Abstract
To expand the breadth and extent of current multiple myeloma (MM)-specific immunotherapy, we have identified various antigens on CD138+ tumor cells from newly diagnosed MM patients (n=616) and confirmed B-cell Maturation Antigen (BCMA) as a key myeloma-associated antigen. The aim of this study is to target the BCMA, which promotes MM cell growth and survival, by generating BCMA-specific memory CD8+ CTL that mediate effective and long-lasting immunity against MM. Here we report the identification of novel engineered peptides specific to BCMA, BCMA72–80 (YLMFLLRKI) and BCMA54–62 (YILWTCLGL), which display improved affinity/stability to HLA-A2 compared to their native peptides and induce highly functional BCMA-specific CTL with increased activation (CD38, CD69) and co-stimulatory (CD40L, OX40, GITR) molecule expression. Importantly, the heteroclitic BCMA72–80 specific CTL demonstrated poly-functional Th1-specific immune activities [IFN-γ/IL-2/TNF-α production, proliferation, cytotoxicity] against MM, which were correlated with expansion of Tetramer+ and memory CD8+ CTL. Additionally, heteroclitic BCMA72–80 specific CTL treated with anti-OX40 (immune agonist) or anti-LAG-3 (checkpoint inhibitor) display increased immune function, mainly by central memory CTL. These results provide the framework for clinical application of heteroclitic BCMA72–80 peptide, alone and in combination with anti-LAG3 and/or anti-OX40 therapy, in vaccination and/or adoptive immunotherapeutic strategies to generate long-lasting anti-tumor immunity in patients with MM or other BCMA expressing tumors.
INTRODUCTION
Despite recent advances in treatment of multiple myeloma (MM) including incorporation of novel therapies into the stem cell transplantation paradigm, ongoing DNA damage and genomic evolution underlie relapse in many patients.1,2 Novel therapeutic approaches with distinct mechanisms of action are therefore needed. The constitutive evolving genetic complexity, coupled with immune responsiveness of B cell malignancies, has stimulated the development of immunotherapeutic options in MM including monoclonal antibodies, bispecific antibodies, immunotoxins, and chimeric antigen receptor T cell (CAR-T).3,4 Although MM patient-specific CAR-T therapy has achieved remarkable deep responses, durability of responses is not establishes and they are labor-intensive, time-consuming, and expensive.5–8 To overcome these limitations, we have developed immunogenic peptides-based cancer vaccines as an off-the-shelf immunotherapy for treating patients more widely and efficiently.9,10 Our peptide-based therapeutic approach does not have limitations of recombinant proteins, mRNA, or DNA-based vaccines, which require the processes of internalization, degradation of protein into optimal immunogenic peptides to HLA, along with additional steps required for suitable translation (for mRNA) or transcription (for DNA). To overcome MHC restriction and treat a more diverse patient population using our well-defined immunogenic peptide vaccine approach, we have pooled peptide cocktails to include major HLA subtypes.11–13 Moreover, we have already shown that lenalidomide can augment peptide vaccine specific immune responses and memory cytotoxic T cell (CTL) activities, setting the stage for combination approaches with checkpoint inhibitors and/or immune agonists. In addition, anti-tumor efficacy triggered by immunogenic peptides can be enhanced by their ability to induce “epitope spreading” upon the generation of effector cells, whereby targeted lysed cancer cells release new antigenic epitopes which are subsequently taken up, processed, and presented by antigen-presenting cells to a new repertoire of CTLs.14,15
B cell maturation antigen (BCMA) is a member of the TNF receptor superfamily 17 (TNFRSF17) and is characterized as a type III trans-membrane protein containing cysteine-rich extracellular domains with a central role in regulating B-cell maturation and differentiation into plasma cells.16–18 As a receptor for the MM cell growth and survival factors, B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), BCMA is required for the survival of MM cells, making it a promising therapeutic target.19,20 Nearly all MM tumor cells express BCMA, and it has been proposed as a marker for identification of tumor cells.21–26 Its selective expression on a subset of mature B and long lived plasma cells further suggest a favorable therapeutic index for BCMA directed treatment approaches. At present BCMA is being targeted by several immunotherapeutic strategies including antibodies (naked antibodies, antibodies-drug conjugates, and bispecific antibodies) and cellular therapies (chimeric antigen receptor T-cells), with promising clinical results even in relapsed refractory MM.27–31 In addition, serum soluble BCMA is elevated among patients with MM and chronic lymphocytic leukemia and can served as a prognostic marker and monitor of clinical response. Finally, most recent studies indicate that BCMA is expressed in non-hematopoietic tissue: BCMA is abnormally expressed in non-small cell lung cancer cell lines and may play a role in the tumors through the ERK1/2 signaling pathway.32,33 These data support targeting BCMA in immunotherapeutic strategies in MM and potentially BCMA expressing solid tumors as well.
In the present study, we developed a peptide-based immunotherapeutic approach targeting BCMA that induces development of antigen-specific CD8+ CTL with effective and long-lasting immunity against MM cells. The identified novel immunogenic native and heteroclitic HLA-A2specific BCMA peptides are capable of eliciting MM-specific immune responses with highly effective anti-tumor activities. Importantly, the heteroclitic BCMA72–80 (YLMFLLRKI) peptide demonstrated the highest level of immunogenicity, with the greatest affinity/stability to HLA-A2 molecule and robust induction of BCMA-specific memory CTL with poly-functional activities against HLA-A2+ patients’ MM cells and MM cell lines. Our studies provide the framework for clinical application of this novel engineered immunogenic BCMA72–80 (YLMFLLRKI) peptide in cancer vaccine and adoptive immunotherapeutic protocols to provide long lasting memory antitumor immunity in patients with MM or BCMA expressing cancers.
MATERIALS AND METHODS
Cell lines
The MM cell lines, MM1S, OPM2, OPM1, H929, OCIMY5, RPMI, U266, KMS1, HSB2, McCAR and ANBL6, and a breast cancer cell line MDA-MB-231 were obtained from ATCC (Manassas, VA). The T2 cell line, a human B and T cell hybrid expressing HLA-A2 molecules, was provided by Dr. J. Molldrem (University of Texas M. D. Anderson Cancer Center, Houston, TX). The cell lines were cultured in DMEM (for MM and T2 cells; Gibco-Life Technologies, Rockville, MD) or Leibovitz’s L-15 (for MDA-MB231; ATCC, Manassas, VA) media supplemented with 10% fetal calf serum (FCS; BioWhittaker, Walkersville, MD), 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco-Life Technologies).
Reagents
Fluorochrome conjugated anti-human monoclonal antibody (mAb) specific to BCMA, HLA-A2, CD3, CD8, CD38, CD40L, CD69, 41BB, CCR7, CD45RO, CD107a, IFN-γ, IL-2, TNF-α, PD1, LAG3, OX40 or GITR was purchased from Becton Dickinson (BD) (San Diego, CA), LifeSpan Bioscience (Seattle, WA) or BioLegend (San Diego, CA). Live/Dead Aqua stain kit was purchased from Molecular Probes (Grand Island, NY). Recombinant human GM-CSF was obtained from Immunex (Seattle, WA); and human IL-2, IL-4, IFN-α, and TNF-α were purchased from R&D Systems (Minneapolis, MN). Heteroclitic hBCMA72–80 (YLMFLLRKI) peptide-specific Tetramer-PE was synthesized by MBL International Corporation (Woburn, MA). Clinical grade mAb to LAG3 or OX40 was provided by Bristol-Myers Squibb (New York, NY).
Synthetic peptides
Native BCMA peptides [BCMA64–72 (LIISLAVFV), BCMA69–77 (AVFVLMFLL), BCMA9–17 (SQNEYFDSL), BCMA72–80 (VLMFLLRKI), BCMA54–62 (AILWTCLGL), BCMA114–120 (ILPRGLEYT)], heteroclitic BCMA peptides [hBCMA72–80 (YLMFLLRKI), hBCMA54–62 (YILWTCLGL), hBCMA9–17 (YQNEYFDSL)] and HIV-Gag77–85 (SLYNTVATL) were synthesized by standard fmoc (9-fluorenylmethyl-oxycarbonyl) chemistry, purified to >95% using reverse-phase chromatography, and validated by mass-spectrometry for molecular weight (Biosynthesis, Lewisville, TX).
HLA-A2 affinity and stability Assays
T2 cells were pulsed overnight with various doses of peptide plus β2-microglobulin (3 µg/ml) (Sigma, St Louis, MO). Following overnight incubation, the cells were stained with HLA-A2-PE mAb and analyzed by flow cytometry. Peptide/HLA-A2 complex stability was measured on peptide loaded T2 cells at 0, 2, 4, 6 and 14 hours post-Brefeldin A treatment by staining with HLA-A2-PE mAb and flow cytometry analyses and shown as an increase in specific HLA-A2 median fluorescence intensity (MFI).
Generation of dendritic Cells
Peripheral blood mononuclear cells (PBMC) were isolated by standard density gradient centrifugation over Ficoll-Paque™ Plus (Amersham Pharmacia Biotech AB, Uppsala Sweden) from leukopaks obtained from multiple HLA-A2+ normal donors. Monocytes isolated from the PBMC were cultured for 7 days in the presence of 1,000 units/ml GM-CSF and 1,000 units/ml IL4 in RPMI-1640 medium (Gibco-Life Technologies) supplemented with 10% FCS. Fresh media plus GM-CSF and IL-4 was added to the cultures every other day. Mature DC (mDC) were obtained on day 7, following 3 additional days incubation with 1,000 units/ml IFN-α plus 10 ng/ml TNF-α.
Induction of heteroclitic BCMA peptide-specific CTL
CD3+ T cells were obtained by negative selection from the HLA-A2+ non-adherent cell fraction post monocyte separation using the EasySep® magnet and Robosep® from StemCell Technologies. Heteroclitic BCMA peptide-specific CTL (hBCMA-CTL) were generated ex vivo by repeated stimulation of the CD3+ T cells with antigen-presenting cells (APC) pulsed with a heteroclitic BCMA peptide. In brief, heteroclitic BCMA72–80 (YLMFLLRKI) or BCMA54–62 (YILWTCLGL) peptide (50 µg/ml)-pulsed APC were irradiated (10 Gy) and used to stimulate T cells at a 1 APC/peptide: 20 T cell ratio. The T cell cultures were restimulated every 7 days and maintained in AIM-V medium supplemented with 10% human AB serum (BioWhittaker) in the presence of IL-2 (50 units/ml).
Phenotypic analysis of BCMA peptide-specific CTL or tumor Cells
Phenotypic characterization was performed on BCMA-CTL after staining with Live/Dead Aqua stain kit and fluorochrome conjugated anti-human mAbs and Tetramer-PE. Alternatively, the MM and breast cancer cell lines were stained with fluorochrome-conjugated BCMA or HLA-A2 mAb to confirm antigen expression on target cells. After staining, the cells were washed, fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
Cell proliferation by Carboxy Fluorescein Succinimidyl Ester (CFSE) tracking
BCMA-CTL were labeled with CFSE (Molecular Probes) and co-incubated with irradiated (10 Gy) tumor cells or peptide-pulsed APC in the presence of IL-2 (10 units/ml). On day 4, 5, 6 or 8 of co-culture, cells were harvested and stained with Live/Dead Aqua stain kit and CD3/CD8/CD45RO/CCR7 mAbs. The level of CD3+CD8+ CTL proliferation was determined as a reduction in CFSE fluorescence intensity, as measured by flow cytometry.
CD107a degranulation and intracellular IFN-γ/IL-2/TNF-α cytokines production
The functional immune activities of BCMA-CTL were measured by CD107a degranulation and Th1-specific cytokines production by flow cytometry. In brief, BCMA-CTL were co-incubated with tumor cells or peptide loaded T2 cells in the presence of CD107a mAb. After 1-hour incubation, CD28/CD49d mAb, Brefeldin A, and Monensin (BD) were added for an additional 5 hours. Cells were harvested, washed in PBS, and incubated with mAbs specific to T cell antigens. After surface staining, cells were washed, fixed/permeabilized, stained with anti-IFN-γ/IL-2/TNF-α mAbs, washed with Perm/Wash solution (BD), fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
Statistical Analysis
Summary results are presented as the mean ± SE. Groups were compared using unpaired Student’s t-test. Differences were considered significant when *p < 0.05.
RESULTS
Heteroclitic BCMA72–80 peptide shows the highest affinity and stability to HLA-A2 molecules.
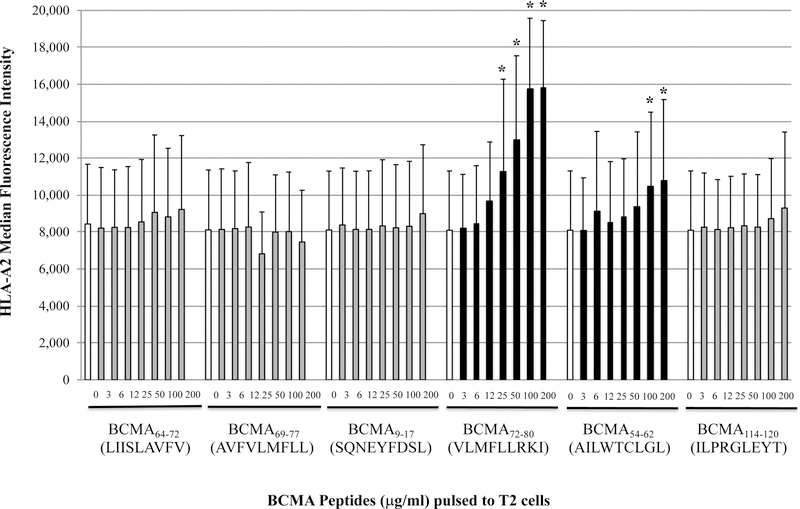
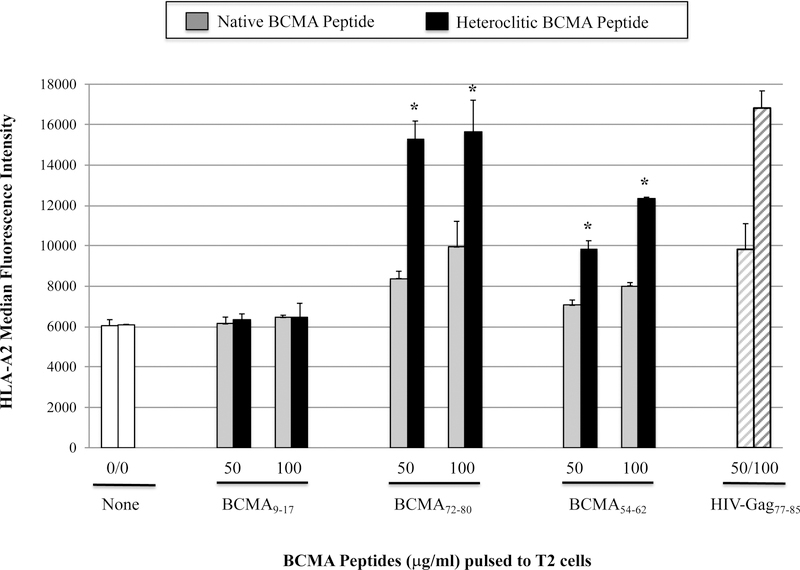
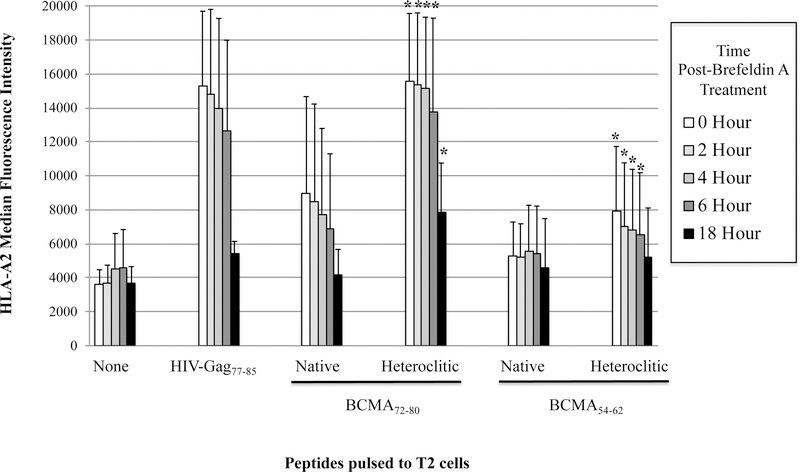
The full length BCMA protein sequence was evaluated to predict epitopes with HLA-A2 affinity, extended half-time disassociation rates, proteasome C terminal cleavage, and TAP transport using various search software programs. Among the six native peptides selected [BCMA64–72 (LIISLAVFV), BCMA69–77 (AVFVLMFLL), BCMA9–17 (SQNEYFDSL), BCMA72–80 (VLMFLLRKI), BCMA54–62 (AILWTCLGL), BCMA114–120 (ILPRGLEYT)], BCMA72–80 (VLMFLLRKI) and BCMA54–62 (AILWTCLGL) showed the highest HLA-A2 binding affinity in a dose-dependent manner, indicated as a significant increase in HLA-A2-specific MFI from baseline (n=3, *p < 0.05) (Figure 1A). Furthermore, the native BCMA72–80 (VLMFLLRKI) and BCMA54–62 (AILWTCLGL) peptides with highest HLA-A2 binding affinity were chosen for modification of their anchor residues to further enhance HLA-A2 affinity. The HLA-A2 nonspecific BCMA9–17 (SQNEYFDSL) peptide was also modified to evaluate whether a change in an anchor motif alters HLA-A2 affinity. Among the engineered peptides designed, heteroclitic BCMA72–80 (YLMFLLRKI) and heteroclitic BCMA54–62 (YILWTCLGL) peptides displayed a significant increase in HLA-A2 affinity than their native peptides (n=3, *p < 0.05). The highest affinity was detected by heteroclitic BCMA72–80 (YLMFLLRKI) in both doses (50 µg/ml, 100 µg/ml) tested, which was close to the binding affinity of the HLA-A2-specific HIV-Gag77–85 (SLYNTVATL) peptide (n=3, *p < 0.05) (Figure 1B). In contrast, replacing the anchor motif in the non-HLA-A2 specific BCMA9–17 (SQNEYFDSL) to heteroclite BCMA9–17 (YQNEYFDSL) did not alter its HLA-A2 affinity status, indicating that improved HLA-A2 affinity is only achieved in HLA-A2-specific peptides. In addition, native BCMA72–80 and BCMA54–62 peptides displayed extended HLA-A2 stability for greater than 6 hours, which was further enhanced in the engineered heteroclitic BCMA72–80 (YLMFLLRKI) and BCMA54–62 (YILWTCLGL) peptides, close to the stability of the HLA-A2-specific HIV-Gag77–85 peptide (n=3, *p < 0.05) (Figure 1C). In summary, the heteroclitic BCMA72–80 peptide had the highest level of HLA-A2 affinity and stability, which was close to the HLA-A2 positive control HIV-Gag77–85 peptide.
Figure 1. Characterization of native or heteroclitic BCMA peptide for their HLA-A2 binding affinity and stability.
T2 cells were pulsed overnight with native or heteroclitic BCMA peptide in serum-fee AIM-V media and stained with HLA-A2-PE mAb for flow cytometry analyses. HLA-A2-specificity of the peptide is shown as an increase in HLA-A2 mean fluorescence intensity (MFI) on T2 cells. HLA-A2 specific HIV-Gag77–85 peptide was used as the positive control. Fig. 1A. Native BCMA peptides (3 – 200 µg/ml) binding affinity to HLA-A2. Fig. 1B. Native and heteroclitic BCMA9–17, BCMA72–80 and BCMA54–62 peptides (50 – 100 µg/ml) binding affinity to HLA-A2. Fig. 1C. Native and heteroclitic BCMA72–80 and BCMA54–62 peptides (50 µg/ml) binding affinity and stability to HLA-A2, post-Brefeldin A treatment (0 – 18 hours). Results represent the mean ± SE of three separate experiments.
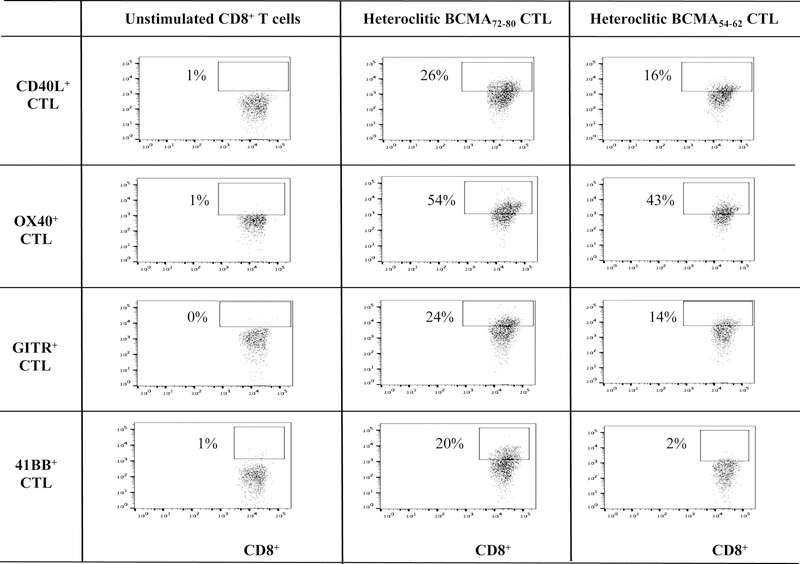
BCMA-specific CTL generated with heteroclitic BCMA72–80 (YLMFLLRKI) or BCMA54–62 (YILWTCLGL) peptide have increased T cell activation and co-stimulatory molecule expression.
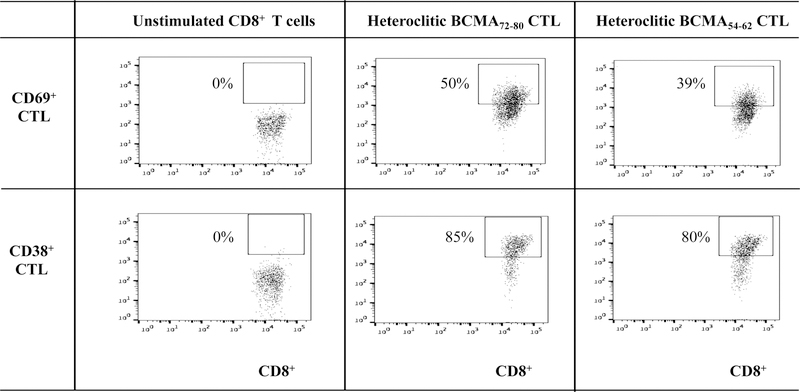
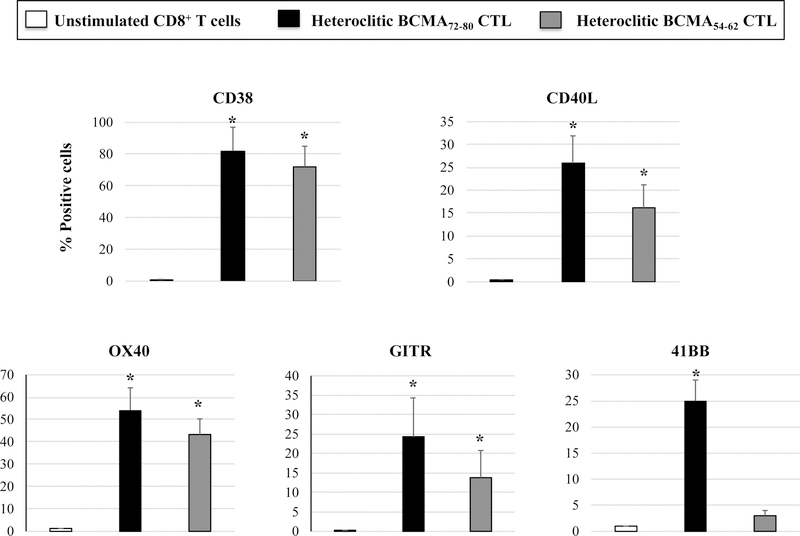
Phenotypic characterization of heteroclitic BCMA72–80 peptide-specific CTL (hBCMA72–80 CTL) or heteroclitic hBCMA54–62 peptide-specific CTL (hBCMA54–62 CTL) was performed after the fourth round of respective peptide stimulation. Both hBCMA72–80 CTL and hBCMA54–62 CTL displayed increased levels of T cell activation markers (CD69, CD38) expression with the highest upregulation on the hBCMA72–80 CTL [Baseline vs. hBCMA72–80 CTL vs. hBCMA54–62 CTL: CD69+ cells – 0% vs. 50% vs. 39%, CD38+ cells – 0% vs. 85% vs. 80%] (Figure 2A). In addition, the hBCMA72–80 CTL showed distinctly higher expression of CD40L, OX30, GITR and 41BB costimulatory molecules than hBCMA54–62 CTL [Baseline vs. hBCMA72–80 CTL vs. hBCMA54–62 CTL: CD40L+ cells – 1% vs. 26% vs. 16%, OX40+ cells – 1% vs. 54% vs. 43%, GITR+ cells – 0% vs. 24% vs. 14%, 41BB+ cells – 1% vs. 20% vs. 2%] (Figure 2B). In summary, hBCMA72–80 CTL and hBCMA54–62 CTL generated from three HLA-A2+ individuals demonstrated similar patterns of T cell activation and co-stimulatory molecule expression, with the highest upregulation seen on the hBCMA72–80 CTL (n=3, *p < 0.05) (Figure 2C).
Figure 2. Upregulation of critical T cell activation and co-stimulatory markers on heteroclitic BCMA72–80 peptide-specific CTL.
CD3+ T cells obtained from HLA-A2+ individual donors were stimulated with heteroclitic BCMA72–80 (YLMFLLRKI) or BCMA54–62 (YILWTCLGL) peptide loaded antigen-presenting cells and analyzed post-4 cycles of peptide stimulation (once/week) for activation and co-stimulatory molecules expression within the gated CD3+CD8+ T cells population by flow cytometry. Fig. 2A. Representative flow cytometry analyses for CD69 and CD38 expression. Fig. 2B. Representative flow cytometry analyses for CD40L, OX30, GITR and 41BB expression. Fig. 2C. Summary (mean ± SE; N=3) of activation and co-stimulatory molecule expression.
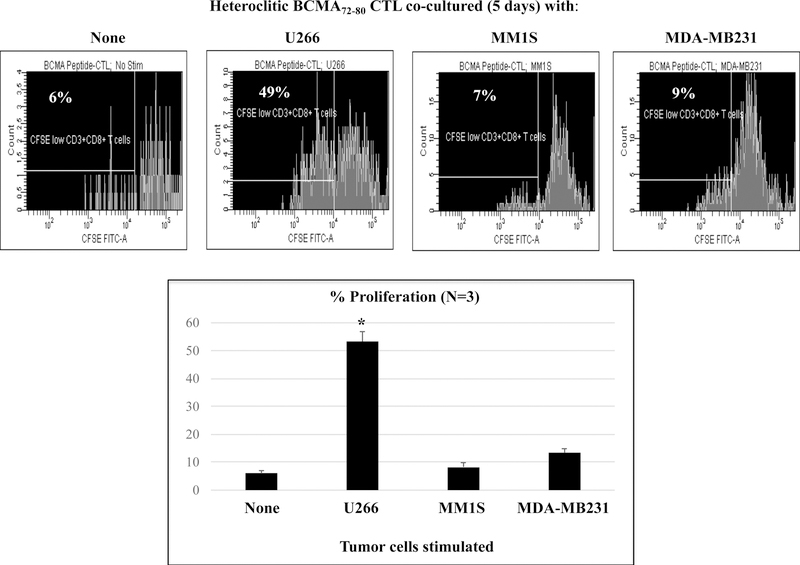
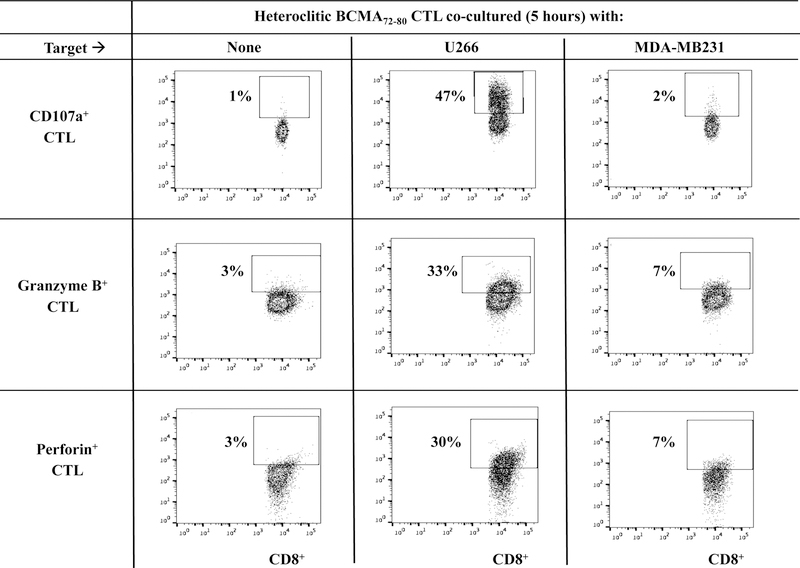
Heteroclitic BCMA72–80 specific CTL display BCMA antigen-specific immune activities in response to MM cell lines.
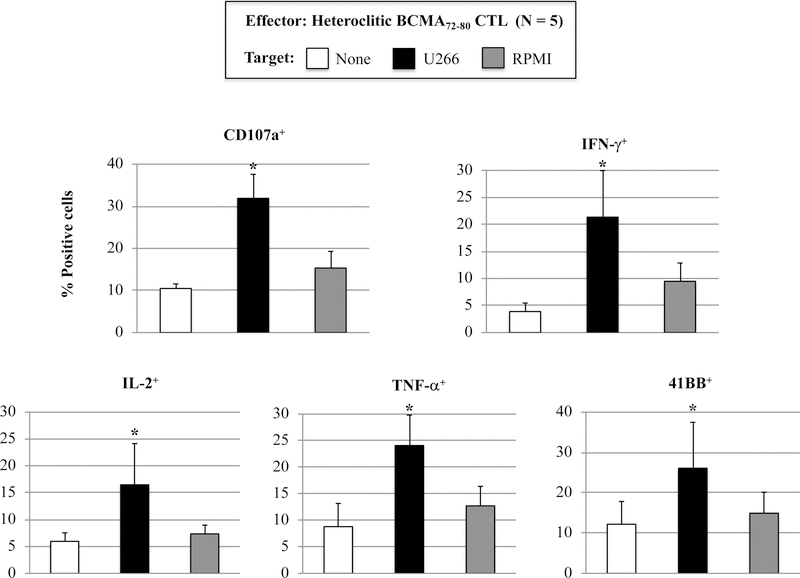
The phenotype and functional immune activities of hBCMA72–80 CTL were assessed after each round of peptide stimulation. A gradual increase in the % CD3+CD8+ T cells (Supplemental Fig. 1) and a corresponding decrease in % CD3+CD4+ T cells (Supplemental Fig. 2) was observed after each round of hBCMA72–80 peptide stimulation (N=3). In parallel, phenotype analyses showed a high expression of BCMA expression (mAb clones used to stain: ANC3B1, VICKY1, 19F2) on the H929, MMIS, U266 and OPM1 myeloma cell lines, but not on the MDA-MB231 breast cancer cell line (Supplemental Fig. 3). The hBCMA72–80 CTL showed significantly (*p < 0.05) higher proliferation of CD3+CD8+ T cells in response to HLA-A2+ BCMA+ U266 (49%) compared to HLA-A2− BCMA+ MM1S (7%), HLA-A2+ BCMA− MDA-MB231 (9%), or media alone (6%) (Figures 3A, Histogram). This HLA-A2-restricted and BCMA antigen-specific CD8+ CTL proliferation was consistently observed in hBCMA72–80 CTL generated from three HLA-A2+ individuals (Figure 3A, Bar graphs; n=3, *p < 0.05). In addition, hBCMA72–80 CTL were further investigated for functional anti-tumor activities against myeloma cells by the upregulation of CD107a degranulation, which is widely accepted as a measure of cytotoxicity which directly correlates with tumor target cell killing by effector cells.34,35 The hBCMA72–80 CTL demonstrated robust CD107a degranulation (47%) and Granzyme B (33%)/Perforin (30%) upregulation in response to HLA-A2+ BCMA+ U266 cells, but not to mismatched HLA-A2+ BCMA− MDAMB231 cells (Figure 3B). In additional experiments, hBCMA72–80 CTL generated from multiple HLA-A2+ individuals consistently demonstrated high levels of IFN-γ/IL-2/TNF-α production, 41BB upregulation, and CD107a degranulation against BCMA+ MM cells in an HLA-A2 restricted manner (n=5, *p < 0.05) (Figure 3C). Thus, these data further demonstrate the MHC restricted and BCMA antigen-specific immune responses by heteroclitic BCMA72–80 peptide generated CTL against myeloma.
Figure 3. HLA-A2 restricted and BCMA antigen-specific immune responses by heteroclitic BCMA72–80 specific CTL against cancer cell lines.
Heteroclitic BCMA72–80 CTL were evaluated for their specific anti-tumor activities. Fig. 3A. Representative flow analyses and summary (mean ± SE; N=3) of CD8+ T cells proliferation (day 5) of h against matched U266 (HLA-A2+ BCMA+) or mis-matcherd MM1S (HLA-A2− BCMA+) and MDA-MB231 (HLA-A2+ BCMA−) cancer cell line. Fig. 3B. Representative flow analyses of CD107a degranulation and Granzyme B/Perforin upregulation by hBCMA72–80 CTL. Fig. 3C. Summary (mean±SE; N=5) of CD107a degranulation, Th1 cytokine (IFN-γ/IL-2/TNF-α) production, and 41BB upregulation in hBCMA72–80 CTL against matched U266 (HLA-A2+ BCMA+) or mis-matched RPMI (HLA-A2− BCMA+) myeloma cell line.
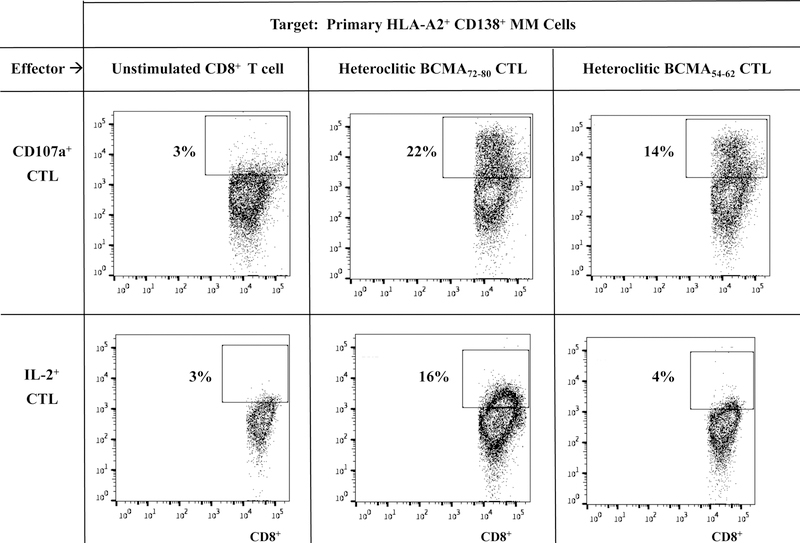
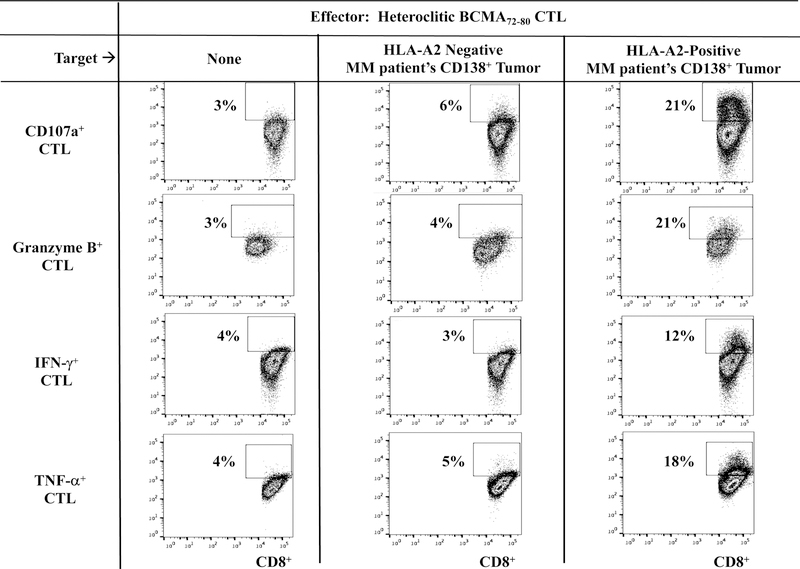
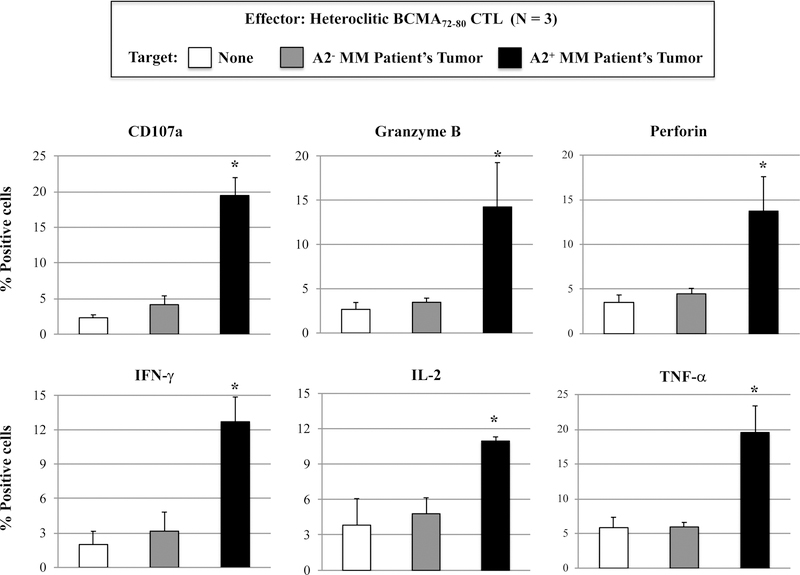
Heteroclitic BCMA72–80 specific CTL demonstrate functional immune responses to primary CD138+ tumor cells from HLA-A2+ patients’ MM cells.
Next, we evaluated the functional activities of hBCMA72–80 CTL against primary CD138+ tumor cells isolated from MM patients. Compared to heteroclitic BCMA54–62 CTL, heteroclitic BCMA7280 CTL displayed more robust anti-MM activities against primary HLA-A2+ MM cells, as measured by CD107a degranulation (hBCMA72–80 CTL vs. hBCMA54–62 CTL: 22 % vs. 14%) and IL-2 production (16% vs. 4%) (Figure 4A). In addition, hBCMA72–80 CTL consistently demonstrated the highest CD107a degranulation, Granzyme B upregulation and IFN-γ/TNF-α Th1 cytokines production in an HLA-A2 restricted manner (Figure 4B). The hBCMA72–80 CTL generated from other HLA-A2+ individuals (n=3) displayed the same pattern of response and showed significant (*p < 0.05) increases in anti-tumor activities (CD107a degranulation, Granzyme B/Perforin upregulation and IFN-γ/IL-2/TNF-α production) against CD138+ tumor cells from MM patients in an HLA-A2-restricted manner (Figure 4C). The robust functional activities by hBCMA72–80 CTL were aligned with their increased upregulation of T cell activation and co-stimulatory molecule expression (Figure 2). Thus, these data provide additional evidence on the great immunogenicity of heteroclitic BCMA72–80 peptide and support its potential clinical application in novel MM treatments.
Figure 4. Functional anti-myeloma activities of heteroclitic hBCMA54–62 specific CTL or heteroclitic hBCMA72–80 specific CTL against primary CD138+ MM tumor cells.
Heteroclitic BCMA peptide-specific CTL were evaluated for their functional activities against primary CD138+ tumor cells obtained from HLA-A2 negative or HLA-A2 positive MM patients. Fig. 4A. Representative flow analyses of CD107a degranulation and IL-2 production by hBCMA72–80 CTL and hBCMA54–62 CTL against HLA-A2+/CD138+ MM cells. Fig. 4B. Representative flow analyses of HLA-A2 restricted CD107a degranulation, Granzyme B upregulation, and IFN-γ/TNF-α cytokines production by hBCMA72–80 CTL in response to CD138+ cells from MM patients. Fig. 4C. Summary (mean ± SE; N=3) of MHC-restricted poly-functional immune responses of hBCMA72–80 CTL against primary CD138+ cells from MM patients.
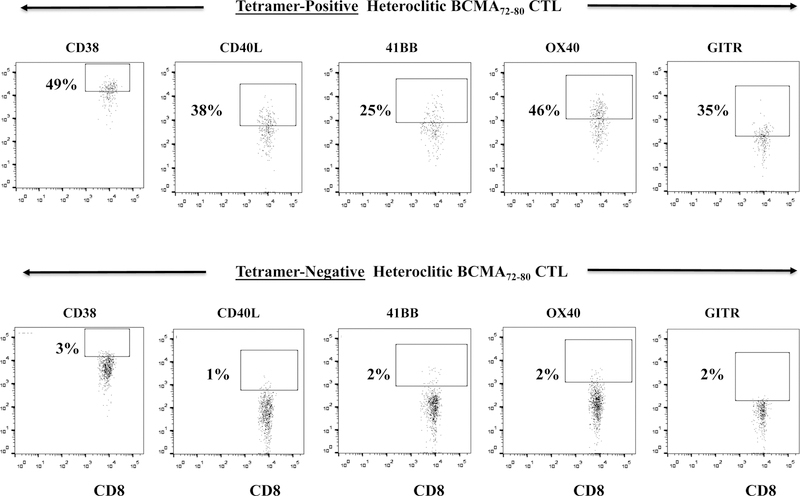
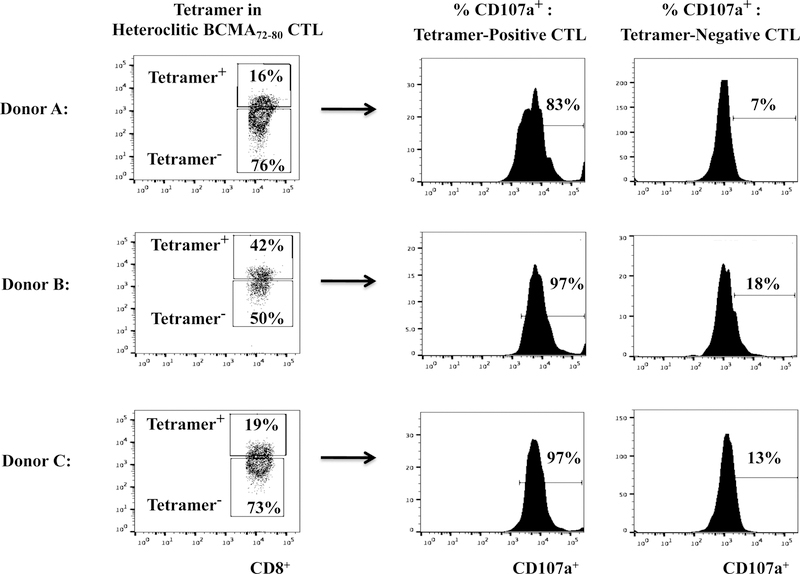
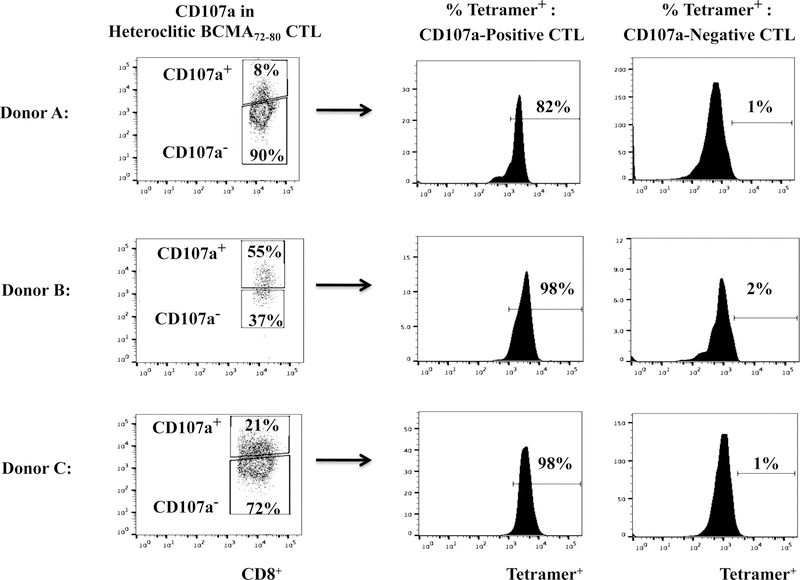
Heteroclitic BCMA72–80 specific CTL are enriched for Tetramer+ CD8+ T cells with robust anti-MM activities.
The hBCMA72–80 CTL were further characterized to define the specific Naïve:Memory CD8+ T cell subsets with anti-MM activities. The Tetramer+ hBCMA72–80 CTL population displayed significantly higher T cell activation and co-stimulatory molecule expression as compared to the non-specific Tetramer− cells (Tetramer+ vs. Tetramer−, CD38+: 49% vs. 3%, CD40L+: 38% vs. 1%, 41BB+: 25% vs. 2%, OX40+: 46% vs. 2%, GITR+: 35% vs. 2%) (Figure 5A). In addition, the Tetramer+ hBCMA72–80 CTL population consistently demonstrated high levels of CD107a degranulation against U266 MM cells (Tetramer+ vs. Tetramer−: Donor A, B, C - 83%, 97%, 97% vs. 6%, 18%, 13%) (Figure 5B). Finally, we evaluated the frequency of Tetramer+ cells within CD107a+ population of hBCMA72–80 CTL. We observed that the majority of T cells inducing antitumor activities (CD107a+: Donor A, B, C - 82%, 98%, 98%) were also within hBCMA72–80 specific Tetramer+ cells (Figure 5C). Therefore, these results confirm that anti-MM activities are indeed induced by the BCMA72–80 peptide specific CTL, which also display high expression of key T cell activation markers and co-stimulatory molecules.
Figure 5. Key antigen expression and functional activities of specific Tetramer+ hBCMA72–80 CTL against MM cells.
Heteroclitic hBCMA72–80 CTL were evaluated for phenotype and anti-MM activities related to Tetramer-positivity. Fig. 5A. Representative flow analyses of key activation (CD38) and costimulatory (CD40L, 41BB, OX40, GITR) molecules expression in hBCMA72–80 peptide specific Tetramer-positive or Tetramer-negative CTL subset. Fig. 5B. Flow cytometry analyses of functional CD107a degranulation by Tetramer-positive or Tetramer-negative cells in hBCMA72–80 CTL (Donor A, Donor B, Donor C; N=3) against HLA-A2+ U266 MM cells. Fig. 5C. Flow cytometry analyses of Tetramer-positivity within the functional CD107a+ hBCMA72–80 CTL (Donor A, Donor B, Donor C; N=3) in response to HLA-A2+ U266 MM cells.
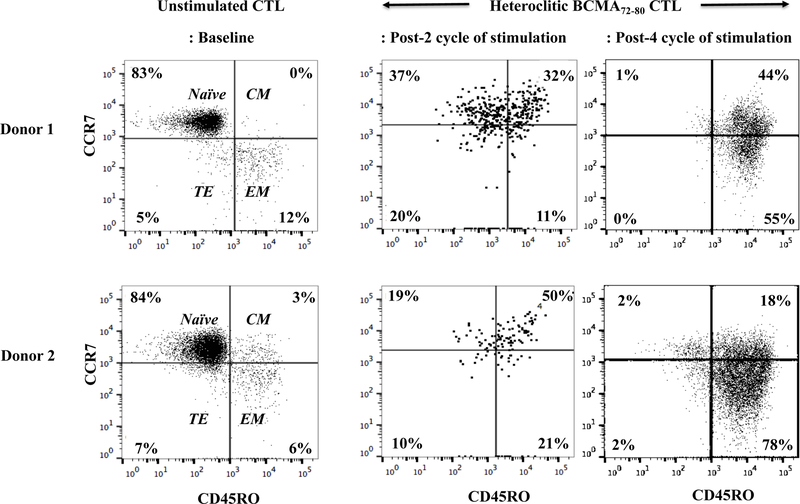
Heteroclitic BCMA72–80 peptide induces MM-specific memory CD8+ CTL with anti-tumor activities.
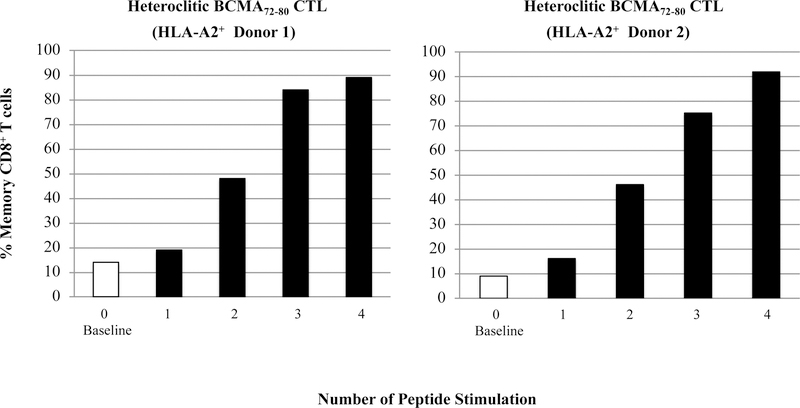
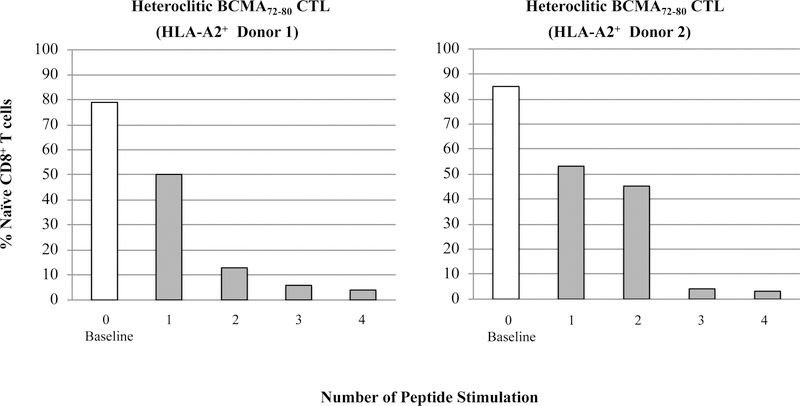
To characterize anti-tumor activities of BCMA peptide-specific CTL, we evaluated the BCMAspecific memory CTL development after each cycle of peptide stimulation. Gradual progressive phenotypic changes were detected within CD8+ T cells of hBCMA72–80 CTL; from naïve (CD45RO−CCR7+) at baseline [Donor 1: 83%, Donor 2: 84%] to central memory (CM; CD45RO+CCR7+) after 2 cycles of peptide stimulation [Donor 1: 32%, Donor 2: 50%], and then to an enriched effector memory (EM; CD45RO+CCR7−) CTL after 4 cycles of stimulation (Donor 1: 55%, Donor 2: 78%) (Figure 6A). Overall, memory CD8+ CTL was gradually developed following each round of heteroclitic BCMA72–80 peptide stimulation (Figure 6B), which was associated with a corresponding decrease in naïve T cells (Figure 6C). These results therefore demonstrate the induction and gradual expansion of memory CTL upon the stimulation of T cells with heteroclitic BCMA72–80 peptide.
Figure 6. Phenotypic characterization of hBCMA72–80 specific memory CTL development upon stimulation with heteroclitic hBCMA72–80 peptide.
The Naïve:Memory phenotype of heteroclitic hBCMA72–80 CTL (Donor 1, Donor 2) was analyzed at baseline (no peptide stimulation) or one week after each cycle of peptide stimulation. Fig. 6A. Flow cytometry analyses of hBCMA72–80 CTL differentiation from naïve into memory CD8+ T cells at baseline, 2 cycles and 4 cycles of BCMA72–80 peptide stimulation. Fig. 6B and Fig. 6C. Summary of changes in memory (CD45RO+CCR7+ and CD45RO+CCR7−) and naïve (CD45ROCCR7+) CD8+ T cells frequency after each cycle of peptide stimulation.
Central memory CD8+ T cells specific to heteroclitic BCMA72–80 demonstrate the greatest anti-MM activities.
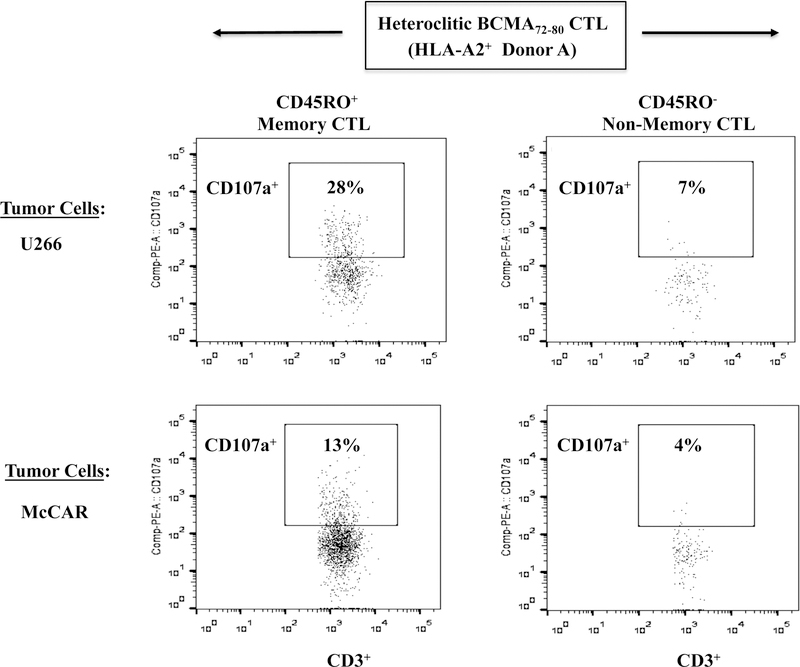
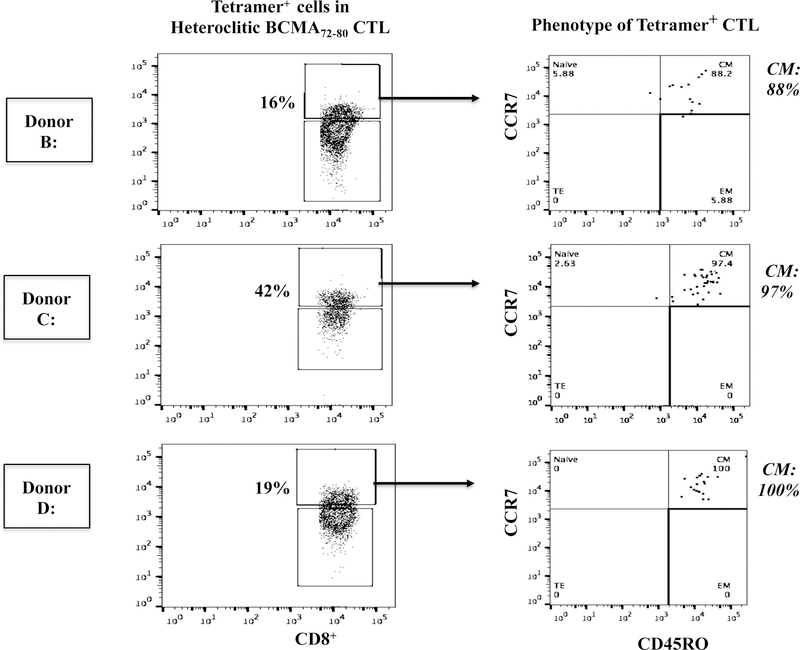
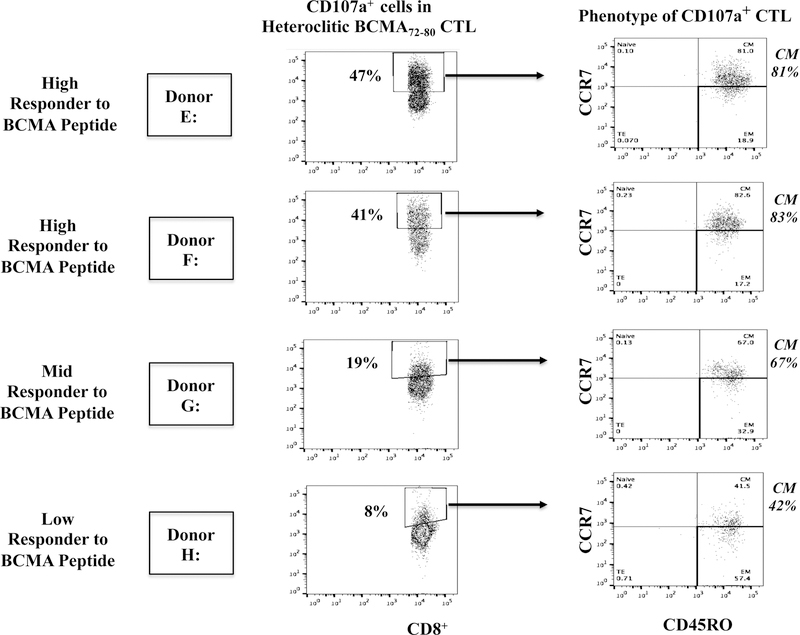
Memory T cells specific to heteroclitic BCMA72–80 peptide were characterized for their immune functional activities. The CD45RO+ memory hBCMA72–80-CTL (Donor A) demonstrated higher CD107a degranulation in response to U266 (CD45RO+ vs. CD45RO-: 28% vs. 7%) and McCAR (13% vs. 4%) (Figure 7A). The hBCMA72–80 CTL displayed highly functional anti-MM activity against target cells, having both high BCMA (U266) and low BCMA expression (McCAR). Additionally, the Tetramer+ cells were predominantly central memory subset, which was consistently seen in the hBCMA72–80 CTL generated from different individuals (% CM of Tetramer+ cells - Donor B: 88%, Donor C: 97%, Donor D: 100%) (Figure 7B). The hBCMA72–80 specific CM subset also demonstrated highly functional immune activities against U266 cells. Importantly, the CD107a degranulation in the hBCMA72–80 specific CTL was directly associated with the frequency of CM cells (% CM within CD107a+ cells - Donor E: 81%, Donor F: 83%, Donor G: 67%, Donor H: 42%) (Figure 7C). Finally, the high responders (Donor E, Donor F) to the heteroclitic BCMA72–80 peptide showed the greatest anti-MM activities and also displayed the highest CM subset frequency as compared to mid-level responder (Donor G) or low-level responder (Donor H). Thus, these results further indicate the distinct enrichment of effector cells with anti-MM activities are predominantly within hBCMA72–80 specific central memory cell subset.
Figure 7. High anti-MM activities by heteroclitic BCMA72–80 peptide-specific central memory CD8+ T cells.
The Naïve:Memory cell subsets within hBCMA72–80 CTL were evaluated for their functional immune responses against HLA-A2+ MM cells (U266, McCAR). Fig. 7A. The CD107a degranulation by CD45RO+ memory cells or CD45RO− non-memory cells in hBCMA72–80 CTL (Donor A) against U266 [BCMA high expression] or McCAR [BCMA low expression] MM cells. Fig. 7B. The Naïve:Memory phenotype of Tetramer+ CTL in hBCMA72–80 CTL (Donor B, Donor C, Donor D). Fig. 7C. The frequency of heteroclitic BCMA72–80 peptide-specific central memory CTL and level of anti-tumor activities against U266 cells by High (Donors E and F), Mid (Donor G) and Low (Donor (H) responders.
Inhibition of LAG3 or stimulation of OX40 enhances anti-MM activities of heteroclitic BCMA72–80 peptide-specific central memory CTL.
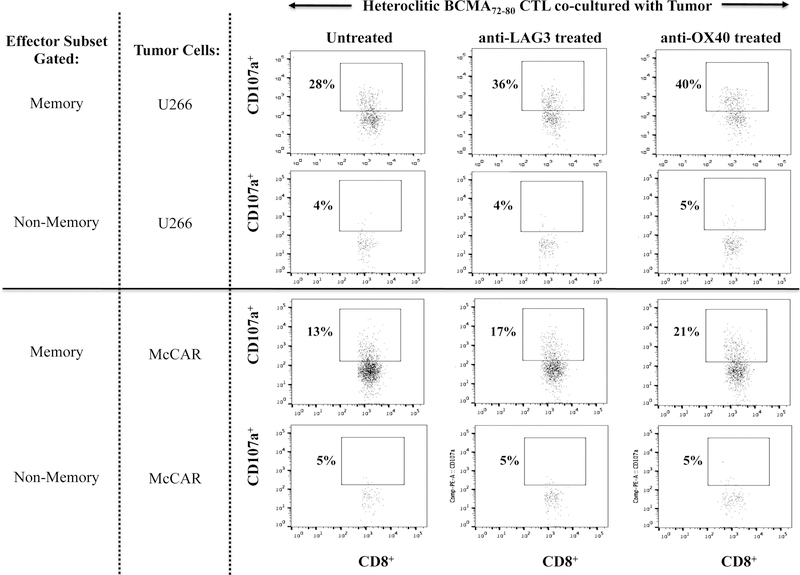
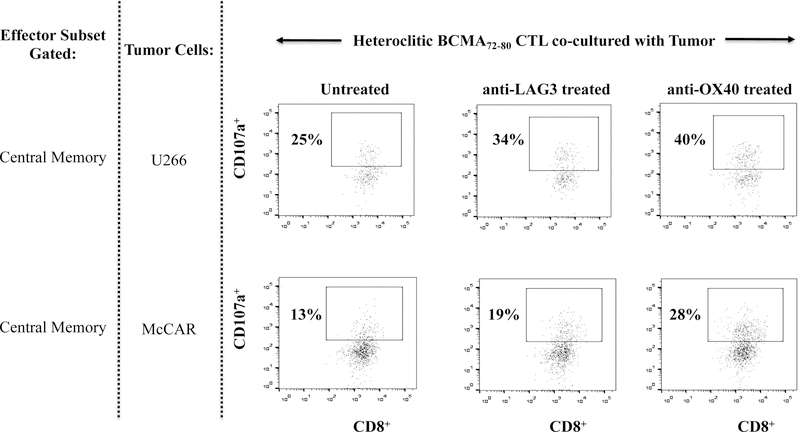
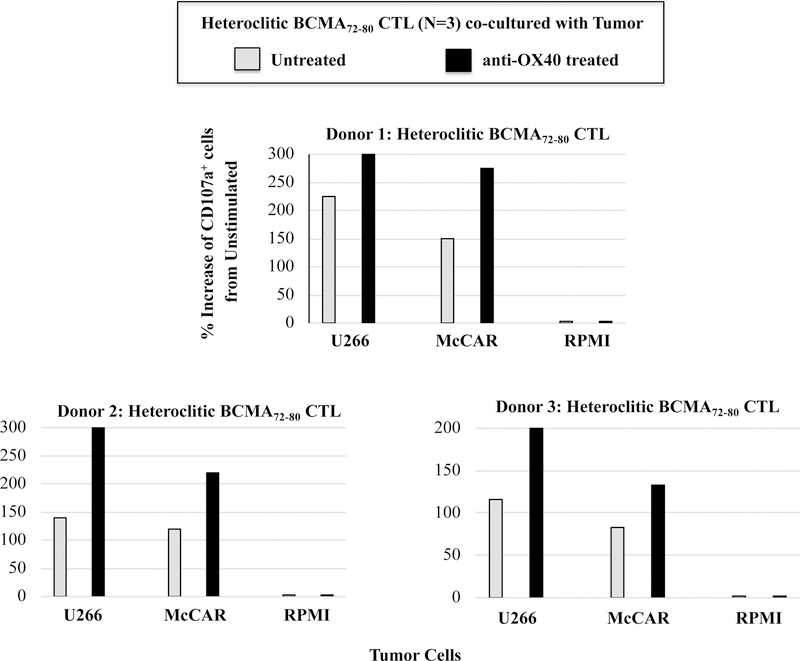
Finally, we evaluated the potential impact of a checkpoint inhibitor (anti-LAG3) or immune agonist (anti-OX40) on the immune function of hBCMA72–80 CTL. Treatment of hBCMA72–80 CTL with either anti-LAG3 or anti-OX40 enhanced the specific cytotoxic activity, especially within the CD45RO+ memory subset against U266 (untreated 28%, anti-LAG3 treated 36%, anti-OX40 treated 40%) and McCAR (untreated 13%, anti-LAG3 treated 17%, anti-OX40 treated 21%) (Figure 8A). The checkpoint inhibitor and immune agonist did not induce the anti-MM responses by CD45RO− non-memory cells within the BCMA-CTL. Lastly, the beneficial effect of antiLAG3 and anti-OX40 was further investigated within the specific central memory CTL subsets. Anti-LAG3 or anti-OX40 treatment had a greater impact on regulating the functional activity of CM subset compared to EM subset within the hBCMA72–80 CTL, as evidenced by their higher CD107a degranulation against U266 MM cells (Figure 8B). Increased anti-tumor activities was observed consistently in the hBCMA72–80 CTL generated from three different HLA-A2+ individuals (Donor 1, Donor 2, Donor 3) upon anti-OX40 treatment, as demonstrated in % increase of CD107a+ CTL, from each individual’s baseline (no peptide stimulated), in HLA-A2 restricted manner (Figure 8C). Therefore, these results support the utility of anti-LAG3 or anti-OX40 to further enhance anti-MM activities of hBCMA72–80 CTL induced from HLA-A2+ individuals.
Figure 8. Enhanced immune function of heteroclitic BCMA72–80 peptide-specific memory CTL in combination with anti-LAG3 or anti-OX40.
Anti-myeloma activities were investigated in treatment of hBCMA72–80 CTL with anti-LAG3 or anti-OX40. Fig. 8A. The CD107a degranulation by CD45RO+ memory cells or CD45RO− non-memory cells in hBCMA72–80 CTL, untreated or treated, against U266 or McCAR cells. Fig. 8B. The CD107a degranulation by central memory (CD45RO+CCR7+) CD3+CD8+ T cells in hBCMA72–80 CTL, untreated or treated, against U266 or McCAR cells. Fig. 8C. The percent increase of CD107a+ cells induced by hBCMA72–80 CTL generated from three different HLA-A2+ donors (N=3), untreated or treated with anti-OX40, against U266, McCAR or RPMI cells.
DISCUSSION
Even in patients with refractory MM relapsing after allo-transplantation, long-lasting responses have been achieved with the infusion of donor lymphocytes (DLI).36,37 These early encouraging results of DLI have provided the framework for evaluation of active-specific immunotherapy approaches to treat MM.38–40 Cancer targeting vaccines, one such active-specific immunotherapy approach, have demonstrated the ability to induce highly effective CD8+ CTL with anti-tumor activities.41–43 The success of vaccination depends on selection of the appropriate patient population, targeting antigens expressed selectively on tumor, and utilizing combination approaches to effectively induce and maintain antigen-specific memory anti-tumor immune responses. We have recently reported on novel immunogenic HLA-A2 and HLA-A24 specific peptides derived from XBP1, CD138 and CS1 antigens, which are highly over-expressed in MM and solid tumors including breast, pancreatic, and colon cancers, and demonstrated their ability to induce the peptides-specific CD8+ CTL with anti-tumor activities against HLA-A2+ or HLA-A24+ tumor cells both in preclinical and clinical studies.11, 44–48 In addition, combination studies of peptide stimulation/vaccination with immune modulatory drugs such as lenalidomide or with histone deacetylase 6 inhibitor ACY241 enhanced the peptides-specific CTL activities against tumor cells. Our previous studies demonstrated that combinations of peptide stimulation with either Lenalidomide or ACY241 augmented antigens-specific CD8+ CTL activity associated with upregulation of transcriptional regulators such as T-bet/Eomes and with activation of AKT, which links antigen-specific CTL differentiation to FOXO, mTOR and Wnt/β-catenin signaling pathways.49,50 Importantly, these effects were confined primarily to antigen-specific CD45RO+ memory CTL, with the most robust increases in IFN-γ and Granzyme B production and CD8+ CTL proliferation in response to tumor cells observed mainly within the specific CM subset.
Due to our encouraging preclinical results, the XBP1/CD138/CS1 multipeptide vaccine has been evaluated, alone and in combination with lenalidomide, in clinical trials to treat patients with smoldering MM (SMM), as well as in combination with anti-PD1 in clinical trials to treat patients with triple negative breast cancer. In patients with SMM, the multipeptide vaccine was well tolerated and immunogenic as a monotherapy, evidenced by enhanced frequency of Tetramer+ CD8+ CTL with IFN-γ production; moreover, combination with lenalidomide triggered higher mean fold increases in CD8+ T cells with tetramer-positivity and IFN–γ production. Importantly, CD45RO+ memory CTL specific to the XBP1/CD138/CS1 peptides were induced by the peptide vaccine, and further enhanced in combination with lenalidomide.51,52 Although stable disease and responses have been observed in SMM, randomized trials are needed to assess whether time to progression from SMM to active disease can be prolonged by the peptide vaccination.
To expand the MM-specific immunotherapy beyond XBP1/CD138/CS1 antigens, we have recently identified additional tumor associated antigens on CD138+ tumor cells from newly diagnosed MM patients (N=616). Here we report on the identification and characterization of a novel immunotherapeutic strategy targeting BCMA, selectively expressed on normal and malignant plasma cells and the target of several current immune treatments in MM. We report on highly immunogenic engineered BCMA-specific nanomers, heteroclitic BCMA72–80 (YLMFLLRKI) and BCMA54–62 (YILWTCLGL), with highly improved HLA-A2 affinity/stability from their native BCMA peptides. These peptides evoke BCMA-specific CTL, increased BCMAspecific Tetramer+ cells, enhanced CD107 degranulation, Th1-type cytokines (IFN-γ/IL-2/TNF-α) production, and proliferation to MM cells in an HLA-A2-restricted manner. Most importantly, the increase of BCMA-specific memory CD8+ CTL, both CM and EM cells, along with the capacity of self-renewal and response to MM cells, strongly support the potential of heteroclitic BCMA peptide in novel vaccination and/or immunotherapeutic approaches in MM. Indeed, we have planned a clinical protocol with heteroclitic BCMA72–80 (YLMFLLRKI) peptide vaccination, harvest and expansion of BCMA-specific CM cells ex vivo, reinfusion of these CM cells as adoptive immunotherapy, and then vaccination with the BCMA peptide as needed thereafter to assure their persistence to effectively treat MM patients. We observed that BCMA-specific memory CD8+ CTL expressed key molecules modulating T cells function, both for co-stimulation and immune suppression. These results are consistent with our previous report of increased expression of these molecules on XBP1/CD138/CS1-specific CTL generated ex vivo by peptides stimulation.11 Importantly, the highest induction of co-stimulatory and immune checkpoint molecules was detected on CM subset within hBCMA72–80 peptide–specific CTL, which is the population that demonstrated the most highly effective poly-functional activities against MM. Importantly, these findings indicated the potential of combination therapy of hBCMA72–80 peptide with checkpoint inhibitors or immune agonists to enhance the functional anti-MM activities of BCMA-specific CTL. This may be particularly relevant, given the recent concerns when combining pembrolizumab (an antibody targeting PD-1) with immunomodulatory drugs lenalidomide or pomalidomide or with daratumumab (anti-CD38 mAb), where toxicities have curtailed studies.53–55 Here, we report on targeting alternative inhibitory receptors and suppressive mechanisms within the MM tumor microenvironment. In particular, LAG3 (CD223) is the third inhibitory receptor to be targeted in the clinic, following CTLA and PD1/PD-L156,57 and was expressed on BCMA-specific CM CTLs. In parallel, immune agonists, especially the costimulatory tumor necrosis factor receptors targeting OX40 (CD134), 41BB (CD137) and GITR (CD357), have received considerable attention for their therapeutic utility in enhancing anti-tumor immune responses; among these, anti-OX40 mAb has recently demonstrated encouraging efficacy in induction of tumor regression by boosting effector T cell expansion and functional anti-tumor activities in several pre-clinical studies.58–60 Importantly, we used a clinical grade anti-LAG3 and anti-OX40 (provided by Bristol-Myers Squibb; New York, NY) to evaluate changes in the functional activities of heteroclitic BCMA72–80 specific CTL to MM cells. Our ex vivo studies demonstrated that both anti-LAG3 (checkpoint inhibitor) and anti-OX40 (immune agonist) increased functional activity specifically of memory CTL within the BCMA-CTL against MM cells, without affecting the activity of non-memory CTL. The impact on function of BCMA-CTL generated from multiple HLA-A2+ individuals’ T cells was greater after treatment with anti-OX40 than anti-LAG3, and greater within their specific CM versus EM subset. These studies provide the framework for scientifically informed combination clinical trials of BCMA peptide-specific immunotherapy with the immune agonist or checkpoint inhibitor.
In summary, we have identified and validated novel immunogenic HLA-A2-specific native and engineered BCMA peptides, which are capable of inducing antigen-specific CD8+ CTL with functional anti-tumor activities against MM cells. These results provide the framework for therapeutic application of these highly immunogenic heteroclitic BCMA peptides in MM patients as vaccines and/or as stimuli for expansion of autologous antigen-specific memory CTL. They further support the potential utility of combinations incorporating BCMA peptide vaccine or BCMA-specific adoptive T cells immunotherapy with anti-OX40 and/or anti-LAG3 to enhance BCMA directed anti-MM responses. Based on the encouraging results presented here, we plan to vaccinate patients with BCMA peptide, treat with BCMA-specific memory T cells expanded ex vivo for adoptive immunotherapy, and then vaccinate again as needed to provide for long term BCMA directed anti-MM immunity.
Supplementary Material
Key Points.
Engineered heteroclitic BCMA-specific peptides identified in this study has an immunotherapeutic potential as a vaccine and adoptive immunotherapy in myeloma patients by inducing the antigens-specific CTL.
Based on the encouraging results presented here, we plan to vaccinate patients with BCMA peptide, treat with BCMA-specific memory T cells expanded ex vivo for adoptive immunotherapy, and then vaccinate again as needed to provide for long term BCMA directed anti-MM immunity.
Footnotes
Conflict of Interest:
No relevant conflicts of interest were disclosed by the authors.
REFERENCES
- 1.Köhler M, Greil C, Hudecek M, Lonial S, Raje N, Wäsch R et al. Current developments in immunotherapy in the treatment of multiple myeloma. Cancer 2018; 124: 2075–2085. [DOI] [PubMed] [Google Scholar]
- 2.Musto P, Anderson KC, Attal M, Richardson PG, Badros A, Hou J et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol 2018; 29: 1074. [DOI] [PubMed] [Google Scholar]
- 3.Falank C, Fairfield H, Reagan MR. Signaling interplay between bone marrow adipose tissue and multiple myeloma cells. Front Endocrinol 2016; 7: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Xu YY, Kalos M et al. Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clin Cancer Res 2014; 20: 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinde P, Fernandes S, Melinkeri S, Kale V, Limaye L. Compromised functionality of monocyte-derived dendritic cells in multiple myeloma patients may limit their use in cancer immunotherapy. Sci Rep 2018; 8: 5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karp Leaf R, Cho HJ, Avigan D. Immunotherapy for multiple myeloma, past, present, and future: monoclonal antibodies, vaccines, and cellular therapies. Curr Hematol Malig Rep 2015; 10: 395–404. [DOI] [PubMed] [Google Scholar]
- 7.Allegra A, Penna G, Innao V, Greve B, Maisano V, Russo S et al. Vaccination of multiple myeloma: Current strategies and future prospects. Crit Rev Oncol Hematol 2015; 96: 339–354. [DOI] [PubMed] [Google Scholar]
- 8.Leaf RK, Stroopinsky D, Pyzer AR, Kruisbeek AM, van Wetering S, Washington A et al. DCOne as an allogeneic cell-based vaccine for multiple myeloma. J Immunother 2017; 40: 315–322. [DOI] [PubMed] [Google Scholar]
- 9.Hos BJ, Tondini E, van Kasteren SI, Ossendorp F. Approaches to improve chemically defined synthetic peptide vaccines. Front Immunol 2018; 9: 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obara W, Kanehira M, Katagiri T, Kato R, Kato Y, Takata R. Present status and future perspective of peptide-based vaccine therapy for urological cancer. Cancer Sci 2018; 109: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae J, Hideshima T, Zhang GL, Zhou J, Keskin DB, Munshi NC et al. Identification and characterization of HLA-A24-specific XBP1, CD138 (Syndecan-1) and CS1 (SLAMF7) peptides inducing antigens-specific memory cytotoxic T lymphocytes targeting multiple myeloma. Leukemia 2018; 32: 752–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundar R, Rha SY, Yamaue H, Katsuda M, Kono K, Kim HS et al. A phase I/Ib study of OTSGC-A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer 2018; 18: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara Y, Okada K, Omori T, Sugimura K, Miyata H, Ohue M et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int J Oncol 2017; 50: 1655–1662. [DOI] [PubMed] [Google Scholar]
- 14.Waki K, Kawano K, Tsuda N, Ushijima K, Itoh K, Yamada A. Plasma levels of High-Mobility Group Box 1 during peptide vaccination in patients with recurrent ovarian cancer. J Immunol Res 2017; 2017: 1423683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamoto S, Yamada T, Terazaki Y, Yoshiyama K, Sugawara S, Takamori S et al. Feasibility study of personalized peptide vaccination for advanced small cell lung cancer. Clin Lung Cancer 2017; 18: e385–e394. [DOI] [PubMed] [Google Scholar]
- 16.Lee L, Bounds D, Paterson J, Herledan G, Sully K, Seestaller-Wehr LM et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br J Haematol 2016; 174: 911–922. [DOI] [PubMed] [Google Scholar]
- 17.Coquery CM, Erickson LD. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol 2012; 32: 287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez E, Smith EJ, Yashar MA, Patil S, Li M, Porter AL et al. The role of B-Cell Maturation Antigen in the biology and management of, and as a potential therapeutic target in, multiple myeloma. Target Oncol 2018; 13: 39–47. [DOI] [PubMed] [Google Scholar]
- 19.Moreaux J, Legouffe E, Jourdan E, Quittet P, Rème T, Lugagne C et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004; 103: 3148–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 2004; 199: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga C, Laubach JP, Anderson KC, Richardson PG. Investigational agents in immunotherapy: a new horizon for the treatment of multiple myeloma. Br J Haematol 2018; 181: 433–446. [DOI] [PubMed] [Google Scholar]
- 22.Terpos E; International Myeloma Society. Multiple Myeloma: Clinical Updates From the American Society of Hematology Annual Meeting, 2017. Clin Lymphoma Myeloma Leuk 2018; 18: 321–334. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez E, Tanenbaum EJ, Patil S, Li M, Soof CM, Vidisheva A et al. The clinical significance of B-cell maturation antigen as a therapeutic target and biomarker. Expert Rev Mol Diagn 2018; 18: 319–329. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 2013; 19: 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellucci R, Alyea EP, Chiaretti S, Wu CJ, Zorn E, Weller E et al. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood 2005; 105: 3945–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 2004; 173: 807–817. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez E, Tanenbaum EJ, Patil S, Li M, Soof CM, Vidisheva A et al. The clinical significance of B-cell maturation antigen as a therapeutic target and biomarker. Expert Rev Mol Diagn 2018; 18: 319–329. [DOI] [PubMed] [Google Scholar]
- 28.Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T Cell Bispecific Antibody EM801 for multiple myeloma treatment. Cancer Cell 2017; 31: 396–410. [DOI] [PubMed] [Google Scholar]
- 29.Hipp S, Tai YT, Blanset D, Deegen P, Wahl J, Thomas O et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 2017; 31: 1743–1751. [DOI] [PubMed] [Google Scholar]
- 30.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016; 128: 1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen AD, Garfall AL, Stadtmauer EA, Lacey SF, Lancaster E, Vogl DT et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Am Soc Hematol 2016; 128: 1147. [Google Scholar]
- 32.Kevin MF, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL et al. Effective targeting of multiple B-Cell Maturation Antigen–expressing hematological malignances by anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum Gene Ther 2018; 29: 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dou H, Yan Z, Zhang M, Xu X. APRIL, BCMA and TACI proteins are abnormally expressed in non-small cell lung cancer. Oncol Lett 2016; 12: 3351–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003; 281: 65–78. [DOI] [PubMed] [Google Scholar]
- 35.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004; 294: 15–22. [DOI] [PubMed] [Google Scholar]
- 36.Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple myeloma. Expert Opin Biol Ther 2017; 17: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 37.Tricot G, Jagannath S, Vesole DH, Bracy D, Desikan KR, Siegel D et al. Hematopoietic stem cell transplants for multiple myeloma. Leuk Lymphoma 1996; 22: 25–36. [DOI] [PubMed] [Google Scholar]
- 38.Rossmann E, Österborg A, Löfvenberg E, Choudhury A, Forssmann U, von Heydebreck A et al. Mucin 1-specific active cancer immunotherapy with tecemotide (L-BLP25) in patients with multiple myeloma: an exploratory study. Hum Vaccin Immunother 2014; 10: 3394–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Xu YY, Kalos M et al. Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clin Cancer Res 2014; 20: 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bae J, Munshi NC, Anderson KC. Immunotherapy strategies in multiple myeloma. Hematol Oncol Clin North Am 2014; 28: 927–943. [DOI] [PubMed] [Google Scholar]
- 41.Berry J, Vreeland T, Trappey A, Hale D, Peace K, Tyler J et al. Cancer vaccines in colon and rectal cancer over the last decade: lessons learned and future directions. Expert Rev Clin Immunol 2017; 13: 235–245. [DOI] [PubMed] [Google Scholar]
- 42.Cao JX, Zhang XY, Liu JL, Li JL, Liu YS, Wang M et al. Validity of combination active specific immunotherapy for colorectal cancer: a meta-analysis of 2993 patients. Cytotherapy 2015; 17: 1746–1762. [DOI] [PubMed] [Google Scholar]
- 43.Randazzo M, Terness P, Opelz G, Kleist C. Active-specific immunotherapy of human cancers with the heat shock protein Gp96-revisited. Int J Cancer 2012; 130: 2219–2231. [DOI] [PubMed] [Google Scholar]
- 44.Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC et al. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma. Leukemia 2011; 25: 1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae J, Tai YT, Anderson KC, Munshi NC. Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders. Br J Haematol 2011; 155: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bae J, Song W, Smith R, Daley J, Tai YT, Anderson KC et al. A novel immunogenic CS1specific peptide inducing antigen-specific cytotoxic T lymphocytes targeting multiple myeloma. Br J Haematol 2012; 157: 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae J, Smith R, Daley J, Mimura N, Tai YT, Anderson KC et al. Myeloma-specific multiple peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma and other plasma cell disorders. Clin Cancer Res 2012; 18: 4850–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bae J, Prabhala R, Voskertchian A, Brown A, Maguire C, Richardson P et al. A multiepitope of XBP1, CD138 and CS1 peptides induces myeloma-specific cytotoxic T lymphocytes in T cells of smoldering myeloma patients. Leukemia 2015; 29: 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae J, Keskin DB, Cowens K, Lee AH, Dranoff G, Munshi NC et al. Lenalidomide polarizes Th1-specific anti-tumor immune response and expands XBP1 antigen-specific central memory CD3+CD8+ T cells against various solid tumors. J Leuk 2015; 3: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae J, Hideshima T, Tai YT, Song Y, Richardson P, Raje N et al. Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors. Leukemia 2018. 10.1038/s41375-018-0062-8. [DOI] [PMC free article] [PubMed]
- 51.Nooka AJ, Wang M, Yee AJ, Thomas SK, O’Donnell EK, Shah JJ et al. Final results of a Phase 1/2a, dose escalation study of Pvx-410 multi-peptide cancer vaccine in patients with Smoldering Multiple Myeloma (SMM). American Society of Hematology 2016; 128: 2124. [Google Scholar]
- 52.Nooka AJ, Wang M, Yee AJ, Kaufman J, Bae J, Peterkin D et al. Safety and Immunogenicity of PVX-410 Vaccine ± lenalidomide in smoldering multiple myeloma. JAMA Oncology 2018; August 16:e183267 In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia 2018; 32: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanpouille-Box C, Lhuillier C, Bezu L, Aranda F, Yamazaki T, Kepp O et al. Trial watch: Immune checkpoint blockers for cancer therapy. Oncoimmunology 2017; 6: e1373237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa R, Costa RB, Talamantes SM, Helenoswki I, Carneiro BA, Chae YK, et al. Analyses of selected safety endpoints in phase 1 and late-phase clinical trials of anti-PD-1 and PD-L1 inhibitors: prediction of immune-related toxicities. Oncotarget 2017; 8: 67782–67789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou G, Noordam L, Sprengers D, Doukas M, Boor PPC, van Beek AA et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology 2018; 7: e1448332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 2017; 276: 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waight JD, Chand D, Dietrich S, Gombos R, Horn T, Gonzalez AM et al. Selective FcγR Co-engagement on APCs modulates the activity of therapeutic antibodies targeting T cell antigens. Cancer Cell 2018; 33: 1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pham Minh N, Murata S, Kitamura N, Ueki T, Kojima M, Miyake T et al. In vivo antitumor function of tumor antigen-specific CTLs generated in the presence of OX40 co-stimulation in vitro. Int J Cancer 2018; 142: 2335–2343. [DOI] [PubMed] [Google Scholar]
- 60.Dushyanthen S, Teo ZL, Caramia F, Savas P, Mintoff CP, Virassamy B et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat Commun 2017; 8: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.