Abstract
Purpose
Fatigue, decreased functionality, and impaired quality of life are some of the most common adverse outcomes of chemo-radiotherapy (CRT). Head and neck cancers (HNC) affect more than half a million individuals globally and its treatment takes a heavy toll on the patient, often affecting their speech, swallowing, and respiratory functions, and as a result they often develop fatigue, depression, and physical inactivity. The purpose of this study was to evaluate the effectiveness of exercise-based rehabilitation on functional capacity, quality of life, fatigue, hemoglobin, and platelet counts in patients with HNC on CRT.
Patients and methods
A randomized controlled trial was conducted on 148 patients with head and neck cancer undergoing CRT to evaluate the effectiveness of exercise on functional capacity measured by the 6-min walk test, quality of life measured by the Medical Outcomes Survey Short Form 36 v2 questionnaire, fatigue by the NCCN (0–10) scale, hemoglobin, and platelets. The control group received standard physical activity recommendations while the exercise group received a structured exercise program of aerobic and active resistance exercises for a period of 11 weeks.
Results
There was a significant improvement in the functional capacity (p < 0.001), quality of life (p < 0.001), and prevention of worsening of fatigue (p < 0.001) in the exercise group. The blood parameters did not show a significant difference between the control group and the exercise group.
Conclusion
Our results elucidate that an 11-week structured exercise program for HNC patients receiving CRT helps in improving their functional capacity and quality of life. It also prevents deterioration of fatigue levels in the exercise group.
Keywords: Exercise, Cancer rehabilitation, Head and neck cancer, Chemo-radiotherapy, Functional capacity, Quality of life
Introduction
Head and neck cancers (HNC) are prevalent in regions of the world with the extensive use of tobacco and alcohol. HNC was ranked as the eighth leading cause of cancer deaths world-wide with a global incidence of more than half a million per year. In India, HNC comprises one-third of the cancer burden [1]. Treatments include surgery, radiotherapy, chemotherapy, or a chemo-radiotherapy (CRT) regimen. The treatment regimen is decided based on the site and stage of cancer. Stage 3, stage 4a, and stage 4b cancer of the laryngeal and oropharyngeal region at our institution are treated with radical CRT with a dose of 70 Gy of radiation given over 35 fractions and cisplatin 45 mg/m2 of body surface area as the chemotherapeutic drug of choice. Speech, swallowing, and respiratory functions are usually adversely affected in HNC patients undergoing radical CRT [2]. Patients with HNC often depend on soft or liquid diet and in some cases even require gastrostomy tube feeding as they develop oral mucositis. Radiation-induced mucositis is a major cause of pain and decreased QoL in patients with HNC [3]. Adverse effects of chemo-radiotherapy for cancer often lead to impairments in functional capacity and altered quality of life (QoL) [4]. With the advent of more dose-intense and multimodal therapies, these adverse effects have become even more intense and distressing [5].
Fatigue and depression have also been found to lead to physical inactivity in HNC patients which can also impair their QoL and functional capacity.
Exercise has been found to improve functional capacity and QoL of patients undergoing chemotherapy, radiotherapy, or combination therapy [6]. There is a growing body of evidence that suggests that regular bouts of physical activity (3–5 h of moderate-intensity walking per week) lead to a 30–50% reduction in the risk of cancer-specific mortality and an all-cause mortality compared with patients who were physically inactive [7]. However, there is a paucity of studies on the role of structured exercise-based cancer rehabilitation program in improving functional capacity and QoL in patients with HNC. There is a dearth of literature regarding the effects of exercise training in patients with HNC receiving concurrent CRT as many such studies mainly focus on breast cancer cases [8]. Only two studies have explored the effect of an exercise program in patients with HNC during radiotherapy (RT) [9, 10], and one during concurrent CRT [11]. Increase in survival rates owing to improvement in cancer care also authenticates the need for rehabilitation programs for this patient population [12].
The primary aim of our study was to evaluate the effectiveness of exercise-based rehabilitation on functional capacity and quality of life among patients with HNC receiving CRT The secondary objective was to study the effect of exercise on fatigue and blood parameters viz., hemoglobin and platelets.
Methodology
Participants
This randomized controlled trial was conducted on 148 patients (131 males and 17 females with mean age of 52.81 ± 10.48 in control group and 52.76 ± 9.65 in exercise group) receiving radical CRT for HNC being treated conservatively at Shirdi Sai Baba Cancer Hospital and Research Centre at Manipal with an Eastern Cooperative Oncology Group (ECOG) Score < 2 included in the study.
Patients whose platelet count was less than 30,000/ul or Hb was less than 8 mg/dl or having severe orthopedic and neurological problems or having any contraindication to exercise testing and training were excluded from the study.
Randomization
Based on a pilot study using mean change in 6-min walk distance, it was estimated that a sample size of 55 was necessary for each intervention group [11]. Considering a dropout rate of 30%, the total sample size required in each group was 74 participants. Block randomization was done with 15 blocks of 10 subjects each. A computerized sequence generation was done for the same and the allocation was concealed.
Intervention
The patients were randomized into control group and exercise group using block randomization. The trial was initiated after approval from the Institutional Ethics Committee with registration number ECR/146/Inst/KA/2013 and written informed consent was acquired from each patient.
Both the control and the exercise group received standard hospital care which included 70 Gy of radiation over 35 fractions and weekly chemotherapy with a cisplatin dose of 45 mg/m2 of body surface area over a period of 7 weeks. The standard hospital care also included low-level laser therapy for the prevention and treatment of oral mucositis.
Control group—The control group was given physical activity recommendations of three 10-min walks during the day for 5 days a week [14]. They were encouraged to remain as active as possible and to follow the standard hospital care.
Exercise group—The exercise group received the intervention in the format described below along with the standard hospital care during the course of CRT for a period of 7 weeks followed by a 4-week home-based exercise program. The exercise adherence was monitored using an exercise log book and by making weekly phone calls to the patient while they were undergoing the home-based exercise program.
The intervention for the exercise group was carried out 5 days/week (except on day of chemotherapy) at an intensity of 3–5/10 RPE (rating of perceived exertion) on modified Borg’s scale. The type of exercises include aerobic (brisk walking, 15–20 min) and active resistance exercise program for the major muscles of upper limb and lower limb done in two sets (1 set = 8 to 15 repetitions) of biceps curl, triceps extension, overhead shoulder flexion, hip flexion, quadriceps (knee extension), and hip abduction.
All patients were monitored daily for any complication during the course of rehabilitation. Total duration of study was 11 weeks, with 7 weeks of exercise training in the hospital during the course of CRT and 4 weeks of home-based exercise program. The adherence was monitored using an exercise log book and by making weekly phone calls when the patient was at home after discharge from the hospital.
Outcome measures
This trial on patients with HNC undergoing CRT was conducted to evaluate the effectiveness of a structured exercise program in comparison with standard physical activity recommendations, on functional capacity, QoL, fatigue, and the blood parameters—hemoglobin and platelets.
Given below are the outcome measures used in this study. These were measured at baseline, 3rd week, and 7th week of CRT. The exercise regimen was supervised at hospital as patients were admitted during course of CRT. At the end of 7 weeks of CRT, patients in the exercise group continued the regimen at home. All outcome measures except blood parameters were measured at the 11th week (i.e., 4 weeks post CRT) during follow-up visit:
- Primary outcome measures
-
•Quality of life was measured using the Medical Outcomes Survey Short Form 36 questionnaire (SF 36) v2 version
-
•Functional capacity was measured using the 6-min walk test
-
•
- Secondary outcome measures
-
•Cancer-related fatigue was measured using the 10 point NCCN Scale [13]0—No fatigue (none)1–3—Mild fatigue4–6—Moderate fatigue7–10—Severe fatigue
-
•Blood parameters evaluated during the course of CRT were hemoglobin and platelet counts.
-
•
Data analysis
Mean and standard deviation (SD) was used to summarize continuous variables. Log transformation was used for transforming skewed continuous variables. Geometric mean and standard deviation was used as a measure of summary and dispersion for log transformed variables. Frequency and percentage was used to summarize categorical variables. Repeated measures ANOVA was used for data analysis of all the outcome measures. Repeated measures ANOVA for platelets and fatigue scores was done after log transformation of the data. SPSS 15 was used for data analysis.
Results
A total of 148 eligible patients were randomized to either control or exercise group. At the end of 11 weeks, there were 62 patients in the control group and 58 in the exercise group. Twelve participants dropped out of the control group and 16 dropped out of the exercise group for various reasons such as depression, severe pneumonia, fatigue, loss of interest, and loss to follow-up. Two participants from the control group died within 3 weeks of the commencement of the study. The CONSORT [15] flow chart in Fig. 1 explains the flow of the participants enrolled in this study from baseline until the end of 11 weeks.
Fig. 1.
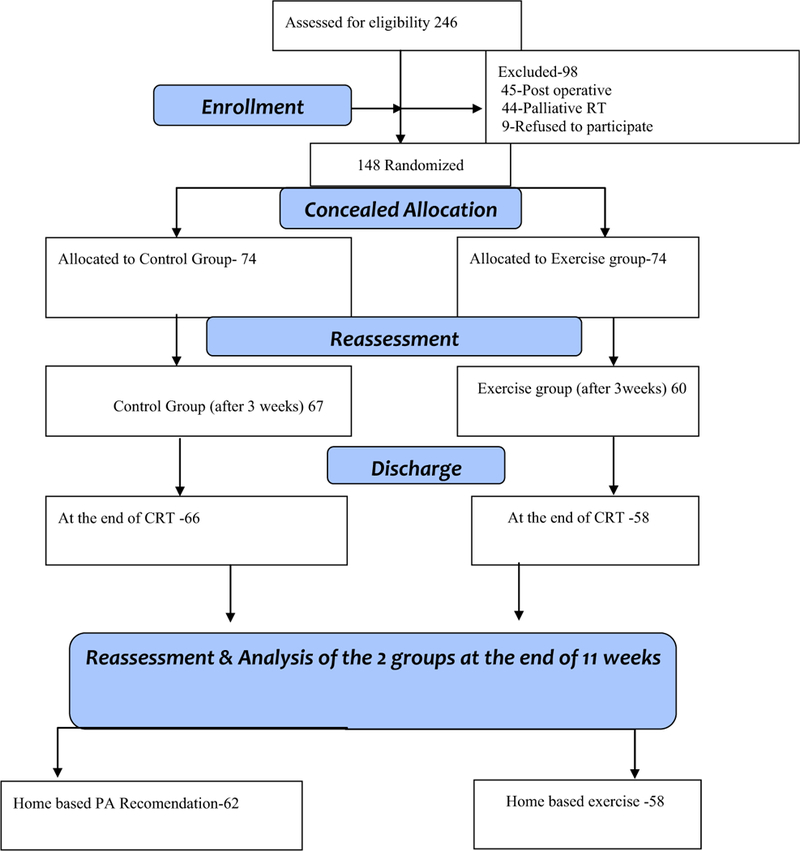
CONSORT flow chart
Results
Demographics
The baseline characteristics of the subjects enrolled in this trial are given in Table 1 and they were homogenous at baseline.
Table 1.
Baseline data of patients
| Control | Exercise | |
|---|---|---|
| Age (mean ± SD, years) | 52.81 ± 10.48 | 52.76 ± 9.65 |
| Sex | Males 63 (85%) | Males 68 (92%) |
| Females 11 (15%) | Females 6 (8%) | |
| BMI (mean ± SD, kg/m2) | 20.58 ± 3.89 | 21.29 ± 3.33 |
| Cancer site | ||
| Oropharyngeal carcinoma | n = 63 | n = 57 |
| Laryngeal carcinoma | n =11 | n = 17 |
| Cancer stage | ||
| Stage 3 | n = 18 | n = 20 |
| Stage 4a | n = 49 | n = 45 |
| Stage 4b | n = 7 | n = 9 |
| ECOG | 0.47 ± 0.05 | 0.81 ± 1.73 |
| Hb (gm/dl) | 12.80 ± 1.81 | 12.85 ± 1.81 |
| Platelets/ul | 2,87,793.93 ± 1.52 | 2,73,758.05 ± 1.49 |
| 6MWD (meters) | 447.32 ± 59.22 | 446.31 ± 62.87 |
| SF-36 (PCS) | 43.51 ± 7.10 | 43.96 ± 7.21 |
| SF-36 (MCS) | 42.63 ± 7.47 | 39.58 ± 9.85 |
| Fatigue scores (0–10) | 2.91 ± 1.84 | 3.70 ± 1.75 |
Functional capacity
The functional capacity of our patients was evaluated by using the 6-min walk test which is a standardized test for the evaluation of the functional capacity in patients with cancer.
Table 2 highlights the 6-min walk distance (6MWD) values of the control and the exercise groups during the course of CRT and after discharge. Both groups were homogenous at baseline in terms of their functional capacity which was evaluated using the 6-min walk test. At the end of the 11-week program, the control group showed a decrease of 73 m whereas the exercise group showed an improvement of 37 m. These changes were statistically significant between the groups. Hence, the results reveal that an 11-week exercise program had a significant effect F(3,345) = 23.67, p = < 0.001 on the functional capacity of head and neck cancer patients receiving CRT.
Table 2.
Six-minute walk distance (meters)—between group analysis
| Baseline | 3 weeks | 7 weeks | 11 weeks | F(df1,df2) | p value | |
|---|---|---|---|---|---|---|
| Control group mean ± SD | 447.32 ± 59.22 | 380.74 ± 105.26 | 354.90 ± 115.66 | 374.52 ± 110.26 | F(3,345) = 23.67 | <0.001 |
| Exercise group mean ± SD | 446.31 ± 62.87 | 437.18 ± 69.75 | 441.72 ± 90.92 | 483.16 ± 88.24 | ||
Figure 2 indicates the pattern of change in the 6MWD of the control and the exercise group patients during the course of CRT and in the span of 4 weeks after CRT. The first time point indicates the measurement of 6MWD at baseline followed by its measures at the 3rd week and 7th week during CRT respectively. The last time point is the 11th week which is 4 weeks after CRT.
Fig. 2.
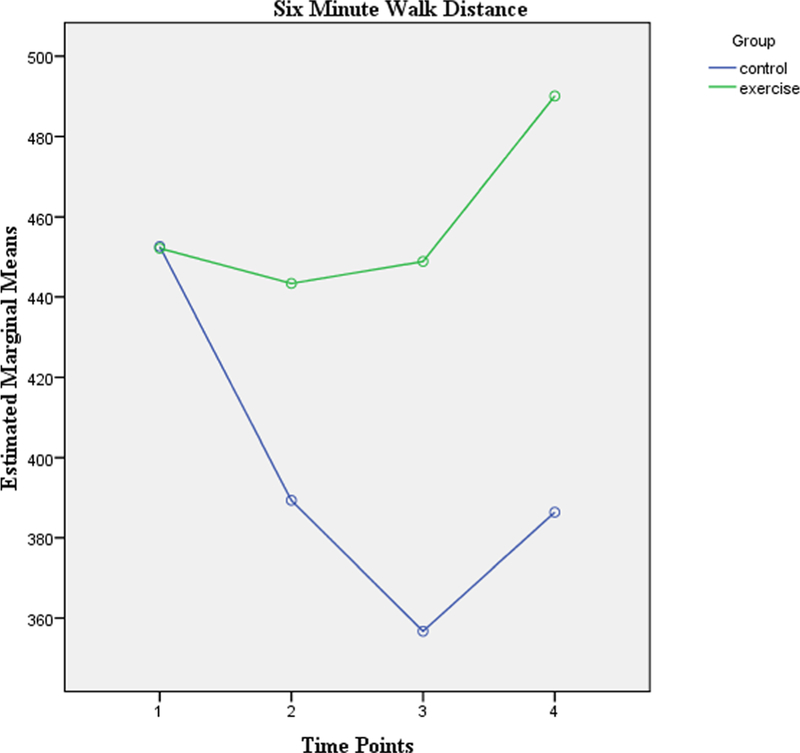
Six-minute walk distance at baseline and end of 3rd, 7th, and 11th week
Quality of life
Quality of life was evaluated using the SF 36 v2 questionnaire which has the Physical Component Score (PCS) and Mental Component Score (MCS). Table 3 depicts the results of the PCS and MCS in both groups in addition to the fatigue scores.
Table 3.
Quality of life and fatigue scores pre-, post, and during CRT
| SF36-PCS | Baseline | 3 weeks | 7 weeks | 11 weeks | F(df1,df2) | p value |
| Control group mean ± SD | 43.51 ± 7.10 | 40.2658 ± 4.74 | 37.57 ± 6.56 | 39.10 ± 4.95 | (3,345) = 21.39 | <0.001 |
| Exercise group mean ± SD | 43.96 ± 7.21 | 42.21 ± 6.36 | 44.7391 ± 7.15 | 48.58 ± 6.63 | ||
| SF36-MCS | ||||||
| Control group | 42.639 ± 7.47 | 37.5385 ± 6.64 | 35.10 ± 7.02 | 36.34 ± 5.20 | F(3,345) = 15.25 | <0.001 |
| Exercise group mean ± SD | 39.58 ± 9.85 | 41.26 ± 8.41 | 40.78 ± 7.79 | 43.99 ± 6.39 | ||
| Fatigue scores | ||||||
| Control group mean ± SD | 2.91 ± 1.84 mild | 4.95 ± 1.33 moderate | 5.41 ± 1.40 moderate | 4.48 ± 1.59 moderate | (3,162) = 11.31 | <0.001 |
| Exercise group mean ± SD | 3.70 ± 1.75 mild | 3.81 ± 1.58 mild | 3.59 ± 1.78 mild | 2.45 ± 1.97 mild | ||
At baseline, both groups were comparable in terms of their Physical Component Scores. The PCS decreased by 10% at the end of 11 weeks in the control group while it increased by 10.5% in the exercise group. All these changes were significant F(3,345) = 21.39, p < 0.001 and show that exercise played an important role in improving the physical component of quality of life in HNC patients receiving CRT.
The pattern of change in the SF 36 PCS of the control and the exercise group patients during the course of CRT and in the span of 4 weeks after CRT helps to infer that exercise helps in improving the physical component of QoL during the course of CRT.
Table 3 also shows the MCS in both groups. The MCS increased by 11.14% following the exercise training program, while in the control group, there was a 14.75% decrease. The changes in mean difference from baseline to the 11th week were statistically significant F(3,345)= 15.25, p < 0.001 These changes between the groups show the beneficial effect of exercise on the mental component of QoL of patients with HNC receiving CRT.
The pattern of change in the SF 36 MCS shows that during the period of CRT (i.e., time points between baseline and 7 weeks), the control group values steeply decline while the exercise group values are significantly better. The last time point is the 11th week which is 4 weeks after CRT. There is significant difference in the mental and physical components of QoL between groups.
Fatigue
Fatigue was evaluated using the NCCN (0–10) scale which has the none, mild, moderate, and severe category of fatigue. Table 3 demonstrates the fatigue scores in the control and exercise groups.
The control group had mild fatigue (2.91 ± 1.84) at baseline which increased to moderate fatigue (4.48 ± 1.59) at the end of 11 weeks with an increase of 53.95%. The exercise group maintained the baseline category of mild fatigue (3.70 ± 1.75) during the course of 11 weeks with a decrease of 33.78% seen in the fatigue values (2.45 ± 1.97) at the end of 11 weeks.
Hemoglobin and platelets
The hemoglobin values of both the exercise and control groups showed a decrease of 14% during the course of CRT The difference of change in means from baseline to the end of 11 weeks was not significant F(2,254) = 0.4, p = 0.61 between the groups.
The platelet values in both groups during the course of CRT showed a decrease of 8.68% in the control group and 12.19% in the exercise group in the platelet count and the changes in mean difference from baseline to the 11th week were not statistically significant F(2,252) = 0.52, p = 0.57.
Figure 3 shows the trend of platelet counts during the course of CRT for exercise and control groups. The first time point indicates the measurement of 6MWD at baseline followed by its measures at the 3rd week and 7th week during CRT respectively.
Fig. 3.
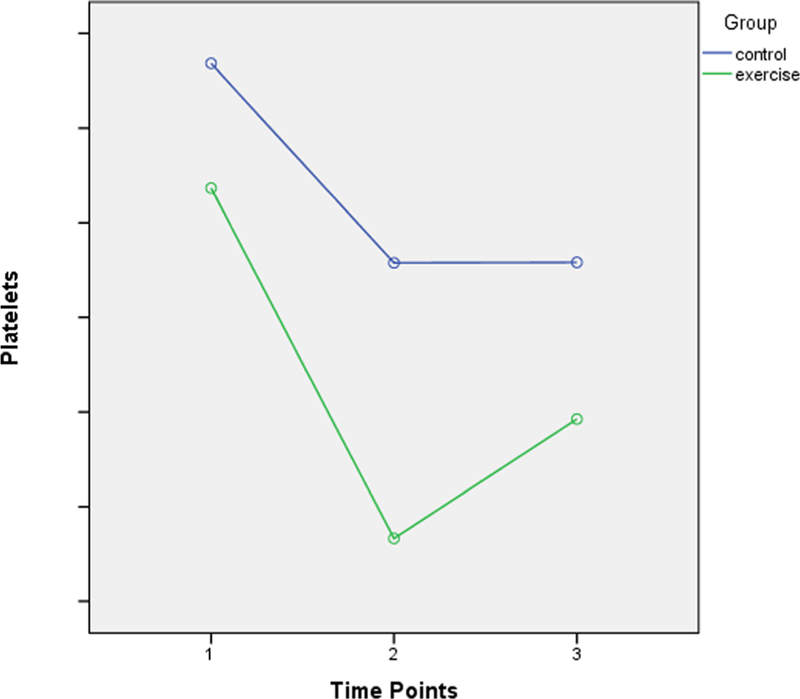
Trend of platelet counts during the course of CRT
Discussion
This trial studied the effectiveness of an 11-week exercise training program in patients with HNC receiving radical CRT. The exercise program used in this study was feasible with an average adherence of 75%. There is a plethora of evidence to advocate for the positive impact of exercise training on functional capacity in patients suffering from cancer of the breast, colon, and prostate [8]. This study aimed to evaluate the effect of exercise training on functional capacity in patients with HNC on active CRT. This study confirms the preliminary evidence obtained from our pilot study that a walking and active exercise program is feasible and well tolerated by patients with HNC undergoing CRT [11]. The exercise intensity for our study was set using rating of perceived exertion (RPE) which can be a simple means of the patients continuing the exercise at home after the end of CRT. This facilitated the patients continuing the exercise program at home for 4 weeks, which resulted in an improvement in the functional capacity and quality of life while preventing worsening of fatigue in the exercise group. Rogers et al. reported that HNC is an understudied cancer survivor group in regard to exercise training and its effect on exercise capacity [10]. This underlines the importance of this study in the field of cancer rehabilitation. Although understudied, there is emerging evidence on the benefits of exercise and physical activity in patients with HNC. This emerging evidence warranted the need to not deprive the control group of an exercise program and this led to us giving them physical activity recommendations during the course of CRT. The end result of this trial on 148 patients has now set the stage for exercise to become a routine part of patients with HNC treated with radical CRT. No adverse events were noticed during the exercise training or during the 6-min walk test. Research shows that over 60% of HNC survivors are likely to be interested in exercise interventions [16].
This study is the largest RCT till date to evaluate the effects of exercise training on patients with HNC patients receiving CRT. A feasibility study done in 2014 reported that traveling back and forth to receive treatment is a barrier to exercising. In our study, the setting enables to circumvent the problem of traveling schedules as the patient is admitted for CRT or at home after the CRT during the period of exercise intervention [17].
The exercise program administered by us was found to be safe and feasible for clinical and research work in this population. This study determines that exercise can be incorporated as a routine part of patient care during the course of CRT in patients with HNC. The results of our study are concurrent with another pilot study which concluded that a 14-week exercise intervention in participants with HNC was feasible and it helped to maintain or improve function or QOL [18]. Some of the unique contributions of our study is the fact that we have been able to elucidate that our supervised exercise regimen significantly improves the functional capacity and quality of life and prevents further fatigue-related decline in stage 3, stage 4a, and stage 4b HNC patients on CRT when compared to the current practice of recommending standard exercise without supervision and follow-up. We further discuss in detail the improvement or maintenance of outcome measures in the exercise group in contrast with the significant decline of the control group in outcome measure values specifically of the functional capacity, quality of life, and fatigue at various time points of this 11-week trial which helps to describe the variation at early-stage and later-stage CRT. A notable limitation of our study was that we could not have a blinded assessor due to the lack of funding. Another limitation was that we could only analyze the blood parameters until the end of CRT and not until the end of 11 weeks due to lack of funding. In general, we would recommend that exercise training is effective in maintaining and improving functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. For further exploration and study, we recommend comparing aerobic versus resistance training to find out the most suitable mode and intensity of exercise for this population and also funded studies that study the impact of exercise on inflammatory markers could be carried out in head and neck cancer patients receiving chemo-radiotherapy.
In conclusion, our findings indicate that the 5 days/week aerobic and active resistance exercise program for the major muscles of upper limb and lower limb are both safe and feasible during chemo-radiotherapy for head and neck cancer. The exercise-based rehabilitation intervention significantly improved the functional capacity and quality of life and prevented deterioration of fatigue levels. In general, we would recommend that patients who undergo CRT for HNC should be prepared and trained in the exercise intervention as it improves patient outcomes and aids in rehabilitation across the spectrum without any notable adverse effects.
Acknowledgements
Dr. Samuel was supported by a junior research fellowship by Department of Science and Technology, Government of India (Ref No: IDP/MED/2011/20) for 2 years & a PhD Scholarship from Manipal Academy of Higher Education for 1 year.
NCI UG1CA189961, NCI R01CA181064 are the current grants supporting Drs. Mustian and Lin.
Footnotes
Compliance with ethical standards
The trial was initiated after approval from the Institutional Ethics Committee with registration number ECR/146/Inst/KA/2013 and written informed consent was acquired from each patient.
Disclaimers This research has been presented in part at the MAASC Annual meeting 2018 at Austria, Vienna. It has not been submitted for publication nor has it been published in whole or in part elsewhere.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Kulkarni MR (2013) Head and neck cancer burden in India. Int J Head Neck Surg 4:29–35 [Google Scholar]
- 2.John S, Hassuji M, Rajashekhar B (2011) Speech and swallowing outcomes in buccal mucosa carcinoma. Indian J Palliat Care 17(3): 238–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Nigudgi S (2013) Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemo-radiotherapy–a randomized controlled trial. Support Care Cancer 21 (5):1421–1428. 10.1007/s00520-012-1684-4 [DOI] [PubMed] [Google Scholar]
- 4.Dimeo FC (2001) Effects of exercise on cancer-related fatigue. Cancer 92:1689–1693 [DOI] [PubMed] [Google Scholar]
- 5.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S (2003) Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer 98:1786–1801 [DOI] [PubMed] [Google Scholar]
- 6.Holmes MD, Chen WY, Feskanich D et al. (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293(20):2479–2486. 10.1001/jama293.20.2479 [DOI] [PubMed] [Google Scholar]
- 7.Van Blarigan EL, Meyerhardt JA (2015) Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol 33(16): 1825–1834. 10.1200/JCO.2014.59.7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson D, Diorio C, Beyene J, Sung L (2014) Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil 93(8):675–686. 10.1097/PHM.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 9.Aghili M, Farhan F, Rade M (2007) A pilot study of the effects of programmed aerobic exercise on the severity of fatigue in cancer patients during external radiotherapy. Eur J Oncol Nurs 11:179–182 [DOI] [PubMed] [Google Scholar]
- 10.Rogers LQ, Anton PM, Fogleman A, Hopkins-Price P, Verhulst S, Rao K, Malone J, Robbs R, Courneya KS, Nanavati P, Mansfield S, Robbins KT (2013) Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck 35(8):1178–1188. 10.1002/hed.23118 [DOI] [PubMed] [Google Scholar]
- 11.Samuel SR, Maiya GA, Babu AS et al. (2013) Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res 137:515–520 [PMC free article] [PubMed] [Google Scholar]
- 12.Pulte D, Brenner H (2010) Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 15(9):994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger AM, Abemethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cella D, Cimprich B, Cleeland C, Eisenberger MA, Escalante CP, Jacobsen PB, Kaldor P, Ligibel JA, Murphy BA, O’Connor T, Pirl WF, Rodler E, Rugo HS, Thomas J, Wagner LI (2010) Cancer-related fatigue. JNCCN J Natl Compr Cancer Netw 8(8):904–931. 10.6004/jnccn.2010.006712 [DOI] [PubMed] [Google Scholar]
- 14. Jones LW, Demark-Wahnefried W (2006) Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol 7:1017–1026. 10.1016/S1470-2045(06)70976-7 [DOI] [PubMed] [Google Scholar]
- 15.Campbell MK, Piaggio G, Elbourne DR, Altman DG (2012)Consort 2010 statement: extension to cluster randomised trials. Bmj 345:5661–5661. 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 16.Midgley AW, Lowe D, Levy AR, Mepani V, Rogers SN (2017) Exercise program design considerations for head and neck cancer survivors. Eur Arch Otorhinolaryngol 275(1): 169–179 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5754417/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cnossen IC, van Uden-Kraan CF, Rinkel RN,et al. (2014). Multimodal guided self-help exercise program to prevent speech, swallowing, and shoulder problems among head and neck cancer patients: a feasibility study. Med Internet Res 6;16(3):e74 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3961811/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao SG, Alexander NB, Jolly S et al. (2015) Maintaining physical activity during head and neck cancer treatment: results of a pilot controlled trial. Head Neck 38:1086–1096 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5304917/ [DOI] [PMC free article] [PubMed] [Google Scholar]


