Concise Summary
This analysis of death certificate data in Washington State revealed that age-standardized abdominal aortic aneurysm (AAA) related mortality has declined from 1996 to 2016 with a notable significant drop in ruptured AAA-related mortality. The death rate trends vary by sex, race, and county.
OBJECTIVE
Management of abdominal aortic aneurysms (AAA) has undergone considerable advances over the last two decades. Our aim was to evaluate AAA-related mortality trends in Washington State (WA) over a 21 year period and to assess variation in AAA-related mortality by sex, race, and county over the same time period. We hypothesized that a significant decline in AAA-related mortality in WA would be noted.
METHODS
Death certificate records were obtained from the WA Department of Health for the period of 1996 to 2016. Records in which AAA was listed as an underlying or an associated cause of death were selected for analysis. Age-standardized mortality rates for each year were calculated using the 2016 WA population as the standard. Mortality trends were compared by sex and race using linear regression. County-specific age-standardized ruptured AAA (rAAA) mortality rates were compared using a Kruskal-Wallis test.
RESULTS
Of the 1,014,039 deaths occurring in WA during the study period, 4,438 (0.4%) had AAA listed as an underlying or associated cause of death (66.1% Male, 94.8% White, mean age of death 79.4 ± 9.3 years). In 64.1% of the cases, AAA was listed as the underlying cause of death. AAA-related mortality rates declined by 62.1% over the 21 years from 5.8 2.2 deaths per 100,000. Notably, there was a statistically significant drop in ruptured AAA (rAAA) related mortality rates (3.2 to 0.95 per 100,000, decline of 0.12 death/100,000/year, 95% CI: 0.11–0.14, r2 = 0.95). Men had a significantly steeper decline in age standardized AAA-related mortality rates with 55% decline (6.5 to 3.0 per 100,000) vs. a 41% decline (2.4 to 1.4 per 100,000) among women. , and were younger at the time of death compared to women (78.1 ± 9.4 vs. 81.9 ± 8.6 years respectively, P<.001), Individuals who were white had a significantly steeper decline in age standardized AAA-mortality rates with a 53% decline (5.3 to 2.5 per 100,000) compared to a 13% decline among individuals who were nonwhite (1.5 to 1.3 per 100,000). Age-standardized rAAA-related mortality rates varied by county (P <.001).
CONCLUSIONS
Age-standardized AAA-related mortality rate has declined in WA between the years 1996 and 2016, with a notable drop in rAAA-related mortality rate. The AAA-related mortality rates decline varied by sex and race. Additionally, rAAA-related mortality rates differed between counties. These observations are first step toward regional population assessments. Future work to understand the sources of variation can influence public health interventions on a state level.
Keywords: Aortic aneurysms, Ruptured aortic aneurysm, Aortic related mortality, Sex differences, Cause of Death/trends
Introduction
Abdominal Aortic aneurysms (AAAs) are a major cause of mortality with an estimated rupture-related mortality risk of 35 – 67%.1 The U.S. Preventive Services Task Force (USPSTF) estimates the prevalence of AAA at 6–7% of men and 2% of women aged 65–75 years old with a smoking history, and 3% in men and <0.6% in women without a smoking history.2 Multiple public health campaigns and changes in management have occurred over the last few decades to reduce this risk in the United States and globally.3 These include atherosclerosis risk factor modification, decreasing smoking rates, implementation of AAA screening programs, and the introduction of endovascular abdominal aortic aneurysm repair (EVAR). A recent meta-analysis demonstrated an overall decrease in AAA prevalence worldwide with a prevalence ranging from 2.2% in the United States to 6.7% in Australia.4 Few studies have closely examined AAA related mortality and geographic variation in mortality in the modern era.5,6 The aim of this study is to evaluate the trends in AAA-related mortality rates in Washington State (WA) over the last two decades and compare these trends by gender, race, and geography. We hypothesized that due to the advances in AAA care made over this time period, a significant decline in AAA-related mortality in WA would be noted.
Methods
The University of Washington Human Subjects Division reviewed this project (STUDY00001645) and determined that this research does not involve human subjects, as defined by federal and state regulations. Therefore, review and approval by the IRB was not required.
Washington State Mortality Data
Publicly available annual mortality data were obtained from the WA State Department of Public Health from 1996 to 2016, inclusive. Mortality data were abstracted from the death certificates for all WA State residents irrespective of the location of death. Data included the underlying cause of death and up to 14 associated causes of death based on established multiple cause of death methodology.7 Causes of death were coded using the 9th revisions of the International Statistical Classification of Diseases (ICD-9) prior to 1999 and the 10th revision (ICD-10) after 1999, as the WA Department of Public Health back-coded all deaths after 1999 to the ICD-10 schema. Additional information included individual-level demographics (age at death, race, gender, city of residence, education level, marital status), location of death, and type of facility where the death occurred (such as home, nursing home, hospital, etc.).
Cohort
Records in which AAA (ICD9 codes 441.3, 441.4 and ICD10 codes 171.3,171.4) appeared as an underlying or associated cause of death were selected for analysis. Contradictions in diagnostic codes were resolved as follows:
Rupture ascertainment: Whenever a diagnostic code for a “rupture” was present (ICD9 codes 441.3; ICD10 codes 171.3), the cause of death was categorized as a “ruptured” AAA death even if the code for a “non-ruptured aneurysm” (ICD9 codes 441.4; ICD10 codes 171.4) appeared on the same death certificate. Additionally, the aneurysm was considered “ruptured” if one or more of the following associated diagnosis codes were also reported: hemoperitoneum (ICD9 568.8; ICD10 K66.1), hemorrhage NOS/not elsewhere classified (ICD9 459.0; ICD10 R58), shock without trauma (ICD9 785.5), shock unspecified (ICD10 R57.9), shock other (ICD10 R57.8), rupture of artery (ICD10 177.2). In contrast, “non-ruptured aneurysm” was coded as such only when none of the rupture codes appeared in conjunction with a “non-ruptured aneurysm” code.
To classify the anatomic extent of the aortic aneurysm, any death with both thoracic aortic aneurysm diagnostic codes—including thoracoabdominal aortic aneurysm codes—(ICD9 codes 441.1, 441.2, 441.6, and 441.7; ICD10 codes 171.1,171.2,171.5, and 171.6) and abdominal or unspecific site aortic aneurysm codes were considered to be of thoracic nature and excluded from analysis.
Statistical analysis
Data were analyzed using SPSS 19.0 for Windows (SPSS, Inc., Chicago, IL) and StatalC version 14 (StataCorp, College Station, Texas). Statistics based on continuous data are presented as means and standard deviation from the mean. Means of continuous data were compared using analysis of variance. Categorical data were compared using the Pearson χ2 test. P-value < .05 was considered significant in all analyses.
Age-standardized mortality rates
Age-Standardized mortality rates were calculated as deaths/100,000 of the population. For each year, the population was segmented into age-groups with a 10-year range (e.g.: age 0–10 years, 11–20 years, 21–30 years, etc.). Age-group-specific mortality rates were calculated using census-derived age distributions as the denominator. The 2016 Washington State population was used as the standard and age-group-specific mortality rates for each other year was weighted accordingly to produce an age-standardized mortality rate. Trends in mortality rates were assessed for all AAA-related deaths and were also subdivided by whether there was rAAA or AAA without mention of rupture. Additionally, trends by sex and race were assessed. Observing no inflexion points in the trend-lines, trends in age-standardized annual mortality rates were evaluated with linear regression using mortality-rate as the dependent variable and year as the independent variable.8 The value of the coefficient represents change in mortality rate/year. Differences in trends were assessed by creating combined linear regression models which included year of death and a categorical variable differentiating the groups of interest, and determining whether an interaction term was statistically significant. Mortality rates were reported without confidence intervals, given that this dataset includes all deaths of WA residents over the reported time period rather than a sampling.
Geographic variation in rAAA assessment
To assess the degree of geographic variability in mortality rates the age-standardized county-specific mortality rates for rAAA were calculated. Time trends for each county were not calculated because of small numbers of deaths in individual years in many of the less populated counties. Therefore, the time-averaged mortality rates used and plotted on a map of WA which included major cities (population > 200,000), level 1 and level 2 trauma centers, and highways in order to visually elicit county-specific variation. A Kruskal-Willis test was used to evaluate whether the age-standardized, county-specific mortality rates differed statistically. To examine the association between rurality and mortality rates the 39 counties of residence were classified using the US Department of Agriculture Rural-Urban Continuum codes and the age-standardized mortality rates for these groups were evaluated.9
Results
The WA population ranged from 5,509,963 in 1996 to 7,183,700 in 2016. Of the 1,014,039 deaths in WA over the 21-year study time (49.6% Male, 90.8% White, mean age of death 73.2 ± 18.5 years), 4,438 (0.4%) were related to AAA (66.1% Male, mean age of death 79.4 ± 9.3 years). The mean age AAA-related mortality increased over the 21-year period from 78.3 ± 9.2 years in 1996 to 80.7 ± 10.3 years in 2016 (P=.003) and AAA-related mortality rate increased with increasing age (Figure 1). Ruptured AAA accounted for 52.3% (N=2,323) of all of the AAA-related mortality. The most commonly reported location for death was the hospital (55.1%) followed by at home (19.8%). An autopsy was performed in 6.7% of the cases and the rate of autopsy remained constant over time. The highest autopsy rate was among those who were younger than 55 years of age at the time of death (28.1%, P<0.001).
Figure 1.

Age distribution of deaths related to abdominal aortic aneurysm in Washington State from 1996–2016
Ruptured AAA was noted as the “underlying” cause of death in 91.3% of the death certificates in which this was listed while AAA without mention of rupture was listed as an “associated” rather than “underlying” cause of death in 65.8% of the cases in which this was listed. The mean age of death from rAAA was younger than the mean age of death in those noted to have an AAA without mention of rupture (78.3 ± 9.5 years vs. 80.7 ± 8.9 years respectively, P< 001). The most commonly associated causes of deaths in both groups were cardiac disease, chronic obstructive pulmonary disease, tobacco use, and essential hypertension as summarized in Table 1.
Table 1.
A comparison of abdominal aortic aneurysm without rupture and ruptured abdominal aortic aneurysm related mortality in Washington State from 1996 to 2016.
| N (%) | Abdominal aortic aneurysm without mention of rupture (N=2,115) | Ruptured abdominal aortic aneurysm (N=2,323) | P |
|---|---|---|---|
| Mean age (± SD) | 80.7±8.9 | 78.3±9.5 | <0.001 |
| Age category | <0.001 | ||
| <55 | 13 (0.6) | 20 (0.9) | |
| ≥ 55 and < 65 | 82 (3.9) | 184 (7.9) | |
| ≥ 65 | 2,020 (95.5) | 2,119 (91.2) | |
| Male | 1,379 (63.2) | 1,556 (67) | 0.21 |
| Race | 0.55 | ||
| White | 2,009 (95) | 2,200 (94.7) | |
| Asian | 42 (2) | 53 (2.3) | |
| Black | 26 (1.2) | 21 (0.9) | |
| Other | 38 (1.8) | 49 (2.1) | |
| Facility type for place of death | <0.001 | ||
| Hospital inpatient | 820 (38.8) | 1,625 (70.0) | |
| Emergency Room or dead on arrival | 106 (5) | 296 (12.7) | |
| Home | 656 (31) | 222 (9.6) | |
| Nursing home and hospice | 470 (22.2) | 141 (6.1) | |
| Other or unknown | 63 (3) | 39 (1.7) | |
| Autopsy | 66 (3.2) | 223 (9.8) | <0.001 |
| Associated diagnosis codes | |||
| None | 104 (4.9) | 567 (24.4) | <0.001 |
| Chronic ischemic heart disease | 1031 (48.7) | 410 (17.6) | <0.001 |
| Chronic obstructive pulmonary disease | 551 (26.1) | 231 (9.9) | <0.001 |
| Tobacco use | 485 (22.9) | 350 (15.1) | <0.001 |
| Essential Hypertension | 526 (24.9) | 419 (18) | <0.001 |
Mortality Trends
There was a 62.1% decrease in age standardized AAA-related mortality during the study period from 5.8 deaths/100,000 in 1996 to 2.2 deaths/100,000 in 2016 (Figure 2). The linear regression trend line had a coefficient of −0.18 deaths/100,000/ year (95% CI −0.16 – −0.19/ r2= 0.95). There was a notable statistically significant drop in rAAA-related mortality which was at 3.2/100,000 in 1996 and decreased to 0.95/100,000 in 2016 thus accounting for a decline of 0.12 death/100,000/year, 95% Cl: 0.11–0.14, r2 = 0.95) as illustrated in Figure 3.
Figure 2.

Age-standardized abdominal aortic aneurysm related mortality in Washington State between 1996 and 2016.
Figure 3.
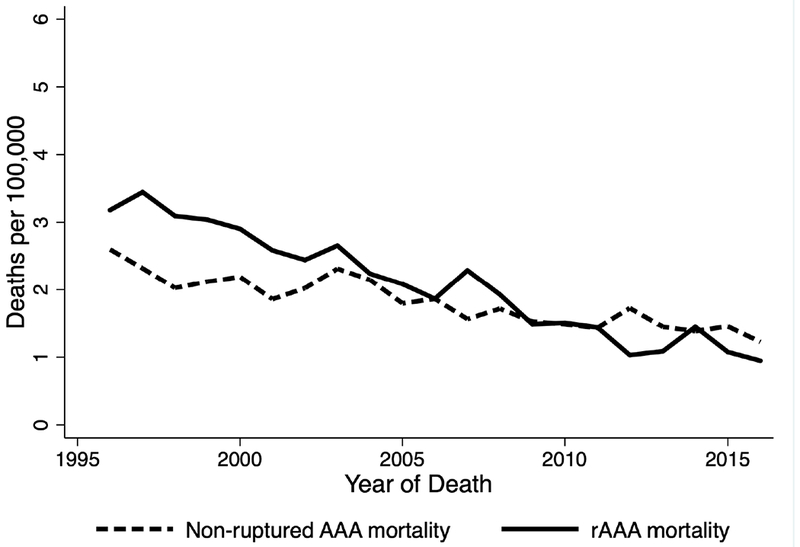
Age-standardized abdominal aortic aneurysm without mention of rupture and ruptured abdominal aortic aneurysm related mortality in Washington State between 1996 and 2016.
Sex differences in mortality rates
Of the 511,586 women who died during the time period, 1,503 women (0.29%) had AAA noted as an underlying or contributing cause of death, of which 767 cases were due to rAAA. On average, the mean age of death was younger among men compared to women with AAA-related mortality (78.1 ± 9.4 vs. 81.9 ± 8.6 years respectively, P<.001), and similarly, with rAAA-related mortality = (76.8 ± 9.5 vs. 81.4 ± 8.7 years respectively, P<.001).
Sex based differences in the rates of decline in age standardized overall AAA and rAAA related mortality were observed between men and women. These differences were statistically significant (P <.001) for both overall AAA and rAAA trend differences. During the study period, there was a 55% decline in AAA-related mortality rates among men (6.5 to 3.0 per 100,000) vs. a 41% decline (2.4 to 1.4 per 100,000) among women. Also, there was a 68% decline in rAAA-related mortality rates among men (3.9 to 1.3 per 100,000) vs. a 51% decline (1.3 to 0.6 per 100,000) among women. (Figure 4).
Figure 4.
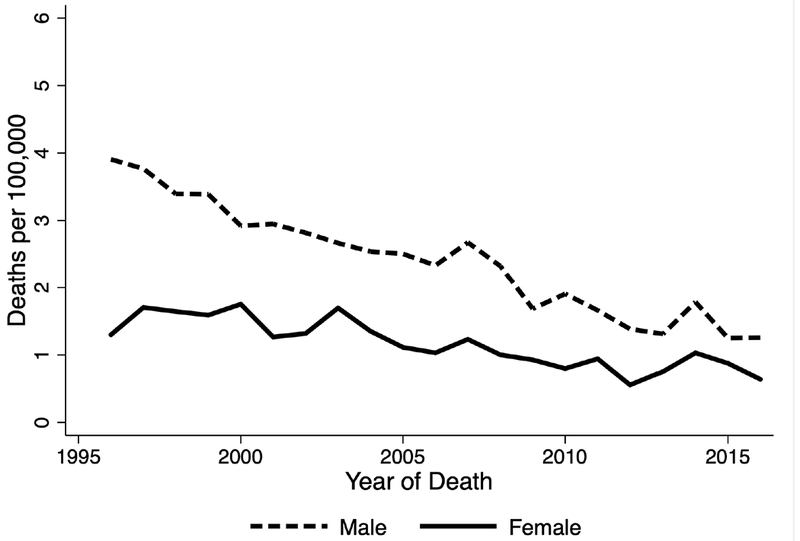
Age-standardized ruptured abdominal aortic aneurysm related mortality rates in Washington State by sex between 1996 and 2016.
Race differences in mortality rates
Of the 92,815 non-white individuals who died during the time period, 229 (0.24%) had AAA noted as an underlying or contributing cause of death. There were no differences in age of death between white and non-white individuals who died with AAA-related mortality (80.7 + 8.7 vs. 80 + 11.8 years respectively, P=.44) or rAAA related mortality 78.4 ± 9.5 vs 76.9 ± 9.7 vs. years respectively, P<.10). However, significant differences (P<0.001) for both overall AAA and rAAA- related mortality trends were observed among white and non-white individuals. The age-standardized AAA-related mortality rate over the study period showed 53% decline among white individuals (5.3 to 2.5 per 00,000) compared to an only 13% decline in among non-white individuals (1.5 to 1.3 per 100,000). Similarly, the age-standardized rAAA-related mortality rate over the study period showed 64% decline in mortality rates among white individuals (2.9 to 1.1 per 100,000) compared to a 20% decline among non-white individuals (0.8 to 0.7 per 100,000).
County level variation in rAAA-related mortality
The median county-specific rAAA-related mortality was 2.3 deaths/100,000 (IQR, 0.3 to 11.8 deaths/100,000, range 0.02– 161.6/100,000). There was statistically significant (P <.001) geographic variation in rAAA-related mortality (Figure 5). When classified using the USD A Rural-Urban Continuum, mortality rates were similar for all groups except for the rural-most areas which appeared to have higher AAA-related mortality, though this analysis does account for a reliability adjustment based on differences in population for each group (Figure 6).
Figure 5.
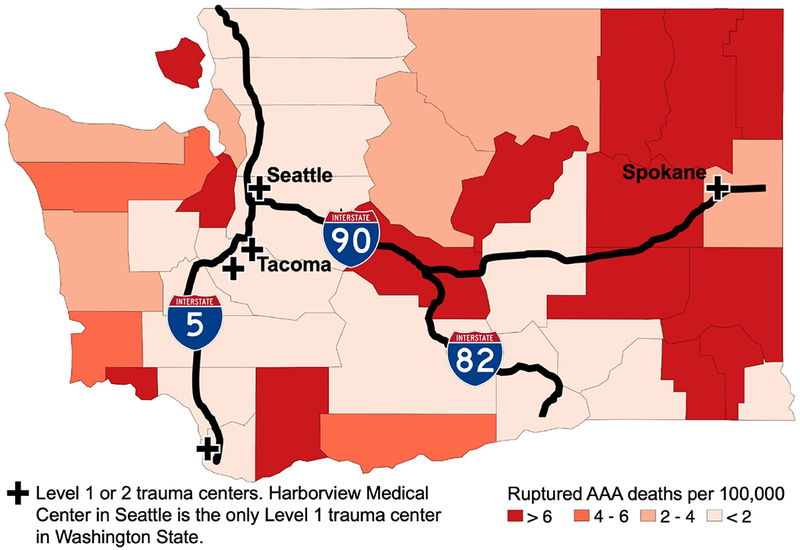
Age-standardized ruptured abdominal aortic aneurysm related mortality in Washington State by county, averaged over 21 years. The major highways and trauma centers are noted.
Figure 6.

Age-standardized, county-specific ruptured abdominal aortic aneurysm related mortality rate in Washington State grouped by USD A Rural-urban continuum codes into 8 categories: (1) Metro areas of 1 million population or more; (2) Metro areas of 250,000 to 1 million population; (3) Metro areas of fewer than 250,000 population; (4) Urban population of 20,000 or more, adjacent to a metro area; (5) Urban population of 20,000 or more, not adjacent to a metro area; (6) Urban population of 2,500 to 19,999, adjacent to a metro area; (7) Urban population of 2,500 to 19,999, not adjacent to a metro area; (8) Completely rural or less than 2,500 urban population, adjacent to a metro area.
Discussion
We observed a decline in AAA related mortality of 62.1% in WA from 1996 to 2016 with a substantial drop in rAAA-related mortality rate. Prior work utilizing the World Health Organization mortality database showed a decline in AAA-related mortality in the United States by 6.7% per year in men and 3.9% per year in women from 1994 to 2010.5 A subsequent study showed a 49.4% decline in aortic aneurysm mortality between 1980 and 2014.8 Thus our findings are in line with prior work demonstrating a decline in AAA-related mortality in the United States.10 The contemporary trend of AAA-related mortality decline has been seen in other countries such as the Netherlands, Finland, New Zealand, and Australia, while in Brazil, a recent study demonstrated an increase in AAA related mortality over similar time periods.11–15
We observed a strong linear relationship in AAA-related mortality over time without any notable inflection points. Thus we speculate that the etiology is multifactorial including an overall decrease in incidence as well as improvements in public health awareness, screening and management. The prevalence of AAAs in the United States is likely reduced through primary prevention measures focused on atherosclerosis risk factor modification including smoking cessation, treatment of hypertension, and statin use.3,16–18 A recent study showed that AAA-related mortality in men mirrors the decline in annual adult per capita cigarette consumption in the United States.3,18 While a reduction in AAA incidence and overall prevalence through risk-factor modification may account for a portion of the AAA-related mortality decline, it would not alone explain the steeper decline observed in rAAA-related mortality compared to “AAA without mention of rupture” related mortality.
Screening for AAA, primarily in men >65 years, has been shown to be associated with reduction in AAA related mortality with an absolute reduction of 4 per 1000.19–21 The Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act was introduced on January 1, 2007 by Medicare covering AAA screening at the time of enrollment in Medicare for men with a history of smoking. The current recommendations by the USPSTF is a one-time screening by abdominal ultrasound for men with a history of smoking or anyone with a family history of AAA. The recent Society for Vascular Surgery (SVS) guidelines recommends extending the recommendation to include women 65 to 75 years of age with a history of tobacco use.2,22,23 Assessment of SAAAVE Act implementation among Medicare enrollees from 2004 to 2008 demonstrated that this was not associated with changes in rates of AAA repair, rAAA, or allcause mortality.24 Interestingly, we did not identify any inflection points in the decline in rAAA-related mortality that correlate with the introduction of the SAAVE Act. This could possibly be a reflection of the underutilization of AAA screening25 or of a delayed effect of screening that not captured in the time period of this analysis.24 An additional source of diagnosis is via the increased utilization of CT scans that may facilitate the incidental diagnosis of AAA, though this link has not been well established.26
Another factor that could lead to reduction on aortic related mortality is related to advances in critical care medicine and the adoption of endovascular aortic aneurysm repair (EVAR) for elective AAA repair and rAAA repair.27 EVAR as a method of operative repair surpassed the open repair technique in 2004–2005.28 While this may suggest that an increase in EVAR has translated to a decrease in AAA-related mortality, the overall number of AAA repairs has remained relatively constant in the United States since the mid-1990s.29 However, we speculate that if case volume remained constant while the prevalence of disease was decreasing due to risk factor modification, the elective repair of AAA would selectively decrease mortality from rAAA by reducing the incidence of rupture.
Differences in age standardized rAAA-related mortality rate decline over time in WA varied by sex and race with women and non-white individuals showing a slower decline in AAA-related mortality rates over time compared to men and white individuals respectively. This finding adds to a growing body of literature showing health care disparities.14,29–33 While our study highlights this disparity, it was not designed to address the causes of the disparities. These disparities are likely a reflection of differences in health care access that can occur at multiple levels including access to care, screening and diagnosis, repair, and outcomes. Future study, which combines clinical and epidemiological data, would help in understanding the drivers of these differences at the state level and elucidate how healthcare systems and policy can adequately address disparities.
Our study demonstrated variation at the county level in rAAA-related mortality in WA. This is consistent with findings of recent epidemiological study that also demonstrated widespread variation at a county level in burden of cardiovascular disease and related-mortality in the United States.8 One relevant question is whether rAAA-related mortality variation is related to access to a nearby hospital. Several studies examining the association between distance traveled to the hospital and survival post rAAA found no association.34–36 In our data, grouping counties based on USDA Rural-Urban Continuum and plotting the mortality rates in did not show a strong pattern. Thus the reasons for this county level variation remain unexplained and warrant further investigation.
There are several limitations to this study related to analysis of death records data. There is a known variation in the reporting of the underlying and associated causes of death in death certificates.34,37 In addition to variation, there is potential for error in ascertaining the cause of death by the health care provider completing the death certificate. A recent study comparing the Vermont Electronic Death Registration System with clinical summaries from medical records noted that 51% of death certificates had “major errors”.35 An additional limitation is relevant to the reason why an “AAA without mention of rupture” is listed as a cause of death. Listing this on a death certificate was puzzling. We adjusted for this by recoding them to rAAA when evidence of hemorrhage is also presented on the death certificate; however, the number of reclassified causes was rather small. It is possible that these codes refer to incidentally noted AAA, cases in which an AAA was noted and not repaired, or possibly a history of AAA repair with death related to complications. It was not possible to ascertain the reasons for this choice of coding. Despite this limitation, we anticipate that cause of death misclassification should be are independent of time and that the mortality trends over time should hold true.36
This work is a first step toward regional AAA assessment. Much remains to be understood in how the relative contributions of AAA prevention strategies, AAA screening, vascular surgery workforce, and operative repair modality influence the overall decline in AAA-related mortality rates. Future work linking death records data to identifiable administrative datasets will likely prove valuable in further understanding the AAA epidemiology, disparities in care, and regional variation. Further clarification of these trends will inform possible areas of focus for health policy and public health interventions.
Conclusions
Age-standardized AAA-related mortality rate has significantly declined over the past 21 years in WA. The reasons for the decline are likely multifactorial and related to risk factor modification, screening, and the evolution in operative repair options. The decline in AAA-related mortality rates vary by sex, race, and county. These observations are first step toward the regional population assessment. Future work to understand the sources of variation can influence public health intervention on a state level.
Article Highlights:
Type of Research
Analysis of death certificate data in Washington State.
Take Home Message
The analysis of death certificate data in Washington State revealed that age-standardized abdominal aortic aneurysm (AAA) related mortality rate has declined from 1996 to 2016, with a notable significant drop in ruptured AAA-related mortality rate. The death rate trends vary by sex, race, and county.
Recommendation
Further study to understand the drivers for the decline in AAA-related mortality rate, sex and race disparities, and regional variability is warranted.
Acknowledgments
Funding
This project was made possible by funding in part supported by the Endovascular Training and Research Fund (SS) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, under Award Number T32DK070555 (MB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
None
Presentation
This work was presented as a podium presentation at the 2017 Western Vascular Society Annual Meeting in Blaine, WA, on September 25, 2017 and at the Pacific Northwest Vascular Society Meeting in Portland, OR, on November 3, 2017.
References
- 1.Stewart B, Khanduri P, McCord C, Ohene-Yeboah M, Uranues S, Vega Rivera F, et al. Global disease burden of conditions requiring emergency surgery. Br J Surg. 2014; 101:9–22. [DOI] [PubMed] [Google Scholar]
- 2.LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014; 161:281–90. [DOI] [PubMed] [Google Scholar]
- 3.Lederle FA. The rise and fall of abdominal aortic aneurysm. Circulation. 2011; 124:1097–9. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population-a meta-analysis. PLoS One. 2013; 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidloff D, Stather P, Dattani N, Bown M, Thompson J, Sayers R, et al. Aneurysm global epidemiology study public health measures can further reduce abdominal aortic aneurysm mortality. Circulation. 2014; 129:747–53. [DOI] [PubMed] [Google Scholar]
- 6.Zettervall SL, Soden PA, Buck DB, Cronenwett JL, Goodney PP, Eslami MH, et al. Significant regional variation exists in morbidity and mortality after repair of abdominal aortic aneurysm. J Vase Surg. 2017; 65:1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamblee RF, Evans MC. New dimensions in cause of death statistics. Am J Public Health. 1982; 72:1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, et al. Trends and Patterns of Geographic Variation in Cardiovascular Mortality Among US Counties, 1980–2014. JAMA. 2017; 317:1976–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker T USDA ERS - Rural-Urban Continuum Codes 2016. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.Cited10/19/2017.
- 10.Schermerhorn ML, Bensley RP, Giles KA, Hurks R, O’Malley AJ, Cotterill P, et al. Changes in Abdominal Aortic Aneurysm Rupture and Short-Term Mortality, 1995–2008. Ann Surg. 2012; 256:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelissen BGL, Herwaarden JA, Pasterkamp G, Moll FL, Vaartjes I. Shifting abdominal aortic aneurysm mortality trends in the Netherlands. J Vase Surg. 2015; 61:642–647. [DOI] [PubMed] [Google Scholar]
- 12.Laine MT, Laukontaus SJ, Kantonen I, Venermo M. Population-based study of ruptured abdominal aortic aneurysm. Br J Surg. 2016; 103:1634–9. [DOI] [PubMed] [Google Scholar]
- 13.Santo AH, Puech-leao P, Krutman M. Trends in aortic aneurysm- and dissection-related mortality in the state of Sao Paulo, Brazil, 1985–2009: multiple-cause-of-death analysis. BMC Public Health. 2012;859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg. 2011; 98:645–51. [DOI] [PubMed] [Google Scholar]
- 15.Norman PE, Spilsbury K, Semmens JB. Falling rates of hospitalization and mortality from abdominal aortic aneurysms in Australia. J Vase Surg. 2011; 53:274–7. [DOI] [PubMed] [Google Scholar]
- 16.Takagi H, Yamamoto H, Iwata K, Goto S, Umemoto T. Effects of statin therapy on abdominal aortic aneurysm growth: A meta-analysis and meta-regression of observational comparative studies. Eur J Vase Endovasc Surg. 2012; 44:287–92. [DOI] [PubMed] [Google Scholar]
- 17.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vase Surg. 2010;52:539–48 [DOI] [PubMed] [Google Scholar]
- 18.Trends in Current Cigarette Smoking Among High School Students and Adults, United States, 1965–2014.Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm.Cited4/30/2018.
- 19.Takagi H, Goto SN, Matsui M, Manabe H, Umemoto T. A further meta-analysis of population-based screening for abdominal aortic aneurysm. J Vase Surg. 2010; 52:1103–8. [DOI] [PubMed] [Google Scholar]
- 20.Guirguis-Blake JM, Beil TL, Sun X, Senger CA, Whitlock EP. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S Preventive Services Task Force. . Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. January (Evidence Syntheses, No. 109.) [PubMed] [Google Scholar]
- 21.Cosford PA, Leng GC, Thomas J. Screening for abdominal aortic aneurysm. Cochrane Database SystRev. 2007; 18:CD002945. [DOI] [PubMed] [Google Scholar]
- 22.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vase Surg. 2018; 67:2–77. e2. [DOI] [PubMed] [Google Scholar]
- 23.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for Abdominal Aortic Aneurysm: A Best-Evidence Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005; 142: 203–11. [DOI] [PubMed] [Google Scholar]
- 24.Shreibati JB, Baker LC, Hlatky MA, Mell MW. Impact of the screening abdominal aortic aneurysms very efficiently (SAAAVE) act on abdominal ultrasonography use among medicare beneficiaries. Arch Intern Med. 2012; 172:1456–62. [DOI] [PubMed] [Google Scholar]
- 25.Mell MW, Hlatky MA, Shreibati JB, Dalman RL, Baker LC. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vase Surg. 2013; 57:1519–1523. e1. [DOI] [PubMed] [Google Scholar]
- 26.Bellolio F, Heien H, Sangaralingham L, Jeffery M, Campbell R, Cabrera D, et al. Increased Computed Tomography Utilization in the Emergency Department and Its Association with Hospital Admission. West J Emerg Med. 2017; 18:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starnes BW, Quiroga E, Hutter C, Tran NT, Hatsukami T, Meissner M, et al. Management of ruptured abdominal aortic aneurysm in the endovascular era. J Vase Surg. 2010; 51:9–18. [DOI] [PubMed] [Google Scholar]
- 28.Zettervall SL, Buck DB, Soden PA, Cronenwett JL, Goodney PP, Eslami MH, et al. Regional variation exists in patient selection and treatment of abdominal aortic aneurysms. J Vase Surg. 2016; 64:921–927. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States : Have we improved outcomes equally between men and women ? J Vase Surg. 2006; 43: 230–8. [DOI] [PubMed] [Google Scholar]
- 30.Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. Ann Surg. 1999; 230:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001–2004. J Vase Surg 2007; 45:891–9. [DOI] [PubMed] [Google Scholar]
- 32.Soden PA, Zettervall SL, Deery SE, Hughes K, Stoner MC, Goodney PP, et al. Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J Vase Surg. 2018; 67:549–556. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deery SE, O’Donnell TFX, Shean KE, Darling JD, Soden PA, Hughes K, et al. Racial disparities in outcomes after intact abdominal aortic aneurysm repair. J Vase Surg. 2018; 67:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith Sehdev a E, Hutchins GM, Sehdev AES, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001; 161:277–84. [DOI] [PubMed] [Google Scholar]
- 35.McGivem L, Shulman L, Carney JK, Shapiro S, Bundock E. Death certification errors and the effect on mortality statistics. Public Health Rep. 2017; 132:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren JR, Milesi C, Grigorian K, Humphries M, Muller C, Grodsky E. Do inferences about mortality rates and disparities vary by source of mortality information? Ann Epidemiol. 2017; 27:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naghavi M, Makela S, Foreman K, O’Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr. 2010; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


