Abstract
Objective
To demonstrate experience and feasibility of a precision medicine approach for patients with unexplained cytopenias, defined as low blood counts in one or more cell lineages, persistent for ≥ 6 months, in the absence of known nutritional, autoimmune, infectious, toxic and neoplastic (secondary) causes.
Patients and Methods:
Patients were evaluated in our clinic from November 8, 2016 and January 12, 2018. After a thorough evaluation of known causes, family history and appropriate clinical assays, genomic evaluation was done in a stepwise manner, through Sanger, targeted and/or whole exome sequencing. Variants were analyzed and discussed in a genomic tumor board attended by clinicians, bioinformaticians, and molecular biologists.
Results:
Sixty-eight patients were evaluated in our clinic and after genomic interrogation, were classified into inherited bone marrow failure syndromes [IBMFS, (n=24, 35%)], cytopenias without a known clinical syndrome which included clonal (CCUS) and idiopathic (ICUS) cytopenias of undetermined significance (n=30, 44%), and patients that did not fit into the above two categories (‘others’, n=14, 21%). A significant family history was found in only 17 (25%) patients (9-IBMFS, 2-CCUS, 6-others), while gene variants were found in 43 (63%) patients [34 (79%) pathogenic; 12-IBMFS, 17-CCUS, 5-others]. Genomic assessment resulted in a change in clinical management in 17 (25%) patients, as evidenced by changes in decisions with regards to therapeutic interventions (n=8, 47%), donor choice (n=6, 35%) and/or choice of conditioning regimen for hematopoietic stem cell transplantation (n=8, 47%).
Conclusion:
We demonstrate clinical utility of a real-world algorithmic precision medicine approach for unexplained cytopenias.
Introduction:
The advent of individualized medicine in recent years has enabled identification of genetic drivers in several human diseases, especially in cancers.1-3 Its relevance in identification of molecular drivers is not only limited to unraveling the underlying disease biology, but also to offer novel insights into targeted therapeutic approaches.4 At Mayo Clinic, experience with precision medicine in solid tumors was shown to have an impact on treatment decisions and clinical care in a minority of patients.5 That being said, genomic approaches are still in their adolescence and face significant challenges such as variability in bioinformatics analyses, intra-tumor heterogeneity and genome variation, limited actionable mutations, access to novel therapeutic agents and barriers to health care delivery.6-8 However, with measured skepticism, it is anticipated that the aforementioned approaches, with wider use and development have the potential to significantly impact clinical care. 8-11
In the field of non-malignant hematology, exome sequencing has enabled identification of age-related clonal hematopoiesis, which has important clinical implications such as elevated independent cardiovascular risk and increased propensity to develop myeloid neoplasms.12-15 Further, precision medicine has been particularly helpful in identification of key molecular drivers associated with inherited bone marrow failure syndromes (IBMFS), such as Fanconi anemia (FA), Diamond Blackfan anemia (DBA), short telomere syndromes (STS), Shwachman-Diamond syndrome, GATA2 haploinsufficiency syndrome, Thrombocytopenia Absent Radii and severe congenital neutropenia (SCN) among others.16-24 Moreover, the experience of the Diagnostic Odyssey service line of the Individualized Medicine Clinic at Mayo Clinic has shown that whole exome sequencing was successful to diagnose such patients with inherited diseases including cytopenias and immunodeficiencies.25 Despite being clinically well characterized, these inherited disorders display variable, often attenuated, clinical manifestations (‘forme fruste’) at differing ages of presentation.17 Variable patterns of inheritance, incomplete penetrance and somatic reversion are some of the known factors responsible for this phenomenon.17,26,27 Therefore in clinical practice, patients with unexplained cytopenias often suffer from delayed and/or inaccurate diagnoses, resulting in the administration of contextually inappropriate therapies such as immunosuppression, hematopoietic stem cell transplantation (HSCT) with inappropriate donors, ionizing radiation and cytotoxic chemotherapy.28,29 We thus felt that a precision medicine evaluation for these patients and their family members had the potential to, not only enable timely and appropriate diagnoses, but also impact therapeutic interventions.
Through this paper, we discuss our experience with a unique service line clinic established to apply precision medicine methods to uncover and annotate gene variants in the context of appropriate clinical associations and prospectively study the natural history of patients with clonal cytopenias, acquired and IBMFS.
Methods:
Our Pre-Myeloid and Bone Marrow Failure clinic was established through support from the Mayo Clinic Center for Individualized Medicine (CIM) and the Division of Hematology, with the vision to be a unique collaborative effort between clinicians, bioinformatics specialists and molecular biologists [Institutional Review Board (IRB) #16-004173 and National Clinical Trials # 02958462 (www.clincialtrials.gov)]. The first patient was consented to our clinic on November 8, 2016, while the last patient was consented on January 12, 2018. Patients were evaluated after an appropriate assessment of known causes by a physician/hematologist. Based on a clinical evaluation which included obtaining a detailed family history and physical exam, and appropriate clinical test results, genomic evaluation was done in a stepwise manner, starting with either a targeted (NGS) or Sanger sequencing, and if negative, research-based whole exome sequencing (WES).
Evaluation of patients:
All patients were referred by clinicians in various disciplines, in particular Hematology, Pulmonary Medicine, Gastroenterology and Pediatrics at Mayo Clinic. We defined ‘unexplained cytopenias’ as low blood counts in one or more cell lineages (red or white blood cell or platelets), persistent for ≥ 6 months (this time interval cut-off was chosen in order to definitively exclude patients with MDS as per the World Health Organization guidelines)30, in the absence of known causes of cytopenias, such as, nutritional deficiencies (including deficiencies of iron, vitamin B12, folate, copper, and pyridoxine), myelodysplastic syndromes (MDS) as defined by the World Health Organization31, effect of drugs/toxins, paroxysmal nocturnal hemoglobinuria, chronic conditions such as hepatitis C, human immunodeficiency virus, liver disease etc. Hence, in order to meet eligibility, a minimum suggested evaluation at the discretion of the referring hematologist included: iron chemistries, vitamin B12, methyl-malonic acid, folate, copper and zinc levels, thyroid stimulating hormone levels, liver function tests, hepatitis B, C and human immunodeficiency virus serologies, bone marrow aspiration and core biopsy along with cytogenetics, paroxysmal nocturnal hemoglobinuria (PNH) flow cytometry*, and an abdominal ultrasound to evaluate spleen size when clinically relevant (*Four patients in our cohort had a minor PNH clone, however their clinical phenotype was not consistent with PNH, and hence they were included in the unexplained cytopenias category). If a non-hematologist placed a referral, a hematologist assessed the charts to ensure adequate evaluation was pursued prior to genetic assessment (eConsult). When necessary, an in-person hematology consult was carried out and the appropriate evaluation was completed.
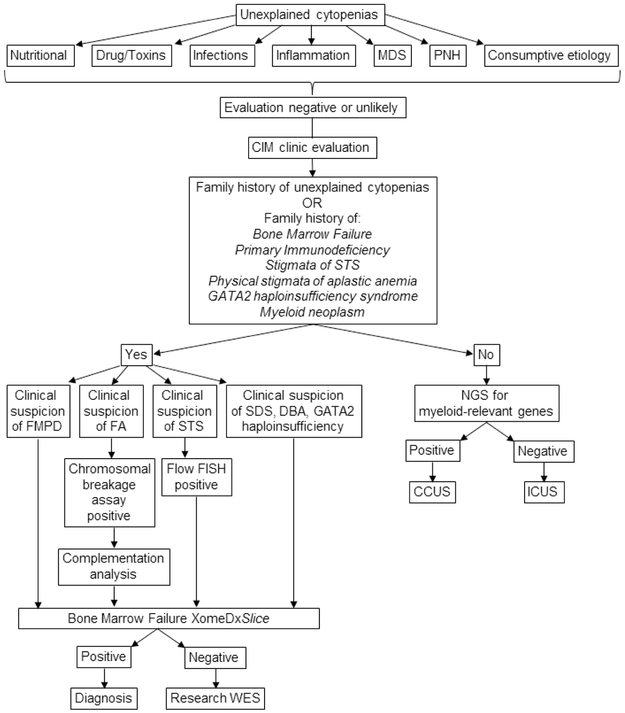
Written informed consent was obtained with the help of a dedicated study coordinator. Once consent was obtained, health related and quality of life information was collected from the medical records and by an in-person interview, survey and clinical encounters. A detailed stepwise diagnostic algorithm for this clinic is outlined in figure 1A.
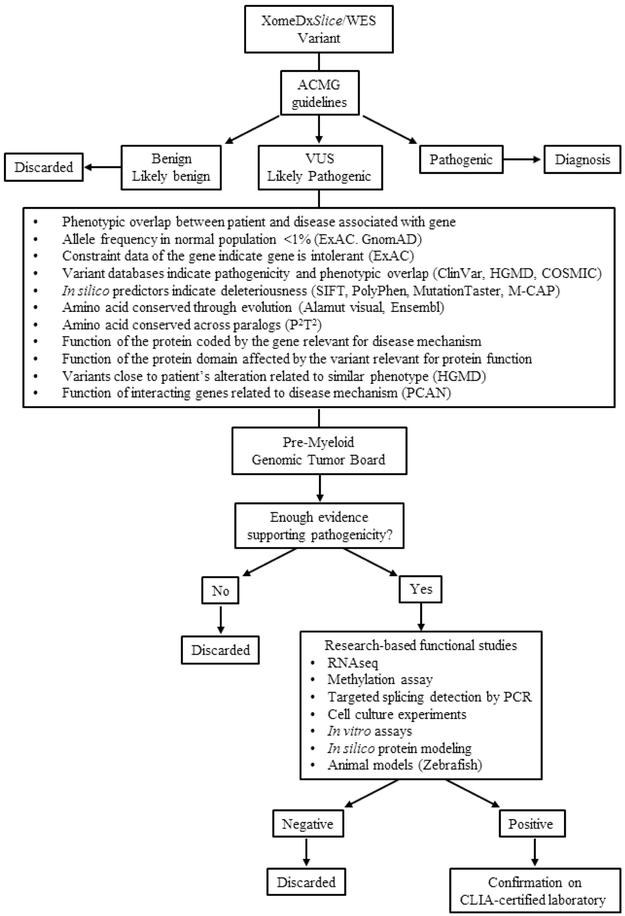
Figure 1:
Figure 1A shows diagnostic algorithm followed by our clinic. Figure 1B shows the variant decision tree adopted by our team. Nutritional causes include deficiencies of iron, B12, copper, folate and pyridoxine. A wide array of drugs, toxins, infections and inflammatory etiology is considered at the discretion of the referring hematologist. Consumptive etiology includes splenomegaly and various causes of intravascular and extravascular hemolysis. Abbreviations: MDS = myelodysplastic syndromes; PNH = paroxysmal nocturnal hemoglobinuria; STS = short telomere syndrome; CIM = center for individualized medicine; SDS = Shwachman-Diamond syndrome; DBA = Diamond-Blackfan anemia, GATA2 = GATA-binding factor 2; FMPD = familial myeloid predisposition syndromes; FA = Fanconi anemia; FLOW-FISH = flow cytometry-fluorescent in-situ hybridization; NGS = next generation sequencing; WES = whole exome sequencing; CCUS = clonal cytopenia of undetermined significance; ICUS = idiopathic cytopenia of undetermined significance; ACMG = American College of Medical Genetics and Genomics; VUS = variant of uncertain significance; HGMD = Human Gene Mutation Database; COSMIC = Catalogue of Somatic Mutations in Cancer; SIFT = Sorting Intolerant From Tolerant; M-CAP = Mendelian Clinically Applicable Pathogenicity score; P2T2 = Protein Panoramic Annotation Tool; PCAN = Phenotype Consensus Analysis; RNAseq = RNA sequencing; PCR = polymerase chain reaction; CLIA = Clinical Laboratory Improvement Amendments.
Collection of samples:
After proper genetic counseling, all enrolled participants, at the time of a clinically indicated blood draw or bone marrow biopsy, were asked to provide 20 ml of bone marrow and/or up to 50 ml of fresh blood, from which bone marrow (BM) and peripheral blood (PB) mononuclear cells (MNC) were isolated, respectively. These cells were then processed to obtain deoxyribonucleic acid (DNA), ribonucleic acid (RNA), chromatin and viably frozen cells for future use. When clinically indicated, after consent, skin biopsies were obtained for fibroblast cultures so as to obtain germline DNA for assessment.
Sequencing:
After an appropriate clinical evaluation, genomic sequencing was done in a stepwise manner, beginning with Sanger or next generation sequencing (NGS) and if negative, then research-based whole exome sequencing was performed (Figure 1A and 1B). Details are mentioned the supplementary methods and supplementary table 1.
Pre-Myeloid Genomic Tumor Board:
Results from this research were summarized and presented at a multidisciplinary Pre-Myeloid Genomic Tumor Board that included physicians, research scientists, pathologists, bioinformaticians and genetic counselors from Mayo Clinic (Rochester, MN). Consensus among these members determined how to proceed with each patient (Figure 1B), similar to previously described for other groups at Mayo Clinic5. If a particular gene variant was not considered relevant after discussion at the tumor board, it was discarded. However, based on available data, if consensus was obtained to support a variant’s role in relationship to a particular phenotype, then additional research based tests were carried out to confirm this association. These studies were specific to the affected gene and included options such as, in silico protein modeling, RNA sequencing, methylation assays, splicing detection by PCR, and/or any other in vitro, cellular or animal model experiments that could help establish the association. Variants considered to be pathogenic were then validated in a CLIA-certified laboratory by Sanger sequencing.
Classification of patients:
Patients were grouped into three different categories: IBFMS, cytopenias without a known clinical syndrome, and an ‘others’ category which included patients who did not fit into either of the two categories. Patients were diagnosed with IBFMS if they had a significant family history defined as two or more first or second degree relatives with hematologic malignancies and/or a solid tumor with a known germline predisposition and/or longstanding cytopenias and/or bleeding history, and/or classical inherited marrow failure syndrome organ manifestations OR were found to have a known IBFMS-related pathogenic variant on genomic assessment. The definition of significant family history was obtained from previously published consensus guidelines for identification of inherited myeloid malignancies.32,33
Impact on clinical management:
In order to assess impact of our genomic assessment on clinical care, we planned for a clinician (hematologist) to retrospectively review the medical records of the patients included in our cohort. Prior to conducting chart review, we defined impact on clinical management as decision to start or not to start a particular therapy, alter donor selection and/or conditioning regimen intensity for HSCT, as described in similar recent study by Alder JK et al. evaluating clinical utility of measuring telomere lengths using flow cytometry fluorescence in-situ hybridization in hospital practice. 20
Charts of all patients were reviewed to assess ‘change in clinical management’ based on genetic results and sub-categorized into: decision to start or not to start a drug, choice of donor and/or conditioning regimen intensity for HSCT.
Results:
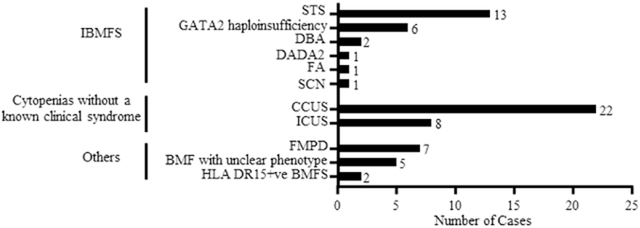
To date, we have identified 68 patients with unexplained cytopenias and evaluated them following the algorithm outlined in figure 1. After a genomic evaluation, 24 (35%) patients were diagnosed with an IBFMS (patients 31-54 in supplementary table 2), 30 (44%) with cytopenias without a known clinical syndrome (patients 1-30 supplementary table 2) and 14 (21%) were classified in the ‘others’ category (patients 55-68 in supplementary table 2) which included patients with familial myeloid predisposition syndromes (FMPD, n=7, 50%, patients 55-61 in supplementary table 2), bone marrow failure syndromes (BMFS) without a clear clinical phenotype (n=5, 36%, patients 62-66 in supplementary table 2) and HLA DR15+ BMFS (n=2, 14%, patients 67 and 68 in supplementary table 2) patients. See table 1 and figure 2 for details.
Table 1:
Table showing demographic, clinical and genetic characteristics of patients included in our cohort (Range or % only provided if n>1).
| Disorder subtype |
No. of patients (N=68) |
Median age of disease onset (Range) |
No. of Males (%) |
Defining clinical features or diagnoses [Number of cases (%)] |
Progression to MDS or AML (%) |
Genetic alterations identified (details in supplementary table 2) |
No. of deaths (%) |
Median overall survival in months (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Inherited bone marrow failure syndromes (n=24) | ||||||||
| Short telomere syndromes a | 13 (54) | 53 (2-74) | 9 (69) | -Pancytopenia (n=6, 47%) -Anemia (n=4, 31%) -Leukopenia (n=2, 15%) (1 neutropenia, 1 lymphopenia) -Thrombocytopenia (n=5, 39%) -IIP (n=5, 39%) -Cirrhosis (n=2, 15%) -DKC clinical triad b (n=2, 15%) |
1 | •TERT - c.2947C>T; p.His983Tyr (n=1) - c.2768C>T; p.Pro923Leu (n=1) •DKC1 (details N/A, n=1) |
4 (31) | 1412 (108, NR) |
| GATA2 haploinsufficiency syndromes | 6 (25) | 34.5 (18-50) | 3 (50) | -Immunodeficiency with/without fungal or viral infections (n=4, 67%) -HPV-driven warts (n=1, 17%) -Pancytopenia (n=1, 17%). |
3 (50) | • GATA2 - c.1339A>C; p.S447R (n=3) - c.1187G>A; p.Arg396Gln (n=1) - c.1192C>T; p.Arg398Trp (n=1) -Deletion (n=1) |
0 | Median NR |
| Diamond-Blackfan anemia | 2 (8) | 36 (31-41) | 2 (100) | -Anemia (n=2, 100%) | 0 | • RPS19 - c.357-1G>A; p.? (n=1) • RPL5 - c.90_01dup; p.Tyr31Phefs*8 (n=1) |
0 | Median NR |
| Fanconi anemia | 1 | 11 | 1 | -Anemia -Short stature -Right thumb hypoplasia -Bilateral hypertrophy of thenar muscles -Absent scaphoid bone -Horseshoe kidney Cutaneous hyper- & hypopigmentation -Café-au-lait macules -Eck webbing High-arched palate -Patent ductus arteriosus |
0 | • FANCG - c.313G>T; p.Glu105* (n=1) |
0 | 241 |
| Deficiency of ADA2 | 1 | 17 | 0 | -Pancytopenia -Cutaneous polyarteritis nodosa -Livedo reticularis -Nodular regenerative -Hyperplasia -Hypogammaglobinemia |
0 | 0 | 0 | 21 |
| Cyclical neutropenia | 1 | 23 | 1 | -Leukopenia with neutropenia | 0 | None | 0 | 77 |
| Cytopenias without a known clinical syndrome (n=30) | ||||||||
| CCUS | 22 (73) | 15 (63) | -Pancytopenia (n=4, 18%) -Anemia (n=12, 55%) -Leukopenia (all neutropenia) (n=6, 27%) -Thrombocytopenia (n=9, 41%) |
6 (27) | • TET2 - c.210 211insC; p.Asn71Glnfs*2 (n=1) - c.4045-1G>A; p.? (n=1) - c.4523_4524del; Ala1508Glufs*69 (n=1) - c.5642A>G; p.His188Arg (n=1) - c.3250C>T; p.Gln1084* (n=1) - c.421_424dup; p.Ser142Cysfs*6 (n=1) - c.3594+2dup; p.? (n=1) • DNMT3A - c.2645G>A; p.Arg882His (n=1) • ASXL1 - c.1758dup; p.Gly587Argfs*32 (n=1) - c.2053G>T; p.Gly685* (n=1) • IDH1 - c.395G>T; p.Arg132Leu (n=1) - c.394C>T; p.Arg132Cys (n=1) • IDH2 - c.419G>A; p.Arg140Gln (n=1) • ZRSR2 - c.376C>T; p.Arg126X (n=1) - c.83dup; p.Lys29Glufs*26 (n=1) - c.787G>T; p.Glu263X (n=1) - c.558-1_558dup; p.Cys187Aspfs*52 (n=1) - c.376C>T; p.Arg126* (n=1) • SRSF2 - c.284C>A; p.Pro95His (n=1) - c.284C>G; p.Pro95Arg (n=2) • U2AF1 - c.470A>C; p.Gln157Pro (n=1) - c.101C>T; p.Ser34Phe (n=1) • SF3B1 - c.2098A>G; p.Lys700Glu (n=1) • JAK2 - c.1849G>T; p.Val617Phe (n=1) • SETBP1 - c.2602G>A; p.Asp868Asn (n=1) • BCOR - c.3448dup; p.Glu1150Glyfs*18 (n=1) • TP53 - c.844C>T; p.Arg282Trp (n=1) - c.455dup; p.Pro153AlafsX28 (n=1) - c.743G>A; p.Arg248Gln (n=1) • PHF6 - c.941T>C; p.Ile314Thr (n=1) |
1 | Median NR | |
| ICUS | 8 (11) | 40.5 (26-74) | 3 (38) | -Pancytopenia (n=1, 13%) -Anemia (n=4, 50%) -Leukopenia (n=3, 38%) -Thrombocytopenia (n=1) |
0 | None | 1 | Median NR |
| Other (n=14) | ||||||||
| Familial myeloid predisposition syndromes | 7 (10) | 24 (6-69) | 4 (57) | -Pancytopenia (n=1) -Thrombocytopenia (n=6, 86%) |
2 (29) | • RUNX1 - c.710G>A; p.Arg273Lys (n=1) - c.351+1G>A; p.? (n=1) • ANKRD26 - 5’UTR exome 1 and exome 2 (n=1) - c.116C>T; p.Gly39Asp (n=1) |
0 | Median NR |
| Bone marrow failure syndromes without a clear clinical phenotype | 5 (7) | 34 (21-65) | 1 | -Pancytopenia (n=4, 80%) -Leukopenia (both neutropenia and monocytopenia) (n=1) |
0 | None | 0 | Median NR |
| HLA DR15+ve bone marrow failure syndrome | 2 (3) | 39.5 (34-45) | 0 (0) | -Pancytopenia (n=2, 100%) | 0 | • BCOR - c.3448dup; p.Glu1150Glyfs*18 (n=1) |
0 | Median NR |
Abbreviations: CCUS = Clonal cytopenias of undetermined significance; ICUS = Idiopathic cytopenias of undetermined significance; STS = Short telomere syndromes; ADA2 = Adenosine deaminase-2; DKC = dyskeratosis congenita; HPV = human papilloma virus; MDS = myelodysplastic syndromes; AML = acute myeloid leukemia; NR = Not reached.
Ten of these 13 patients were a part of a previously published cohort of 17 patients;36
DKC clinical triad includes dysplastic nails, lacy reticular skin pigmentation and oral leukoplakia. Variants labeled with “p.?” refer to intronic alterations close to a splicing donor/acceptor predicted to affect mRNA splicing, but not confirmed with a functional test.
Figure 2:
Figure representing a visual description of the different diagnoses included in our cohort of patients with unexplained cytopenias. We divided the patients into three primary categories; IBMFS (n=24, 35%), cytopenias without a known clinical syndrome (n=30, 44%) and other patients which did not fit either of the two categories (n=14, 21%). Abbreviations: IBMFS = inherited bone marrow failure syndromes; STS = short telomere syndromes; DBA = Diamond-Blackfan anemia; DADA2 = deficiency of adenosine deaminase-2; SCN = severe congenital neutropenia; FA: Fanconi’s anemia; ICUS = idiopathic cytopenias of undetermined significance; CCUS = clonal cytopenias of undetermined significance; FMPD = familial myeloid predisposition; BMFS = bone marrow failure syndromes.
I. IBMFS: Of the 68 patients, 24 (35%) primary index patients were identified with IBMFS; median age 34.5 (range: 2-74) years, with 16 (67%) being males. The most common diagnoses under this category included short telomere syndromes (STS) [(n=13, 54%; patients 31-43 in supplementary table 2), GATA2 haploinsufficiency syndromes (n=6, 25%; patients 44-49 in supplementary table 4), DBA (n=2, 8%; patients 52 and 53 in supplementary table 2) and one patient each with FA, deficiency of ADA2 (DADA2) and SCN (patients 54, 50 and 51 in suppl. table 2 respectively). Of note, 10 of 13 STS patients were a part of a previously published cohort34, while the DADA2 patient (#50 in supplementary table 2) has been published previously.25,35 Significant family history was found only in 9 (38%) patients (3-STS, 4-GATA2, 1-DADA2, 1-FA), while 13 (54%) patients (9-STS, 1-GATA2, 1-DADA2, 1-SCN, 1-FA) had an IBMFS-associated physical abnormality such as Idiopathic Interstitial Pneumonia (IIP, n=7, 54%), unexplained cirrhosis (n=4, 31%), oral leukoplakia (n=1), lacy skin pigmentation (n=1), nasolacrimal duct obstruction (n=1), human papilloma virus-driven warts (n=1), nodular regenerative hyperplasia (n=1), short stature (n=1), resulting in a definite diagnosis in 7 (29%) patients. Genomic variants were found in 14 (58%) patients (Sanger-7, NGS-6, WES-1), of which 12 (50%) patients had pathogenic variants in the following genes: GATA2 (n=6, 5 pathogenic variants, while 1 patient had GATA2 gene deletion), TERT (n=2), DKC1 (n=1), RPS19 (n=1), RPL5 (n=1) and FANCG (n=1) (details in supplementary table 2 and 4), while VUS in TERC, CSF3R, RTEL1, ALG2, COG1 and LRBA genes were found in 3 (13%) patients. Among the 20 (83%) patients tested through gene sequencing (Sanger-8, NGS-11, WES-3, two patients underwent both Sanger and NGS), pathogenic variants were identified in 12 (60%) patients (7-Sanger, 4-NGS, 1-WES).Change in clinical management: Definitive evidence of altered clinical management was found in 10 (42%) patients, of which, an appropriate reduced intensity conditioning HSCT regimen was selected in 7 (70%) patients; in 5 (50%) patients, donor selection was altered, and in 3 (30%) patients, decision was made to start new therapy such as danazol (n=2, both with STS diagnosis), and adalimumab plus intravenous immunoglobulin (n=1, patient with DADA2 diagnosis). Six patients had altered clinical management involving more than one of the aforementioned categories (please refer to table 2 for details). At a median follow-up of 64 (range: 0-1416) months, there were 4 (17%) deaths, while 3 (13%) patients clonally evolved into myelodysplastic syndromes (two with underlying diagnosis of GATA2 haploinsufficiency, while 1 with STS) and 2 (8%) patients progressed to acute myeloid leukemia (AML, both with germline GATA2 haploinsufficiency syndromes).
II. Cytopenias without a known clinical syndrome: Based on the presence or absence of a presumed clonal abnormality, two diagnoses were included in this category (n=30, 44%; patients 1-30 in suppl. table 4), namely clonal (CCUS, n=22, 73%) and idiopathic (ICUS, n=8, 27%) cytopenias of undetermined significance. Median age at onset of cytopenias was 63.5 (range: 6-79) years with 18 (60%) males. Significant family history of unexplained cytopenias or hematologic malignancies was identified in 2 (all CCUS) patients (1 each of aplastic anemia and polycythemia vera). Among 28 (93%) tested patients, genomic abnormalities were found in 21 (75%) patients (all through NGS-based gene panel testing), all with CCUS diagnoses, which included 17 (61%) patients with pathogenic variants (11 with more than one variant), 4 (14%) with variants of uncertain significance, and 7 (25%) with chromosomal abnormalities. One CCUS patient had both a cytogenetic abnormality (13q-) and a minor PNH clone (PNH antigen loss in 3.62% granulocytes, 0.06% red blood cells and 4.5% monocytes) without evidence of dysplasia on bone marrow morphology. By definition, patients in whom both cytogenetic and pathogenic variants were not identified and clinical features of a bone marrow failure syndrome/MDS were not apparent, were classified as ICUS. Individual pathogenic variant details are mentioned in table 1 and supplementary tables 2 and 3, while VUS details are outlined in supplementary table 4. Change in clinical management: Evidence of altered clinical management due to our precision genomics approach was documented in 5 (17%) patients. This included starting specific therapies in 3 patients such as hypomethylating agent therapy (n=1, after discovery of a myeloid-relevant pathogenic variant), and immunosuppressive therapy (IST) or granulocyte-colony stimulating factor support (n=2, after exclusion of myeloid-relevant pathogenic variants). On the other hand, in 1 patient we decided to avoid the use of IST due to the detection of myeloid clonal evolution, as evidenced by the presence of myeloid-relevant pathogenic variants (in IDH1, SRSF2 and TP53 genes) and finally, 1 patient underwent reduced intensity conditioning HSCT due to concern for myeloid clonal evolution with a pathogenic variant in the SETBP1 gene [(additional details in table 2)]. At a median follow-up of 25 (range: 2-290) months, there were 2 (7%) deaths and 6 (20%) patients (all with CCUS) that underwent myeloid clonal evolution (5-MDS, 1-chronic myelomonocytic leukemia).
III. Others category: Patients who did not fit into either of the two aforementioned categories were classified in the “others” category (n=14, 21%), with the most frequent diagnoses being FMPD (n=7, 50%; with variants identified in the following genes: DDX41 (n=1), RUNX1 (n=2), ANKRD26 5 ’ UTR (n=1), ANKRD26 (n=1), in 3 patients variants were not identified despite a highly suggestive family history, patients 55-61 in suppl. table 4), BMFS without a clear clinical phenotype (n=5, 36%; patients 62-66 in suppl. table 4), and HLA DR15+ve BMFS (n=2, 14%; patients 67 and 68 in suppl. table 4) patient. Family history was significant in 6 (43%) patients (all FMPD). A PNH clone was identified through peripheral blood flow cytometric immunophenotyping in 3 (21%) patients (all BMFS without a clear clinical phenotype) with median partial antigen loss in 0.01 (range: 0.01-0.13) % erythrocytes, median complete antigen loss in 2.14 (range: 0.03-3.23) % erythrocytes, 31.41 (range: 2.35-62.55) % granulocytes and 10.57 (range: 2.45-65.96) % monocytes; however other clinical/laboratory criteria in these patients were insufficient for a diagnosis of PNH. Among tested patients [n=13 (93%), Sanger-3, NGS-9, WES-2; 1 patients underwent both Sanger and NGS], genetic abnormalities were identified in 8 (62%) patients [Sanger-3, NGS-3, WES-2], 5 (63%) pathogenic variants [RUNX1 (n=2), ANKRD26 5’UTR (n=1), ANKRD26 (n=1), BCOR (n=1)]. Of note, one patient each of RUNX-1 mutated and ANKRD26 associated thrombocytopenia have been previously published.36,37 Details on variants are mentioned in supplementary table 2, 3 and 4. Change in clinical management: Altered clinical management was seen in 2 (14%) patients, of which in one we chose to treat with equine anti-thymocyte globulin plus cyclosporine and prednisone in a HLA DR15+ BMFS patient with a minor PNH clone (after exclusion of myeloid-relevant pathogenic variants, details in table 2), while in the other patient, as we had identified a FMPD an appropriate sibling donor was identified after making sure that the donor did not have the same variant. At a median follow-up of 35 (range: 7-598) months, there were no deaths and 2 transformations to myeloid neoplasms (1-MDS, 1-CMML, both FMPD with alterations in ANKRD26 5’UTR exon 1/2 and ANKRD26 coding sequence respectively (c. 116C>T)).
Table 2:
Clinical and genomic characteristics of the subset of patients who underwent a change in clinical managementa (n=17, 25%).
| Case no. |
Diagnosis | Age | Sex | Clinical features | Pathogenic variants | Change in clinical managementa |
|---|---|---|---|---|---|---|
| 36 | STS | 3 | M | Oral leukoplakia, lacrimal duct stenosis, lacy skin pigmentation, pancytopenia | • DKC1 (details unknown) | Started on danazol, subsequently underwent allogeneic RIC allogeneic PBSCT (reduced intensity conditioning chosen due to defect in telomere-related genes) |
| 37 | STS | 58 | M | Pancytopenia | • TERC n.214G>C, noncoding variant | Underwent RIC allogeneic HSCT, RIC chosen due to STS diagnosis |
| 38 | STS | 17 | M | Pancytopenia | • TERT c.2768C>T; p.Pro923Leu | Consideration of RIC allogeneic regimen for HSCT, related donors being screened for the same variant |
| 39 | STS | 63 | M | Pancytopenia, IIP | None identified | Started on danazol after FLOW FISH results. |
| 44 | GATA2 haploinsuffici ency |
38 | M | Pancytopenia, invasive fungal sinusitis | • GATA2 c.1339A>C (p.Ser447Arg) | Donor selection and reduced intensity conditioning for HSCT |
| 45 | GATA2 haploinsuffici ency |
31 | F | Pancytopenia | • GATA2 c.1339A>C, (p.Ser447Arg) | Donor selection for HSCT |
| 46 | GATA2 haploinsuffici ency |
35 | M | Immunodeficiency, MAC infection, NK cell dysfunction | • GATA2 c. 1187G>A, (p.Arg396Gln) | Intensity of conditioning for HSCT |
| 47 | GATA2 haploinsuffici ency |
18 | M | Pancytopenia, HPV-driven warts | • GATA2 deletion | Intensity of conditioning for HSCT, donor selection |
| 48 | GATA2 haploinsuffici ency |
50 | F | Pancytopenia, RSV pneumonitis, aspergillus pulmonary infection | • GATA2 c. 1192C>T (p.Arg398Trp) | RIC regimen chosen for HSCT |
| 50 | DADA2 | 17 | F | Pancytopenia, cutaneous polyarteritis nodosa, livedo reticularis, nodular regenerative hyperplasia, hypogammaglobinemia | • ADA2 c.37_39 del; p.Lys13del and c. 1159C>A; p.Asn328Lys | Therapy with adalimumab, IVIG, donor selection for HSCT |
| 13 | CCUS | 61 | F | Anemia, leukopenia | • TP53 c.578A>G, p.His193Arg • SRSF2 c.284C>G, p.Pro95Arg •IDH1 c.395G>T, p.Arg132Leu |
No immunosuppression used due to evidence for clonal hematopoiesis, not consistent with aplastic anemia |
| 14 | CCUS | 45 | F | Pancytopenia | 46,XX,del(13)(q12q14)[12]/46,XX[8] [no mutations identified] |
Decision to proceed with immunosuppression after exclusion of somatic variants on NGS and presence of a minor PNH clone. |
| 15 | CCUS | 74 | M | Pancytopenia | • PHF6 c.941T>C; p.Ile314Thr • TP53 c.743G>A; p.Arg248Gln |
HMA used to abrogate cytopenias and transfusion dependency |
| 25 | ICUS | 30 | F | Leukopenia with neutropenia | None identified | G-CSF support after excluding myeloid-relevant mutations |
| 28 | CCUS | 6 | M | Severe congenital neutropenia, anemia | • SETBP1 c. 2602G>A; p.Asp868Asn | RIC allogeneic HSCT due to clonal evolution |
| 58 | Familial myeloid predisposition syndrome |
29 | F | Thrombocytopenia | • RUNX1 c.710G>A; p.Arg273Lys | Donor selection for HSCT |
| 68 | HLA DR15+ BMF syndrome |
45 | F | Pancytopenia | • BCOR c.3448dup (p.Glu1150Glyfs*18) | Start of immunosuppressive therapy |
Abbreviations: STS = short telomere syndrome; DADA2 = deficiency of adenosine deaminase-2; : CCUS = clonal cytopenias of undetermined significance; ICUS = idiopathic cytopenias of undetermined significance; HLA = human leukocyte antigen; BMF = bone marrow failure; F = female; M = male; IIP = idiopathic interstitial pneumonia; MAC = mycobacterium avium complex; NK = natural killer; HPV = human papilloma virus; RSV = respiratory syncytial virus; NGS = next generation sequencing; FLOW FISH = flow cytometry fluorescence in-situ hybridization; MDS = myelodysplastic syndromes; AML = acute myeloid leukemia; G-CSF = granulocyte-colony stimulating factor; RIC = reduced intensity conditioning; HSCT = hematopoietic stem cell transplantation; IVIG = intravenous immunoglobulin.
Change in clinical management was defined as start or change of a new therapy, impact on donor selection and/or conditioning regimen for hematopoietic stem cell transplantation as per Alder JK et al. in ref. 20.
In summary, 68 patients were evaluated in our clinic and after genomic interrogation, were classified into IBMFS, (n=24, 35%), cytopenias without a known clinical syndrome which included both CCUS and ICUS (n=30, 44%) patients, and patients that did not fit into the above two categories (n=14, 21%). Significant family history defined as per the aforementioned definition was ascertained in only 17 (25%) patients (9-IBMFS, 2-CCUS, 6-other patients), while gene variants were found in 43 (63%) patients [34 (79%) pathogenic; 12-IBMFS, 17-CCUS, 5-other]. A retrospective chart review was conducted and concluded a change in clinical management in 17 (25%) patients after a genomic assessment, as evidenced by changes in decisions with regards to therapeutic interventions (n=8, 47%), donor choice (n=6, 35%) and/or choice of conditioning regimen for hematopoietic stem cell transplantation (n=8, 47%).
Discussion:
Precision medicine is playing a major role in our understanding of various malignant and non-malignant disorders. BMFS are often caused by inherited or somatic genomic alterations, with several variants already well established as pathogenic.16,34,38-42 As reiterated in previous studies, our study confirms the clinical and genetic heterogeneity of IBMFS.17,34,38,43-45 Although germline nature is established by the presence of genetic variants in non-hematopoietic cells such as skin fibroblasts and screening of affected and unaffected family members, family history may be noticeably absent, as demonstrated in our cohort and other studies.16,46 Further, some of these conditions may have attenuated clinical presentations, well into adulthood.16,17 Hence, a high index of clinical suspicion, with employment of latest sequencing technologies would not only enable timely diagnosis, but also avoid subjecting patients to inadvertent therapies such as immunosuppression, myeloablative doses of chemotherapy, alkylating agents and ionizing radiation. Even when a definite diagnosis was not reached, based on the risk of developing a malignancy, patients received a tailored follow-up plan (e.g. CCUS). Moreover, in patients where there is a clinical need for allogeneic HSCT, screening family members for the same gene variants is important to choose appropriate donors.47-49 On the other hand, molecular characterization of marrow failure states can help direct personalized therapies. One such example is the role of NGS in acquired BMFS/AA, where the detection of clonal hematopoiesis in the form of mutations involving BCOR, BCORL1 and PIG-A predict response to IST, whereas the presence of mutations involving DNMT3A, ASXL1 and TET2, predict incomplete responses/refractoriness to IST50,51 Additional targeted/specific therapies available once an appropriate diagnosis is made include, TNA-alpha inhibitors for patients with DADA2 syndromes52, danazol for STS patients with BMF53-55, and G-CSF therapy for heterozygous variants in gene encoding for neutrophil elastase (ELANE) in patients with SCN.56 Besides therapy for cytopenias, prophylactic therapies to prevent infections, such as azithromycin to prevent mycobacterium avium complex infections in patients with GATA2 haploinsufficiency syndromes, are also supported by consensus guidelines.28
Significant challenges for this approach include difficulty in associating clinical signs and symptoms with variants (especially, if not previously described) and the possibility that not all causal genes for IBMFS have hitherto been discovered. Analyzing WES and RNAseq data from patients with a striking clinical presentation, yet negative results on targeted sequencing, is expected to uncover additional genomic associations, especially with novel approaches such as pathway analysis or studying paralog genes from other organisms.57 We are already pursuing fUrther research-based options in our clinic performing functional studies on several VUS uncovered using this approach that will help us conclude the clinical relevance of these variants. This is a work in progress that will need to also be evaluated in the future for its applicability. However, as demonstrated in our cohort, this methodology is not foolproof and fails to identify candidate genomic drivers in all patients despite a suggestive clinical phenotype. This is likely due to inherent technical limitations of WES such as failure to pick up copy number variations58, variations in promoter regions59 and abnormalities in the epigenome. Hence, in addition to sequencing, incorporation and clinical validation of novel molecular biology techniques such as reduced representative bisulfite sequencing (RRBS) to analyze methylation patterns, chromatin immunoprecipitation sequencing (ChIP-seq) to study chromatin dynamics, novel sequencing technologies for enhanced detection of deletions, duplications and subtelomeric region abnormalities is currently underway at our institution.
Conclusion:
A precision genomics approach for patients with unexplained cytopenias has the potential to alter clinical management, at least in a subset of patients. Despite limitations, these novel technologies are expected to enable personalized care and direct therapies. Future studies such as ours would enable adoption of this streamlined genomics approach for patients with unexplained cytopenias with an anticipated positive impact on clinical management.
Supplementary Material
Acknowledgement:
We acknowledge ‘The Gerstner Family Career Development Award’, Mayo Clinic Center for Individualized Medicine and CTSA Grant number KL2 TR000136 from the National Center for Advancing Translational Science for providing grant support. Text within this manuscript does not necessarily represent the official views of the U.S National Institutes of Health and authors are solely responsible for its contents.
Abbreviations:
- IBMFS
Inherited Bone Marrow Failure Syndromes
- FA
Fanconi’s Anemia
- DBA
Diamond-Blackfan Anemia
- STS
Short Telomere Syndromes
- SCN
Severe Congenital Neutropenia
- NGS
Next Generation Sequencing
- FMPD
Familial Myeloid Predisposition
- BMFS
Bone Marrow Failure Syndromes
- WES
Whole Exome Sequencing
- MDS
myelodysplastic syndromes
- HSCT
hematopoietic stem cell transplantation
- IRB
Institutional Review Board
- PNH
Paroxysmal Nocturnal Hemoglobinuria
- CIM
Center for Individualized Medicine
- BM
Bone marrow
- PB
Peripheral Blood
- MNC
Mononuclear Cell
- DNA
deoxyribonucleic acid
- RNA
ribonucleic acid
- CLIA
Clinical Laboratory Improvement Amendments
- PCR
Polymerase Chain Reaction
- VCF
Variant Call Format
- ACMG
American College of Medical Genetics and Genomics
- VUS
Variant of Uncertain Significance
- DADA2
Deficiency of Adenosine Deaminase-2
- CCUS
Clonal Cytopenia of Undetermined Significance
- ICUS
Idiopathic Cytopenias of Undetermined Significance
- AML
Acute Myeloid Leukemia
- MDS
Myelodysplastic Syndromes
- IST
Immunosuppressive Therapy
- OS
Overall Survival
- CI
Confidence Interval
- NR
Not reached
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Aronson SJ, Rehm HL. Building the foundation for genomics in precision medicine. Nature. 2015;526(7573):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roychowdhury S, Chinnaiyan AM. Translating genomics for precision cancer medicine. Annu Rev Genomics Hum Genet. 2014;15:395–415. [DOI] [PubMed] [Google Scholar]
- 3.Roden DM, Tyndale RF. Genomic medicine, precision medicine, personalized medicine: what's in a name? Clin Pharmacology Ther. 2013;94(2):169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RL, Settleman J. From cancer genomics to precision oncology--tissue's still an issue. Cell. 2014;157(7):1509–1514. [DOI] [PubMed] [Google Scholar]
- 5.Bryce AH, Egan JB, Borad MJ, et al. Experience with precision genomics and tumor board, indicates frequent target identification, but barriers to delivery. Oncotarget. 2017;8(16):27145–27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson TJ. Genome variation and personalized cancer medicine. J Internal Med. 2013;274(5):440–450. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Mischel PS, Cavenee WK. Precision cancer therapy is impacted by oncogene-dependent epigenome remodeling [published online ahead of print on March 20, 2017]. NPJ Precis Oncol. 2017;1(1):1 https://doi:10.1038/s41698-017-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meric-Bernstam F, Brusco L, Shaw K, et al. Feasibility of Large-Scale Genomic Testing to Facilitate Enrollment Onto Genomically Matched Clinical Trials. J Clin Oncol. 2015;33(25):2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordero P, Ashley EA. Whole-genome sequencing in personalized therapeutics. Clin Pharmacology Ther. 2012;91(6):1001–1009. [DOI] [PubMed] [Google Scholar]
- 10.Le Tourneau C, Delord JP, Goncalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015; 16(13): 1324–1334. [DOI] [PubMed] [Google Scholar]
- 11.Collins FS, Varmus H. A New Initiative on Precision Medicine. New Engl J Med. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steensma DP. Clinical Implications of Clonal Hematopoiesis. Mayo Clin Proc. 2018;93(8): 1122–1130. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluteau O, Sebert M, Leblanc T, et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood. 2018;131(7):717–732. [DOI] [PubMed] [Google Scholar]
- 17.West AH, Churpek JE. Old and new tools in the clinical diagnosis of inherited bone marrow failure syndromes. Hematology Am Soc Hematol Educ Program. 2017;2017(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muramatsu H, Okuno Y, Yoshida K, et al. Clinical utility of next-generation sequencing for inherited bone marrow failure syndromes. Genet Med. 2017;19(7):796–802. [DOI] [PubMed] [Google Scholar]
- 19.Ghemlas I, Li H, Zlateska B, et al. Improving diagnostic precision, care and syndrome definitions using comprehensive next-generation sequencing for the inherited bone marrow failure syndromes. J Med Genet. 2015;52(9):575–584. [DOI] [PubMed] [Google Scholar]
- 20.Alder JK, Hanumanthu VS, Strong MA, et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc Natl Acad Sci U S A. 2018;115(10):E2358–e2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wlodarski MW, Hirabayashi S, Pastor V, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127(11):1387–1397; quiz 1518. [DOI] [PubMed] [Google Scholar]
- 22.Dhanraj S, Matveev A, Li H, et al. Biallelic mutations in DNAJC21 cause Shwachman-Diamond syndrome. Blood. 2017;129(11):1557–1562. [DOI] [PubMed] [Google Scholar]
- 23.Mirabello L, Macari ER, Jessop L, et al. Whole-exome sequencing and functional studies identify RPS29 as a novel gene mutated in multicase Diamond-Blackfan anemia families. Blood. 2014;124(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albers CA, Paul DS, Schulze H, et al. Compound inheritance of a low-frequency regulatory SNP and a rare null mutation in exon-junction complex subunit RBM8A causes TAR syndrome. Nature genetics. 2012;44(4):435–439, s431-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazaridis KN, Schahl KA, Cousin MA, et al. Outcome of Whole Exome Sequencing for Diagnostic Odyssey Cases of an Individualized Medicine Clinic: The Mayo Clinic Experience. Mayo Clin Proc. 2016;91(3):297–307. [DOI] [PubMed] [Google Scholar]
- 26.Soulier J, Leblanc T, Larghero J, et al. Detection of somatic mosaicism and classification of Fanconi anemia patients by analysis of the FA/BRCA pathway. Blood. 2005;105(3): 1329–1336. [DOI] [PubMed] [Google Scholar]
- 27.Wilson DB, Link DC, Mason PJ, Bessler M. Inherited bone marrow failure syndromes in adolescents and young adults. Ann Med. 2014;46(6):353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquet M, Bellanne-Chantelot C, Tavitian S, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121(5):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2017: 106–108. [Google Scholar]
- 31.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 32.Churpek JE, Lorenz R, Nedumgottil S, et al. Proposal for the clinical detection and management of patients and their family members with familial myelodysplastic syndrome/acute leukemia predisposition syndromes. Leuk Lymphoma. 2013;54(1):28–35. [DOI] [PubMed] [Google Scholar]
- 33.How I diagnose and manage individuals at risk for inherited myeloid malignancies. Blood. 2016; 128(14): 1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangaonkar AA, Ferrer A, Pinto EVF, et al. Clinical Correlates and Treatment Outcomes for Patients With Short Telomere Syndromes. Mayo Clin Proc. 2018;93(7):834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez Santiago TM, Zavialov A, Saarela J, et al. Dermatologic Features of ADA2 Deficiency in Cutaneous Polyarteritis Nodosa. JAMA Dermatol. 2015;151(11):1230–1234. [DOI] [PubMed] [Google Scholar]
- 36.Perez Botero J, Chen D, Cousin MA, et al. Clinical characteristics and platelet phenotype in a family with RUNX1 mutated thrombocytopenia. Leuk Lymphoma. 2017;58(8):1963–1967. [DOI] [PubMed] [Google Scholar]
- 37.Perez Botero J, Chen D, He R, et al. Clinical and laboratory characteristics in congenital ANKRD26 mutation-associated thrombocytopenia: A detailed phenotypic study of a family. Platelets. 2016;27(7):712–715. [DOI] [PubMed] [Google Scholar]
- 38.Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124(18):2775–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonassaint NL, Guo N, Califano JA, Montgomery EA, Armanios M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell. 2013;12(2):319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117(21):5607–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangaonkar AA, Patnaik MM. Short Telomere Syndromes in Clinical Practice: Bridging Bench and Bedside. Mayo Clin Proc. 2018;93(7):904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu AP, Johnson KD, Falcone EL, et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013; 121(19):3830–3837, s3831-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dokal I, Vulliamy T. Inherited bone marrow failure syndromes. Haematologica. 2010;95(8):1236–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armanios M, Chen JL, Chang YP, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102(44):15960–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drazer MW, Kadri S, Sukhanova M, et al. Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Adv. 2018;2(2):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alter BP. Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Hematology Am Soc Hematol Educ Program. 2017;2017(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietz AC, Mehta PA, Vlachos A, et al. Current Knowledge and Priorities for Future Research in Late Effects after Hematopoietic Cell Transplantation for Inherited Bone Marrow Failure Syndromes: Consensus Statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietz AC, Orchard PJ, Baker KS, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone marrow Transplant. 2011;46(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood. 2014;124(17):2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogawa S Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128(3):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michniacki TF, Hannibal M, Ross CW, et al. Hematologic Manifestations of Deficiency of Adenosine Deaminase 2 (DADA2) and Response to Tumor Necrosis Factor Inhibition in DADA2-Associated Bone Marrow Failure. J Clin Immunol. 2018;38(2):166–173. [DOI] [PubMed] [Google Scholar]
- 53.Townsley DM, Dumitriu B, Liu D, et al. Danazol Treatment for Telomere Diseases. N Engl J Med. 2016;374(20): 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khincha PP, Bertuch AA, Gadalla SM, Giri N, Alter BP, Savage SA. Similar telomere attrition rates in androgen-treated and untreated patients with dyskeratosis congenita. Blood Adv. 2018;2(11):1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khincha PP, Wentzensen IM, Giri N, Alter BP, Savage SA. Response to androgen therapy in patients with dyskeratosis congenita. Br J Haematol. 2014;165(3):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donini M, Fontana S, Savoldi G, et al. G-CSF treatment of severe congenital neutropenia reverses neutropenia but does not correct the underlying functional deficiency of the neutrophil in defending against microorganisms. Blood. 2007;109(11):4716–4723. [DOI] [PubMed] [Google Scholar]
- 57.Kupiec M Biology of telomeres: lessons from budding yeast. FEMS Microbiology Rev. 2014;38(2):144–171. [DOI] [PubMed] [Google Scholar]
- 58.Waespe N, Dhanraj S, Wahala M, et al. The clinical impact of copy number variants in inherited bone marrow failure syndromes [published online ahead of print May 10, 2017]. NPJ Genomic Med. 2017;2 http://doi:10.1038/s41525-017-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science. 2015;349(6255):1483–1489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.