Abstract
Hypoxia inducible factor 1alpha (HIF1α) is a key regulator of oxygen homeostasis and its target genes mediate adaptive, protective and pathological processes. The role of HIF1α in neuronal survival is controversial and the brain maturation stage is important in determining its function in brain ischemia or hypoxia ischemia (HI). In this study, we used neuron-specific HIF1α knockout mice at postnatal day 9 (P9) and immature cortical neurons (day 7-8 in vitro) treated with HIF1α inhibitor 2-methoxyestradiol (2ME2) or stabilizer dimethyloxalylglycine (DMOG) to examine the function of neuronal HIF1α in neonatal HI in vivo (Vannucci model) and in vitro (oxygen glucose deprivation, OGD). Inhibition of HIF1α with 2ME2 in primary neurons or deletion of neuronal HIF1α in P9 mice increased both necrotic and apoptotic cell death following HI as evaluated by the protein levels of spectrin breakdown products at 145/150kD and 120kD, at 24hr after HI. DMOG attenuated neuronal death right after OGD. Acute pharmacological manipulation of HIF1α synchronously regulated the expression of its targets vascular endothelial growth factor (VEGF) and erythropoietin (Epo) in the same manner The in vivo findings agree with our previous data using the same HIF1α deficient mice at an earlier age. The present study confirms the role of neuronal HIF1α signaling in the endogenous protective responses following hypoxia ischemia in the developing brain.
Keywords: brain, development, hypoxia/ischemia, hypoxia inducible factor 1alpha
Introduction
Hypoxic-Ischemic Encephalopathy (HIE) is an important complication at birth and a significant cause of neonatal death. Moderate to severe HIE are usually associated with poor neurological outcomes including motor impairment or cognitive deficits [1]. Strategies that boost endogenous brain protection or stimulate regenerative repair hold the promise of translating research into clinical practice. One such strategy is targeting the transcription factor hypoxia-inducible factor 1 (HIF1), which plays a critical role in regulating cellular adaptation to hypoxia and oxidative stress [2-4]. HIF1 consists of two subunits, HIF1α and HIFβ. HIFβ does not respond to changes in oxygen tension, while HIF1α stability, subcellular localization, as well as transcriptional activity are especially affected by the oxygen level [5,6]. In normoxic conditions HIF1α is rapidly degraded by the prolyl hydroxylase (PHD)-dependent proteasomal system, however, under hypoxia, HIF1α protein degradation is prevented [7,8]. Upon its accumulation and phosphorylation, HIF1α dimerizes with HIFβ, translocates into the nucleus and promotes the transcription of hypoxia response elements (HRE)-containing target genes that are involved in energy metabolism, redox homeostasis, erythropoiesis, vascular remodeling and other processes [9,10].
Induction of HIF1α has been reported in the ischemic penumbra in adult rats and mice, as well as in the neonatal brain following hypoxia-ischemia (HI) [11-15]. The role of HIF1α in mediating protective or injury mechanisms remains controversial, as HIF1α has been shown to upregulate both pro- and anti-survival genes [16-19]. The discrepancies are related to the model of brain injury, the timing and duration of HIF1α being stimulated or inhibited, the pathways that are activated downstream of HIF1α, the cell type in which HIF1α is induced, as well as the intercellular communication to transmit the HIF1α signaling. Selective loss of HIF1α function in astrocytes leads to different outcomes from neuron-specific inactivation of HIF1α [20]. Using Cre-loxP-mediated conditional knockout approach, we have shown that deficiency in forebrain neuronal HIF1α caused more severe cortical injury compared to their wildtype (WT) littermates without HIF1α deletion [21]. However, the underlying mechanisms are not clear. In this study, to further dissect out the specific contribution of HIF1α in neuronal cell death/survival, we used pharmacological strategies with a known HIF1α inhibitor 2-methoxyestradiol (2ME2) or a PHD inhibitor dimethyloxalylglycine (DMOG) that stabilizes HIF1α, to manipulate HIF1α levels in immature primary cortical neurons. We investigated whether acute manipulation of HIF1α expression has any impact on neuronal viability, as well as its effects on HIF1α substrates vascular endothelial growth factor (VEGF) and erythropoietin (Epo), both of which are proven to be beneficial to brain repair in rodent models of neonatal HI and neonatal stroke [22-26].
The maturation stage of the brain at the time of the HI insult is another important contributor to determine injury evolution, recovery and repair. Our previous work indicates that HIF1α is upregulated in postnatal day (P) 7 mice following neonatal HI [21,27], in P10 rats in a neonatal stroke model [28], and after treatment with desferoxamine, an iron chelator that could stabilize HIF1α, in neonatal stroke [29] or in primary hippocampal cultures undergoing oxygen glucose deprivation (OGD), an in vitro paradigm of hypoxia ischemia [30]. HIF1α deficient mice have increased brain injury at P7 in response to HI [21]. At the same age, hypoxic preconditioning alleviates brain damage in the WT mice, but not in the HIF1α knockout mice [27]. These studies support the role of HIF1α in the endogenous protective mechanisms and that HIF1α is important for hypoxic preconditioning in the neonatal brain. P7 mouse brain is equivalent to human brain at late-preterm, while the age of P9-10 is more adequate to mimic human brain of full term infants and therefore more relevant for studies of HIE. We chose to use P9 neuron-specific HIF1α knockout mice for the HI model in this study to confirm its consistency with the in vitro data and the results from the P7 mice.
Materials and Methods
All animal experiments were approved by the University of California San Francisco (UCSF) institutional animal care and use committee. C57BL/6 mice at embryonic day 15 (Charles River Laboratories Inc., Wilmington, MA) were used for primary cortical neuronal culture. The mice that carry homozygous loxP-flanking exon 2 of HIF1α alleles were bred with heterozygous mice expressing Cre recombinase under the control of the calcium/calmodulin-dependent kinase II α (CaMKII α) promoter to produce mice with a forebrain predominant, neuron-specific deletion of HIF1α (knock out, KO), as well as their wild-type (WT) littermates without the deletion [31-34]. All mice negative by PCR for the Cre gene were considered WT. Both sexes were used for neonatal hypoxia ischemia procedure at P9.
Neonatal Brain Hypoxic-Ischemic (HI) Injury
Neonatal HI was performed as previously described with the Vannucci procedure [35]. At P9, the pups were anesthetized with isoflurane (2-3% isoflurane/balance oxygen) and the left common carotid artery was electrocauterized. Animals were allowed to recover for one hour with their dam and then exposed to 60 minutes of hypoxia in a humidified chamber at 37°C with 10% oxygen/balance nitrogen. Sham-operated control animals received isoflurane anesthesia and exposure of the left common carotid artery without electrocauterization or hypoxia. HI and sham animals were returned to their dams until they were euthanized.
Primary cortical neuronal culture
Brains were removed from embryonic day 15 C57BL/6 mice [36] and maintained in high glucose Dulbecco's Modified Eagle Medium (DMEM) with 25μM of 2-mercaptoethanol at 4°C during dissection. Cortices were dissected and dissociated in trypsin (0.05% with EDTA) and DNAse (10 μg/ml) and then resuspended in neurobasal (NB) medium with 2% B27 and 1mM glutamine supplement (Gibco, Rockville, MD). The cells were plated onto poly-D-lysine precoated 96-well plates or 35-mm dishes (Corning Life Sciences, Tewksbury, MA) at a density of 1 × 105 cells/cm2. The cultures were kept at 37°C in a humidified incubator with a 5% CO2 atmosphere. Three days after plating, half of the medium was replaced with the NB medium with 5-Fluoro-2’- deoxyuridine (4 μg/ml) and Uridine (20 μg/ml) to inhibit growth of non-neuronal cells. The neurons were used on 7–8 day in vitro (DIV) for oxygen-glucose deprivation (OGD) experiments.
Oxygen-glucose deprivation and drug treatment
Briefly, the medium was washed out twice and replaced with NB medium without glucose (UCSF Cell Culture Facility) supplemented with B27/glutamine. Cultures were transferred to a humidified Modular incubator chamber (Billups-Rothenberg Inc., Del Mar, CA) and flushed by a gas mixture consisting of 95% N2/5% CO2 for 5 min. The chamber was then sealed and kept in 37°C incubator for 6 hours. Oxygen concentration was maintained at 0.3–0.5% monitored by an oxygen analyzer (MSA medical products, Pittsburgh, PA) throughout the experiment. OGD was ended by changing the medium to regular NB medium with glucose and returning the cells back to normoxic incubator. The control cells underwent the same medium change but with regular NB medium and was placed in nomoxic incubator [36]. 2ME2 (Sigma-Aldrich Corp, MO) was constituted in DMSO as a stock solution and further diluted in the culture medium to a final concentration of 50μM. DMSO was diluted in the same manner to a final of 0.5% as the vehicle control. DMOG (Sigma) was dissolved in the medium and used at 25μM final concentration. The drugs were maintained during OGD and for 24hr after OGD.
Extraction of nuclear/cytoplasmic fractions
The cortices from HIF-1α WT and KO mice were dissected at 24 hours after HI procedure, snap frozen and stored at −80 °C until use. Nuclear and cytoplasmic proteins were extracted with the NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s protocol. Briefly, the tissue was homogenized in 250 μl ice-cold CER I buffer with the 100x Halt™ protease and phosphatase inhibitors (Pierce Biotechnology). After incubation with 13.75μl CER II buffer and vortex, the sample was centrifuged for 10 min at 16,000xg at 4 °C and the supernatant was saved as the cytoplasmic extract. The pellet was resuspended in 100 μl ice-cold NER buffer, shaked at 1,500 rpm in the cold room for 20 min, and centrifuged at 16,000xg at 4 °C for 30 min. The resultant supernatant was considered as the nuclear extract. The cytoplasmic and nuclear protein aliquots were stored at −80 °C until use. The protein concentrations were measured by BCA assay kit (Pierce). The nuclear and cytosol protein was prepared from primary cortical neurons using the same kit.
Western blotting
For Western blot analysis, an equal amount of protein samples (25-30μg of cytoplasmic, 10-15μg of nuclear protein) was applied to 4-12% Bis-Tris SDS polyacrylamide gel electrophoresis and transferred to polyvinyl difluoride membrane. The blots were probed with the following primary antibodies overnight at 4°C: rabbit anti-HIF1α (Noves Biologicals LLC, Littleton, CO); rabbit anti-Epo (Santa Cruz Biotechnology Inc.; Dallas, Texas); rabbit anti-VEGF (Santa Cruz Biotechnology Inc.); rabbit anti-histone 3 (Cell Signaling Technology, Boston, MA); mouse anti-α spectrin (Millipore, Billerica, MA) and β-actin (Santa Cruz Biotechnology Inc.). Horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary IgG antibodies (Santa Cruz Biotechnology Inc.) were used, and signal was visualized with enhanced chemiluminescence (Amersham, GE Healthcare). Image J software was used to measure the mean optical densities (OD) and the areas of protein signal on radiographic film after scanning.
LDH assay
This assay was carried out with the primary neurons cultured on the 96 well plates in 100 μl of medium. Neuronal cell death was quantified by a colorimetric cytotoxicity detection kit (Roche, Indianapolis, IN) based on the measurement of lactate dehydrogenase (LDH) activity released from the damaged/dead cells into the culture medium. Fifty μl of medium was collected immediately after OGD (0hr) from the control or OGD-treated wells to represent cell death during the 6 hour of OGD, and after medium change, again, at 24 hours after OGD to indicate cell death during 24hrs of reoxygenation. At the end of 24hr, the medium was completely changed and the plates were frozen at −80°C for at least 2 hours and then thawed. Another 50 μl of medium was collected to serve as the leftover survived cells following OGD. The percentage of cell death was expressed as: (LDH value at 0hr + at 24hr)/(LDH value at 0hr + at 24hr + after freeze-thaw cycle) x100.
Statistical Analysis
LDH results and data of optical densities of Western Blotting are presented as mean ± SD and were evaluated statistically using SAS Wilcoxon-Mann-Whitney test. Differences were considered significant at p < 0.05.
Results
Pharmacological manipulation of HIF1α protein in primary cortical neurons
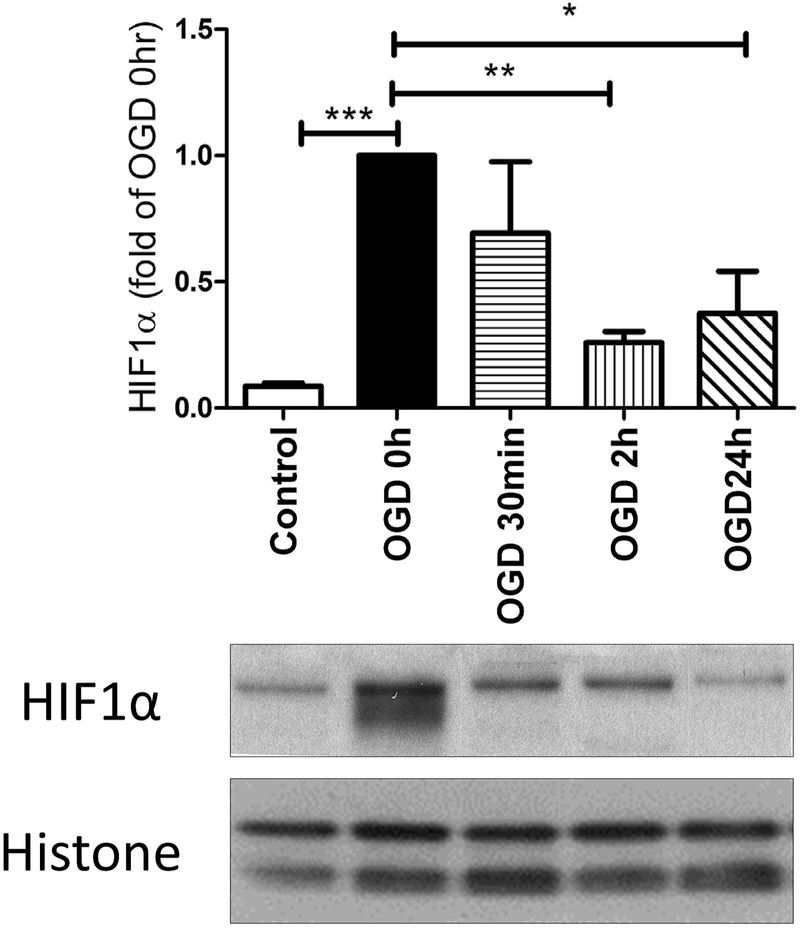
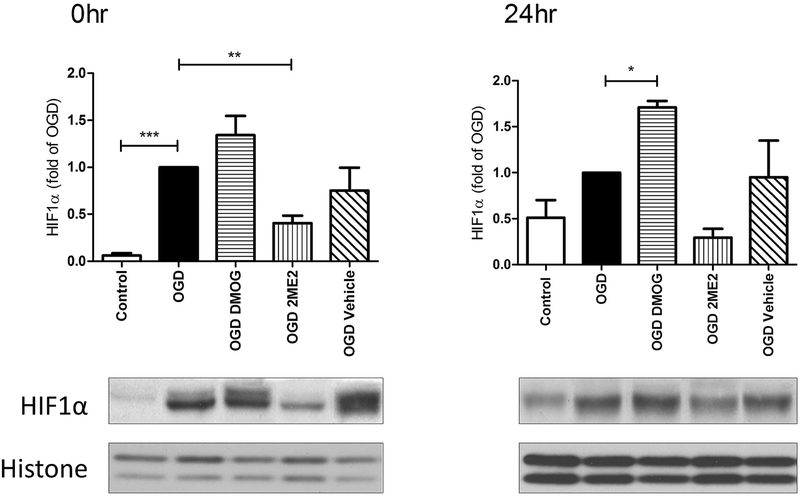
The expression of HIF1α protein was measured by Western Blotting at different time points following OGD, an in vitro model of hypoxia-ischemia. Fig. 1 showed that HIF1α accumulated during the 6 hours of OGD (measured immediately after OGD, i.e. OGD 0h) and was degraded upon reoxygenation. HIF1α expression decreased over time within 24hr. 2ME2, but not the vehicle control (0.5% DMSO), inhibited the increase of HIF1α during OGD, while DMOG stabilized the protein for at least 24hrs (Fig. 2). HIF1α was maintained at a lower level at 24hr with 2ME2 treatment although the difference with OGD alone was not significant (Fig. 2).
Fig. 1: The time course of HIF1α expression after OGD in primary cortical neurons.
HIF1α expression peaked immediately after OGD (0hr) and decreased over time after reoxygenation. (* p< 0.05, ** p< 0.01 vs. OGD 0hr; *** p< 0.001 vs. control cells; n= 4-5)
Fig. 2: Manipulation of HIF1α protein by 2ME2 and DMOG.
2ME2 inhibited HIF1α expression at 0hr, while DMOG stabilized HIF1α for at least 24hr. (* p< 0.05, ** p< 0.01 vs. OGD 0hr; *** p< 0.001 vs. control cells; vehicle was 0.5% DMSO. n= 4-6)
HIF1α inhibition enhanced necrotic and apoptotic cell death after OGD
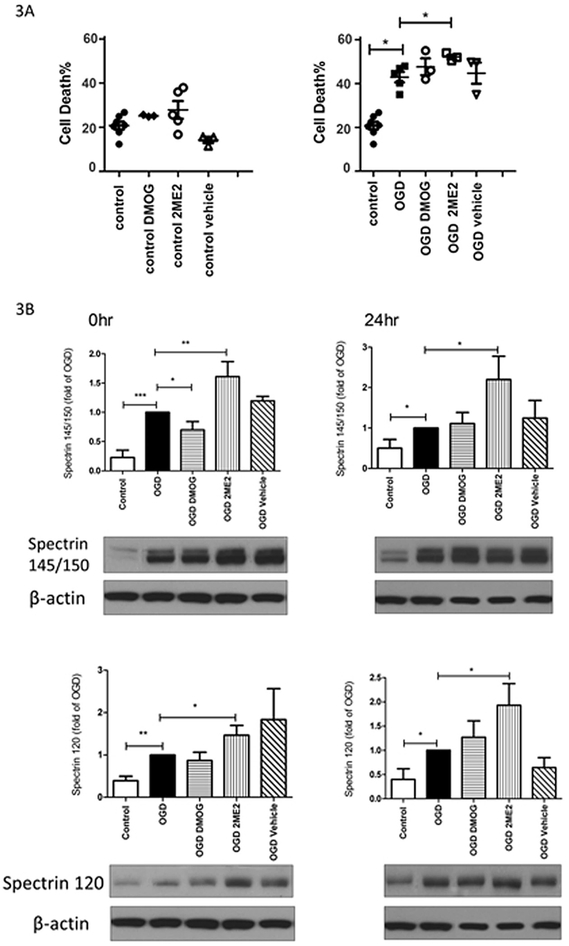
To study the role of HIF1α in the endogenous protective responses following OGD, the HIF1α inhibitor 2ME2 or stabilizer DMOG was added during and after OGD to determine their effects on cell viability. The LDH analysis (Fig. 3A) showed that 2ME2 at 50μM or DMOG at 25μM was not toxic to the neurons under the control normoxic condition. OGD increased cell death from 20.03 ± 2.64% (control) to 41.35 ± 5.76% (OGD) at 24hr thereafter (p<0.05 vs. control). HIF1α inhibition further augmented the neuronal death (53.57 ± 1.98%, p<0.05 vs. OGD) suggesting that HIF1α is involved in protective mechanisms after OGD. There was no difference following DMOG treatment compared to OGD alone. Increased spectrin cleavage by calcium-sensitive proteases such as calpain and caspases has been used as an indicator of necrotic and apoptotic cell death. Spectrin cleavage leads to elevated generation of spectrin breakdown products (SBDPs) of 145/150kD (indicative of necrotic and excitotoxic neuronal death) and of 120kD (represents apoptotic death) [37]. As shown in Fig. 3B, OGD induced the production of SBDPs at 145/150kD and 120kD at 0hr and 24hr after OGD (*p<0.05, **p<0.0l, ***p<0.00l vs. control), which was further increased by HIF1α inhibition with 2ME2 (*p<0.05, **p<0.0l vs. OGD alone). These data were consistent with the LDH results. On the contrary, stabilizing HIF1α with DMOG attenuated the expression of SBDPs at 145/150kD immediately after OGD, but not at 24hr post-OGD.
Fig. 3: HIF1α inhibition enhanced necrotic and apoptotic cell death after OGD.
3A: 2ME2 exposure during and after OGD caused more cell death than OGD alone by LDH assay (right). The drugs were not toxic to control cells (left). * p<0.05, n= 5-7. 3B: 2ME2 increased the levels of SBDP 145/150KD and SBDP 120KD at 0hr and 24hr after OGD compared to the OGD alone group. DMOG reduced SBDP 145/150KD expression right after OGD. (* p< 0.05, ** p< 0.01, *** p< 0.001; n= 4-5)
Epo and VEGF expression following OGD
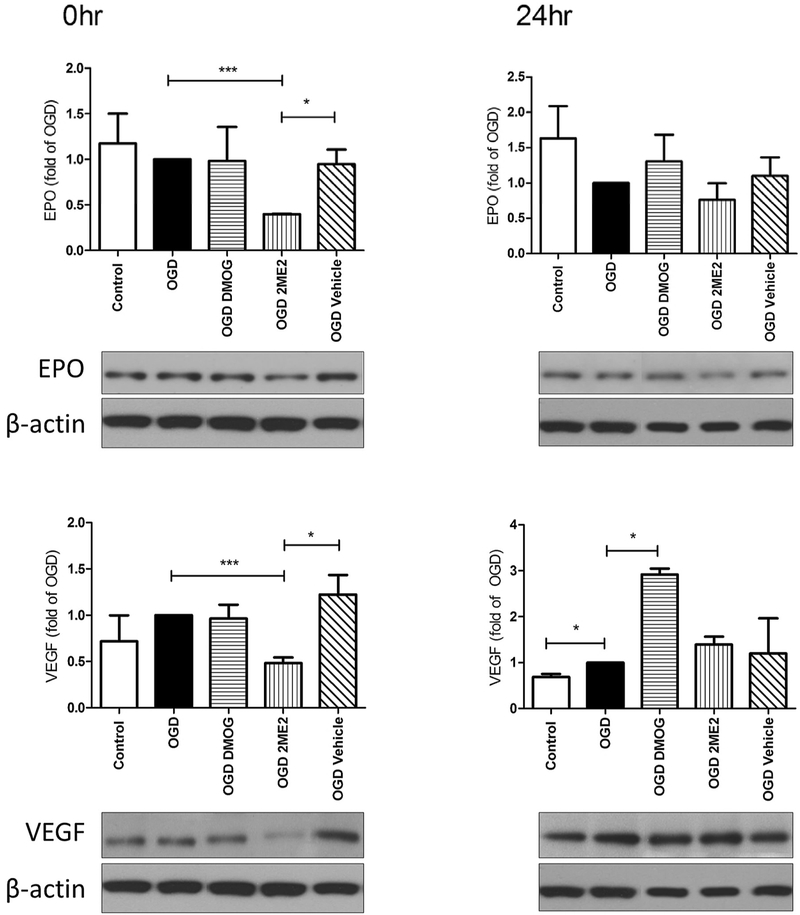
Epo and VEGF are known HIF1α substrates that are involved in neuroprotection in the rodent model of neonatal hypoxia-ischemia and neonatal stroke [22,38]. In our in vitro conditions, VEGF expression was slightly increased at 24hr after OGD (p<0.05, OGD vs. control, Fig. 4), while Epo was not induced at these early time points. HIF1α inhibition with 2ME2 diminished the levels of Epo and VEGF immediately after OGD (p<0.001, OGD 2ME2 vs. OGD alone, Fig. 4), but not at 24hrs thereafter. DMOG, however, increased the expression of VEGF at 24hr after OGD (p<0.05, OGD DMOG vs. OGD alone, Fig. 4).
Fig. 4: EPO and VEGF expression following OGD.
2ME2, but not the vehicle control, diminished Epo and VEGF expression at 0hr compared to OGD alone group (***p< 0.001). DMOG increased VEGF levels at 24hr after OGD. (* p< 0.05) n= 3-5
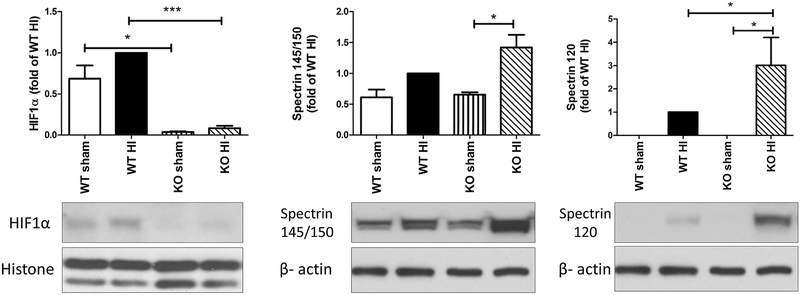
Neuronal specific HIF1α knockout mice have more severe brain injury after neonatal HI
We have shown previously that HIF1α deficient mice have exacerbated brain injury at P7 than their WT littermates following HI [21]. At a more mature stage of P9, these mice are similarly more vulnerable to HI brain damage. The expression of HIF1α protein was confirmed to be minimal in the HIF1α KO mice at 24 hours after HI (Fig. 5, * p< 0.05, WT sham vs. KI sham, *** p<0.001, WT HI vs. KI HI). Compared to the WT pups, the neuron-specific HIF1α KO mice had significantly higher levels of apoptotic cell death evaluated by the expression of SBDPs at 120kD (Fig. 5, * p< 0.05, WT HI vs. KI HI; KO sham vs. KO HI). These data agree with our observation when the mice were used at P7 in the same HI model.
Fig. 5: Neuron-specific HIF1α KO mice had more severe brain injury than the WT mice at P9 after HI.
HIF1α expression was abolished in the KO sham and HI-injured animals (left panel). The levels of SBDP 145/150KD and SBDP 120KD were significantly higher in the KO mice than those in the WT animals at 24hr post-HI (right panels). *: p< 0.05, ***: p< 0.001, n=4-7.
Discussion
Using genetic and acute pharmacological manipulation of HIF1α, we examined the function of neuronal HIF1α and its key effectors that may protect the neonatal brain or the immature neurons against HI injury in vivo and in vitro. We demonstrated that inhibition of HIF1α with 2ME2 in cortical neurons or deletion of neuronal HIF1α in P9 mice increased both necrotic and apoptotic cell death following hypoxia-ischemia, confirming the role of neuronal HIF1α signaling in HI neuroprotection in the developing brain. The in vivo findings are in accordance with our previous data using the same HIF1α deficient mice at P7 [21].
2-methoxyestradiol (2ME2) is an endogenous metabolite of estradiol and is the most commonly employed HIF1α inhibitor. The mechanism for its inhibition on HIF1α in a human tumor cell line and human umbilical vein endothelial cells is that 2ME2 inhibits HIF1αmRNA translation/de novo synthesis, and its association with microtubules and thereby blocks HIF1 nuclear accumulation and its transcriptional activity [39-41]. In adult rodent brain, administration of 2ME2 before or early after the insult protected against global ischemia [42] or middle cerebral artery occlusion (MCAO)-induced brain damage [43,44], except that one study reported that 2ME2 given 10min after ischemia exacerbated apoptotic death in the CA1 region of the hippocampus following global ischemia [45]. 2ME2 treatment at later time points (8hr after MCAO) was ineffective or even detrimental [46]. This was also true in neonatal HI where 2ME2 showed protection by reducing brain edema and infarct volume when used 5min after HI, whereas the protection was lost if 2ME2 was administrated 3hr after HI [47,48]. It is believed that HIF1α induction at early phase activates pro-death genes involved in apoptosis and autophagy, while late stage HIF1α increases substrates including VEGF that improve functional recovery and brain repair [28,46]. In our primary cortical neuronal cultures, 2ME2 effectively inhibited HIF1α accumulation during the 6hr of OGD. As a consequence, neurons with 2ME2 treatment had significantly more cell death as evaluated by two different cell viability assays (LDH measurement and the levels of SBDPs). The increased neuronal death was not due to 2ME2 toxicity, as at the dose of 50μM it did not cause additional death under normoxia when incubated for 30 hrs. On the other hand, when HIF1α was stabilized with the PHD inhibitor DMOG, while the LDH assay did not show any changes in cell death, it reduced the levels of SBDPs at 145/150kD right after OGD. DMOG was used at 25μM since the higher concentrations (tested at 50, 100 and 150μM) were toxic to primary neurons. These pharmacological data support the protective effects of HIF1α in immature neurons, while in the in vivo models, 2ME2 may regulate HIF1α in glial cells or other cell populations that collectively modify the overall pattern and severity of cell death. Furthermore, although 2ME2 is relatively specific for HIF1α inhibition, DMOG, as a competitive inhibitor of PHD used for HIF1α stabilization, also increases HIF2α expression. DMOG is a synthetic analogue of α- ketoglutarate and a dimethyl ester of N-oxalylglycine, therefore has multiple pharmacological effects including direct inhibition of mitochondrial function precedes HIF target gene expression [49]. A more specific strategy for HIF1α gain of function study is needed to further clarify its roles in neonatal brain hypoxia-ischemia.
The expression of VEGF and Epo was measured in the primary neurons to identify the HIF1α-mediated protective signaling pathways. They are important HIF1α target substrates implicated in angiogenesis and neurogenesis respectively, therefore, have been a focus of investigation for promoting recovery and regenerative repair after brain ischemia in rodents and human [50-53]. Under culture conditions, VEGF was upregulated at 24hr after OGD suggesting that it could be a downstream event following HIF1α activation, while Epo expression remained unchanged in the first 24hr after the insult. In a rat neonatal stroke model, we demonstrated that HIF1α and VEGF are co-localized in neurons with a similar time course of expression [28]. Epo is increased early in neurons, but later in astrocytes [29]. Recent studies indicate that HIF 2α has emerged as the transcription factor that regulates Epo synthesis in kidney, liver and brain as well [54-56]. Epo transcription and secretion are increased by hypoxia more in cortical astrocytes where HIF2α is located than those in the neurons [57]. This may explain why we did not observe changes in Epo expression in our primary neurons, or alternatively, Epo might be induced after 24hrs in our in vitro system. It’s not clear whether Epo expression in different cell type or at different timing has distinct significance, but we found that exogenous Epo given early or delayed after transient neonatal stroke improves histological and behavioral outcomes in P10 rats [38,58]. Interestingly, despite that VEGF and Epo were not enhanced right after 6hr of OGD, 2ME2 reduced their levels at this early time point, in parallel with its inhibition on HIF1α expression. On the contrary, overexpression of HIF1α by DMOG at 24hr after OGD was accompanied by an increase in VEGF at the same time. The correlated timing and pattern of changes suggest that VEGF, and perhaps Epo, are the possible HIF1α downstream mediators for neuroprotection, however, we cannot make this conclusion without inhibition of VEGF or Epo signaling. In our transient neonatal stroke model, treatment with SU5416, a VEGF receptor antagonist, enhanced cell death and limited angiogenesis [59], implying the role of VEGF in the recovery and repair in brain ischemia. VEGF is also involved in subventricular zone (SVZ) remodeling following neonatal HI, where VEGF A and C are produced by glial progenitor cells and astrocytes. VEGF A increases astrocyte proliferation and contributes to astrogliogenesis, whereas VEGF-C enhances the proliferation of oligodendrocyte progenitors, thereby promoting myelination and white matter regeneration after the injury [60].
The results from neuron-specific HIF1α knockout mice are in agreement with our in vitro data with HIF1α inhibition in primary neurons. It also confirms our previous findings that these mice have overall increased brain injury at P7 after neonatal HI [21] suggesting that the HIF1α responses are similar in this range of developing stage (P7-P9). The KO mice showed significantly higher level of spectrin 120kD than the WT pups after HI, but not as much of spectrin 145/150kD, indicating that HIF1α pathways might protect the cells against apoptosis, rather than the necrotic cell death in our neonatal HI model.
Taken together, we demonstrated in vitro and in vivo that HIF1α signaling mediates the endogenous protective responses after neonatal brain hypoxia-ischemia. It plays a key role in promoting neuronal survival by upregulation of its target protective or repair genes, including VEGF and Epo, or possibly via other molecular pathways. Identification of proteins involved in stabilization and activation of HIF1α could be advantageous in neonatal brain ischemic conditions, and may aid to discover new therapeutic approaches to neonatal HI.
Acknowledgements
This study was supported by NIH National Institute of Neurological Disorders and Stroke (5R35NS097299 to Dr. Ferriero; RO1NS084057 to Dr. Jiang).
Footnotes
The authors declare they have no conflicts to disclose.
References
- 1.Millar LJ, Shi L, Hoerder-Suabedissen A, Molnar Z: Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front Cell Neurosci 2017;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL: Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–594. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL: HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 2001;13:167–171. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL: Life with oxygen. Science 2007;318:62–64. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW: Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 2004;36:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Sharp FR, Bernaudin M: HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 2004;5:437–448. [DOI] [PubMed] [Google Scholar]
- 7.Salceda S, Caro J: Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 1997;272:22642–22647. [DOI] [PubMed] [Google Scholar]
- 8.Metzen E, Ratcliffe PJ: HIF hydroxylation and cellular oxygen sensing. Biol Chem 2004;385:223–230. [DOI] [PubMed] [Google Scholar]
- 9.Chavez A, Miranda LF, Pichiule P, Chavez JC: Mitochondria and hypoxia-induced gene expression mediated by hypoxia-inducible factors. Ann N Y Acad Sci 2008;1147:312–320. [DOI] [PubMed] [Google Scholar]
- 10.Majmundar AJ, Wong WJ, Simon MC: Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 2010;40:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez JC, LaManna JC: Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci 2002;22:8922–8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F: The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev 2009;62:99–108. [DOI] [PubMed] [Google Scholar]
- 13.Trollmann R, Gassmann M: The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain Dev 2009;31:503–509. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR: Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 1999;11:4159–4170. [DOI] [PubMed] [Google Scholar]
- 15.Singh N, Sharma G, Mishra V: Hypoxia inducible factor-1: its potential role in cerebral ischemia. Cell Mol Neurobiol 2012;32:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acker T, Acker H: Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol 2004;207:3171–3188. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Ostrowski RP, Obenaus A, Zhang JH: Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury. Exp Neurol 2009;216:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kietzmann T, Knabe W, Schmidt-Kastner R: Hypoxia and hypoxia-inducible factor modulated gene expression in brain: involvement in neuroprotection and cell death. Eur Arch Psychiatry Clin Neurosci 2001;251:170–178. [DOI] [PubMed] [Google Scholar]
- 19.Piret JP, Mottet D, Raes M, Michiels C: Is HIF-1alpha a pro- or an anti-apoptotic protein? Biochem Pharmacol 2002;64:889–892. [DOI] [PubMed] [Google Scholar]
- 20.Vangeison G, Carr D, Federoff HJ, Rempe DA: The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci 2008;28:1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon RA, Osredkar D, Lee CL, Jiang X, Mu D, Ferriero DM: HIF-1 alpha-deficient mice have increased brain injury after neonatal hypoxia-ischemia. Dev Neurosci 2009;31:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM: Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res 2013;4:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiseler SJ, Morland C: The Janus Face of VEGF in Stroke. Int J Mol Sci 2018;19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osredkar D, Sall JW, Bickler PE, Ferriero DM: Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis 2010;38:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu YW, Gonzalez FF: Erythropoietin: a novel therapy for hypoxic-ischaemic encephalopathy? Dev Med Child Neurol 2015;57 Suppl 3:34–39. [DOI] [PubMed] [Google Scholar]
- 26.Jain A, Kratimenos P, Koutroulis I, Jain A, Buddhavarapu A, Ara J: Effect of Intranasally Delivered rh-VEGF165 on Angiogenesis Following Cerebral Hypoxia-Ischemia in the Cerebral Cortex of Newborn Piglets. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheldon RA, Lee CL, Jiang X, Knox RN, Ferriero DM: Hypoxic preconditioning protection is eliminated in HIF-1alpha knockout mice subjected to neonatal hypoxia-ischemia. Pediatr Res 2014;76:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM: Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis 2003;14:524–534. [DOI] [PubMed] [Google Scholar]
- 29.Mu D, Chang YS, Vexler ZS, Ferriero DM: Hypoxia-inducible factor 1alpha and erythropoietin upregulation with deferoxamine salvage after neonatal stroke. Exp Neurol 2005;195:407–415. [DOI] [PubMed] [Google Scholar]
- 30.Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM: A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci Lett 2005;379:96–100. [DOI] [PubMed] [Google Scholar]
- 31.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC: Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 2007;27:6320–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragatsis I, Zeitlin S: CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis 2000;26:133–135. [DOI] [PubMed] [Google Scholar]
- 33.Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, Johnson RS, Lipton SA, Barlow C: Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci 2005;25:4099–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS: Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 2000;60:4010–4015. [PubMed] [Google Scholar]
- 35.Sheldon RA, Sedik C, Ferriero DM: Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res 1998;810:114–122. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X, Mu D, Manabat C, Koshy AA, Christen S, Tauber MG, Vexler ZS, Ferriero DM: Differential vulnerability of immature murine neurons to oxygen-glucose deprivation. Exp Neurol 2004;190:224–232. [DOI] [PubMed] [Google Scholar]
- 37.Yan XX, Jeromin A, Jeromin A: Spectrin Breakdown Products (SBDPs) as Potential Biomarkers for Neurodegenerative Diseases. Curr Transl Geriatr Exp Gerontol Rep 2012;1:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YS, Mu D, Wendland M, Sheldon RA, Vexler ZS, McQuillen PS, Ferriero DM: Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res 2005;58:106–111. [DOI] [PubMed] [Google Scholar]
- 39.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P: 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 2003;3:363–375. [DOI] [PubMed] [Google Scholar]
- 40.Mueck AO, Seeger H: 2-Methoxyestradiol--biology and mechanism of action. Steroids 2010;75:625–631. [DOI] [PubMed] [Google Scholar]
- 41.Yu T, Tang B, Sun X: Development of Inhibitors Targeting Hypoxia-Inducible Factor 1 and 2 for Cancer Therapy. Yonsei Med J 2017;58:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin XY, Pan J, Wang XQ, Ma JF, Ding JQ, Yang GY, Chen SD: 2-methoxyestradiol attenuates autophagy activation after global ischemia. Can J Neurol Sci 2011;38:631–638. [DOI] [PubMed] [Google Scholar]
- 43.Chen C, Hu Q, Yan J, Lei J, Qin L, Shi X, Luan L, Yang L, Wang K, Han J, Nanda A, Zhou C: Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem 2007;102:1831–1841. [DOI] [PubMed] [Google Scholar]
- 44.Cheng YL, Park JS, Manzanero S, Choi Y, Baik SH, Okun E, Gelderblom M, Fann DY, Magnus T, Launikonis BS, Mattson MP, Sobey CG, Jo DG, Arumugam TV: Evidence that collaboration between HIF-1alpha and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol Dis 2014;62:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou D, Matchett GA, Jadhav V, Dach N, Zhang JH: The effect of 2-methoxyestradiol, a HIF-1 alpha inhibitor, in global cerebral ischemia in rats. Neurol Res 2008;30:268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh SH, Ou LC, Gean PW, Hung JJ, Chang WC: Selective inhibition of early--but not late--expressed HIF-1alpha is neuroprotective in rats after focal ischemic brain damage. Brain Pathol 2011;21:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, Jadhav V, Tang J, Zhang JH: HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiol Dis 2008;31:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Jadhav V, Tang J, Zhang JH: HIF-1 alpha inhibition ameliorates neonatal brain damage after hypoxic-ischemic injury. Acta Neurochir Suppl 2008;102:395–399. [DOI] [PubMed] [Google Scholar]
- 49.Zhdanov AV, Okkelman IA, Collins FW, Melgar S, Papkovsky DB: A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression. Biochim Biophys Acta 2015;1847:1254–1266. [DOI] [PubMed] [Google Scholar]
- 50.Alexander ML, Hill CA, Rosenkrantz TS, Fitch RH: Evaluation of the therapeutic benefit of delayed administration of erythropoietin following early hypoxic-ischemic injury in rodents. Dev Neurosci 2012;34:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM: Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci 2009;31:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juul SE, Comstock BA, Heagerty PJ, Mayock DE, Goodman AM, Hauge S, Gonzalez F, Wu YW: High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): A Randomized Controlled Trial - Background, Aims, and Study Protocol. Neonatology 2018;113:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traudt CM, McPherson RJ, Bauer LA, Richards TL, Burbacher TM, McAdams RM, Juul SE: Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev Neurosci 2013;35:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haase VH: Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013;27:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A: Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci 2002;22:10291–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeo EJ, Cho YS, Kim MS, Park JW: Contribution of HIF-1alpha or HIF-2alpha to erythropoietin expression: in vivo evidence based on chromatin immunoprecipitation. Ann Hematol 2008;87:11–17. [DOI] [PubMed] [Google Scholar]
- 57.Chavez JC, Baranova O, Lin J, Pichiule P: The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 2006;26:9471–9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF: Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis 2016;93:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimotake J, Derugin N, Wendland M, Vexler ZS, Ferriero DM: Vascular endothelial growth factor receptor-2 inhibition promotes cell death and limits endothelial cell proliferation in a neonatal rodent model of stroke. Stroke 2010;41:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW: Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res 2013;4:158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]