Abstract
Background:
While increased levels of circulating inflammatory cytokines in chronologically aged humans have been linked to the development of aging-associated chronic disorders (e.g., cardiovascular disease, type II diabetes, osteoporosis and Alzheimer’s disease), approaches that reduce circulating cytokines are not yet available. In chronologically aged mice, we recently demonstrated that epidermal dysfunction largely accounts for age-associated elevations in circulating cytokine levels, and that improving epidermal function reduced circulating cytokine levels.
Objective:
We performed a pilot study to determine whether improving epidermal function reduces circulating proinflammatory cytokine levels in aged humans.
Methods:
Thirty-three aged humans were treated twice-daily for 30 days, with ≈3 ml of an emollient, previously shown to improve epidermal function, while untreated, aged humans and a cohort of young volunteers served as controls. Changes in epidermal function and levels of three key, age-related, plasma cytokines (IL-1β, IL-6 and TNFα) were measured at baseline and after treatment, using Luminex 200™ system.
Results:
We also found significantly higher baseline levels of IL-1β, IL-6 and TNFα in aged vs. young humans (p<0.001), as previously reported. Topical applications of the barrier repair emollient significantly enhanced epidermal permeability barrier function (p<0.01) and stratum corneum hydration (p<0.05). In parallel, circulating levels of IL-1β and IL-6 normalized, while TNFα levels declined substantially.
Conclusion:
The results of this preliminary study suggest that a larger clinical trial should be performed to confirm whether improving epidermal function also can reduce circulating proinflammatory cytokine levels in aged humans, while also possibly attenuating the downstream development of chronic inflammatory disorders in the aged humans.
Keywords: Aging, Age Skin, Barrier Function, Stratum Corneum Hydration, Inflammation, IL-1β, IL-6, TNFα, Systemic Disorders
Introduction
Aged humans exhibit chronic, subclinical systemic inflammation, commonly termed ‘inflammaging’, potentially linked to the downstream emergence of several age-associated chronic disorders (e.g., atherosclerosis, type II diabetes, osteoporosis, Alzheimer’s disease)1–3. While the etiology of this age-associated, systemic inflammation remains uncertain, our recent studies have shown that both aged human and mouse skin display sustained abnormalities in epidermal permeability barrier homeostasis, stratum corneum (SC) hydration, and elevations in SC pH4–6, each of which has been shown to independently provoke cutaneous inflammation. We showed further that disruption of the epidermal permeability barrier provokes 1) an increase in cutaneous cytokine production; and 2) an increase in serum cytokine levels, independent of hepatic or T cell involvement7. Moreover, chronologically aged mice display elevations in both cutaneous and circulating levels of cytokines; and directly pertinent to this study, improving epidermal function in aged mice reduced cutaneous, as well as circulating levels of three key, age-associated cytokines7. In this proof-of-concept preliminary pilot study, we assessed whether improving epidermal function with an emollient, containing a mixture of lipids that mimics the components of normal SC, lowers circulating levels of these same pro-inflammatory cytokines in aged humans.
Materials and Methods
Human Subjects and Treatment Protocol:
Based on the results of our prior study in aged humans, we performed N power calculations for required number of subjects, which showed that 25 subjects should suffice to detect significant reductions in circulating levels of IL-1β, IL-6 and TNFα. But because of an anticipated 20% case loss, we initially enrolled 30+ volunteers in both groups. Therefore, a total of 63 aged volunteers, and 11 young volunteers (age 32.7 ± 0.9) were enrolled (Table 1). None of the subjects had a history of or visible signs of inflammatory skin diseases, or other known inflammatory disease. All stopped the use of topical skin care products, including soaps, for at least seven days prior to study entry. All of the aged subjects resided in the same assisted living facility, and alternately assigned to either the treated or untreated control group in order of registration (Fig 1). Because a previous study had shown that topical applications of Atopalm® (Supplemental Table 1), an over-the-counter, triple lipid formulation (Neopharm, South Korea), improves epidermal function in humans8, we chose this formulation here. The entire skin surface of the treated, aged subjects was treated topically with ≈3ml of this formulation twice-daily for one month, while the parallel, aged cohort remained untreated. To ensure consistency in the volume of cream applied, as well as the timing of each application, all topical applications were performed by designated, trained staff. The untreated, young control group were recruited from the residents of Dalian City. This pilot study was carried out during the early Spring (i.e., from March 14 to April 14, 2017), to prove the concept that improvements in epidermal function can lower circulating levels of cytokines. Because of the preliminary nature of this pilot study, we did compare the efficacy of Atopalm® to other products.
Table 1.
Demographic Data of Human Subjects
| Age Group | Assigned Treatment | Gender | Number | Age Range (median) | Hydration | TEWL | pH |
|---|---|---|---|---|---|---|---|
| Young Controls | Untreated | Females | 9 | 29–38 (32) | 32.73 ± 0.94 | 17.70 ± 1.16 | 5.47 ± 0.13 |
| Males | 2 | ||||||
| Aged | Untreated | Females | 23 | 58–93 (73) | 28.63 ± 1.25 | 12.48 ± 0.91 | 5.61 ± 0.15 |
| Males | 7 | ||||||
| Treated | Females | 21 | 58–95 (82) | 26.24 ± 1.16 | 13.66 ± 0.78 | 5.65 ± 0.16 | |
| Males | 12 |
Figure 1.
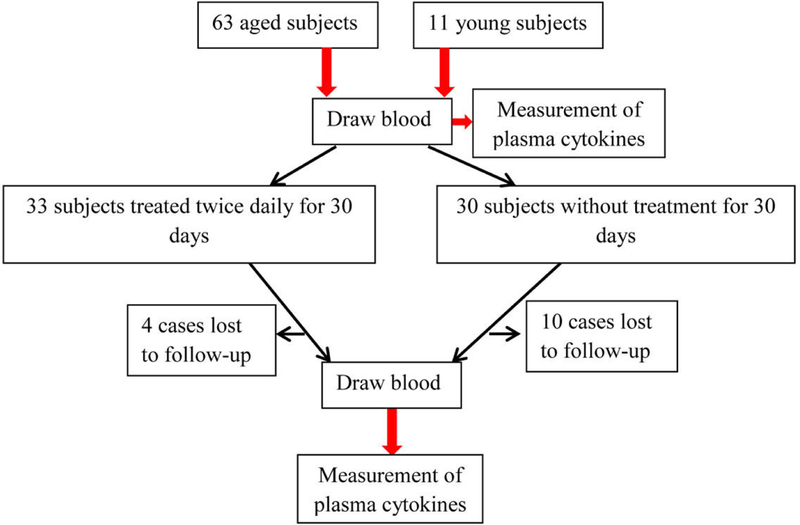
Flowchart of Subject Recruitment and Treatment
This human research protocol was approved by the Institutional Review Boards of Dalian Skin Disease Hospital, and registered with the Chinese Clinical Trials Registry (ChiCTR-RPC-16007717, “Role of epidermal permeability barrier function in systemic inflammation”), dated January 1, 2016, in accordance with the Helsinki principles, and informed consent was obtained from participants prior to trial entry.
Measurement of Plasma Cytokines:
Immediately prior to study entry and at the end of the study, blood samples were collected from both aged cohorts, and the young, control subjects for assessments of changes in circulating levels of the key, age-associated inflammatory markers. Cytokine levels were measured with Human Cytokine/Chemokine Magnetic Bead Panel (Item # HCYTOMAG-60k-05) (Millipore, Billerica, MA, USA), using Luminex 200tm system (Austin, TX, USA).
Measurements of Epidermal Function:
A multifunctional skin physiology monitor (MPA5, Courage–Khazaka Electronic GmbH, Köln, Germany) was used to measure SC hydration (capacitance), skin surface pH and transepidermal water loss (TEWL) on the right forearm prior to and at the end of this study. Measurements were obtained in a room maintained at temperature of 18±2 oC and relative humidities of 60±4%.
Subject Exclusion:
One young control subject was excluded because his baseline cytokine levels were > 80 times higher than the next highest in the same cohort. The Q test determined that exclusion was appropriate (Q=0.99).
After treatment, IL-6 levels in one subject in the untreated, aged group was 3 times higher than the next highest level. The Q test again determined that exclusion of this subject was appropriate (Q=0.7138).
Statistics:
All data are expressed as the mean ± SEM, using GraphPad Prism 4 software (San Diego, CA, USA) for statistical analyses. A paired t test was used to determine the differences in epidermal function between pre- and post-treatment. Unpaired t test with Welch’s correction was used to determine the significances of differences between young and aged group, and between treated and untreated aged group. A one-way ANOVA with Dunnett’s Multiple Comparison Test was used to determine the significant differences between treated and untreated aged vs. young groups. In cases of missing data, last observation carried forward imputation method was also used to analyze the data. Because missing data in the present study was “Missing at Random”, and is usually ignorable, data were not analyzed by multiple imputation; instead, a common “complete case analysis” was used. Significances are indicated in figure legends.
Results
Topical Applications of Physiologic Lipid Containing Formulation Improve Epidermal Functions
Aged humans display multiple functional abnormalities, including elevations in SC pH, decreased SC hydration and impaired epidermal permeability barrier homeostasis4–6. Our previous studies showed that topical applications of this formulation improved SC hydration and epidermal permeability barrier homeostasis in both humans and mice8,9. As shown in Fig. 2, 30 days of treatment with Atopalm® markedly increased SC hydration, while also significantly lowering surface pH vs. pretreatment levels. Although transepidermal water loss rates declined in both treated and untreated groups, a more significant reduction was observed in the treated group (p<0.01, versus pretreatment). These results indicate that this formulation improves key epidermal functions in aged humans.
Figure 2. Topical Treatments with Physiologic Lipid Containing Formulation Improve Epidermal Function in Aged Humans.
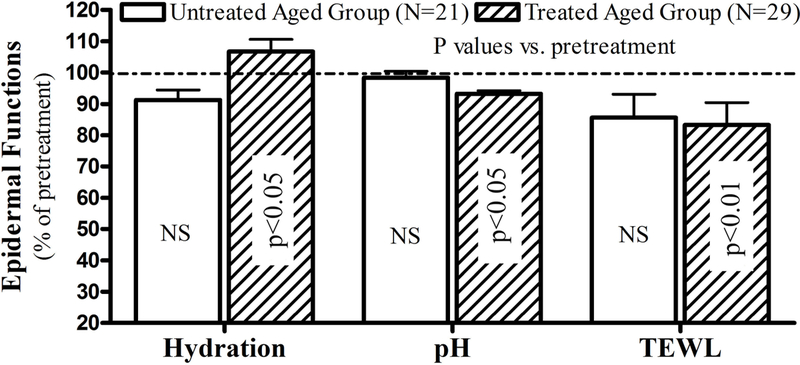
Subjects and treatments were treated as described in Materials and Methods and Fig 1. Data were expressed as percent of pretreatment, setting pretreatment levels as 100%. Number of subject and p values are indicated in the figure
Topical Applications of Physiologic Lipid Containing Formulation Reduce Circulating Levels of IL-1β, IL-6 and TNFα in Aged Humans
Aged humans exhibit elevated circulating levels of several cytokines10, but of these cytokines, IL-1β, IL-6 and TNFα are considered most strongly associated with the development of age-associated chronic disorders11. Hence, we next compared basal levels (pretreatment) of these three cytokines in the plasma of aged vs. young humans (Table 1). As shown in Fig. 3a, basal levels of plasma IL-1β, IL-6 and TNFα were significantly elevated in chronologically aged individuals vs. young human controls (see also12). We next assessed whether repeated, topical applications of the physiologic lipid containing formulation reduce circulating levels of IL-1β, IL-6 and TNFα in aged humans. After 30 days of twice-daily topical treatments, circulating levels of IL-1β and IL-6 decreased significantly in the treated aged cohort vs. the untreated aged controls (Fig. 3b). Surprisingly, these topical treatments reduced circulating levels of IL-1β and IL-6 to levels comparable to young controls (IL-1β: 13.8±1.4 in treated aged vs. 10.5 ± 2.2 in young humans; IL-6: 5.7 ± 0.9 in treated aged vs. 5.1 ± 0.9 in young humans). Though levels of TNFα declined by over 40% in comparison to untreated aged humans, the difference did not attain statistical significance (p=0.1032 vs. untreated aged controls). In consideration of missing data, we also analyzed data using the ‘last observation carried forward’ method. The trend of changes in cytokine levels (Suppl Fig 1) were similar to that described above. Together, these results suggest that improvements in epidermal function can largely normalize circulating levels of three pro-inflammatory cytokines that are strongly associated with chronologic aging.
Figure 3. Circulating Levels of Inflammatory Cytokines in Chronically Aged Humans at Baseline and Post-Treatment.
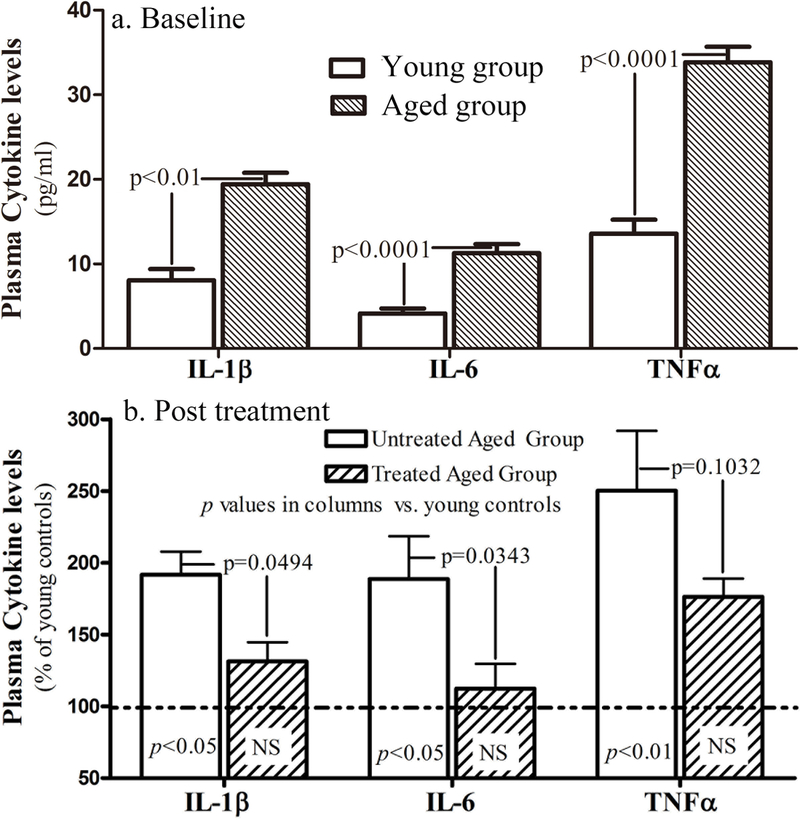
a: Baseline levels of circulating inflammatory cytokines in young vs. aged humans. Significances are indicated in the figure. N=10 for young group; N=57 for aged group b: Changes in circulating levels of three inflammatory cytokines following topical applications of physiologic lipid containing formulation. The significances are indicated in the figures (N=29 in treated group; N=20 in untreated controls [N=19 for IL-6]).
Discussion
Among circulating cytokines known to be elevated in the elderly, IL-1β and IL-6 are considered of particular importance in the pathogenesis of age-associated disorders11. For example, serum levels of both IL-1β and IL-6 increase in several age-associated disorders, including cardiovascular disease, Alzheimer’s disease, and type II diabetes11. Moreover, elevated IL-6 levels have been linked to the development of other age-associated disorders, including lymphoma and osteoporosis12, and further associated with increased mortality in community-dwelling, aged adults13. Finally, very recent studies have demonstrated that canakinumab, an antibody directed against IL-1β, decreases cardiac events, independent of its effects on serum lipids14. Taken together, alleviation of systemic inflammation could in theory prevent and/or mitigate the development of certain age-associated chronic disorders, linked to “inflammaging”.
Although a pathogenic role of ‘inflammaging’ in the development of systemic disorders in aged humans is widely acknowledged1–3,15,16, the possibility that the responsible cytokines could originate in the skin has not yet been explored. Yet, because of its sheer size, the skin is worthy of consideration as a potential contributor to systemic inflammation. In support of this potentially new paradigm, circulating levels of cytokines increase in inflammatory dermatoses that display prominent epidermal functional abnormalities, including atopic dermatitis and psoriasis, and both of these disorders are linked to the downstream development of chronic systemic disorders, such as atherosclerosis and type II diabetes. However, whether aging causes inflammation or vice versa is not clear. At least in the skin, we postulated that aging compromises epidermal functions, leading to productions of pro-inflammatory cytokines because a) aged skin displays multiple functional abnormalities, including compromised permeability barrier homeostasis, elevated SC pH and reductions in SC hydration, which all can increase pro-inflammatory cytokine production, and b) improvements in epidermal function can lower levels of pro-inflammatory cytokines in both the skin and circulation7. Despite the evidence from this pilot study, this hypothesis remains to be validated in future studies.
As early as age 50, chronologically aged humans exhibit multiple cutaneous functional abnormalities, which persist into advanced aging4–6, paralleled by sustained elevations in several markers of cutaneous inflammation10. Because these elevations in cytokine expression could eventually provoke systemic inflammation, improvements in epidermal function could in theory prevent and/or mitigate systemic inflammation. Chronologically aged mice, with no clinical evidence of inflammation, exhibit cutaneous functional abnormalities5,6, which are accompanied by elevated levels of certain cytokines in the skin and circulation7. Pertinently, disruption of epidermal permeability barrier function by repeated tape-stripping, and following acute exposure to erythemogenic UV-B provoke increases in the levels of cutaneous and circulating cytokines, including IL-1β, IL-6 and TNFα17,18.
Our recent studies showed that improving epidermal functions in chronologically aged mice skin normalizes not only cutaneous, but also serum levels of cytokines7. In this pilot study, we show that topical applications of a physiologic lipid-containing emollient, known to improve epidermal function in humans8, normalized circulating levels of key pro-inflammatory cytokines, closely associated with the development of chronic diseases in otherwise normal aged humans.
Yet, while the present pilot study provides evidence that topical applications of a physiologic lipid containing emollient reduces circulating levels of certain cytokines in aged humans, the clinical significance of the present findings need to be addressed in proper controlled clinical trials. Whether this physiologic lipid containing emollient also lowers levels of other inflammatory markers, such as amyloid A, also remains to be explored. Other remaining questions include: whether other forms of topical therapy, which also improve epidermal functions, such as petrolatum or other emollients, are also effective; and what surface area of the body needs to be treated in order to lower circulating cytokine levels? Ultimately, it will be important to determine whether sustained improvements in epidermal functions can delay or attenuate the downstream development of chronic age-associated systemic disorders.
Supplementary Material
Supplemental Figure 1. Circulating Levels of Inflammatory Cytokines Analyzed by Last Observation Carried Forward Method. a: Baseline levels of circulating inflammatory cytokines in young vs. aged humans. Significances are indicated in the figure. N=10 for young group; N=63 for aged group b: Changes in circulating levels of three inflammatory cytokines following topical applications of physiologic lipid containing formulation. N=10 for young group; N-33 for treated aged group and N=30 for untreated aged group.
Acknowledgements
The authors gratefully acknowledge the editorial assistance of Ms. Joan Wakefield.
Funding sources: This work was supported by the National Natural Science Foundation of China (81430037, 81573075), and NIH grants AR061106, AR051930 and AG028492, administered by the Northern California Institute for Research and Education, with resources from the Research Service, Department of Veterans Affairs. Neither these sponsors not manufacturer of Atopalm® cream played a role in designing this study, writing this article; or in the decision to submit this manuscript for publication.
Footnotes
Conflicts of interest: PME and MQM serve as consultants to Neopharm, Ltd., South Korea. An invention disclosure has been filed with the UCSF Office of Innovation, Technology & Alliances for the concept of preventing/treating systemic disorders using strategies that improve epidermal function.
REFERENCES
- 1.Deleidi M, Jäggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci 2015; 9,172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014; 69,S4–9. [DOI] [PubMed] [Google Scholar]
- 3.Nadrowski P, Chudek J, Skrzypek M, et al. Associations between cardiovascular disease risk factors and IL-6 and hsCRP levels in the elderly. Exp Gerontol 2016; 85,112–117. [DOI] [PubMed] [Google Scholar]
- 4.Man MQ, Xin SJ, Song SP, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol 2009; 22,190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol 2007; 127,2847–56. [DOI] [PubMed] [Google Scholar]
- 6.Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest 1995; 95,2281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu L, Mauro TM, Dang E, et al. Epidermal Dysfunction Leads to an Age-Associated Increase in Levels of Serum Inflammatory Cytokines. J Invest Dermatol 2017; 137,1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong S, Lee SH, Park BD, Wu Y, Man G, Man MQ. Comparison of the Efficacy of Atopalm(®) Multi-Lamellar Emulsion Cream and Physiogel(®) Intensive Cream in Improving Epidermal Permeability Barrier in Sensitive Skin. Dermatol Ther (Heidelb) 2016; 6,47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man MQM, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol 1996; 106,1096–1101. [DOI] [PubMed] [Google Scholar]
- 10.Puzianowska-Kuźnicka M, Owczarz M, Wieczorowska-Tobis K, et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing 2016; 13,21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 2010; 17,314–21. [DOI] [PubMed] [Google Scholar]
- 12.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000; 51,245–70. [DOI] [PubMed] [Google Scholar]
- 13.Lee JK, Bettencourt R, Brenner D, Le TA, Barrett-Connor E, Loomba R. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One 2012; 7,e34218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377,1119–1131. [DOI] [PubMed] [Google Scholar]
- 15.Monti D, Ostan R, Borelli V, Castellani G, Franceschi C. Inflammaging and human longevity in the omics era. Mech Ageing Dev 2017;165:129–138. [DOI] [PubMed] [Google Scholar]
- 16.Fougère B, Boulanger E, Nourhashémi F, Guyonnet S, Cesari M. Chronic Inflammation: Accelerator of Biological Aging. J Gerontol A Biol Sci Med Sci 2017;72:1218–1225. [DOI] [PubMed] [Google Scholar]
- 17.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005; 2005,273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf P, Gruber-Wackernagel A, Rinner B, et al. Phototherapeutic hardening modulates systemic cytokine levels in patients with polymorphic light eruption. Photochem Photobiol Sci 2013;12,166–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Circulating Levels of Inflammatory Cytokines Analyzed by Last Observation Carried Forward Method. a: Baseline levels of circulating inflammatory cytokines in young vs. aged humans. Significances are indicated in the figure. N=10 for young group; N=63 for aged group b: Changes in circulating levels of three inflammatory cytokines following topical applications of physiologic lipid containing formulation. N=10 for young group; N-33 for treated aged group and N=30 for untreated aged group.


