Abstract
Objective:
Moderate alcohol consumption has been found to be associated with lower risk of coronary heart disease and myocardial infarction, which share similar risk factors and pathophysiology with chronic kidney disease (CKD). However, there is inconsistent evidence on the association between alcohol consumption and CKD.
Design:
Prospective cohort study.
Setting:
The Atherosclerosis Risk in Communities (ARIC) study, a community-based cohort of 4 sites in the U.S.
Subjects:
12,692 participants aged 45–64 years.
Intervention:
We categorized participants into 6 alcohol consumption categories: never drinkers, former drinkers, ≤1 drink/week, 2–7 drinks/week, 8–14 drinks/week, and ≥15 drinks/week based on food frequency questionnaire responses at visit 1 (1987–1989).
Main outcome measure:
Incident CKD was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2 accompanied by ≥25% estimated glomerular filtration rate decline, a kidney disease-related hospitalization or death, or end-stage renal disease.
Results:
During a median follow-up of 24 years, there were 3,664 cases of incident CKD. Current drinkers were more likely to be men, whites, and to have a higher income level and education level. After adjusting for total energy intake, age, sex, race-center, income, education level, health insurance, smoking, and physical activity, there was no significant association between being a former drinker and risk of incident CKD. Participants who drank ≤1 drink/week, 2–7 drinks/week, 8–14 drinks/week, and ≥15 drinks/week had, respectively, a 12% (HR: 0.88, 95% CI: 0.79–0.97), 20% (HR: 0.80, 95% CI: 0.72–0.89), 29% (HR: 0.71, 95% CI: 0.62–0.83), and 23% (HR: 0.77, 95% CI: 0.65–0.91) lower risk of CKD compared to never drinkers.
Conclusion:
Consuming a low or moderate amount of alcohol may lower the risk of developing CKD. Therefore, moderate consumption of alcohol may not likely be harmful to the kidneys.
Keywords: alcohol, diet, beverages, kidney, renal disease
Introduction
Long-term, excessive alcohol consumption may increase the risk of adverse health outcomes such as alcohol use disorders, liver cirrhosis, some cancers, and injuries (1, 2). Moderate alcohol consumption (up to 1 drink/day for women and up to 2 drinks/day for men) has been found to be associated with reduced risk of coronary heart disease (CHD) (3–5). This may be attributed to alcohol increasing high-density lipoprotein (HDL) cholesterol levels, which could increase transport rate of lipoproteins and lipoprotein lipase activity, therefore preventing CHD (6, 7). Because CHD and chronic kidney disease (CKD) share many risk factors and pathophysiology, it is possible that moderate alcohol consumption may also reduce the risk of CKD. However, the relationship is complex as alcohol increases blood pressure and therefore may increase the risk of hypertension, a major risk factor for CKD (8, 9). On the other hand, alcohol may be protective of CHD, myocardial infarction, and diabetes, which are also key risk factors of CKD (5, 10–12).
Several observational studies have examined the association between moderate alcohol consumption and CKD; however, the results have been inconsistent. Some studies have found no association between moderate alcohol consumption and renal function (13–16) while others have found a protective association (17–21). Many of these studies were conducted in homogeneous populations of only whites or males or had relatively small sample sizes (13, 14, 16, 18, 19). More evidence is needed on the relationship between alcohol and CKD in a larger, more diverse population to allow for broader generalizability. Therefore, we aimed to characterize the relationship between alcohol consumption and CKD risk in a community-based population of middle-aged, predominantly black and white men and women.
Methods
Study Population and Design
We conducted a prospective analysis of the Atherosclerosis Risk in Communities (ARIC) study, a community-based cohort of 15,792 middle-aged (45–64 years) predominantly black and white men and women (22). Study participants were enrolled in 1987–1989 from 4 U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Five follow-up study visits have occurred since baseline and visit 7 is ongoing. An ethics committee at each site approved the study protocol and study participants provided informed consent at each study visit.
We excluded participants who were Asian or Indian (n=48) and blacks from Washington County, Maryland (n=33) and Minneapolis, Minnesota (n=22) due to small numbers. We additionally excluded participants with missing data for smoking (n=15), body mass index (BMI) (n=23), total energy intake (n=26), education level (n=17), income (n=905), health insurance (n=15), hypertension (n=66), diabetes (n=113), or serum creatinine (n=19), and participants with CHD (n=701), heart failure (n=550), or CKD at baseline [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (n=149)]. We additionally excluded participants with missing alcohol data (n=48), high self-reported alcohol consumption despite normal liver enzyme level indicating potential misreporting (n=28), former drinkers misclassified as current drinkers (reported being a former and current drinker; n=23), participants with extreme total energy intakes (women: <500 or >3,500 kcal/d; men: <700 or >4,500 kcal/d; n=297), and participants missing more than 15 food frequency questionnaire items (n=2). Our final analytic sample included 12,692 participants.
Measurement of Alcohol Consumption
Alcohol consumption was assessed at visit 1 (1987–89) using a 66-item semi-quantitative validated food frequency questionnaire, modified from the Willett questionnaire (23–25). The questionnaire was administered in-person by a trained interviewer, who asked participants to report if they currently drink alcoholic beverages and, if so, how often in the last year they consumed wine (4-ounce), beer (12-ounce), and hard liquor (1 ½-ounce shots) each week. Participants who responded that they did not currently drink were then asked if they ever drank alcohol. Participants who responded that they did not drink in the present or past were classified as never drinkers and those who reported that they did not drink in the present but did in the past were classified as former drinkers. For current drinkers, we calculated total alcohol consumption (drinks/week) by summing the number of drinks reported for wine, beer, and liquor. Current drinkers were then divided into categories based on total drinks consumed per week: ≤1 drink/week, 2–7 drinks/week, 8–14 drinks/week, and ≥15 drinks/week.
Ascertainment of Kidney Disease
Blood creatinine was measured using the modified kinetic Jaffé method and used to calculate eGFR with the 2009 Chronic Kidney Disease Epidemiology Collaboration equation (26–28). Incident CKD cases were defined by meeting at least one of the following criteria: 1) eGFR <60 mL/min/1.73 m2 accompanied by ≥25% eGFR decline, 2) kidney disease-related hospitalization or death based on International Classification of Diseases (ICD)-9/10 codes identified through active surveillance and linkage to the National Death Index, or 3) end-stage renal disease (ESRD) identified by linkage to the U.S. Renal Data System registry (29).
As a sensitivity analysis for the composite definition of incident CKD, we identified cases of CKD using visit-based measures exclusively: eGFR<60 mL/min/1.73 m2 at a subsequent study visit accompanied by ≥30% eGFR decline relative to baseline.
Measurement of Covariates
At baseline, demographic and lifestyle characteristics, socioeconomic status, and health history were ascertained using a questionnaire administered by trained interviewers (22). Race and study center were combined into one variable given the non-uniform distribution of blacks and whites across study centers. Education level was categorized as less than high school, high school or equivalent, and college or more education. Household income was categorized as <$24,000/year, $24,000-$49,999/year, and ≥$50,000/year. Participants were asked whether they had health insurance (yes/no). Participants were categorized into never, former, or current smokers. Physical activity was assessed using a modified Baecke questionnaire (30, 31). A physical activity index score (1-lowest, 5-highest) was calculated based on intensity and time dedicated to exercise during leisure time. Dietary Approaches to Stop Hypertension (DASH) diet score (8-lowest, 40-highest) was based on a previously developed index by Fung et al (32). The score was calculated based on 8 components, where higher intakes of fruits, vegetables, whole grains, nuts and legumes, and low-fat dairy and lower intakes of red/processed meat, sweetened beverages, and sodium were rewarded more points (32).
Clinical factors included diabetes, hypertension, BMI, and baseline eGFR. Diabetes was defined at baseline as fasting blood glucose ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, self-reported history of diagnosed diabetes, or use of diabetes medication in the preceding 2 weeks. Fasting glucose was collected at baseline and measured by the modified hexokinase/glucose-6-phosphate dehydrogenase method. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication in the past 2 weeks. Three seated measurements of blood pressure were taken by a certified technician using a random-zero sphygmomanometer after a five-minute resting period. The average of the second and third blood pressure readings was used in the analysis. BMI was calculated as weight in kilograms divided by height in meters squared.
Statistical Analysis
Descriptive statistics were used to characterize the study population according to alcohol consumption category. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between alcohol and incident CKD using Cox proportional hazards regression models. Time to event accumulated from visit 1 (1987–89) through December 31, 2016. Our minimally adjusted model (model 1) included total energy intake. In model 2, our main model, we additionally adjusted for age, sex, race-center, income, education level, health insurance, smoking, and physical activity. In model 3, we further adjusted for potential mediators (diabetes status, hypertension status, BMI, and baseline eGFR). We performed competing risk analyses to account for non-CKD related deaths for the composite definition of CKD and to account for all-cause mortality for the visit-based definition of CKD according to the method of Fine and Gray (33). We conducted continuous analyses among current drinkers using restricted cubic splines with knots at 1, 7, and 14 drinks/week.
For sensitivity analyses, we examined the association using ≤1 drink/week as the reference group since the risk profile of never drinkers may be different compared to that of light alcohol drinkers. We tested interaction terms for potential effect modifiers (sex, race, diabetes, hypertension, smoking) using the likelihood ratio test and conducted stratified analyses by subgroup. All p-values were 2-tailed, and statistical significance was set a priori at P<0.05. All analyses were performed using Stata software (version 14.0; StataCorp, College Station, Texas, USA).
Results
Baseline Characteristics
Of the 12,692 participants included in our study, 25% were classified as never drinkers, 18% were former drinkers, 23% consumed ≤1 drink/week, 20% consumed 2–7 drinks/week, 8% consumed 8–14 drinks/week, and 6% consumed ≥15 drinks/week. Compared to never drinkers, current drinkers were more likely to be men, white, have at least a college education, have income ≥$50,000/year, have health insurance, and be a current or former smoker (Table 1). Never drinkers were more likely to have a higher BMI, diabetes, hypertension, and higher baseline eGFR relative to current drinkers. Among current drinkers, those who consumed higher amounts of alcohol (≥15 drinks/week) were more likely to be black, had less than high school education, hypertension, higher total energy intake levels, and lower DASH diet scores.
Table 1.
Baseline Characteristics According to Categories of Alcohol Consumption
| Alcohol Consumption Category | ||||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Never drinker (n=3,118) | Former drinker (n=2,239) | ≤1 drink/week (n=2,960) | 2–7 drinks/week (n=2,592) | 8–14 drinks/week (n=1,029) | ≥15 drinks/week (n=754) |
| Age, years | 54 ± 6 | 54 ± 6 | 54 ± 6 | 54 ± 6 | 54 ± 6 | 54 ± 6 |
| Female, n (%) | 2,411 (77) | 1,085 (48) | 1,906 (64) | 1,236 (48) | 333 (32) | 118 (16) |
| Race, n (%) | ||||||
| White | 1,756 (56) | 1,566 (69) | 2,693 (91) | 2,159 (83) | 875 (85) | 607 (81) |
| Black | 1,362 (44) | 673 (31) | 267 (9) | 433 (17) | 154 (15) | 147 (20) |
| Education level, n (%) | ||||||
| <High school | 943 (30) | 799 (36) | 383 (13) | 334 (13) | 157 (15) | 140 (19) |
| High school or equivalent | 1,302 (42) | 869 (39) | 1,339 (45) | 1,064 (41) | 385 (37) | 302 (40) |
| ≥College | 873 (28) | 571 (26) | 1,238 (42) | 1,194 (46) | 487 (47) | 312 (41) |
| Annual household income, n (%) | ||||||
| <$24,000 | 1,656 (53) | 1,094 (49) | 730 (25) | 611 (24) | 231 (22) | 229 (30) |
| $24,000–$49,999 | 1,053 (34) | 797 (36) | 1,287 (43) | 1,022 (39) | 370 (36) | 285 (38) |
| ≥$50,000 | 409 (13) | 348 (16) | 943 (32) | 959 (37) | 428 (42) | 240 (32) |
| Health insurance, n (%) | 2,687 (86) | 1,948 (87) | 2,835 (96) | 2,434 (94) | 960 (93) | 672 (89) |
| Center, n (%) | ||||||
| Forsyth County, North Carolina | 890 (29) | 573 (26) | 852 (29) | 611 (24) | 238 (23) | 200 (27) |
| Jackson, Mississippi | 1,290 (41) | 569 (25) | 192 (6) | 375 (14) | 142 (14) | 121 (16) |
| Minneapolis, Minnesota | 138 (4) | 385 (17) | 1,108 (37) | 1,055 (41) | 404 (39) | 244 (32) |
| Washington County, Maryland | 800 (26) | 712 (32) | 808 (27) | 551 (21) | 245 (24) | 189 (25) |
| Smoking status, n (%) | ||||||
| Never smoker | 2,187 (70) | 679 (30) | 1,361 (46) | 860 (33) | 238 (23) | 102 (14) |
| Former smoker | 482 (15) | 901 (40) | 894 (30) | 996 (38) | 432 (42) | 302 (40) |
| Current smoker | 449 (14) | 659 (29) | 705 (24) | 736 (28) | 359 (35) | 350 (46) |
| Physical activity index score (1–5)* | 2.3 ± 0.7 | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.6 ± 0.8 | 2.6 ± 0.8 | 2.5 ± 0.9 |
| BMI, kg/m2 | 28.6 ± 6 | 28.0 ± 6 | 27.1 ± 5 | 26.6 ± 5 | 26.4 ± 4 | 26.6 ± 4 |
| Systolic BP, mmHg | 123 ± 20 | 121 ± 18 | 117 ± 17 | 119 ± 18 | 122 ± 18 | 126 ± 19 |
| HDL cholesterol, mg/dL | 52.9 ± 16 | 48.0 ± 15 | 51.2 ± 16 | 53.6 ± 18 | 56.0 ± 20 | 54.5 ± 19 |
| Diabetes, n (%) | 445 (14) | 330 (15) | 215 (7) | 169 (7) | 78 (8) | 48 (6) |
| Fasting glucose, mg/dL | 108.4 ± 40 | 109.6 ± 39 | 102.3 ± 27 | 103.0 ± 24 | 105.1 ± 25 | 105.3 ± 22 |
| Hypertension, n (%) | 1,216 (39) | 760 (34) | 717 (24) | 677 (26) | 308 (30) | 273 (36) |
| Anti-hypertensive medication, n (%) | 1,003 (32) | 641 (29) | 619 (21) | 524 (20) | 233 (23) | 166 (22) |
| Baseline eGFR, mL/min/1.73 m2 | 106 ± 15 | 103 ± 15 | 102 ± 13 | 102 ± 13 | 102 ± 14 | 104 ± 14 |
| Total energy intake, kcal/day | 1,540 ± 568 | 1,670 ± 645 | 1,587 ± 592 | 1,631 ± 603 | 1,692 ± 591 | 1,888 ± 644 |
| DASH diet score (8–40) | 24.1 ± 5 | 23.6 ± 5 | 24.8 ± 5 | 24.2 ± 5 | 23.5 ± 5 | 22.1 ± 5 |
Note: Values for categorical variables are given as number (percentage); for continuous variables, mean ± standard deviation. 1 drink is defined as a 4-ounce glass of wine, a 12-ounce beer, or 1.5-ounce shot of hard liquor.
Abbreviations: BMI, body mass index; BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; kcal, kilocalories; kg/m2, kilogram per meter squared; mg/dL, milligrams per deciliter; mmHg, millimeters of mercury
Physical activity index score was calculated based on intensity and time of sport and non-sport exercise during leisure time; 1 is lowest and 5 is highest.
Alcohol Consumption and Incident Chronic Kidney Disease
There were 3,664 cases of incident CKD (composite definition) during a median follow-up of 24 years. Approximately 51% of cases were detected from in-person visits, 42% through ICD-9/10 codes, and 7% through linkage to the USRDS registry. In all 3 models, participants who drank alcohol had significantly lower risks of CKD compared to never drinkers (Table 2). There was no significant association for former drinkers. In our main model (model 2), adjusted for total energy intake, age, sex, race-center, income, education level, health insurance, smoking, and physical activity, participants who drank ≤1 drink/week, 2–7 drinks/week, 8–14 drinks/week, and ≥15 drinks/week had, respectively, a 12% (HR: 0.88, 95% CI: 0.79–0.97), 20% (HR: 0.80, 95% CI: 0.72–0.89), 29% (HR: 0.71, 95% CI: 0.62–0.83) and 23% (HR: 0.77, 95% CI: 0.65–0.91) lower risk of CKD compared to never drinkers. In model 3, which additionally adjusted for potential mediators (diabetes, hypertension, BMI, baseline eGFR), the estimates remained approximately the same. The risk of CKD per each additional drink/day after accounting for the competing risk of non-CKD death was similar to the main results (subdistribution HR: 0.90, 95% CI: 0.85–0.95).
Table 2.
Risk for Incident Chronic Kidney Disease by Alcohol Consumption Category
| Alcohol Consumption Category | ||||||
|---|---|---|---|---|---|---|
| Never drinker (n=3,118) | Former drinker (n=2,239) | ≤1 drink/week (n=2,960) | 2–7 drinks/week (n=2,592) | 8–14 drinks/week (n=1,029) | ≥15 drinks/week (n=754) | |
| Incident Chronic Kidney Disease (composite definition) | ||||||
| # events (rate*) | 1,007 (15) | 724 (16) | 810 (12) | 683 (12) | 247 (11) | 193 (13) |
| Model 1 | 1 (reference) | 1.11 (1.01–1.22) | 0.80 (0.73–0.88) | 0.77 (0.70–0.85) | 0.74 (0.64–0.85) | 0.90 (0.77–1.05) |
| Model 2 | 1 (reference) | 1.00 (0.90–1.11) | 0.88 (0.79–0.97) | 0.80 (0.72–0.89) | 0.71 (0.62–0.83) | 0.77 (0.65–0.91) |
| Model 3 | 1 (reference) | 0.96 (0.86–1.06) | 0.86 (0.78–0.95) | 0.82 (0.73–0.91) | 0.72 (0.62–0.83) | 0.80 (0.68–0.95) |
| Incident Chronic Kidney Disease (visit-based definition) | ||||||
| # events (rate*) | 525 (12) | 314 (11) | 462 (11) | 389 (10) | 115 (8) | 87 (9) |
| Model 1 | 1 (reference) | 0.94 (0.82–1.08) | 0.84 (0.74–0.95) | 0.77 (0.68–0.88) | 0.59 (0.48–0.73) | 0.76 (0.61–0.96) |
| Model 2 | 1 (reference) | 0.93 (0.80–1.08) | 0.91 (0.79–1.04) | 0.80 (0.69–0.92) | 0.62 (0.50–0.77) | 0.72 (0.56–0.92) |
| Model 3 | 1 (reference) | 0.87 (0.75–1.00) | 0.86 (0.75–0.99) | 0.78 (0.68–0.91) | 0.61 (0.49–0.76) | 0.74 (0.58–0.95) |
Note: Unless otherwise mentioned, all estimates are reported as hazard ratio (HR) and 95% confidence interval (CI). 1 drink is defined as a 4-ounce glass of wine, a 12-ounce beer, or 1.5-ounce shot of hard liquor.
Crude incidence rate per 1000 person-years
Model 1: Adjusted for total energy intake
Model 2: Model 1+additionally adjusted for age, sex, race-center, income, education level, health insurance, smoking, and physical activity
Model 3: Model 2+additionally adjusted for diabetes status, hypertension status, body mass index, and baseline estimated glomerular filtration rate
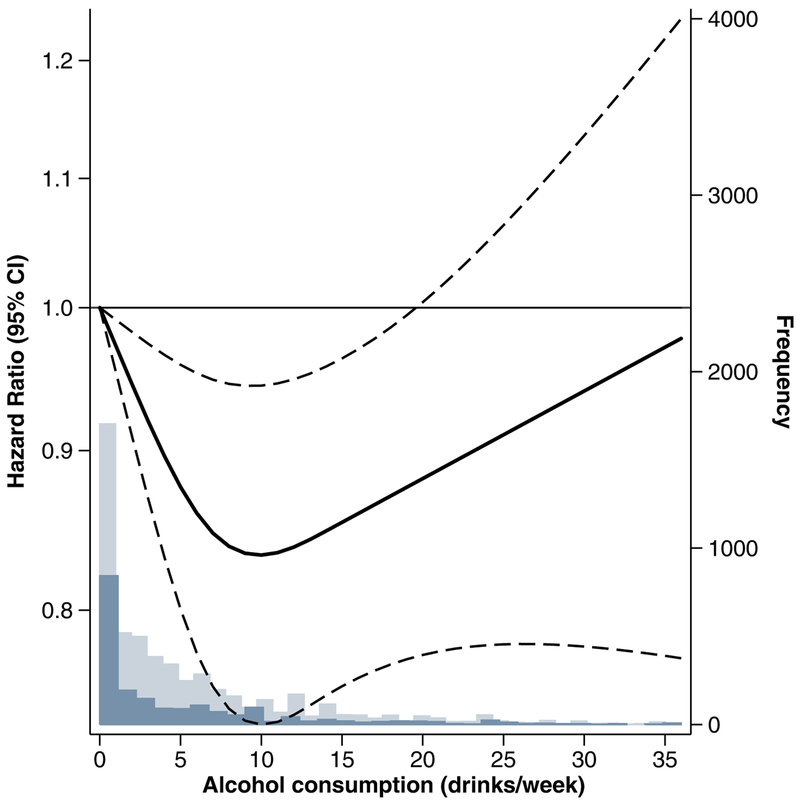
In a continuous analysis among current drinkers, there was a linear decrease in CKD risk from 1 drink/week to 10 drinks/week (Figure 1). For alcohol consumption between 10 drinks/week and 20 drinks/week, there was still a reduced risk of CKD, but the association started to approach the null. For alcohol consumption greater than 20 drinks/week, the association was no longer statistically significant.
Figure 1.
Restricted Cubic Spline Plot of Adjusted Hazard Ratios for Incident Chronic Kidney Disease (Composite) by Alcohol Consumption among Current Drinkers Bold line represents hazard ratios adjusted for total energy intake, age, sex, race-center, income, education level, health insurance, smoking, and physical activity (Model 2) modeled using restricted cubic spline terms with knots at 1, 7, and 14 drinks/week. Dashed lines represent 95% confidence intervals. Dark blue bars represent the frequency of alcohol consumption among those who developed CKD over follow-up. Light blue bars represent the frequency of alcohol consumption among those who did not develop CKD over follow-up.
Sensitivity Analyses
In a sensitivity analysis using a visit-based definition of CKD (eGFR<60 mL/min/1.73 m2 and ≥30% eGFR decline), we found similar results to the main analyses (Table 2). In model 2, participants who drank 2–7 drinks/week, 8–14 drinks/week, and ≥15 drinks/week had, respectively, a 20% (HR: 0.80, 95% CI: 0.69–0.92), 38% (HR: 0.62, 95% CI: 0.50–0.77), and 28% (HR: 0.72, 95% CI: 0.56–0.92) lower risk of CKD compared to never drinkers. The risk of CKD per each additional drink/day after accounting for the competing risk of all-cause mortality was similar to the results from the main analysis (subdistribution HR: 0.88, 95% CI: 0.82–0.95). In another sensitivity analysis using the ≤1 drink/week as the reference, never drinkers (HR: 1.14, 95% CI: 1.03–1.26) and former drinkers (HR: 1.14, 95% CI: 1.03–1.27) had a higher risk of CKD. Participants who drank 8–14 drinks/week had a lower risk of CKD compared to those who drank ≤1 drink/week (HR: 0.82, 95% CI: 0.71–0.94) (Table 3).
Table 3.
Risk for Incident Chronic Kidney Disease by Alcohol Consumption Category Using ≤1 Drink/week as Reference Category
| Alcohol Consumption Category | ||||||
|---|---|---|---|---|---|---|
| Never drinker (n=3,118) | Former drinker (n=2,239) | ≤1 drink/week (n=2,960) | 2–7 drinks/week (n=2,592) | 8–14 drinks/week (n=1,029) | ≥15 drinks/week (n=754) | |
| Incident Chronic Kidney Disease (composite definition) | ||||||
| # events (rate*) | 1,007 (15) | 724 (16) | 810 (12) | 683 (12) | 247 (11) | 193 (13) |
| Model 1 | 1.25 (1.14–1.37) | 1.39 (1.26–1.54) | 1 (reference) | 0.97 (0.87–1.07) | 0.92 (0.80–1.07) | 1.12 (0.96–1.31) |
| Model 2 | 1.14 (1.03–1.26) | 1.14 (1.03–1.27) | 1 (reference) | 0.91 (0.82–1.01) | 0.82 (0.71–0.94) | 0.88 (0.75–1.04) |
| Model 3 | 1.16 (1.05–1.28) | 1.11 (1.00–1.23) | 1 (reference) | 0.94 (0.85–1.05) | 0.83 (0.72–0.96) | 0.93 (0.79–1.10) |
Note: Unless otherwise mentioned, all estimates are reported as hazard ratio (HR) and 95% confidence interval (CI). 1 drink is defined as a 4-ounce glass of wine, a 12-ounce beer, or 1.5-ounce shot of hard liquor.
Crude incidence rate per 1000 person-years
Model 1: Adjusted for total energy intake
Model 2: Model 1+additionally adjusted for age, sex, race-center, income, education level, health insurance, smoking, and physical activity
Model 3: Model 2+additionally adjusted for diabetes status, hypertension status, body mass index, and baseline estimated glomerular filtration rate
Stratified Analyses
There were no significant interactions by sex (p=0.2), race (p=0.9), diabetes (p=0.2), or hypertension (p=0.7) for the association between alcohol consumption and CKD. There was evidence of interaction by smoking status (p<0.001). The association seemed to be stronger among smokers compared to non-smokers (Supplemental Table).
Discussion
In this prospective analysis conducted in the ARIC study, we found that alcohol consumption ranging from <1 drink/week to ≥15 drinks/week was associated with lower risk of incident CKD compared to never drinkers after adjusting for confounders. When we additionally adjusted for potential mediators in Model 3, results were only slightly attenuated, suggesting that these factors (diabetes, hypertension, BMI, and baseline eGFR) do not fully explain the association between alcohol and CKD. Diet quality may also have been an important confounder as people who consumed higher levels of alcohol may have also consumed poorer quality diets. However, additional adjustment for the DASH diet score did not change our estimates.
The findings of our study echo previous studies that found an inverse association between alcohol intake and CKD (17–20), although these studies were conducted in select populations with limited generalizability. The present analysis, which was conducted in a community-based cohort of black and white men and women from four U.S. centers, helps to address this gap. An analysis in the Physicians’ Health Study included 11,023 apparently healthy men and found that participants who drank ≥7 drinks/week had a 29% lower odds (odds ratio, OR: 0.71, 95% CI: 0.55–0.92) of elevated creatinine levels (≥1.5 mg/dL) and a 24% lower odds (OR: 0.76, 95% CI: 0.64–0.91) of reduced eGFR (≤55 mL/min) compared to participants who drank ≤1 drink/week (18). However, this study did not have information on never or former drinkers and was conducted among only men.
A meta-analysis conducted by Cheungpasitporn et al. included 20 studies in their analyses to assess the relationship between high alcohol consumption and CKD, ESRD, and proteinuria (34). The authors found a significant inverse association between high alcohol consumption and CKD (risk ratio, RR: 0.83, 95% CI: 0.71–0.98) and no significant associations with ESRD or proteinuria. However, the definition of high alcohol consumption was not consistent across studies included in the meta-analysis. Further, there was heterogeneity in defining CKD among the studies.
While our study found an inverse association between alcohol consumption and CKD, several previous studies have found no association (13–16). Studies from the Cardiovascular Health Study and Nurses’ Health Study found no significant association between moderate alcohol consumption and kidney function decline; however, they did not examine incident CKD (14, 15). In the Cardiovascular Health Study, kidney function decline was defined as eGFR loss >3 mL/min/1.73 m2/year. The results were also null but this may have been due to a relatively short mean follow-up of 5.6 years and study participants were older; therefore, the etiologically relevant time window may have passed (15). In the Nurses’ Health Study, kidney function decline was defined as ≥25% eGFR decline. The odds ratios of eGFR decline were protective for all categories of alcohol consumption compared to never drinkers but were not significant, which may have been due to low power (14).
The biological mechanism of alcohol on CKD may be similar to the mechanism between alcohol and CHD since they share similar pathways. Previous studies have attributed the protective effect of alcohol on CHD to increasing HDL cholesterol (5, 6, 35). The exact mechanisms of HDL cholesterol on CHD are not fully understood; however, it has been proposed that HDL cholesterol may increase transport rate of lipoproteins and lipoprotein lipase activity (7). Since low HDL cholesterol levels may increase the risk of renal dysfunction, the inverse association between alcohol and CKD may be mediated by HDL cholesterol (6, 36). In our cross-sectional analyses, higher HDL cholesterol levels were observed for those who consumed more alcohol. The observed association may also be mediated by type 2 diabetes since alcohol lowers risk of type 2 diabetes (10, 12). In our study, there was a lower prevalence of diabetes among current alcohol drinkers relative to never drinkers. Since diabetes is a major risk factor for CKD, alcohol may therefore lower the risk of diabetic nephropathy and arteriosclerosis associated with type 2 diabetes (37). It has also been suggested from autopsy data in the Honolulu Heart Study that alcohol intake may prevent hyalinization of the renal arterioles (38).
There are limitations in our study that must be acknowledged. First, alcohol consumption was self-reported, which is subject to reporting bias and may have been under-reported. However, if reported alcohol consumption was indeed lower than true consumption and non-differential by CKD status given that alcohol intake was assessed prior to outcome ascertainment, we would expect our estimates to be conservative. Second, drinking habits may have changed over time. We attempted to address reverse causality by excluding participants with CKD or CHD at baseline. Another limitation is that we did not have baseline measures for albuminuria and proteinuria, which would have been useful markers of kidney damage. Further, we did not have confirmation of an eGFR<60 mL/min/1.73 m2 with a second reading after at least 90 days and the outcome definition did not account include proteinuria. Another limitation is that there may have been residual confounding due to incomplete adjustment of covariates. However, in the ARIC study, participants were extensively characterized with respect to demographic, socioeconomic, and behavioral factors and we adjusted for these covariates in multivariable regression models.
A strength of our study was the large community-based population with a relatively large number of kidney events and therefore enough power to detect significant associations. Our population includes both men and women and blacks and whites, allowing generalizability to other populations. Our study also had a lengthy follow-up time, with a median of 24 years, which is longer than previous studies. We also conducted several sensitivity analyses such as a visit-based definition of CKD, subgroup analyses, and using the lowest alcohol consumption group as the reference group. Through these sensitivity analyses, we were able to demonstrate the robustness and consistency of our findings.
In summary, our large prospective cohort study of 12,692 blacks and whites in the U.S. found a significant and consistent inverse association between alcohol consumption and incident CKD. The Dietary Guidelines for Americans 2015–2020 state that individuals should not start drinking alcohol for any reason and if alcohol is consumed, women are recommended to drink up to 1 drink/day and men are recommended to drink up to 2 drinks/day (39). Furthermore, the Global Burden of Disease Study 2016 suggested that even a low amount of alcohol consumption may be associated with increased global disease burden (40). Therefore, our findings must be considered in the context of all the potential benefits and harms of alcohol.
Supplementary Material
Acknowledgements:
The authors thank the staff and participants of the ARIC study for their important contributions.
Support: Ms. Hu was supported by a grant from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (training grant T32 HL007024). Dr. Rebholz was supported by a mentored research scientist development award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782) and by a grant from the National Heart, Lung, and Blood Institute (R21 HL143089). The Atherosclerosis Risk in Communities (ARIC) study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: EAH, ML, SDR, MEG, LMS, JC, CMR declare that they have no relevant financial interests.
Practical Application
Consuming a moderate amount of alcohol (up to 1 drink/day for women and up to 2 drinks/day for men) may not be harmful to the kidneys.
References
- 1.National Institute on Alcohol Abuse and Alcoholism. 10th Special Report to the U.S. Congress on Alcohol and Health. Rockville, MD: US Dept of Health and Human Services; 2000. [Google Scholar]
- 2.World Health Organization. Global Status Report on Alcohol and Health 2014. 2014.
- 3.Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta-analysis. Addiction. 2012;107(7):1246–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319(7224):1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329(25):1829–34. [DOI] [PubMed] [Google Scholar]
- 7.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parekh RS, Klag MJ. Alcohol: role in the development of hypertension and end-stage renal disease. Curr Opin Nephrol Hypertens. 2001;10(3):385–90. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertension. 2001;37(5):1242–50. [DOI] [PubMed] [Google Scholar]
- 10.Ajani UA, Hennekens CH, Spelsberg A, Manson JE. Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Arch Intern Med. 2000;160(7):1025–30. [DOI] [PubMed] [Google Scholar]
- 11.Knott C, Bell S, Britton A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More Than 1.9 Million Individuals From 38 Observational Studies. Diabetes Care. 2015;38(9):1804–12. [DOI] [PubMed] [Google Scholar]
- 12.Conigrave KM, Rimm EB. Alcohol for the prevention of type 2 diabetes mellitus? Treat Endocrinol. 2003;2(3):145–52. [DOI] [PubMed] [Google Scholar]
- 13.Foster MC, Hwang SJ, Massaro JM, Jacques PF, Fox CS, Chu AY. Lifestyle factors and indices of kidney function in the Framingham Heart Study. Am J Nephrol. 2015;41(4–5):267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight EL, Stampfer MJ, Rimm EB, Hankinson SE, Curhan GC. Moderate alcohol intake and renal function decline in women: a prospective study. Nephrol Dial Transplant. 2003;18(8):1549–54. [DOI] [PubMed] [Google Scholar]
- 15.Menon V, Katz R, Mukamal K, Kestenbaum B, de Boer IH, Siscovick DS, et al. Alcohol consumption and kidney function decline in the elderly: alcohol and kidney disease. Nephrol Dial Transplant. 2010;25(10):3301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol. 2006;164(3):263–71. [DOI] [PubMed] [Google Scholar]
- 17.White SL, Polkinghorne KR, Cass A, Shaw JE, Atkins RC, Chadban SJ. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dial Transplant. 2009;24(8):2464–72. [DOI] [PubMed] [Google Scholar]
- 18.Schaeffner ES, Kurth T, de Jong PE, Glynn RJ, Buring JE, Gaziano JM. Alcohol consumption and the risk of renal dysfunction in apparently healthy men. Arch Intern Med. 2005;165(9):1048–53. [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi Y, Omori H, Onoue A, Mihara S, Ogata Y, Katoh T. Association between frequency of drinking alcohol and chronic kidney disease in men. Environ Health Prev Med. 2012;17(3):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds K, Gu D, Chen J, Tang X, Yau CL, Yu L, et al. Alcohol consumption and the risk of end-stage renal disease among Chinese men. Kidney Int. 2008;73(7):870–6. [DOI] [PubMed] [Google Scholar]
- 21.Buja A, Scafato E, Baggio B, Sergi G, Maggi S, Rausa G, et al. Renal impairment and moderate alcohol consumption in the elderly. Results from the Italian Longitudinal Study on Aging (ILSA). Public Health Nutr. 2011;14(11):1907–18. [DOI] [PubMed] [Google Scholar]
- 22.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 24.Shimakawa T, Sorlie P, Carpenter MA, Dennis B, Tell GS, Watson R, et al. Dietary intake patterns and sociodemographic factors in the atherosclerosis risk in communities study. ARIC Study Investigators. Prev Med. 1994;23(6):769–80. [DOI] [PubMed] [Google Scholar]
- 25.Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T, Folsom AR. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutrition Research. 1996;16(5):735–45. [Google Scholar]
- 26.Lustgarten JA, Wenk RE. Simple, rapid, kinetic method for serum creatinine measurement. Clin Chem. 1972;18(11):1419–22. [PubMed] [Google Scholar]
- 27.Parrinello CM, Grams ME, Couper D, Ballantyne CM, Hoogeveen RC, Eckfeldt JH, et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61(7):938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grams ME, Rebholz CM, McMahon B, Whelton S, Ballew SH, Selvin E, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64(2):214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. [DOI] [PubMed] [Google Scholar]
- 31.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29(7):901–9. [DOI] [PubMed] [Google Scholar]
- 32.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 34.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Brabec BA, O’Corragain OA, Edmonds PJ, et al. High alcohol consumption and the risk of renal damage: a systematic review and meta-analysis. QJM. 2015;108(7):539–48. [DOI] [PubMed] [Google Scholar]
- 35.Zakhari S Alcohol and the cardiovascular system: molecular mechanisms for beneficial and harmful action. Alcohol Health Res World. 1997;21(1):21–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14(8):2084–91. [DOI] [PubMed] [Google Scholar]
- 37.Wakabayashi I, Kobaba-Wakabayashi R, Masuda H. Relation of drinking alcohol to atherosclerotic risk in type 2 diabetes. Diabetes Care. 2002;25(7):1223–8. [DOI] [PubMed] [Google Scholar]
- 38.Burchfiel CM, Tracy RE, Chyou PH, Strong JP. Cardiovascular risk factors and hyalinization of renal arterioles at autopsy. The Honolulu Heart Program. Arterioscler Thromb Vasc Biol. 1997;17(4):760–8. [DOI] [PubMed] [Google Scholar]
- 39.US Department of Health and Human Services, US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans May 3, 2018. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 40.G. B. D. Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.