Abstract
Background:
Radiotherapy outcomes are limited by toxicity in the healthy tissues surrounding the irradiated tumor. Recent pre-clinical studies have shown that irradiations with electrons or photons delivered at so called FLASH dose rates (i.e. >40 Gy/s) dramatically reduce adverse side effects in the normal tissues while being equally efficient for tumor control as irradiations at conventional dose rates (3-5 cGy/s). In the case of protons however, FLASH effects have not been investigated partially because of the limited availability of facilities that can achieve such high dose rates.
Methods:
Using a novel irradiation platform, we measured acute and long-term biological effects in normal human lung fibroblasts (IMR90) exposed to therapeutically relevant doses of 4.5 MeV protons (LET =10 keV/μm) delivered at dose rates spanning four orders of magnitude. Endpoints included clonogenic cell survival, γH2AX foci formation, induction of premature senescence (β-gal), and the expression of the pro-inflammatory marker TGFß.
Results:
Proton dose rate had no influence on the cell survival, but for the highest dose rate used (i.e. 1000 Gy/s) foci formation saturated beyond 10 Gy. In the progeny of irradiated cells, an increase in dose (20 Gy vs. 10 Gy) and dose rate (1000 Gy/s vs. 0.05 Gy/s) positively affected the number of senescence cells and the expression of TGFß1.
Conclusions:
In normal lung fibroblasts proton dose rate had little impact on acute effects, but significantly influenced the expression of long-term biological responses in vitro. Compared to conventional dose rates, protons delivered at FLASH dose rates mitigated such delayed detrimental effects.
Keywords: Proton FLASH dose rate, Proton FLASH therapy, Proton FLASH irradiation, Proton radiotherapy, Proton dose rate effects
BACKGROUND
It is estimated that each year in the United States almost 500,000 cancer patients receive radiation alone or in combination with chemotherapy (1). In radiotherapy, the dose delivered to the tumor is limited by the toxicity to the surrounding healthy tissue. As the number of cancer survivors increases each year, there is a need to develop novel treatment modalities that eradicate the tumor while preventing or mitigating radiation-induced normal tissue injury.
Pre-clinical studies have shown that the induction of lung fibrosis in mice exposed to 4.5 MeV electrons (2) delivered at dose rates >40 Gy/s, also known as FLASH therapy, occurred at higher dose compared to irradiations at conventional dose rates; while fibrogenesis was observed 24 weeks after exposure to 17 Gy delivered at conventional dose rates, 30 Gy delivered as FLASH elicited only rare fibrotic patches at the same time point (3). Additionally, sparing of brain tissue at dose rates above 100 Gy/s was proven by measuring brain toxicity and cognitive functions in mice after whole brain irradiations with X rays (4). The advantages of FLASH dose rate irradiations over conventional radiation modalities have been recently extended to higher mammals such as cat-cancer patients (5).
In the case of protons however, FLASH therapy studies have been limited by the availability of irradiators that can provide such dose rates (6–10). For example, proton beams for radiotherapy that deliver as much as 5 Gy/s are already considered as ultra-high dose rate beams (11). While such dose rates are still below those required to elicit the FLASH effect, new laser plasma accelerators, which are already considered as the next generation of cost effective tools for radiotherapy, will be able to surpass the FLASH dose rates by several orders of magnitude (12). The need to characterize the biological effects of protons irradiation on normal tissues is also driven by the rapid adoption of proton radiotherapy and the increase of long-term survivors. The extent of the normal tissue toxicity following proton radiotherapy is still controversial. In fact, recent studies have shown that among younger men with prostate cancer, proton radiation was associated with a significant reduction in urinary damage but increased bowel toxicity (13).
We have developed a unique FLASH dose rate proton irradiator that can deliver therapeutically relevant doses at dose rates from 0.025 Gy/s up to 1500 Gy/s. The average linear energy transfer (LET) of the proton beams is around 10 keV/um, which is comparable to that of the typical spread out Bragg peak (SOBP) of a therapeutic proton beam (14).
Despite encouraging pre-clinical results using radiation delivered as FLASH, the molecular mechanisms behind the observed sparing effects of normal tissues remain elusive. In irradiated cells, physiological levels of free radicals may increase for days and months after the initial exposure due to perturbations in oxidative metabolism (15), and these processes occur not only in the irradiated cells but also in their progeny (16, 17). In tissues, a chronic radiation-induced inflammatory response may increase the risk of late effects such as organ fibrosis and other degenerative diseases including cancer (16). Here we investigated acute and long-term effects in normal human lung fibroblasts (IMR90) exposed in vitro to protons delivered at dose rates ranging from 0.05 Gy/s to 1000 Gy/s. In particular, we assessed clonogenic cell survival immediately after irradiation and γH2AX foci formation 30 minutes post-exposure; delayed effects such as premature cell senescence and expression of the pro-inflammatory marker TGFβ were measured in the progeny of irradiated cells.
MATERIAL AND METHODS
The proton FLASH irradiator
The new FLASH irradiator at the Radiological Research Accelerator Facility (RARAF) can deliver an intense pulse of protons accelerated by the HVE 5.5 MV Singletron accelerator (High Voltage Engineering Europa B.V., Amersfoort, Netherlands) to a circular spot with a diameter of around 11 mm (18). The variation in beam intensity across the spot is less than 3%. The dose rate can be adjusted to any value between 0.025 Gy/s to 1500 Gy/s by changing the proton beam current. The beam current, and thus the instantaneous dose rate, is controlled by a thin (3 mm) custom made parallel plate ionization chamber whose electrodes are made of 6-μm thick aluminized mylar foil. The chamber is operated in a current mode and positioned right after the vacuum exit aperture (10 mm diameter) and 10 mm in front of the samples. The exit aperture is covered by a 2.9-μm thick havar foil that allows for ions to exit into the atmosphere, traverse the ionization chamber and reach the samples mounted on an automated motorized stage (19). During the experiment, the beam was deflected off the target by a 1000 V potential difference applied between two 40-cm long deflector plates mounted approximately seven meters away from the vacuum window on the FLASH beamline. The irradiations were performed by sending a square voltage pulse (generated by the waveform generator) with a well-defined time length to discharge the beam deflector and allow for the unobstructed beam passage towards the target. In that way the “beam ON” time (ranging from a one misillisecond to several tens of milliseconds) was precisely controlled and outside of the duration of the discharging pulse the beam was prevented of hitting the sample. The product of the “beam ON” time and the dose rate determined the total dose to which the sample was exposed. Unlaminated EBT3 films (GafChromic™) were used first to calibrate the system and later as an absolute dosimeter to assure that the correct dose was delivered during the irradiations (20). As a part of the dosimetry protocol, the film was irradiated immediately before the samples with the same beam current (monitored by the ionization chamber) and for several “beam ON” times equal to those that were later used to expose the samples. Films were scanned 48 h post-irradiation and the applied doses were determined from the change in the optical density of the film. The calibration of the unlaminated EBT3 film response was performed at the RARAF track segment facility where we have an established dosimetry protocol for ion beam irradiations of biological samples (20).
Cells
Normal human diploid lung fibroblasts (IMR90) were obtained from the American Type Culture Collection (ATCC® CCL-186). Cells at passage 10-12 were grown in Eagle’s Minimum Essential Medium (CellGro) supplemented with 12.5% heat-inactivated (56°C, 30 min) fetal calf serum, 400 mM L-alanyl-L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich Corp.). The cells were maintained at 37° C in a humidified incubator with 5% CO2 in air. For each experiment the cells were seeded 24 h before irradiation. At the time of irradiation (and for the mock-irradiated samples) the medium was removed from the cells. To take into account of the variability of the irradiation time depending on dose and dose rate, at any one time we irradiated a number of wells such that the total time the cells were left without media did not exceed 15 minutes. Similarly for each endpoint, the sham-irradiated cells were left without medium for 15 minutes.
Clonogenic cell survival
Immediately following irradiation to 0, 0.5, 1, 2, 5 or 10 Gy of 4.5 MeV protons delivered at 0.05, 100 or 1000 Gy/s, the cells were trypsinized and seeded in 60-mm dishes at numbers that resulted in ~ 100 clonogenic cells per dish (21). Three replicates were done for each experimental point. After an incubation period of ~12 days, the cells were rinsed with PBS, fixed in 95% ethanol and stained with crystal violet. We counted colonies consisting of at least 50 cells.
Immunolabeling of γH2AX and TGFβ1
We seeded approximately 1000 cells per well 24 h before irradiation. Induction of γH2AX foci formation was assessed 30 min post-irradiation whereas TGFβ1 was assessed 24 h and one month after exposure (for the latter time point, the medium was changed once a week). Briefly, 1) the cells were washed in PBS and fixed with 2% paraformaldehyde/PBS at room temperature (RT) for 20 min; 2) they were permeabilized with methanol at −20° C for 20 min; 3) unspecific binding of antibody was blocked in 0.2% bovine serum albumin (BSA)/PBS at RT for 15 min; 3) the samples were incubated with a solution of 1:200 anti-γH2AX (Serl39) antibody (Cell Signaling 2577) or 1:100 anti-TGFβ1 (Abeam 92486) in 0.2% BSA/PBS in 0.2% BSA/PBS at RT for 45-60 min and 4) subsequently incubated at RT for 45 min in anti-rabbit Alexa555 antibody (Invitrogen A21429) 1:800 in 0.2% BSA/PBS. Between each step, the cells were washed three times in PBS for 5 min with gentle shaking. The samples were mounted on slides with VectaShield Mounting Medium with 5 mg/ml of 4′,6-diamidino-2-phenylindole (DAPI) (Vector Lab Inc. H-1200) and examined at the Olympus IX70 fluorescent microscope equipped with filters and a Photometrics® PVCAM high-resolution, high-efficiency digital camera. For each sample we acquired 40× images of several random fields of view. For any cell, image analysis (22) yielded to the ratio of the γH2AX-conjugated Alexa555 fluorescence intensity and the fluorescence intensity of the whole nucleus (DAPI). In the case of TGFβ1, Image J was used to measure the TGFβ1-conjugated Alexa555 fluorescence intensity.
Senescence
We seeded approximately 1000 cells per well 24 h before irradiation. Immediately after irradiation the cells were re-seeded at low density (~1000 cells) in 6-well plates and the medium was changed once a week for one month. We measured the percentage of senescent cells one month after irradiation using the β-galactosidase staining kit (Abcam ab102534) following the manufacturer instructions. The samples were observed under a 10× objective at the bright field Olympus IX70 microscope and for each field of view we counted the percentage of β-gal positive cells divided by the total number of cells in at least three random fields of view per treatment.
Statistical Analysis
The results are reported as the mean ± SEM and for each endpoint the values were normalized to that measured in sham-treated cells. Comparison between treatment groups and respective controls was performed using the Student’s t test. Analysis of variance (ANOVA) was conducted to compare multiple linear regressions. A p value of ≤ 0.05 was considered significant. Each graph represents the combined results of at least three separate experiments.
RESULTS
The effect of protons dose rate on clonogenic cell survival
Limited data are available on the survival of normal cells exposed to protons delivered at ultra-high dose rates. To characterize the clonogenic cell survival depending on the proton dose rate, cells were exposed to different doses delivered at 0.05, 100 or 1000 Gy/s. The survival curves for all three dose rates followed a typical exponential decay trend with the dose. Although a slight difference between the low (0.05 Gy/s) and the two ultra-high dose rates (100 and 1000 Gy/s) can be observed at the highest dose tested (10 Gy) (Fig. 1), the trends were not statistically different, as calculated by analysis of variance (ANOVA). In particular, for each dose rate, the survival fraction (SF) values were natural log (ln) transformed to bring the error distribution closer to normal (23). We applied linear regression using the normalized ln(SF) values as the dependent variable and proton dose (D, Gy) as the independent variable. Using this approach, the SF were fitted to first-order kinetics according to the equation ln(SF)= −kD. The regressions were performed with the intercept set to zero, which corresponds to 100% survival at the zero radiation dose. Bootstrap 99% confidence intervals for the parameter k were calculated using ANOVA, which resulted in a F-ratio of 0.05 (p= 0.95). These results indicate that proton dose rate had little impact on the clonogenic survival of irradiated normal cells.
Fig. 1: The effect of protons dose rate on clonogenic cell survival.
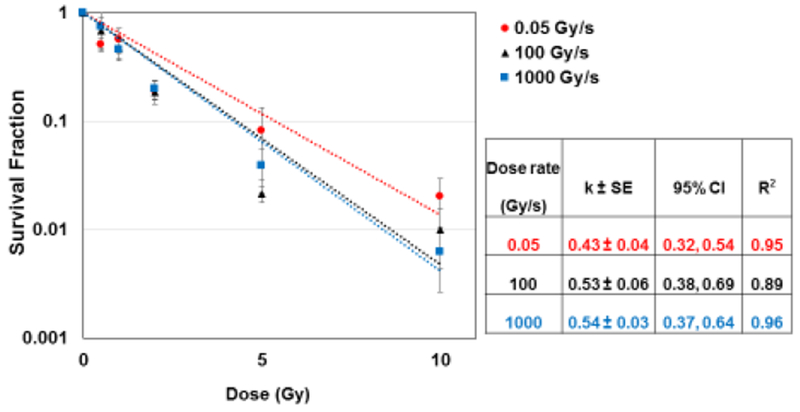
Survival fraction of normal human lung fibroblasts (IMR90) exposed to different doses of 4.5 MeV protons delivered at 0.05, 100 or 1000 Gy/s. For all dose rates, the survival fraction (SF) decreased with the dose D (Gy) as ln(SF)= −kD. The table summarizes the value of k, the 95% confidence interval (upper, lower) and correlation coefficient for the linear regressions applied to each dose rate.
The effect of proton dose rate on DNA damage
The reduced adverse long-term effects of FLASH irradiation observed in normal tissues compared to conventional dose rates (2, 5) may be explained by the different type and/or amount of the induced DNA damage. To test this hypothesis in cells, we measured γH2AX foci formation 30 minutes after exposure to 0, 1, 2, 5, 10 or 20 Gy of protons delivered at 0.05, 100 or 1000 Gy/s (Fig. 2). We observed a statistically significant reduction (p< 0.05) of foci fluorescence intensity at the highest dose (20 Gy) when the protons were delivered at the highest dose rate (1000 Gy/s). In fact, foci formation increased by ~13-folds when the protons were delivered at 0.05 Gy/s or 100 Gy/s. In contrast, the increase was ~ 8-fold when the protons were delivered at 1000 Gy/s. At this dose rate, there might be a possible saturation for foci formation beyond 10 Gy (Fig.2).
Fig. 2: The effect of proton dose rate on DNA damage.
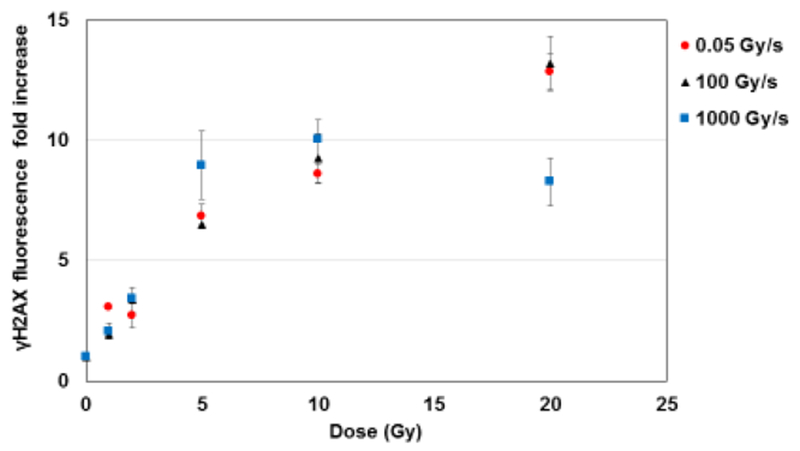
Fold change of γH2AX foci fluorescence intensity (normalized to controls) in normal human lung fibroblasts (IMR90) 30 minutes after exposure to different doses of 4.5 MeV protons delivered at 0.05, 100 or 1000 Gy/s.
Our results indicate that the initial formation of DNA damage foci was positively influenced by the proton dose rate only at relatively high doses (20 Gy), and that the effect was observed only at the highest dose rate tested (1000 Gy/s).
The effect of proton dose rate on premature cell senescence
We measured the percentage of β-galactosidase (β-gal) positive IMR90 cells one month after exposure to 20 Gy of 4.5 MeV protons delivered at different dose rates (Fig. 3); we found that compared to 0.05 Gy/s, the FLASH dose rates of 100 Gy/s or 1000 Gy/s led to a reduced number of senescence cells (p= 0.036 and p= 0.01 for 100 Gy/s and 1000 Gy/s, respectively). The results suggest that in cells irradiated in vitro the proton dose rate modulates long-term effects such as senescence.
Fig.3: The effect of proton dose rate on premature cell senescence.
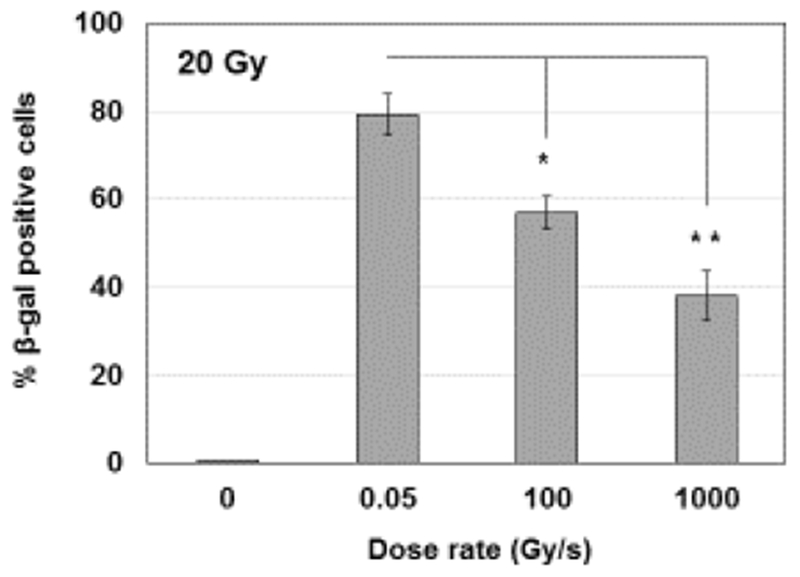
Percentage of β-gal positive normal human lung fibroblasts (IMR90) one month after exposure to 20 Gy of 4.5 MeV protons delivered at 0.05, 100 or 1000 Gy/s. *p< 0.05, **p = 0.01
The effect of proton dose rate on the expression of the inflammatory marker TGFβ
We assessed the impact of proton dose rate on the induction of TGFβ1 by measuring its fluorescent intensity in cells exposed to 20 Gy of protons delivered at low (0.2 Gy/s) or high dose rates (1000 Gy/s); 24 h after exposure we found that while protons delivered at low dose rate induced a ~6.5-fold increase in TGFβ1 fluorescent intensity compared to sham-irradiated cells while protons delivered at 1000 Gy/s resulted in a 1.8-fold increase (Fig. 4). Remarkably, the statistically significant (p< 0.05) differential effect persisted up to a month after exposure when protons delivered at low dose rate induced a ~2.7-fold increase compared to controls while protons delivered at 1000 Gy/s yielded only a ~0.4-fold increase (Fig. 4). These results suggest that protons delivered as FLASH may mitigate the expression of markers typically associated to radiation-induced inflammatory responses.
Fig.4: The effect of proton dose rate on the expression of the inflammatory marker TGFβ.

TGFβ1 fluorescence intensity fold increase in normal human lung fibroblasts (IMR90) 24 h and one month after exposure to 20 Gy of 4.5 MeV protons delivered at 0.2 or 1000 Gy/s. *p< 0.05
DISCUSSION
Using a novel irradiation platform, here we investigated in vitro acute and long-term biological effects induced in normal cells by protons delivered at conventional or at ultra-high dose rates. Previous studies on the biological effects induced by proton irradiation delivered at different dose rates involved extremely high dose-rates (7–9, 24) (109 Gy/s) delivered in nanoseconds, which are much higher than those found to elicit the FLASH effects with electrons. Moreover, the conventional irradiation times used for comparison with the nanosecond irradiations typically lasted 100 ms, which is stil too short to be categorized as FLASH. Finally, the biological endpoints focused exclusively on acute effects like cell survival (24) or DNA damage (9).
In this work, we tested the hypothesis that the beneficial effects observed in normal tissues when radiation is delivered as FLASH as opposed to conventional dose rates may be due to a gain in cell survival. However, in our system this was not the case (Fig. 1). The dose rate however may influence the complexity and/or the extent of the induced DNA damage. To test this notion, we compared the induction of γH2AX foci formation in cells 30 minutes following exposure to protons delivered at different dose rates. It appeared that proton dose rate had an impact on the formation of the foci only at relatively high doses (20 Gy). At the highest dose rate tested (1000 Gy/s), foci fluorescence intensity appeared to saturate at doses beyond 10 Gy and at 20 Gy it resulted in a statistically significant reduction (p< 0.05) in foci induction compared to similar doses delivered at relatively lower dose rates (Fig. 2). In agreement with a previous study in HeLa cells showing differential G2 arrest after proton FLASH irradiation compared to conventional dose rates (24), one can speculate that the proton dose rate modulates the complexity of the induced damage; if the observed results would persists for at least 24 h protons delivered at FLASH dose rates may generate a smaller number of clustered DNA damage.
Despite having little impact on acute cell damage, the proton dose rate may influence the manifestation of delayed harmful effects. Indeed, exposure to radiation induces a perturbation in the cellular oxidative metabolism that may persist long after the initial exposure in the irradiated cells and their progeny (25). In tissues, radiation-induced chronic inflammatory responses may increase the risk of late effects such as organ fibrosis and other oxidative stress-driven degenerative diseases including cancer (16). Radiation-induced premature cell senescence has been proposed as a potential mechanistic link between radiation-induced metabolic oxidative stress and prolonged tissue injury (26). In cells we found that one month after exposure to 20 Gy of protons delivered at FLASH dose rates the number of senescence cells was reduced compared to that induced by a relatively lower dose rate (Fig. 4). Remarkably, senescent cells release pro-inflammatory molecules that may further contribute to the progression of cell and tissue injury, with TGFβ1 being one of the major players in modulating such signals. Here, we found that while protons delivered at the low dose rate of 0.2 Gy/s increased the level of TGFβ1 fluorescent intensity compared to sham-irradiated cells 24 h after exposure, the extent of the levels of TGFβ were significantly lower when the protons were delivered at the relatively higher dose rate of 1000 Gy/s; the differential effects persisted in the progeny of the irradiated cells for up to one month after exposure (Fig. 4).
In summary, our data support the concept that in normal cells exposed in vitro proton dose rate affects long-term outcomes, which may be triggered by a radiation-induced inflammatory response. Here we propose that proton dose rate, despite having little influence on acute effects such as cell survival and DNA damage, may affect the long-term balance between anti- and pro-inflammatory molecules; radiation delivered at conventional dose rate may tip the balance toward the latter leading eventually to the chronic inflammation and degenerative injury observed in normal tissues after conventional radiation therapy. Here we show that delivering protons at ultra-high dose rates can mitigate such adverse delayed responses in normal cells.
Our approach will allow systematic studies on the role of proton dose rate on the induction of biological effects, which is still not fully understood. Indeed, our irradiation platform allows for highly customizable end-station and sample holders making it well suited for studying dose rate effects induced by protons in vitro and in vivo. The ability to use models of increasing biological complexity (cells, 3-D tissues and small animal models) allows for strategic translational studies. In addition, the drastic increase of the dose rate to 100 Gy/s and above needed for proton FLASH therapy would allow to shorten the irradiation time from minutes to tens of milliseconds with the additional benefit of solving the issue of organ motion, which notably increases the challenge for the clinical use of proton beams (27, 28). Opportunely paired with similar studies in tumor cell lines, FLASH therapy has the potential to enhance the therapeutic ratio by mitigating the adverse effects of proton irradiation on healthy cells.
HIGHLIGHTS.
This is the first study on long term effects of proton irradiations at FLASH dose rate in vitro.
Proton dose rate has little impact on acute effects in normal cells.
Cell survival and γH2AX foci formation depend weakly on proton dose rate.
Protons delivered at FLASH dose rates mitigate long-term adverse effects in normal cells.
Increase in proton dose rate reduces the number of senescent cells and cellular levels of TGFβ1.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) under grant no. 5P41EB002033.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
On behalf of the authors of the manuscript entitled “Biological Effects in Normal Cells Exposed to FLASH Protons” (Manuela Buonanno, Veljko Grilj and David J. Brenner), I certify that we have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Contributor Information
Veljko Grilj, Email: vg2400@cumc.columbia.edu.
David J. Brenner, Email: djb3@cumc.columbia.edu.
BIBLIOGRAPHY
- 1.Citrin DE. Recent Developments in Radiotherapy. New Engl J Med 2017;377(22):2200–1. [DOI] [PubMed] [Google Scholar]
- 2.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014;6(245). [DOI] [PubMed] [Google Scholar]
- 3.Jayson GG, Parsons BJ, Swallow AJ. Mechanism of the Fricke Dosimeter. Int J Radiat Phys Ch 1975;7(2-3):363–70. [Google Scholar]
- 4.Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol 2018. [DOI] [PubMed] [Google Scholar]
- 5.Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The advantage of Flash radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res 2018. June 6. doi: 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- 6.Schmid TE, Dollinger G, Beisker W, Hable V, Greubel C, Auer S, et al. Differences in the kinetics of gamma-H2AX fluorescence decay after exposure to low and high LET radiation. Int J Radiat Biol 2010;86(8):682–91. [DOI] [PubMed] [Google Scholar]
- 7.Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, et al. The effectiveness of 20 mev protons at nanosecond pulse lengths in producing chromosome aberrations in human-hamster hybrid cells. Radiation Res 2011;175(6):719–27. [DOI] [PubMed] [Google Scholar]
- 8.Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, et al. Relative biological effectiveness of pulsed and continuous 20 MeV protons for micronucleus induction in 3D human reconstructed skin tissue. Radiother Oncol 2010;95(1):66–72. [DOI] [PubMed] [Google Scholar]
- 9.Zlobinskaya O, Dollinger G, Michalski D, Hable V, Greubel C, Du G, et al. Induction and repair of DNA double-strand breaks assessed by gamma-H2AX foci after irradiation with pulsed or continuous proton beams. Radiat Environ Biophy 2012;51(1):23–32. [DOI] [PubMed] [Google Scholar]
- 10.Zlobinskaya O, Siebenwirth C, Greubel C, Hable V, Hertenberger R, Humble N, et al. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat Res 2014;181(2):177–83. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura T, Egashira Y, Nishio T, Matsumoto Y, Wada M, Koike S, et al. Apparent absence of a proton beam dose rate effect and possible differences in RBE between Bragg peak and plateau. Med Phys 2010;37(10):5376–81. [DOI] [PubMed] [Google Scholar]
- 12.Karsch L, Beyreuther E, Enghardt W, Gotz M, Masood U, Schramm U, et al. Towards ion beam therapy based on laser plasma accelerators. Acta Oncol 2017;56(11):1359–66. [DOI] [PubMed] [Google Scholar]
- 13.Pan HY, Jiang J, Hoffman KE, Tang C, Choi SL, Nguyen QN, et al. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men With Prostate Cancer. Cancer. J Clin Oncol 2018;36(18):1823–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paganetti H Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Physi Med Biol 2014;59(22):R419–R72. [DOI] [PubMed] [Google Scholar]
- 15.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012;327(1-2):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 2011;711(1-2):193–201. [DOI] [PubMed] [Google Scholar]
- 17.Buonanno M, de Toledo SM, Pain D, Azzam EI. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat Res 2011;175(4):405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird RP, Rohrig N, Colvett RD, Geard CR, Marino SA. Inactivation of synchronized Chinese Hamster V79 cells with charged-particle track segments. Radiat Res 1980;82(2):277–89. [PubMed] [Google Scholar]
- 19.Colvett RD, Rohrig N. Biophysical studies with spatially correlated ions. 2. Multiple scattering, experimental facility, and dosimetry. Radiat Res 1979;78(2):192–209. [PubMed] [Google Scholar]
- 20.Grilj V, Brenner DJ, LET dependent response of GafChromic films investigated with MeV ion beams. Phys Med Biol 2018;63(24):245021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med 1956;103(5):653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner HC, Brenner DJ, Chen Y, Bertucci A, Zhang J, Wang H, et al. Adapting the gamma-H2AX assay for automated processing in human lymphocytes. 1. Technological aspects. Radiat Res 2011;175(3):282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keene ON. The Log Transformation Is Special. Stat Med 1995;14(8):811–9. [DOI] [PubMed] [Google Scholar]
- 24.Auer S, Hable V, Greubel C, Drexler GA, Schmid TE, Belka C, et al. Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat Oncol 2011;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012;327(1-2):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Citrin DE, Shankavaram U, Horton JA, Shield W, Zhao SP, Asano H, et al. Role of Type II Pneumocyte Senescence in Radiation-Induced Lung Fibrosis. Jnci-J Natl Cancer I. 2013;105(19):1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Ruysscher D, Sterpin E, Haustermans K, Depuydt T. Tumour Movement in Proton Therapy: Solutions and Remaining Questions: A Review. Cancers (Basel). 2015;7(3):1143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen AM, Pawlicki T, Dong L, Fourkal E, Buyyounouski M, Cengel K, et al. An evidence based review of proton beam therapy: the report of ASTRO’s emerging technology committee. Radiother Oncol 2012; 103(1):8–11. [DOI] [PubMed] [Google Scholar]


