorylation inhibition for AD. Another interesting hypothesis was recently raised with the discovery of an extracellular pool of CRMP2, whereby extracellular CRMP2 acted as an agonist for NMDA receptors [138,139]. In AD, emerging evidence suggests that the propagation of the disease relies on the trans-synaptic transfer of pathological Tau and other prion-like proteins [140]. This raises the hypothesis that trans-synaptic transfer of phosphorylated CRMP2 in AD could be participating in this prion-like propagation of the pathology. More studies will be needed to understand the mechanisms and consequences of extracellularly localized CRMP2.
c. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) or Charcot disease is a neurodegenerative disorder affecting mostly motor neurons. Genetic rodent models of ALS exist to mimic familial manifestations of ALS; for instance, by introducing a G93A mutation in the superoxide dismutase 1 (SOD1) gene. Because CRMP2 phosphorylation by Cdk5 is involved in axonal degeneration, it was hypothesized that CRMP2 phosphorylation would be important for ALS. Genetic suppression of CRMP2 phosphorylation on S522 in the SOD1 G93A mouse model delayed the aggravation of the motor symptoms observed in this ALS model [141]. Similar to the phenotype observed in multiple sclerosis, suppressing CRMP2 phosphorylation protected the motor axons from degeneration [141]. Thus, targeting CRMP2 phosphorylation on S522 could be beneficial for ALS.
d. Stroke: NMDARs, Kainate, AMPARs, and NCX – all link to CRMP2
NMDA:
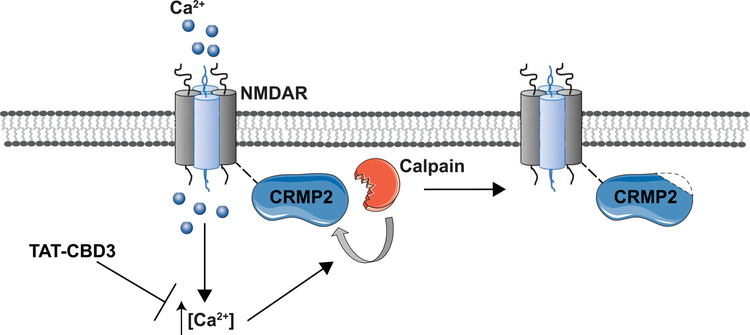
During excitotoxicity, CRMP2 is cleaved into a smaller, 58kDa form via calpain, which then downregulates NMDA receptor function [142–144]. Interestingly, CRMP2 was also identified as an interactor of NMDA receptors, with a preferential interaction for GluN2B-containing NMDARs [145]. As with N-type CaV2.2 channels, a HIV-1 transduction motif (TAT) coupled to the CRMP2-derived calcium binding domain 3 (CBD3) (TAT-CBD3) peptide reduces NMDAR-mediated Ca2+ influx in cultured neurons and post-synaptic NMDA receptor-mediated currents in cortical slices, emphasizing the ability of CRMP2 to regulate NMDAR function and trafficking (Figure 4)[146–148]. Specifically, this was mediated by an activity-dependent down-regulation of GluN2B-containing NMDAR surface expression in dendritic spines, providing neuroprotection against excitotoxic neuronal loss in multiple rodent models [148,147,146,149].
Figure 4. Overexcitation by glutamate induces an NMDAR-mediated increase in intracellular calcium.
The massive influx of calcium in neurons leads to hyper-activation of deleterious signaling pathway, and thus excitotoxicity. Among the enzymes activated, calpain, a Ca2+ activated protease, cleaves CRMP2. TAT-CBD3 is proposed to prevent the increase in intracellular calcium concentration, preventing CRMP2 cleavage and enhancing NMDAR internalization and thus providing neuroprotection. Adapted from Brittain and colleagues [146].
Though several studies have reported a biochemical interaction between CRMP2 and NMDA receptors and a functional regulation of NMDA receptors by CRMP2, it is not known if this interaction is direct or is mediated through other proteins [145–147]. Peptide-array experiments have revealed two possible binding-sites for CRMP2 in the C-terminus of the GluN2B subunit of NMDARs [146]. Notably, as recently demonstrated, CRMP2 may also bind on the extracellular portion of NMDA receptors and act as an atypical neurotransmitter, thereby triggering Ca2+ influx [138,139]. Where CRMP2 originates from in the extracellular medium is not well studied though because the extracellular CRMP2 used in the above experiment was derived from the media of degenerated sciatic nerves, and so the relevance of this finding under physiological conditions remains to be confirmed. Notwithstanding, this novel finding sheds new light on the effects of CRMP2 in NMDAR regulation, with the implication that the action of CRMP2-based peptides could also involve an extracellular component [138,139].
Kainate:
Kainate receptors are ionotropic receptor to the neurotransmitter glutamate. Although they are ubiquitously expressed in adults, their function is less understood compared to the function of ionotropic glutamate receptors AMPA and NMDA. As discussed above in the context of neuronal maturation and elongation, an interaction between CRMP2 and the GluK5 subunit of kainate receptors was identified by coimmunoprecipitation and GST pull down assay, participating in the regulation of Ser9 pGSK3β and Thr555 pCRMP2 [105,112]. The same study proposed that higher concentrations of kainates rely on CRMP2’s interaction with the N-type CaV2.2 channel, as downstream effects were blocked by the N-type CaV2.2 blocker ω-conotoxin [105]. However, downstream effects were also sensitive to tetrodotoxin (TTX) which could indicate involvement of TTX-sensitive voltage-gated sodium channels, such as NaV1.7, a known partner of CRMP2 [105]. As such, further studies to parse out the distinct role of kainite receptors in CRMP2 regulation should be pursued.
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR):
CRMP2 was identified as a possible substrate for PKA-mediated phosphorylation through immunoprobing coupled with mass spectrometry and was suggested to be involved in AMPA receptor up-regulation in cocaine-sensitization [150]. The extent of this interaction remains to be fully explored.
Sodium-calcium exchanger (NCX):
CRMP2 has been identified as a regulator of the Na+/Ca2+ exchanger 3 (NCX3) function, which is involved in regulation of Ca2+ and Na+ neuronal homeostasis [147]. The cell-permeant TAT-CBD3 peptide stabilized this NCX3-CRMP2 interaction, thereby reversibly inducing NCX3 internalization. Moreover, TAT-CDB3 was able to inhibit NCX3-mediated calcium flux both in reverse (influx of Ca2+ toward the intracellular compartment) and forward (extrusion of Ca2+ toward the extracellular compartment) mode [147].
Overall, CRMP2 is critical for Ca2+ homeostasis and the proper recycling of various membrane receptors. Dysregulation of these properties, such as that occurring during stroke, impairs neuronal survival.
e. Chronic pain
In chronic neuropathic pain, presynaptic sensitization occurs in the spinal cord. The pre-synaptic terminal from the dorsal root ganglia sensory neurons is burdened with gain of function of voltage-gated Ca2+ and Na+ channels. CRMP2 S522 phosphorylation and SUMOylation are regulatory events of these channels’ pre-synaptic localization [12,13]. In chronic neuropathic pain, CRMP2 phosphorylation on S522 was augmented in the sensory neurons and at the pre-synaptic terminals concomitantly with its SUMOylation[12,13]. Thus, increased CRMP2 phosphorylation and SUMOylation appears as a major pathological event driving neuropathic pain.
i. CRMP2 and Ca2+ influx
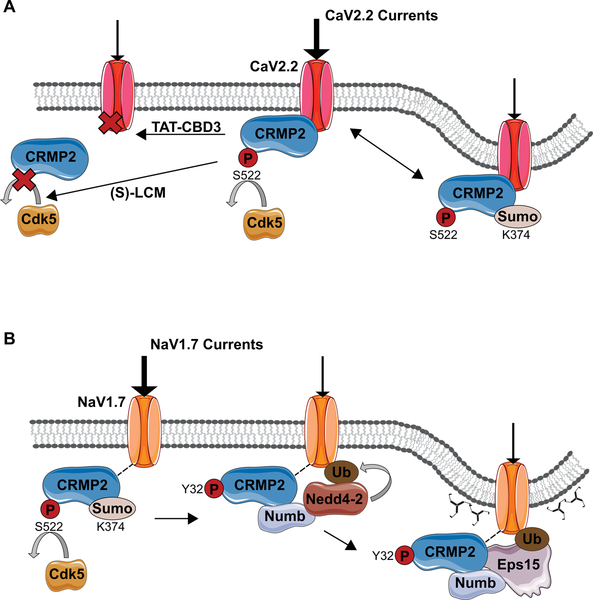
The N-type presynaptic Ca2+ channel CaV2.2 is the predominant source of Ca2+ influx required for neurotransmitter release at immature synapses [151]. Initially discovered in a proteomics screen of CaV2.2-interacting partners, CRMP2 was subsequently shown to co-localize with CaV2.2 at synapses and to coexist in a protein complex [152,153]. Direct interaction of the two proteins was confirmed using a short fifteen amino acid peptide from CRMP2, which when conjugated to the cell penetrant peptide from the TAT protein of HIV (designated TAT-CBD3), abrogated the interaction between CRMP2 and CaV2.2. As such, TAT-CBD3 reduces CaV2.2 plasma membrane content, Ca2+ current density, and excitatory synaptic transmission in vitro and in vivo [154,155]. Specifically, CRMP2 exerts a regulatory activity on CaV2.2 surface expression and CaV2.2-mediated Ca2+ currents by modulating synaptic vesicle recycling through three regions of calcium binding domains [153,5]. Through modulation of CaV2.2, CRMP2 controls glutamate release in developing neurons as well as calcitonin gene-related peptide (CGRP) in sensory neurons [153,156]. In physiological conditions, CRMP2’s interaction with CaV2.2 is finely tuned by post-translational modifications. Opposite to its effect on CRMP2 binding to tubulin and Numb, CRMP2 phosphorylation by Cdk5 enhances CaV2.2-mediated Ca2+ currents, whereas blocking this phosphorylation via (S)-lacosamide, decreases CaV2.2-mediated Ca2+ currents (Figure 5A)[112,157,90]. Similarly, SUMOylation of CRMP2 at Lys 374 also decreases CaV2.2-mediated calcium influx in sensory neurons [124]. As such, CRMP2 is a major regulator of CaV2.2-mediated Ca2+ current through a series of reversible post-translational modifications.
Figure 5. Regulation of voltage-gated calcium channel CaV2.2 and voltage-gated sodium channel NaV1.7 by CRMP2 post translational modifications.
(A) Regulation of CaV2.2. SUMOylation of CaV2.2 at K374 induces internalization of the channel and thus reduction of CaV2.2 mediated currents. Small molecules and peptides have also been shown to regulates CaV2.2. TAT-CB3 peptide downregulates CaV2.2 by preventing its interaction with CRMP2, while (S)-lacosamide ((S)-LCM)) has a similar effect by preventing phosphorylation of CRMP2 at S522 by Cdk5. Notably, preventing Cdk5-mediated phosphorylation also prevents downstream event such as phosphorylation by GSK3β at T509 and T514 as well as CRMP2 SUMOylation. (B) Blocking CRMP2 SUMOylation a K374 residue, as well as preventing the upstream event Cdk5-mediated phosphorylation at S522, causes a reduction of NaV1.7 membrane content and currents. Fyn-mediated phosphorylation of CRMP2 at Y32 favors binding with Numb and recruitment of Nedd4–2 which mono-ubiquitinates NaV1.7. Numb then recruits the endocytic machinery necessary for clathrin-mediated endocytosis. Adapted from Dustrude [46] and Moutal and co-workers [90].
ii. CRMP2 and sodium channel regulation
CRMP2 regulation of NaV1.7 was studied based on the observation that the anti-epileptic drug (R)-lacosamide (R)-LCM) modulates voltage-gated sodium channels in a slow-inactivated state via its binding to CRMP2 [158,81]. CRMP2 and NaV1.7 coexist in the same protein complex but whether there is a direct interaction between the two proteins is still unclear[81,46,159]. However, blocking a single SUMOylation site (K374) on CRMP2 reduces surface expression and current density of sodium channel NaV1.7, likely via endocytic mechanisms (Figure 5B)[47,45,46,160,44,161]. As SUMOylation of CRMP2 at K374 requires CRMP2 phosphorylation by Cdk5 [46]. Blocking Cdk5 mediated phosphorylation of CRMP2 impaired NaV1.7 surface localization and currents in rat and human DRG neurons [46]. However, Fyn-mediated CRMP2 Y32 phosphorylation was also necessary to negatively regulate NaV1.7 density and current; it is involved in the recruitment of the molecular machinery necessary to tag NaV1.7 for clathrin-mediated endocytosis and transfer toward recycling endosomes [46]. Specifically, upon loss of CRMP2 SUMOylation, its affinity for Numb acts as a scaffold between the ubiquitin-ligase Nedd4–2, marking NaV1.7 for endocytosis and the endocytic machinery, including the Eps15 protein [103,162,46].
iii. Neurofibromatosis type 1
Neurofibromatosis type 1 (NF1) is a genetic autosomal dominant disease arising from loss of function mutations in the Nf1 gene [163]. NF1 is a multi-systemic disease characterized by among other things, spontaneous tumor generation, cognitive disorders and idiopathic pain [163,164]. Neurofibromin, the protein encoded by NF1, interacts with CRMP2 and regulates its phosphorylation during neuronal polarity establishment [165]. Upon loss of neurofibromin, CRMP2 phosphorylation by Cdk5 is increased [165]. This elicited a decrease of CRMP2-dependent neurite outgrowth [165]. In sensory neurons, deleting the CRMP2 interacting domain on neurofibromin, increased CRMP2 phosphorylation level (S522) which led to sensitization of the voltage gated ion channels CaV2.2 and NaV1.7 [166,11], an observation replicated in a porcine model of NF1 [167]. Using Nf1 gene editing in sensory neurons in vivo induced a hyperalgesic phenotype in rats [11]. This hypersensitivity to a noxious heat stimulus was dependent on CRMP2 expression [10]. More precisely, hyperalgesia developed by the rats was dependent on the CRMP2-neurofibromin interface that regulated CRMP2 interaction with Syntaxin1A [168,169]. In addition, inhibiting CRMP2 phosphorylation by Cdk5 with (S)-lacosamide was sufficient to reverse CaV2.2 and NaV1.7 sensitization, increased nociceptive neurotransmitter (CGRP) release and the hyperalgesic phenotype of the rats [11,10,169]. Whether other CRMP2 post-translational modifications are altered in NF1 remain to be explored. These studies showed that CRMP2 dysregulation underlies the hypersensitivity to pain reported by NF1 patients. Whether CRMP2 influences any other phenotypes in NF1 has not been investigated.
f. Spinal cord injury
Oshima and colleagues reported that the phosphorylation level of CRMP2 was enhanced after spinal cord injury (SCI) spinal cord [170]. Their data demonstrated that inhibition of CRMP2 phosphorylation (i) promotes axonal regeneration and/or sprouting of motor and sensory axons, (ii) stabilizes microtubules in astrocytes to inhibit fibrous scar formation after SCI, and (iii) reduces infiltration of inflammatory cells. Using CRMP2 KI/KI mice where CRMP2 phosphorylation site for Cdk5 was eliminated by replacing Ser-522 with an inactive Ala residue [6], this study demonstrated the existence of a permissive environment for axonal growth due to reduction of microtubule destabilization in white matter axons, inflammation, and scarring, hypothesizing that inhibition of CRMP2 phosphorylation, by controlling both inhibitory factors and neurotrophic responses to injury, would make an ideal target for repairing SCI.
Nearly half of SCI patients develop neuropathic pain [171] and recent data suggest that the presence of autoantibodies to CRMP2 at 16 days post-SCI increases the odds of developing neuropathic pain within six months of injury by 9.5 times [172]. How CRMP2 autoantibodies are related to development of neuropathic pain is at present unknown but may involve CRMP2-mediated sprouting of neurites and axonal growth cones in neurons mediating pain or a block of function of CRMP2 by endocytosed autoantibodies. Autoantibodies to CRMP2 have also been reported in patients with autoimmune retinopathy and cancer-associated retinopathy [173] as well as in maternal autoantibody-related autism [174]but the outcome of these to the disease or clinical symptoms is largely unknown.
g. Psychiatric diseases: bipolar disorder
CRMP2 dysregulation has also been implicated in neuropsychiatric diseases. In Down’s Syndrome and depression, CRMP2 levels are increased in the cortex [175,176]. Also, CRMP2 gene alterations were correlated with autistic syndromes and schizophrenia [177,20,178,21]. In bipolar disorders, CRMP2 was identified as the main target of lithium chloride, a common therapeutic used for psychiatric diseases. Lithium chloride inhibits both GSK3β and Cdk5, thus leading to a drastic reduction of CRMP2 phosphorylation. In cells from patients with bipolar disorders, CRMP2 phosphorylation by Cdk5 was increased [22]. These observations show that the alteration of CRMP2 phosphorylation is a pathological feature in bipolar disorder. Disruption of the well-known risk gene for schizophrenia, ankyrin 3 (ANK3), has been reported to lead to increased GSK3β activity, followed by increased pCRMP2 and altered microtubule dynamics [179].
h. Cancer
CRMP2 has recently been implicated in cancer progression, with CRMP2 S522 or T514 phosphorylation increased in the tumors of several cancer variants, including glioblastoma, breast, lung, small intestinal neuroendocrine tumors, and lymphoma [180,27,181,23,182]. One particular study found that many cancer variants express the specific CRMP2A isoform phosphorylated at S522 [180]. Although phosphorylated CRMP2 is dissociated from the microtubule network, it was found in the nucleus of cancers cells [182,180]. This ultimately increases Rho-ROCK-mediated CRMP2 signaling, when present at low levels, resulting in non-polar cells with greater motility [183]. Targeting pCRMP2 may be a viable cancer therapy as well, as (S)-LCM treatment decreases glioblastoma cell proliferation and tumor growth in vivo [27]. Inhibiting Fer-mediated CRMP2 phosphorylation stabilized the microtubule network, thus enhancing the ability of cytotoxic compounds, such as paclitaxel, to bind to microtubules [23,27].
i. Neuronal Ceroid Lipofuscinosis
Neuronal ceroid lipofuscinosis (NCL; Batten disease) is a rare, lysosomal storage disorder presenting with visual, motor, and memory/learning decline, followed by early death [184,185]. Progression varies by the 13 genetic subtypes and specific genetic mutations, and therapeutic strategies have been limited due to the unknown functions of many of the NCL gene products [186]. Interestingly, a number of years ago the ER-associated Ceroid-lipofuscinosis neuronal protein 6 (CLN6) protein was found to interact with CRMP2 in a yeast 2-hybrid screening [15]. This study also reported that CLN6 deficient mouse neurons were less mature and died much earlier than their wild type counterparts. Expanding on that report, data from our group has identified a mechanism of this CLN6-CRMP2 interaction, where CLN6, CRMP2, and KLC4 form a unique complex that aids in anterograde transport of ER-derived vesicles in the axon (manuscript in preparation). We found that CLN6 acts as a molecular barcode on ER derived vesicles, transporting these vesicles to the distal end of the axon through CRMP2’s association with KLC4. In the absence of CLN6, vesicle transport and neurite branching is disrupted, while the CRMP2 modulator LKE is able to rescue many of these disturbances (manuscript in preparation). These data suggest that targeting CRMP2 may prove to be beneficial in CLN6-Batten disease. Whether other NCL proteins are directly involved in CRMP2-mediated transport remains to be studied.
6. Future directions
CRMP2 is widely involved in cytoskeletal remodeling through a number of posttranslational modifications including phosphorylation, SUMOylation, oxidation and O-GlcNAcylation. These modifications control CRMP2 interaction with tubulin heterodimers in a variety of cell types. In neurons, CRMP2 is essential for regulating cell division, neuronal migration, polarity, and Ca2+ homeostasis. There are convincing studies demonstrating CRMP2 expression in OLs and OPCs, suggesting a role for CRMP2 in OL maturation, process extension, and retraction. In contrast, evidence for CRMP2 in other glia is limited, but was suggested by RNA-seq analyses. While CRMP2 expression level is rarely altered, increases of CRMP2 post-translational modification state were found in multiple diseases such neurodegenerative, neuropsychiatric, chronic pain and cancer. The dysregulation of CRMP2 phosphorylation appears to be a pathological event contributing to the progression of diseases. Multiple studies pointed at the therapeutic potential of inhibiting CRMP2 phosphorylation and SUMOylation. In the future, efforts to develop small molecules able to manipulate CRMP2 post-translational modification state would allow for a better interrogation of the role of these events in diseased states. For instance, in chronic pain, the CRMP2 phosphorylation inhibitor (S)-lacosamide allowed for the characterization of CRMP2 S522 phosphorylation involvement in pain, migraine, brain cancer and NF1 in vivo [89,187,90,11]. Novel molecules are emerging with a therapeutic potential for stroke [188] or with blood-brain barrier penetration abilities [188,49]. While interesting, these compounds were the result of serendipitous findings. More studies are needed to identify and understand the structural “hotspots” on CRMP2 amenable for small molecule development.
Acknowledgements –
This work was supported by a National Institutes of Health Awards (1R01NS098772, 1R01DA042852, and 1R01AT009716 to RK, R01NS082283 to JMW, and R01HL135112 to PFV); a Neurofibromatosis New Investigator Award from the Department of Defense Congressionally Directed Military Medical Research and Development Program (NF1000099 to RK); and funding from the Synodos for NF1 program at the Children’s Tumor Foundation to JMW; and a research award from the Children’s Tumor Foundation (2015-04-009A) to RK and JMW. A.M. was supported by a Young Investigator’s Award from the Children’s Tumor Foundation.
Footnotes
Conflict of interest – There is no conflict of interest for any of the authors.
References
- 1.Wang LH, Strittmatter SM (1996) A family of rat CRMP genes is differentially expressed in the nervous system. The Journal of neuroscience : the official journal of the Society for Neuroscience 16 (19):6197–6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan F, Thiele CJ, Li Z (2014) Collapsin response mediator proteins: Potential diagnostic and prognostic biomarkers in cancers (Review). Oncol Lett 7 (5):1333–1340. doi: 10.3892/ol.2014.1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ip JP, Fu AK, Ip NY (2014) CRMP2: functional roles in neural development and therapeutic potential in neurological diseases. Neuroscientist 20 (6):589–598. doi: 10.1177/1073858413514278 [DOI] [PubMed] [Google Scholar]
- 4.Makihara H, Nakai S, Ohkubo W, Yamashita N, Nakamura F, Kiyonari H, Shioi G, Jitsuki-Takahashi A, Nakamura H, Tanaka F, Akase T, Kolattukudy P, Goshima Y (2016) CRMP1 and CRMP2 have synergistic but distinct roles in dendritic development. Genes to cells : devoted to molecular & cellular mechanisms 21 (9):994–1005. doi: 10.1111/gtc.12399 [DOI] [PubMed] [Google Scholar]
- 5.Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K (2002) CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nature cell biology 4 (8):583–591. doi: 10.1038/ncb825 [DOI] [PubMed] [Google Scholar]
- 6.Yamashita N, Ohshima T, Nakamura F, Kolattukudy P, Honnorat J, Mikoshiba K, Goshima Y (2012) Phosphorylation of CRMP2 (collapsin response mediator protein 2) is involved in proper dendritic field organization. The Journal of neuroscience : the official journal of the Society for Neuroscience 32 (4):1360–1365. doi: 10.1523/JNEUROSCI.5563-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crews L, Ruf R, Patrick C, Dumaop W, Trejo-Morales M, Achim CL, Rockenstein E, Masliah E (2011) Phosphorylation of collapsin response mediator protein-2 disrupts neuronal maturation in a model of adult neurogenesis: Implications for neurodegenerative disorders. Molecular neurodegeneration 6:67. doi: 10.1186/1750-1326-6-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai J, Takaya R, Piao W, Goshima Y, Ohshima T (2016) Deletion of Crmp4 attenuates CSPG-induced inhibition of axonal growth and induces nociceptive recovery after spinal cord injury. Mol Cell Neurosci 74:42–48. doi: 10.1016/j.mcn.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 9.Yoshida H, Watanabe A, Ihara Y (1998) Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer’s disease. The Journal of biological chemistry 273 (16):9761–9768 [DOI] [PubMed] [Google Scholar]
- 10.Moutal A, Cai S, Luo S, Voisin R, Khanna R (2018) CRMP2 is necessary for Neurofibromatosis type 1 related pain. Channels 12 (1):47–50. doi: 10.1080/19336950.2017.1370524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moutal A, Yang X, Li W, Gilbraith KB, Luo S, Cai S, Francois-Moutal L, Chew LA, Yeon SK, Bellampalli SS, Qu C, Xie JY, Ibrahim MM, Khanna M, Park KD, Porreca F, Khanna R (2017) CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain 158 (12):2301–2319. doi: 10.1097/j.pain.0000000000001002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moutal A, Dustrude ET, Largent-Milnes TM, Vanderah TW, Khanna M, Khanna R (2017) Blocking CRMP2 SUMOylation reverses neuropathic pain. Molecular psychiatry. doi: 10.1038/mp.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moutal A, Luo S, Largent-Milnes TM, Vanderah TW, Khanna R (2018) Cdk5-mediated CRMP2 phosphorylation is necessary and sufficient for peripheral neuropathic pain. Neurobiology of Pain. doi: 10.1016/j.ynpai.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charrier E, Reibel S, Rogemond V, Aguera M, Thomasset N, Honnorat J (2003) Collapsin response mediator proteins (CRMPs): involvement in nervous system development and adult neurodegenerative disorders. Molecular neurobiology 28 (1):51–64. doi: 10.1385/MN:28:1:51 [DOI] [PubMed] [Google Scholar]
- 15.Benedict JW, Getty AL, Wishart TM, Gillingwater TH, Pearce DA (2009) Protein product of CLN6 gene responsible for variant late-onset infantile neuronal ceroid lipofuscinosis interacts with CRMP-2. J Neurosci Res 87 (9):2157–2166. doi: 10.1002/jnr.22032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duplan L, Bernard N, Casseron W, Dudley K, Thouvenot E, Honnorat J, Rogemond V, De Bovis B, Aebischer P, Marin P, Raoul C, Henderson CE, Pettmann B (2010) Collapsin response mediator protein 4a (CRMP4a) is upregulated in motoneurons of mutant SOD1 mice and can trigger motoneuron axonal degeneration and cell death. The Journal of neuroscience : the official journal of the Society for Neuroscience 30 (2):785–796. doi: 10.1523/JNEUROSCI.5411-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim NK, Hung LW, Pang TY, McLean CA, Liddell JR, Hilton JB, Li QX, White AR, Hannan AJ, Crouch PJ (2014) Localized changes to glycogen synthase kinase-3 and collapsin response mediator protein-2 in the Huntington’s disease affected brain. Hum Mol Genet 23 (15):4051–4063. doi: 10.1093/hmg/ddu119 [DOI] [PubMed] [Google Scholar]
- 18.Togashi K, Hasegawa M, Nagai J, Tonouchi A, Masukawa D, Hensley K, Goshima Y, Ohshima T (2018) Genetic suppression of CRMP2 phosphorylation improves outcome in MPTP-induced Parkinson’s model mice. Genes to cells : devoted to molecular & cellular mechanisms. doi: 10.1111/gtc.12651 [DOI] [PubMed] [Google Scholar]
- 19.Petratos S, Ozturk E, Azari MF, Kenny R, Lee JY, Magee KA, Harvey AR, McDonald C, Taghian K, Moussa L, Mun Aui P, Siatskas C, Litwak S, Fehlings MG, Strittmatter SM, Bernard CC (2012) Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation. Brain : a journal of neurology 135 (Pt 6):1794–1818. doi: 10.1093/brain/aws100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabares-Seisdedos R, Rubenstein JL (2009) Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Molecular psychiatry 14 (6):563–589. doi: 10.1038/mp.2009.2 [DOI] [PubMed] [Google Scholar]
- 21.Pham X, Song G, Lao S, Goff L, Zhu H, Valle D, Avramopoulos D (2016) The DPYSL2 gene connects mTOR and schizophrenia. Transl Psychiatry 6 (11):e933. doi: 10.1038/tp.2016.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobe BTD, Crain AM, Winquist AM, Calabrese B, Makihara H, Zhao WN, Lalonde J, Nakamura H, Konopaske G, Sidor M, Pernia CD, Yamashita N, Wada M, Inoue Y, Nakamura F, Sheridan SD, Logan RW, Brandel M, Wu D, Hunsberger J, Dorsett L, Duerr C, Basa RCB, McCarthy MJ, Udeshi ND, Mertins P, Carr SA, Rouleau GA, Mastrangelo L, Li J, Gutierrez GJ, Brill LM, Venizelos N, Chen G, Nye JS, Manji H, Price JH, McClung CA, Akiskal HS, Alda M, Chuang DM, Coyle JT, Liu Y, Teng YD, Ohshima T, Mikoshiba K, Sidman RL, Halpain S, Haggarty SJ, Goshima Y, Snyder EY (2017) Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis. Proceedings of the National Academy of Sciences of the United States of America 114 (22):E4462–E4471. doi: 10.1073/pnas.1700111114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada K, Ishikawa T, Nakamura F, Shimizu D, Chishima T, Ichikawa Y, Sasaki T, Endo I, Nagashima Y, Goshima Y (2014) Collapsin response mediator protein 2 is involved in regulating breast cancer progression. Breast Cancer 21 (6):715–723. doi: 10.1007/s12282-013-0447-5 [DOI] [PubMed] [Google Scholar]
- 24.Meyronet D, Massoma P, Thivolet F, Chalabreysse L, Rogemond V, Schlama A, Honnorat J, Thomasset N (2008) Extensive expression of collapsin response mediator protein 5 (CRMP5) is a specific marker of high-grade lung neuroendocrine carcinoma. Am J Surg Pathol 32 (11):1699–1708. doi: 10.1097/PAS.0b013e31817dc37c [DOI] [PubMed] [Google Scholar]
- 25.Shih JY, Yang SC, Hong TM, Yuan A, Chen JJ, Yu CJ, Chang YL, Lee YC, Peck K, Wu CW, Yang PC (2001) Collapsin response mediator protein-1 and the invasion and metastasis of cancer cells. J Natl Cancer Inst 93 (18):1392–1400 [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Pang J, Li LY, Liu WP, Di JM, Sun QP, Fang YQ, Liu XP, Pu XY, He D, Li MT, Su ZL, Li BY (2010) Expression profiling identifies new function of collapsin response mediator protein 4 as a metastasis-suppressor in prostate cancer. Oncogene 29 (32):4555–4566. doi: 10.1038/onc.2010.213 [DOI] [PubMed] [Google Scholar]
- 27.Moutal A, Villa LS, Yeon SK, Householder KT, Park KD, Sirianni RW, Khanna R (2018) CRMP2 Phosphorylation Drives Glioblastoma Cell Proliferation. Mol Neurobiol 55 (5):4403–4416. doi: 10.1007/s12035-017-0653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moutal A, Honnorat J, Massoma P, Desormeaux P, Bertrand C, Malleval C, Watrin C, Chounlamountri N, Mayeur ME, Besancon R, Naudet N, Magadoux L, Khanna R, Ducray F, Meyronet D, Thomasset N (2015) CRMP5 Controls Glioblastoma Cell Proliferation and Survival through Notch-Dependent Signaling. Cancer Res 75 (17):3519–3528. doi: 10.1158/0008-5472.CAN-14-0631 [DOI] [PubMed] [Google Scholar]
- 29.Khanna R, Wilson SM, Brittain JM, Weimer J, Sultana R, Butterfield A, Hensley K (2012) Opening Pandora’s jar: a primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neuron, and central disorders. Future Neurol 7 (6):749–771. doi: 10.2217/fnl.12.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brot S, Rogemond V, Perrot V, Chounlamountri N, Auger C, Honnorat J, Moradi-Ameli M (2010) CRMP5 interacts with tubulin to inhibit neurite outgrowth, thereby modulating the function of CRMP2. The Journal of neuroscience : the official journal of the Society for Neuroscience 30 (32):10639–10654. doi: 10.1523/JNEUROSCI.0059-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quach TT, Wilson SM, Rogemond V, Chounlamountri N, Kolattukudy PE, Martinez S, Khanna M, Belin MF, Khanna R, Honnorat J, Duchemin AM (2013) Mapping CRMP3 domains involved in dendrite morphogenesis and voltage-gated calcium channel regulation. Journal of cell science 126 (Pt 18):4262–4273. doi: 10.1242/jcs.131409 [DOI] [PubMed] [Google Scholar]
- 32.Tan M, Cha C, Ye Y, Zhang J, Li S, Wu F, Gong S, Guo G (2015) CRMP4 and CRMP2 Interact to Coordinate Cytoskeleton Dynamics, Regulating Growth Cone Development and Axon Elongation. Neural Plast 2015:947423. doi: 10.1155/2015/947423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponnusamy R, Lohkamp B (2013) Insights into the oligomerization of CRMPs: crystal structure of human collapsin response mediator protein 5. Journal of neurochemistry 125 (6):855–868. doi: 10.1111/jnc.12188 [DOI] [PubMed] [Google Scholar]
- 34.Hedgecock EM, Culotti JG, Thomson JN, Perkins LA (1985) Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev Biol 111 (1):158–170 [DOI] [PubMed] [Google Scholar]
- 35.Li W, Herman RK, Shaw JE (1992) Analysis of the Caenorhabditis elegans axonal guidance and outgrowth gene unc-33. Genetics 132 (3):675–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM (1995) Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376 (6540):509–514. doi: 10.1038/376509a0 [DOI] [PubMed] [Google Scholar]
- 37.Minturn JE, Fryer HJ, Geschwind DH, Hockfield S (1995) TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. The Journal of neuroscience : the official journal of the Society for Neuroscience 15 (10):6757–6766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamajima N, Matsuda K, Sakata S, Tamaki N, Sasaki M, Nonaka M (1996) A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene 180 (1–2):157–163 [DOI] [PubMed] [Google Scholar]
- 39.Byk T, Dobransky T, Cifuentes-Diaz C, Sobel A (1996) Identification and molecular characterization of Unc-33-like phosphoprotein (Ulip), a putative mammalian homolog of the axonal guidance-associated unc-33 gene product. The Journal of neuroscience : the official journal of the Society for Neuroscience 16 (2):688–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuasa-Kawada J, Suzuki R, Kano F, Ohkawara T, Murata M, Noda M (2003) Axonal morphogenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. The European journal of neuroscience 17 (11):2329–2343 [DOI] [PubMed] [Google Scholar]
- 41.Balastik M, Zhou XZ, Alberich-Jorda M, Weissova R, Ziak J, Pazyra-Murphy MF, Cosker KE, Machonova O, Kozmikova I, Chen CH, Pastorino L, Asara JM, Cole A, Sutherland C, Segal RA, Lu KP (2015) Prolyl Isomerase Pin1 Regulates Axon Guidance by Stabilizing CRMP2A Selectively in Distal Axons. Cell Rep 13 (4):812–828. doi: 10.1016/j.celrep.2015.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 34 (36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bretin S, Reibel S, Charrier E, Maus-Moatti M, Auvergnon N, Thevenoux A, Glowinski J, Rogemond V, Premont J, Honnorat J, Gauchy C (2005) Differential expression of CRMP1, CRMP2A, CRMP2B, and CRMP5 in axons or dendrites of distinct neurons in the mouse brain. The Journal of comparative neurology 486 (1):1–17. doi: 10.1002/cne.20465 [DOI] [PubMed] [Google Scholar]
- 44.Francois-Moutal L, Dustrude ET, Wang Y, Brustovetsky T, Dorame A, Ju W, Moutal A, Perez-Miller S, Brustovetsky N, Gokhale V, Khanna M, Khanna R (2018) Inhibition of the Ubc9 E2 SUMO-conjugating enzyme-CRMP2 interaction decreases NaV1.7 currents and reverses experimental neuropathic pain. Pain 159 (10):2115–2127. doi: 10.1097/j.pain.0000000000001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dustrude ET, Perez-Miller S, Francois-Moutal L, Moutal A, Khanna M, Khanna R (2017) A single structurally conserved SUMOylation site in CRMP2 controls NaV1.7 function. Channels 11 (4):316–328. doi: 10.1080/19336950.2017.1299838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dustrude ET, Moutal A, Yang X, Wang Y, Khanna M, Khanna R (2016) Hierarchical CRMP2 posttranslational modifications control NaV1.7 function. Proceedings of the National Academy of Sciences of the United States of America 113 (52):E8443–E8452. doi: 10.1073/pnas.1610531113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dustrude ET, Wilson SM, Ju W, Xiao Y, Khanna R (2013) CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking. The Journal of biological chemistry 288 (34):24316–24331. doi: 10.1074/jbc.M113.474924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myllykoski M, Baumann A, Hensley K, Kursula P (2017) Collapsin response mediator protein 2: high-resolution crystal structure sheds light on small-molecule binding, post-translational modifications, and conformational flexibility. Amino Acids 49 (4):747–759. doi: 10.1007/s00726-016-2376-z [DOI] [PubMed] [Google Scholar]
- 49.Lawal M, Olotu FA, Soliman MES (2018) Across the blood-brain barrier: Neurotherapeutic screening and characterization of naringenin as a novel CRMP-2 inhibitor in the treatment of Alzheimer’s disease using bioinformatics and computational tools. Comput Biol Med 98:168–177. doi: 10.1016/j.compbiomed.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 50.Olguin-Albuerne M, Moran J (2018) Redox Signaling Mechanisms in Nervous System Development. Antioxid Redox Signal 28 (18):1603–1625. doi: 10.1089/ars.2017.7284 [DOI] [PubMed] [Google Scholar]
- 51.Chew LA, Khanna R (2018) CRMP2 and voltage-gated ion channels: potential roles in neuropathic pain. Neuronal Signal 2 (1). doi: 10.1042/NS20170220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leney AC, El Atmioui D, Wu W, Ovaa H, Heck AJR (2017) Elucidating crosstalk mechanisms between phosphorylation and O-GlcNAcylation. Proceedings of the National Academy of Sciences of the United States of America 114 (35):E7255–E7261. doi: 10.1073/pnas.1620529114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole RN, Hart GW (2001) Cytosolic O-glycosylation is abundant in nerve terminals. Journal of neurochemistry 79 (5):1080–1089 [DOI] [PubMed] [Google Scholar]
- 54.Cnops L, Van de Plas B, Arckens L (2004) Age-dependent expression of collapsin response mediator proteins (CRMPs) in cat visual cortex. The European journal of neuroscience 19 (8):2345–2351. doi: 10.1111/j.0953-816X.2004.03330.x [DOI] [PubMed] [Google Scholar]
- 55.Cnops L, Hu TT, Burnat K, Van der Gucht E, Arckens L (2006) Age-dependent alterations in CRMP2 and CRMP4 protein expression profiles in cat visual cortex. Brain research 1088 (1):109–119. doi: 10.1016/j.brainres.2006.03.028 [DOI] [PubMed] [Google Scholar]
- 56.Ricard D, Stankoff B, Bagnard D, Aguera M, Rogemond V, Antoine JC, Spassky N, Zalc B, Lubetzki C, Belin MF, Honnorat J (2000) Differential expression of collapsin response mediator proteins (CRMP/ULIP) in subsets of oligodendrocytes in the postnatal rodent brain. Molecular and cellular neurosciences 16 (4):324–337. doi: 10.1006/mcne.2000.0888 [DOI] [PubMed] [Google Scholar]
- 57.Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S (2018) Molecular Architecture of the Mouse Nervous System. Cell 174 (4):999–1014 e1022. doi: 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricard D, Rogemond V, Charrier E, Aguera M, Bagnard D, Belin MF, Thomasset N, Honnorat J (2001) Isolation and expression pattern of human Unc-33-like phosphoprotein 6/collapsin response mediator protein 5 (Ulip6/CRMP5): coexistence with Ulip2/CRMP2 in Sema3a- sensitive oligodendrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience 21 (18):7203–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez-Gamba A, Leal MC, Maarouf CL, Richter-Landsberg C, Wu T, Morelli L, Roher AE, Castano EM (2012) Collapsin response mediator protein-2 phosphorylation promotes the reversible retraction of oligodendrocyte processes in response to non-lethal oxidative stress. Journal of neurochemistry 121 (6):985–995. doi: 10.1111/j.1471-4159.2012.07742.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syed YA, Abdulla SA, Kotter MR (2017) Studying the Effects of Semaphorins on Oligodendrocyte Lineage Cells. Methods Mol Biol 1493:363–378. doi: 10.1007/978-1-4939-6448-2_26 [DOI] [PubMed] [Google Scholar]
- 61.Syed YA, Hand E, Mobius W, Zhao C, Hofer M, Nave KA, Kotter MR (2011) Inhibition of CNS remyelination by the presence of semaphorin 3A. The Journal of neuroscience : the official journal of the Society for Neuroscience 31 (10):3719–3728. doi: 10.1523/jneurosci.4930-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piaton G, Aigrot MS, Williams A, Moyon S, Tepavcevic V, Moutkine I, Gras J, Matho KS, Schmitt A, Soellner H, Huber AB, Ravassard P, Lubetzki C (2011) Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain : a journal of neurology 134 (Pt 4):1156–1167. doi: 10.1093/brain/awr022 [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA (2016) Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89 (1):37–53. doi: 10.1016/j.neuron.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamata T, Subleski M, Hara Y, Yuhki N, Kung H, Copeland NG, Jenkins NA, Yoshimura T, Modi W, Copeland TD (1998) Isolation and characterization of a bovine neural specific protein (CRMP-2) cDNA homologous to unc-33, a C. elegans gene implicated in axonal outgrowth and guidance. Brain research Molecular brain research 54 (2):219–236 [DOI] [PubMed] [Google Scholar]
- 65.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA (2016) New tools for studying microglia in the mouse and human CNS. Proceedings of the National Academy of Sciences of the United States of America 113 (12):E1738–1746. doi: 10.1073/pnas.1525528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirbec H, Marmai C, Duroux-Richard I, Roubert C, Esclangon A, Croze S, Lachuer J, Peyroutou R, Rassendren F (2018) The microglial reaction signature revealed by RNAseq from individual mice. Glia 66 (5):971–986. doi: 10.1002/glia.23295 [DOI] [PubMed] [Google Scholar]
- 67.Hensley K, Christov A, Kamat S, Zhang XC, Jackson KW, Snow S, Post J (2010) Proteomic identification of binding partners for the brain metabolite lanthionine ketimine (LK) and documentation of LK effects on microglia and motoneuron cell cultures. The Journal of neuroscience : the official journal of the Society for Neuroscience 30 (8):2979–2988. doi: 10.1523/JNEUROSCI.5247-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kotaka K, Nagai J, Hensley K, Ohshima T (2017) Lanthionine ketimine ester promotes locomotor recovery after spinal cord injury by reducing neuroinflammation and promoting axon growth. Biochemical and biophysical research communications 483 (1):759–764. doi: 10.1016/j.bbrc.2016.12.069 [DOI] [PubMed] [Google Scholar]
- 69.Hensley K, Gabbita SP, Venkova K, Hristov A, Johnson MF, Eslami P, Harris-White ME (2013) A derivative of the brain metabolite lanthionine ketimine improves cognition and diminishes pathology in the 3 x Tg-AD mouse model of Alzheimer disease. J Neuropathol Exp Neurol 72 (10):955–969. doi: 10.1097/NEN.0b013e3182a74372 [DOI] [PubMed] [Google Scholar]
- 70.Zhou LS, Zhao GL, Liu Q, Jiang SC, Wang Y, Zhang DM (2015) Silencing collapsin response mediator protein-2 reprograms macrophage phenotype and improves infarct healing in experimental myocardial infarction model. Journal of inflammation (London, England) 12:11. doi: 10.1186/s12950-015-0053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradke F, Dotti CG (2000) Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol 10 (5):574–581 [DOI] [PubMed] [Google Scholar]
- 72.Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K (2001) CRMP-2 induces axons in cultured hippocampal neurons. Nature neuroscience 4 (8):781–782. doi: 10.1038/90476 [DOI] [PubMed] [Google Scholar]
- 73.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K (2005) GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120 (1):137–149. doi: 10.1016/j.cell.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 74.Wang LH, Strittmatter SM (1997) Brain CRMP forms heterotetramers similar to liver dihydropyrimidinase. Journal of neurochemistry 69 (6):2261–2269 [DOI] [PubMed] [Google Scholar]
- 75.Niwa S, Nakamura F, Tomabechi Y, Aoki M, Shigematsu H, Matsumoto T, Yamagata A, Fukai S, Hirokawa N, Goshima Y, Shirouzu M, Nitta R (2017) Structural basis for CRMP2-induced axonal microtubule formation. Sci Rep 7 (1):10681. doi: 10.1038/s41598-017-11031-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kimura T, Watanabe H, Iwamatsu A, Kaibuchi K (2005) Tubulin and CRMP-2 complex is transported via Kinesin-1. Journal of neurochemistry 93 (6):1371–1382. doi: 10.1111/j.1471-4159.2005.03063.x [DOI] [PubMed] [Google Scholar]
- 77.Muller-Reichert T, Chretien D, Severin F, Hyman AA (1998) Structural changes at microtubule ends accompanying GTP hydrolysis: information from a slowly hydrolyzable analogue of GTP, guanylyl (alpha,beta)methylenediphosphonate. Proceedings of the National Academy of Sciences of the United States of America 95 (7):3661–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chae YC, Lee S, Heo K, Ha SH, Jung Y, Kim JH, Ihara Y, Suh PG, Ryu SH (2009) Collapsin response mediator protein-2 regulates neurite formation by modulating tubulin GTPase activity. Cell Signal 21 (12):1818–1826. doi: 10.1016/j.cellsig.2009.07.017 [DOI] [PubMed] [Google Scholar]
- 79.Wilson SM, Khanna R (2015) Specific binding of lacosamide to collapsin response mediator protein 2 (CRMP2) and direct impairment of its canonical function: implications for the therapeutic potential of lacosamide. Mol Neurobiol 51 (2):599–609. doi: 10.1007/s12035-014-8775-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson SM, Moutal A, Melemedjian OK, Wang Y, Ju W, Francois-Moutal L, Khanna M, Khanna R (2014) The functionalized amino acid (S)-Lacosamide subverts CRMP2-mediated tubulin polymerization to prevent constitutive and activity-dependent increase in neurite outgrowth. Frontiers in cellular neuroscience 8:196. doi: 10.3389/fncel.2014.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y, Brittain JM, Jarecki BW, Park KD, Wilson SM, Wang B, Hale R, Meroueh SO, Cummins TR, Khanna R (2010) In silico docking and electrophysiological characterization of lacosamide binding sites on collapsin response mediator protein-2 identifies a pocket important in modulating sodium channel slow inactivation. The Journal of biological chemistry 285 (33):25296–25307. doi: 10.1074/jbc.M110.128801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sumi T, Imasaki T, Aoki M, Sakai N, Nitta E, Shirouzu M, Nitta R (2018) Structural Insights into the Altering Function of CRMP2 by Phosphorylation. Cell Struct Funct 43 (1):15–23. doi: 10.1247/csf.17025 [DOI] [PubMed] [Google Scholar]
- 83.Hu S, Zhu L (2018) Semaphorins and Their Receptors: From Axonal Guidance to Atherosclerosis. Front Physiol 9:1236. doi: 10.3389/fphys.2018.01236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito Y, Oinuma I, Katoh H, Kaibuchi K, Negishi M (2006) Sema4D/plexin-B1 activates GSK-3beta through RRas GAP activity, inducing growth cone collapse. EMBO Rep 7 (7):704–709. doi: 10.1038/sj.embor.7400737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arbeille E, Reynaud F, Sanyas I, Bozon M, Kindbeiter K, Causeret F, Pierani A, Falk J, Moret F, Castellani V (2015) Cerebrospinal fluid-derived Semaphorin3B orients neuroepithelial cell divisions in the apicobasal axis. Nature communications 6:6366. doi: 10.1038/ncomms7366 [DOI] [PubMed] [Google Scholar]
- 86.Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K, Ihara Y, Mikoshiba K, Kolattukudy P, Honnorat J, Goshima Y (2005) Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes to cells : devoted to molecular & cellular mechanisms 10 (2):165–179. doi: 10.1111/j.1365-2443.2005.00827.x [DOI] [PubMed] [Google Scholar]
- 87.Cole AR, Causeret F, Yadirgi G, Hastie CJ, McLauchlan H, McManus EJ, Hernandez F, Eickholt BJ, Nikolic M, Sutherland C (2006) Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. The Journal of biological chemistry 281 (24):16591–16598. doi: 10.1074/jbc.M513344200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown M, Jacobs T, Eickholt B, Ferrari G, Teo M, Monfries C, Qi RZ, Leung T, Lim L, Hall C (2004) Alpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. The Journal of neuroscience : the official journal of the Society for Neuroscience 24 (41):8994–9004. doi: 10.1523/JNEUROSCI.3184-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moutal A, Chew LA, Yang X, Wang Y, Yeon SK, Telemi E, Meroueh S, Park KD, Shrinivasan R, Gilbraith KB, Qu C, Xie JY, Patwardhan A, Vanderah TW, Khanna M, Porreca F, Khanna R (2016) (S)-lacosamide inhibition of CRMP2 phosphorylation reduces postoperative and neuropathic pain behaviors through distinct classes of sensory neurons identified by constellation pharmacology. Pain 157 (7):1448–1463. doi: 10.1097/j.pain.0000000000000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moutal A, Francois-Moutal L, Perez-Miller S, Cottier K, Chew LA, Yeon SK, Dai J, Park KD, Khanna M, Khanna R (2016) (S)-Lacosamide Binding to Collapsin Response Mediator Protein 2 (CRMP2) Regulates CaV2.2 Activity by Subverting Its Phosphorylation by Cdk5. Molecular neurobiology 53 (3):1959–1976. doi: 10.1007/s12035-015-9141-2 [DOI] [PubMed] [Google Scholar]
- 91.Uchida Y, Ohshima T, Yamashita N, Ogawara M, Sasaki Y, Nakamura F, Goshima Y (2009) Semaphorin3A signaling mediated by Fyn-dependent tyrosine phosphorylation of collapsin response mediator protein 2 at tyrosine 32. The Journal of biological chemistry 284 (40):27393–27401. doi: 10.1074/jbc.M109.000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng Y, Sethi R, Mangala LS, Taylor C, Goldsmith J, Wang M, Masuda K, Karaminejadranjbar M, Mannion D, Miranda F, Herrero-Gonzalez S, Hellner K, Chen F, Alsaadi A, Albukhari A, Fotso DC, Yau C, Jiang D, Pradeep S, Rodriguez-Aguayo C, Lopez-Berestein G, Knapp S, Gray NS, Campo L, Myers KA, Dhar S, Ferguson D, Bast RC Jr., Sood AK,von Delft F, Ahmed AA(2018) Tuning microtubule dynamics to enhance cancer therapy by modulating FER-mediated CRMP2 phosphorylation. Nature communications 9 (1):476. doi: 10.1038/s41467-017-02811-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shapovalova Z, Tabunshchyk K, Greer PA (2007) The Fer tyrosine kinase regulates an axon retraction response to Semaphorin 3A in dorsal root ganglion neurons. BMC Dev Biol 7:133. doi: 10.1186/1471-213X-7-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Varrin-Doyer M, Vincent P, Cavagna S, Auvergnon N, Noraz N, Rogemond V, Honnorat J, Moradi-Ameli M, Giraudon P (2009) Phosphorylation of collapsin response mediator protein 2 on Tyr-479 regulates CXCL12-induced T lymphocyte migration. The Journal of biological chemistry 284 (19):13265–13276. doi: 10.1074/jbc.M807664200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, Ohno S, Nakamura F, Goshima Y (2002) Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35 (5):907–920 [DOI] [PubMed] [Google Scholar]
- 96.Cole AR, Knebel A, Morrice NA, Robertson LA, Irving AJ, Connolly CN, Sutherland C (2004) GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. The Journal of biological chemistry 279 (48):50176–50180. doi: 10.1074/jbc.C400412200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morimura R, Nozawa K, Tanaka H, Ohshima T (2013) Phosphorylation of Dpsyl2 (CRMP2) and Dpsyl3 (CRMP4) is required for positioning of caudal primary motor neurons in the zebrafish spinal cord. Dev Neurobiol 73 (12):911–920. doi: 10.1002/dneu.22117 [DOI] [PubMed] [Google Scholar]
- 98.Niisato E, Nagai J, Yamashita N, Nakamura F, Goshima Y, Ohshima T (2013) Phosphorylation of CRMP2 is involved in proper bifurcation of the apical dendrite of hippocampal CA1 pyramidal neurons. Dev Neurobiol 73 (2):142–151. doi: 10.1002/dneu.22048 [DOI] [PubMed] [Google Scholar]
- 99.Gu Y, Ihara Y (2000) Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. The Journal of biological chemistry 275 (24):17917–17920. doi: 10.1074/jbc.C000179200C000179200[pii] [DOI] [PubMed] [Google Scholar]
- 100.Lin PC, Chan PM, Hall C, Manser E (2011) Collapsin response mediator proteins (CRMPs) are a new class of microtubule-associated protein (MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction. The Journal of biological chemistry 286 (48):41466–41478. doi: 10.1074/jbc.M111.283580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jauffred B, Llense F, Sommer B, Wang Z, Martin C, Bellaiche Y (2013) Regulation of centrosome movements by numb and the collapsin response mediator protein during Drosophila sensory progenitor asymmetric division. Development 140 (13):2657–2668. doi: 10.1242/dev.087338 [DOI] [PubMed] [Google Scholar]
- 102.Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K (2003) CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nature cell biology 5 (9):819–826. doi: 10.1038/ncb1039 [DOI] [PubMed] [Google Scholar]
- 103.Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP (2000) Numb is an endocytic protein. J Cell Biol 151 (6):1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quach TT, Duchemin AM, Rogemond V, Aguera M, Honnorat J, Belin MF, Kolattukudy PE (2004) Involvement of collapsin response mediator proteins in the neurite extension induced by neurotrophins in dorsal root ganglion neurons. Molecular and cellular neurosciences 25 (3):433–443. doi: 10.1016/j.mcn.2003.11.006 [DOI] [PubMed] [Google Scholar]
- 105.Marques JM, Rodrigues RJ, Valbuena S, Rozas JL, Selak S, Marin P, Aller MI, Lerma J (2013) CRMP2 tethers kainate receptor activity to cytoskeleton dynamics during neuronal maturation. The Journal of neuroscience : the official journal of the Society for Neuroscience 33 (46):18298–18310. doi: 10.1523/JNEUROSCI.3136-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fang WQ, Ip JP, Li R, Ng YP, Lin SC, Chen Y, Fu AK, Ip NY (2011) Cdk5-mediated phosphorylation of Axin directs axon formation during cerebral cortex development. The Journal of neuroscience : the official journal of the Society for Neuroscience 31 (38):13613–13624. doi: 10.1523/JNEUROSCI.3120-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ (2006) PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci 119 (Pt 5):951–957. doi: 10.1242/jcs.02801 [DOI] [PubMed] [Google Scholar]
- 108.Jiang H, Guo W, Liang X, Rao Y (2005) Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell 120 (1):123–135. doi: 10.1016/j.cell.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 109.Sarhan AR, Szyroka J, Begum S, Tomlinson MG, Hotchin NA, Heath JK, Cunningham DL (2017) Quantitative Phosphoproteomics Reveals a Role for Collapsin Response Mediator Protein 2 in PDGF-Induced Cell Migration. Sci Rep 7 (1):3970. doi: 10.1038/s41598-017-04015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshima Y, Kaibuchi K (2000) Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. The Journal of biological chemistry 275 (31):23973–23980. doi: 10.1074/jbc.M001032200 [DOI] [PubMed] [Google Scholar]
- 111.Quarta S, Camprubi-Robles M, Schweigreiter R, Matusica D, Haberberger RV, Proia RL, Bandtlow CE, Ferrer-Montiel A, Kress M (2017) Sphingosine-1-Phosphate and the S1P3 Receptor Initiate Neuronal Retraction via RhoA/ROCK Associated with CRMP2 Phosphorylation. Front Mol Neurosci 10:317. doi: 10.3389/fnmol.2017.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arimura N, Menager C, Kawano Y, Yoshimura T, Kawabata S, Hattori A, Fukata Y, Amano M, Goshima Y, Inagaki M, Morone N, Usukura J, Kaibuchi K (2005) Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Molecular and cellular biology 25 (22):9973–9984. doi: 10.1128/MCB.25.22.9973-9984.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arimura N, Hattori A, Kimura T, Nakamuta S, Funahashi Y, Hirotsune S, Furuta K, Urano T, Toyoshima YY, Kaibuchi K (2009) CRMP-2 directly binds to cytoplasmic dynein and interferes with its activity. Journal of neurochemistry 111 (2):380–390. doi: 10.1111/j.1471-4159.2009.06317.x [DOI] [PubMed] [Google Scholar]
- 114.Kawano Y, Yoshimura T, Tsuboi D, Kawabata S, Kaneko-Kawano T, Shirataki H, Takenawa T, Kaibuchi K (2005) CRMP-2 is involved in kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Molecular and cellular biology 25 (22):9920–9935. doi: 10.1128/MCB.25.22.9920-9935.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rahajeng J, Giridharan SS, Naslavsky N, Caplan S (2010) Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. The Journal of biological chemistry 285 (42):31918–31922. doi: 10.1074/jbc.C110.166066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pace PE, Peskin AV, Konigstorfer A, Jasoni CJ, Winterbourn CC, Hampton MB (2018) Peroxiredoxin interaction with the cytoskeletal-regulatory protein CRMP2: Investigation of a putative redox relay. Free Radic Biol Med 129:383–393. doi: 10.1016/j.freeradbiomed.2018.10.407 [DOI] [PubMed] [Google Scholar]
- 117.Morinaka A, Yamada M, Itofusa R, Funato Y, Yoshimura Y, Nakamura F, Yoshimura T, Kaibuchi K, Goshima Y, Hoshino M, Kamiguchi H, Miki H (2011) Thioredoxin mediates oxidation-dependent phosphorylation of CRMP2 and growth cone collapse. Sci Signal 4 (170):ra26. doi: 10.1126/scisignal.2001127 [DOI] [PubMed] [Google Scholar]
- 118.Gellert M, Venz S, Mitlohner J, Cott C, Hanschmann EM, Lillig CH (2013) Identification of a dithioldisulfide switch in collapsin response mediator protein 2 (CRMP2) that is toggled in a model of neuronal differentiation. The Journal of biological chemistry 288 (49):35117–35125. doi: 10.1074/jbc.M113.521443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Booze ML, Hansen JM, Vitiello PF (2016) A novel mouse model for the identification of thioredoxin-1 protein interactions. Free Radic Biol Med 99:533–543. doi: 10.1016/j.freeradbiomed.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson C, Gonzalez-Billault C (2015) Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front Cell Neurosci 9:381. doi: 10.3389/fncel.2015.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu X, Wicki-Stordeur LE, Sanchez-Arias JC, Liu M, Weaver MS, Choi CSW, Swayne LA (2018) Probenecid Disrupts a Novel Pannexin 1-Collapsin Response Mediator Protein 2 Interaction and Increases Microtubule Stability. Frontiers in cellular neuroscience 12:124. doi: 10.3389/fncel.2018.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saitoh F, Hagiwara H, Wakatsuki S, Araki T (2018) Carboxymethylation of CRMP2 is associated with decreased Schwann cell myelination efficiency. Neurosci Res. doi: 10.1016/j.neures.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 123.Dai X, Sun Z, Liang R, Li Y, Luo H, Huang Y, Chen M, Su Z, Xiao F (2015) Recombinant Nogo-66 via soluble expression with SUMO fusion in Escherichia coli inhibits neurite outgrowth in vitro. Appl Microbiol Biotechnol 99 (14):5997–6007. doi: 10.1007/s00253-015-6477-5 [DOI] [PubMed] [Google Scholar]
- 124.Ju W, Li Q, Wilson SM, Brittain JM, Meroueh L, Khanna R (2013) SUMOylation alters CRMP2 regulation of calcium influx in sensory neurons. Channels 7 (3):153–159. doi: 10.4161/chan.24224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, Sun YE, Coon JJ, Peters EC, Hsieh-Wilson LC (2007) Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nature chemical biology 3 (6):339–348. doi: 10.1038/nchembio881 [DOI] [PubMed] [Google Scholar]
- 126.Zhang JN, Koch JC (2017) Collapsin response mediator protein-2 plays a major protective role in acute axonal degeneration. Neural regeneration research 12 (5):692–695. doi: 10.4103/1673-5374.206631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hensley K, Kursula P (2016) Collapsin Response Mediator Protein-2 (CRMP2) is a Plausible Etiological Factor and Potential Therapeutic Target in Alzheimer’s Disease: Comparison and Contrast with Microtubule-Associated Protein Tau. J Alzheimers Dis 53 (1):1–14. doi: 10.3233/JAD-160076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shah K, Lahiri DK (2017) A Tale of the Good and Bad: Remodeling of the Microtubule Network in the Brain by Cdk5. Mol Neurobiol 54 (3):2255–2268. doi: 10.1007/s12035-016-9792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hensley K, Venkova K, Christov A, Gunning W, Park J (2011) Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Molecular neurobiology 43 (3):180–191. doi: 10.1007/s12035-011-8166-4 [DOI] [PubMed] [Google Scholar]
- 130.Quach TT, Honnorat J, Kolattukudy PE, Khanna R, Duchemin AM (2015) CRMPs: critical molecules for neurite morphogenesis and neuropsychiatric diseases. Molecular psychiatry 20 (9):1037–1045. doi: 10.1038/mp.2015.77 [DOI] [PubMed] [Google Scholar]
- 131.Williamson R, van Aalten L, Mann DM, Platt B, Plattner F, Bedford L, Mayer J, Howlett D, Usardi A, Sutherland C, Cole AR (2011) CRMP2 hyperphosphorylation is characteristic of Alzheimer’s disease and not a feature common to other neurodegenerative diseases. Journal of Alzheimer’s disease : JAD 27 (3):615–625. doi: 10.3233/JAD-2011-110617 [DOI] [PubMed] [Google Scholar]
- 132.Cole AR, Noble W, van Aalten L, Plattner F, Meimaridou R, Hogan D, Taylor M, LaFrancois J, Gunn-Moore F, Verkhratsky A, Oddo S, LaFerla F, Giese KP, Dineley KT, Duff K, Richardson JC, Yan SD, Hanger DP, Allan SM, Sutherland C (2007) Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer’s disease progression. Journal of neurochemistry 103 (3):1132–1144. doi: 10.1111/j.1471-4159.2007.04829.x [DOI] [PubMed] [Google Scholar]
- 133.Isono T, Yamashita N, Obara M, Araki T, Nakamura F, Kamiya Y, Alkam T, Nitta A, Nabeshima T, Mikoshiba K, Ohshima T, Goshima Y (2013) Amyloid-beta(2)(5)(−)(3)(5) induces impairment of cognitive function and long-term potentiation through phosphorylation of collapsin response mediator protein 2. Neurosci Res 77 (3):180–185. doi: 10.1016/j.neures.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 134.Xing H, Lim YA, Chong JR, Lee JH, Aarsland D, Ballard CG, Francis PT, Chen CP, Lai MK (2016) Increased phosphorylation of collapsin response mediator protein-2 at Thr514 correlates with beta-amyloid burden and synaptic deficits in Lewy body dementias. Molecular brain 9 (1):84. doi: 10.1186/s13041-016-0264-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watamura N, Toba J, Yoshii A, Nikkuni M, Ohshima T (2016) Colocalization of phosphorylated forms of WAVE1, CRMP2, and tau in Alzheimer’s disease model mice: Involvement of Cdk5 phosphorylation and the effect of ATRA treatment. J Neurosci Res 94 (1):15–26. doi: 10.1002/jnr.23674 [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Yin H, Li J, Zhang Y, Han B, Zeng Z, Qiao N, Cui X, Lou J, Li J (2013) Amelioration of beta-amyloid-induced cognitive dysfunction and hippocampal axon degeneration by curcumin is associated with suppression of CRMP-2 hyperphosphorylation. Neuroscience letters 557 Pt B:112–117. doi: 10.1016/j.neulet.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 137.Yang Z, Kuboyama T, Tohda C (2017) A Systematic Strategy for Discovering a Therapeutic Drug for Alzheimer’s Disease and Its Target Molecule. Frontiers in pharmacology 8:340. doi: 10.3389/fphar.2017.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castillo C, Martinez JC, Longart M, Garcia L, Hernandez M, Carballo J, Rojas H, Matteo L, Casique L, Escalona JL, Rodriguez Y, Rodriguez J, Hernandez D, Balbi D, Villegas R (2018) Extracellular Application of CRMP2 Increases Cytoplasmic Calcium through NMDA Receptors. Neuroscience 376:204–223. doi: 10.1016/j.neuroscience.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 139.Moutal A, Khanna R (2018) Unconventional Signaling by Extracellular CRMP2: Possible Role as an Atypical Neurotransmitter? Neuroscience 376:224–226. doi: 10.1016/j.neuroscience.2018.02.025 [DOI] [PubMed] [Google Scholar]
- 140.Mudher A, Colin M, Dujardin S, Medina M, Dewachter I, Alavi Naini SM, Mandelkow EM, Mandelkow E, Buee L, Goedert M, Brion JP (2017) What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol Commun 5 (1):99. doi: 10.1186/s40478-017-0488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Numata-Uematsu Y, Wakatsuki S, Nagano S, Shibata M, Sakai K, Ichinohe N, Mikoshiba K, Ohshima T, Yamashita N, Goshima Y, Araki T (2018) Inhibition of collapsin response mediator protein-2 phosphorylation ameliorates motor phenotype of ALS model mice expressing SOD1G93A. Neurosci Res. doi: 10.1016/j.neures.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 142.Chung MA, Lee JE, Lee JY, Ko MJ, Lee ST, Kim HJ (2005) Alteration of collapsin response mediator protein-2 expression in focal ischemic rat brain. Neuroreport 16 (15):1647–1653 [DOI] [PubMed] [Google Scholar]
- 143.Liu W, Zhou XW, Liu S, Hu K, Wang C, He Q, Li M (2009) Calpain-truncated CRMP-3 and −4 contribute to potassium deprivation-induced apoptosis of cerebellar granule neurons. Proteomics 9 (14):3712–3728. doi: 10.1002/pmic.200800979 [DOI] [PubMed] [Google Scholar]
- 144.Bretin S, Rogemond V, Marin P, Maus M, Torrens Y, Honnorat J, Glowinski J, Premont J, Gauchy C (2006) Calpain product of WT-CRMP2 reduces the amount of surface NR2B NMDA receptor subunit. Journal of neurochemistry 98 (4):1252–1265. doi: 10.1111/j.1471-4159.2006.03969.x [DOI] [PubMed] [Google Scholar]
- 145.Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ (2007) NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 27 (31):8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brittain JM, Pan R, You H, Brustovetsky T, Brustovetsky N, Zamponi GW, Lee WH, Khanna R (2012) Disruption of NMDAR-CRMP-2 signaling protects against focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Channels (Austin) 6 (1):52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brustovetsky T, Pellman JJ, Yang XF, Khanna R, Brustovetsky N (2014) Collapsin response mediator protein 2 (CRMP2) interacts with N-methyl-D-aspartate (NMDA) receptor and Na+/Ca2+ exchanger and regulates their functional activity. The Journal of biological chemistry 289 (11):7470–7482. doi: 10.1074/jbc.M113.518472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brittain JM, Chen L, Wilson SM, Brustovetsky T, Gao X, Ashpole NM, Molosh AI, You H, Hudmon A, Shekhar A, White FA, Zamponi GW, Brustovetsky N, Chen J, Khanna R (2011) Neuroprotection against traumatic brain injury by a peptide derived from the collapsin response mediator protein 2 (CRMP2). The Journal of biological chemistry 286 (43):37778–37792. doi: 10.1074/jbc.M111.255455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang H, Kang E, Wang Y, Yang C, Yu H, Wang Q, Chen Z, Zhang C, Christian KM, Song H, Ming GL, Xu Z (2016) Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nature communications 7. doi: 10.1038/ncomms11773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME (2009) Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. Journal of neurochemistry 110 (1):363–377. doi: 10.1111/j.1471-4159.2009.06140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Catterall WA, Few AP (2008) Calcium channel regulation and presynaptic plasticity. Neuron 59 (6):882–901. doi: 10.1016/j.neuron.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 152.Khanna R, Zougman A, Stanley EF (2007) A proteomic screen for presynaptic terminal N-type calcium channel (CaV2.2) binding partners. Journal of biochemistry and molecular biology 40 (3):302–314 [DOI] [PubMed] [Google Scholar]
- 153.Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R (2009) An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. The Journal of biological chemistry 284 (45):31375–31390. doi: 10.1074/jbc.M109.009951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285 (5433):1569–1572 [DOI] [PubMed] [Google Scholar]
- 155.Wilson SM, Brittain JM, Piekarz AD, Ballard CJ, Ripsch MS, Cummins TR, Hurley JH, Khanna M, Hammes NM, Samuels BC, White FA, Khanna R (2011) Further insights into the antinociceptive potential of a peptide disrupting the N-type calcium channel-CRMP-2 signaling complex. Channels (Austin) 5 (5):449–456. doi: 10.4161/chan.5.5.17363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chi XX, Schmutzler BS, Brittain JM, Hingtgen CM, Nicol GD, Khanna R (2009) Regulation of N-type voltage-gated calcium (CaV2.2) channels and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. JCell Sci 23:4351–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brittain JM, Wang Y, Eruvwetere O, Khanna R (2012) Cdk5-mediated phosphorylation of CRMP-2 enhances its interaction with CaV2.2. FEBS letters 586 (21):3813–3818. doi: 10.1016/j.febslet.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 158.Sheets PL, Heers C, Stoehr T, Cummins TR (2008) Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. The Journal of pharmacology and experimental therapeutics 326 (1):89–99. doi: 10.1124/jpet.107.133413 [DOI] [PubMed] [Google Scholar]
- 159.Kanellopoulos AH, Koenig J, Huang H, Pyrski M, Millet Q, Lolignier S, Morohashi T, Gossage SJ, Jay M, Linley JE, Baskozos G, Kessler BM, Cox JJ, Dolphin AC, Zufall F, Wood JN, Zhao J (2018) Mapping protein interactions of sodium channel NaV1.7 using epitope-tagged gene-targeted mice. The EMBO journal 37 (3):427–445. doi: 10.15252/embj.201796692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Francois-Moutal L, Dustrude ET, Wang Y, Brustovetsky T, Dorame A, Ju W, Moutal A, Perez-Miller S, Brustovetsky N, Gokhale V, Khanna M, Khanna R (2018) Inhibition of the Ubc9 E2 SUMO-conjugating enzyme-CRMP2 interaction decreases NaV1.7 currents and reverses experimental neuropathic pain. Pain. doi: 10.1097/j.pain.0000000000001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Francois-Moutal L, Scott DD, Perez-Miller S, Gokhale V, Khanna M, Khanna R (2018) Chemical shift perturbation mapping of the Ubc9-CRMP2 interface identifies a pocket in CRMP2 amenable for allosteric modulation of Nav1.7 channels. Channels (Austin) 12 (1):219–227. doi: 10.1080/19336950.2018.1491244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Laedermann CJ, Decosterd I, Abriel H (2014) Ubiquitylation of voltage-gated sodium channels. Handbook of experimental pharmacology 221:231–250. doi: 10.1007/978-3-642-41588-3_11 [DOI] [PubMed] [Google Scholar]
- 163.Creange A, Zeller J, Rostaing-Rigattieri S, Brugieres P, Degos JD, Revuz J, Wolkenstein P (1999) Neurological complications of neurofibromatosis type 1 in adulthood. Brain : a journal of neurology 122 (Pt 3):473–481 [DOI] [PubMed] [Google Scholar]
- 164.Ferner RE, Thomas M, Mercer G, Williams V, Leschziner GD, Afridi SK, Golding JF (2017) Evaluation of quality of life in adults with neurofibromatosis 1 (NF1) using the Impact of NF1 on Quality Of Life (INF1-QOL) questionnaire. Health and quality of life outcomes 15 (1):34. doi: 10.1186/s12955-017-0607-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Patrakitkomjorn S, Kobayashi D, Morikawa T, Wilson MM, Tsubota N, Irie A, Ozawa T, Aoki M, Arimura N, Kaibuchi K, Saya H, Araki N (2008) Neurofibromatosis type 1 (NF1) tumor suppressor, neurofibromin, regulates the neuronal differentiation of PC12 cells via its associating protein, CRMP-2. The Journal of biological chemistry 283 (14):9399–9413. doi: 10.1074/jbc.M708206200 [DOI] [PubMed] [Google Scholar]
- 166.Moutal A, Dustrude ET, Khanna R (2017) Sensitization of Ion Channels Contributes to Central and Peripheral Dysfunction in Neurofibromatosis Type 1. Mol Neurobiol 54 (5):3342–3349. doi: 10.1007/s12035-016-9907-1 [DOI] [PubMed] [Google Scholar]
- 167.White KA, Swier VJ, Cain JT, Kohlmeyer JL, Meyerholz DK, Tanas MR, Uthoff J, Hammond E, Li H, Rohret FA, Goeken A, Chan CH, Leidinger MR, Umesalma S, Wallace MR, Dodd RD, Panzer K, Tang AH, Darbro BW, Moutal A, Cai S, Li W, Bellampalli SS, Khanna R, Rogers CS, Sieren JC, Quelle DE, Weimer JM (2018) A porcine model of neurofibromatosis type 1 that mimics the human disease. JCI Insight 3 (12). doi: 10.1172/jci.insight.120402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moutal A, Wang Y, Yang X, Ji Y, Luo S, Dorame A, Bellampalli SS, Chew LA, Cai S, Dustrude ET, Keener JE, Marty MT, Vanderah TW, Khanna R (2017) Dissecting the role of the CRMP2-neurofibromin complex on pain behaviors. Pain 158 (11):2203–2221. doi: 10.1097/j.pain.0000000000001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moutal A, Sun L, Yang X, Li W, Cai S, Luo S, Khanna R (2018) CRMP2-Neurofibromin Interface Drives NF1-related Pain. Neuroscience 381:79–90. doi: 10.1016/j.neuroscience.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nagai J, Owada K, Kitamura Y, Goshima Y, Ohshima T (2016) Inhibition of CRMP2 phosphorylation repairs CNS by regulating neurotrophic and inhibitory responses. Experimental neurology 277:283–295. doi: 10.1016/j.expneurol.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 171.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ (2003) A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103 (3):249–257 [DOI] [PubMed] [Google Scholar]
- 172.Hergenroeder GW, Redell JB, Choi HA, Schmitt L, Donovan W, Francisco GE, Schmitt K, Moore AN, Dash PK (2018) Increased Levels of Circulating Glial Fibrillary Acidic Protein and Collapsin Response Mediator Protein-2 Autoantibodies in the Acute Stage of Spinal Cord Injury Predict the Subsequent Development of Neuropathic Pain. Journal of neurotrauma 35 (21):2530–2539. doi: 10.1089/neu.2018.5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Adamus G, Bonnah R, Brown L, David L (2013) Detection of autoantibodies against heat shock proteins and collapsin response mediator proteins in autoimmune retinopathy. BMC Ophthalmol 13:48. doi: 10.1186/1471-2415-13-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, Hertz-Picciotto I, Pessah IN, Van de Water J (2013) Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry 3:e277. doi: 10.1038/tp.2013.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lubec G, Nonaka M, Krapfenbauer K, Gratzer M, Cairns N, Fountoulakis M (1999) Expression of the dihydropyrimidinase related protein 2 (DRP-2) in Down syndrome and Alzheimer’s disease brain is downregulated at the mRNA and dysregulated at the protein level. J Neural Transm Suppl 57:161–177 [DOI] [PubMed] [Google Scholar]
- 176.Khawaja X, Xu J, Liang JJ, Barrett JE (2004) Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: Implications for depressive disorders and future therapies. J Neurosci Res 75 (4):451–460. doi: 10.1002/jnr.10869 [DOI] [PubMed] [Google Scholar]
- 177.Ozgen HM, Staal WG, Barber JC, de Jonge MV, Eleveld MJ, Beemer FA, Hochstenbach R, Poot M (2009) A novel 6.14 Mb duplication of chromosome 8p21 in a patient with autism and self mutilation. J Autism Dev Disord 39 (2):322–329. doi: 10.1007/s10803-008-0627-x [DOI] [PubMed] [Google Scholar]
- 178.Liu Y, Pham X, Zhang L, Chen PL, Burzynski G, McGaughey DM, He S, McGrath JA, Wolyniec P, Fallin MD, Pierce MS, McCallion AS, Pulver AE, Avramopoulos D, Valle D (2014) Functional variants in DPYSL2 sequence increase risk of schizophrenia and suggest a link to mTOR signaling. G3 (Bethesda) 5 (1):61–72. doi: 10.1534/g3.114.015636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Garza JC, Qi X, Gjeluci K, Leussis MP, Basu H, Reis SA, Zhao WN, Piguel NH, Penzes P, Haggarty SJ, Martens GJ, Poelmans G, Petryshen TL (2018) Disruption of the psychiatric risk gene Ankyrin 3 enhances microtubule dynamics through GSK3/CRMP2 signaling. Transl Psychiatry 8 (1):135. doi: 10.1038/s41398-018-0182-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grant NJ, Coates PJ, Woods YL, Bray SE, Morrice NA, Hastie CJ, Lamont DJ, Carey FA, Sutherland C (2015) Phosphorylation of a splice variant of collapsin response mediator protein 2 in the nucleus of tumour cells links cyclin dependent kinase-5 to oncogenesis. BMC Cancer 15:885. doi: 10.1186/s12885-015-1691-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Couderc C, Bollard J, Coute Y, Massoma P, Poncet G, Lepinasse F, Hervieu V, Gadot N, Sanchez JC, Scoazec JY, Diaz JJ, Roche C (2015) Mechanisms of local invasion in enteroendocrine tumors: identification of novel candidate cytoskeleton-associated proteins in an experimental mouse model by a proteomic approach and validation in human tumors. Mol Cell Endocrinol 399:154–163. doi: 10.1016/j.mce.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 182.Tahimic CG, Tomimatsu N, Nishigaki R, Fukuhara A, Toda T, Kaibuchi K, Shiota G, Oshimura M, Kurimasa A (2006) Evidence for a role of Collapsin response mediator protein-2 in signaling pathways that regulate the proliferation of non-neuronal cells. Biochemical and biophysical research communications 340 (4):1244–1250. doi: 10.1016/j.bbrc.2005.12.132 [DOI] [PubMed] [Google Scholar]
- 183.Pilotte J, Kiosses W, Chan SW, Makarenkova HP, Dupont-Versteegden E, Vanderklish PW (2018) Morphoregulatory functions of the RNA-binding motif protein 3 in cell spreading, polarity and migration. Sci Rep 8 (1):7367. doi: 10.1038/s41598-018-25668-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geraets RD, Koh S, Hastings ML, Kielian T, Pearce DA, Weimer JM (2016) Moving towards effective therapeutic strategies for Neuronal Ceroid Lipofuscinosis. Orphanet J Rare Dis 11:40. doi: 10.1186/s13023-016-0414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cooper JD, Tarczyluk MA, Nelvagal HR (2015) Towards a new understanding of NCL pathogenesis. Biochimica et biophysica acta 1852 (10 Pt B):2256–2261. doi: 10.1016/j.bbadis.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 186.Mole SE, Cotman SL (2015) Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochimica et biophysica acta 1852 (10 Pt B):2237–2241. doi: 10.1016/j.bbadis.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moutal A, Eyde N, Telemi E, Park KD, Xie JY, Dodick DW, Porreca F, Khanna R (2016) Efficacy of (S)-Lacosamide in preclinical models of cephalic pain. Pain Rep 1 (1). doi: 10.1097/PR9.0000000000000565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abe H, Jitsuki S, Nakajima W, Murata Y, Jitsuki-Takahashi A, Katsuno Y, Tada H, Sano A, Suyama K, Mochizuki N, Komori T, Masuyama H, Okuda T, Goshima Y, Higo N, Takahashi T (2018) CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage. Science 360 (6384):50–57. doi: 10.1126/science.aao2300 [DOI] [PubMed] [Google Scholar]
- 189.Giraudon P, Nicolle A, Cavagna S, Benetollo C, Marignier R, Varrin-Doyer M (2013) Insight into the role of CRMP2 (collapsin response mediator protein 2) in T lymphocyte migration: the particular context of virus infection. Cell Adh Migr 7 (1):38–43. doi: 10.4161/cam.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]