Abstract
Aims
The aim of this study was to present a new method for removing Sodium dodecyl sulfate (SDS) detergent from decellularized bovine pericardium using vacuum.
Materials and Methods
The cows’ pericardia were collected and decellularized. The samples were incubated with SDS1% for 48 h at 40 °C. To perform vacuum washing (VW: negative pressure was used to wash and remove detergents), every decellularized tissue was cut in 75mm diameter and fixed via a stainless-steel ring with 60mm diameter in the center of filtration Buchner Funnel which was connected to glass filtration flask The system was connected to a vacuum pump by a hose, and a negative pressure of -100 mmHg was applied for 15 min. Then, the samples were shaken and washed at 40-rpm in 100 ml of distilled water for 45 min. This process was repeated for samples of each group (6 times for sample VW6h, 12 times for sample VW12h, and 24 times for sample VW24h). At the end of every cycle, the effluent was collected to take a sample for SDS measurement. The normal washing (NW) group containing distilled water (NWd) and PBS (Phosphate buffered saline) (NWp) were used to wash and remove detergents. SDS measurements, MTT Assay, histological and tensile test, to compare two methods were used.
Results
The highest SDS in the effluent was in groups VW12h and VW24h (P ≤ 0.001) and the lowest residual SDS in scaffold was in two groups of VW12h and VW24h (P ≤ 0.001). MTT assay showed that cell survival in the VW12h and VW24h groups was higher than other groups and there' was no significant difference between cell survival in the VW12h and VW24h groups. Histological study showed destruction of tissue in the VW24h group. The results of the tensile test were shown that the native group had the highest module and the lowest amount was the VW24h sample which was reported with P ≤ 0.001 significance for all groups.
Conclusion
VW12h can be used as an effective method for SDS removal from decellularized pericardium which morphologically demonstrated a good structure in ECM.
Keywords: Cell biology, Cell culture, Cytotoxicity, Extracellular matrix, Stem cells research, Regenerative medicine, Pericardium, Acellular, Toxicity, Sodium dodecyl sulfate
1. Introduction
Bovine pericardium is one of the perfect substances used to manage critical heart disease such as heart valve defects, aortic annulus root infections, and ventricular septal disorders [1]. up to now, several types of decellularized bovine pericard scaffolds have been used in tissue engineering to repair certain defects [1]. Veritas Collagen Matrix®, a kind of a commercial scaffold, is made from bovine pericardium for reconstruction and renewal of the ventricle and pelvic defects [2]. The bovine pericardium has also been used as a commercial product called Tutopatch®, employed in the repair of abdominal hernias. Peri-Guard® is also used as a prosthesis for pericardial closure and soft tissue deficiency such as abdominal defects (femoral, inguinal, diaphragmatic, lumbar, scrotal, and umbilical) along with intra-cardiac and great vessel repair [3, 4]. Another application of decellularized bovine pericardia as a dura matter fixer in some brain surgery is integra® [5]. Decellularized tissues and organs are significantly used in tissue engineering and regenerative medicine. The degree and efficacy of cell removal from decellularized tissue depend on the type of tissue and the methods for cell removal. There are some methods for tissue decellularization including physical, chemical, and enzymatic processes. Each of these methods affects the composition, structure, and mechanical characteristics of the tissues [6, 7]. Ionic and non-ionic detergents such as sodium deoxycholate, triton – X, and sodium dodecyl sulfate (SDS) are very common in chemical methods for decellularization [8]. The ionic detergents are effective for resolving and removal both nuclear wall and cytoplasmic membrane [6, 8, 9].
The SDS is one of the most widely used ionic detergents in tissue decellularization. It is efficient in the removal of cellular components and debris from the tissue. In comparison with the other detergents, SDS removes nuclear remnants and cytoplasmic proteins efficiently [6], but it has a cytotoxic effect on the cell so it needs extensive washing process [10]. An event that occurs after decellularization is the removal of the remaining detergent as debris inside of the decellularized tissue, which is difficult to remove from the tissue due to the adhesion of these materials to the structure of the tissues. In biological studies and in tissue engineering, scientists most of the time use detergents to decellularization of tissue to produce some scaffolds [11, 12, 13, 14]. In spite of good removal effects of SDS on cells and the debris from decellularized tissue, the detergent remaining in the tissue after rinsing with water or Phosphate-buffered saline (PBS) has some undesirable effects on recellularization of the scaffold. A study indicated that the residual detergent had a side effect (metabolic activity and cytotoxicity) on endothelial cells [15]. In a study on porcine intestine and evaluation of various chemical methods for decellularization, the results showed that the SDS had a highly toxic effect on the recellularization process [16]. For washing decellularized tissues and SDS removal, there are some methods in the literature. For example, in a study, the pericardia were washed with distilled water twice for 12 h and then washed in PBS 3 times by changing the PBS every 8 h [15, 17]. In another study, decellularized tissues were rinsed in distilled water for 15 min by agitation (300rpm) which was repeated 4 times and again washed in distilled water for 24h via (300rpm) agitation. Again, it was rinsed in distilled water for 15 min by agitation (300rpm) to remove the remaining detergent. Finally, the samples were washed 24h in distilled water [11]. According to the literature, 48 h is the minimal time for SDS removal from decellularized tissue [15, 17, 18, 19]. In any case, removing the rest of SDS is one of the critical problems in decellularization methods [15]. All of them are time and material consuming. In this research, our aim was to introduce a new method to remove SDS from decellularized bovine pericardium by vacuum as a rapid and efficient SDS removal method.
2. Materials and methods
2.1. The bovine pericardia
Two-year-old male calves of the Dashtyari breed were selected (Iran-Shahrekord slaughterhouse) and the pericardia were collected after sacrificing the animals. After removing adipose tissue, the left part of pericardia was isolated and all of the samples were put in a glass bottle containing 500cc PBS so transferred to the tissue engineering laboratory.
2.2. Design
In this research we had six groups: the native tissues as control (C group), the decellularized tissues with SDS 1% and normal washing with distillated water (NWd group), the decellularized tissues with SDS 1% and normal washing with PBS (NWp group), the decellularized tissue with SDS 1% and 6-hour vacuum washing (VW6h group), the decellularized tissues with SDS 1% and 12-hour vacuum washing (VW12h group) and the decellularized tissues with SDS 1% and 24-hour vacuum washing (VW24h group).
2.3. Decellularization method
For decellularization of samples, all of them were placed in roller bottles (hybridization Incubator GFL-7610), and incubated in SDS1% (Biochem CAS: 151-21-3) for 48 h at 40 °C every 12 h, the detergent was changed (Fig. 1A) [1].
Fig. 1.
Prepared pericardium in 75 mm diameter (A) and decellularized Pericardium before cutting (B).
2.4. Normal washing methods (NWd and NWp)
After the decellularization process, samples of the NWd group were washed in distilled water at 4 °C for 12 h twice. Then the tissues were washed for 24 h by distilled water. Distilled water exchanged every 8 h. The volume of used water was 500 cc per wash for each sample. Samples of the NWp group were washed in distilled water at 4 °C for 12 h twice. Then, the tissues were washed for 24 h by PBS and the PBS exchanged every 8 h. The volume of the used PBS was 500 cc per wash for each sample. The normal washing groups were considered as the control group.
2.5. Vacuum washing method (VW)
For performing VW method, we developed an apparatus containing a filtration Buchner Funnel with 65 mm diameter, glass filtration flask 250 CC, vacuum pump (Millipore Model WPG222050) and a hose. To perform VW, every decellularized tissue was cut in 75mm diameter (Fig. 1B) and fixed on a stainless-steel ring with 60 mm diameter. Then, it was put in the funnel connected to the filtration flask. The system was connected to the vacuum pump by a silicon hose and 10 ml of distilled water add-on tissue to prevent drying of pericardium during suction. Specifically, vacuum was applied for 15 min, and after that, the samples were shaken and washed at 40 rpm (on shaker Rotomix Model: 3476) in 100 ml of distilled water for 45 min. This process was repeated for the samples of each group (6 times for sample VW6h, 12 times for sample VW12h, and 24 times for sample VW24h). At the end of every cycle, the effluent was collected to take a sample for SDS measurement.
2.6. SDS measurements in the effluent
To determine SDS in the effluent of each group, we used the methylene blue (MB) SDS examination. SDS molecules could be combined with MB, where the combination is soluble in chloroform. The amount of SDS could be measured at 650 nm by spectrophotometer.
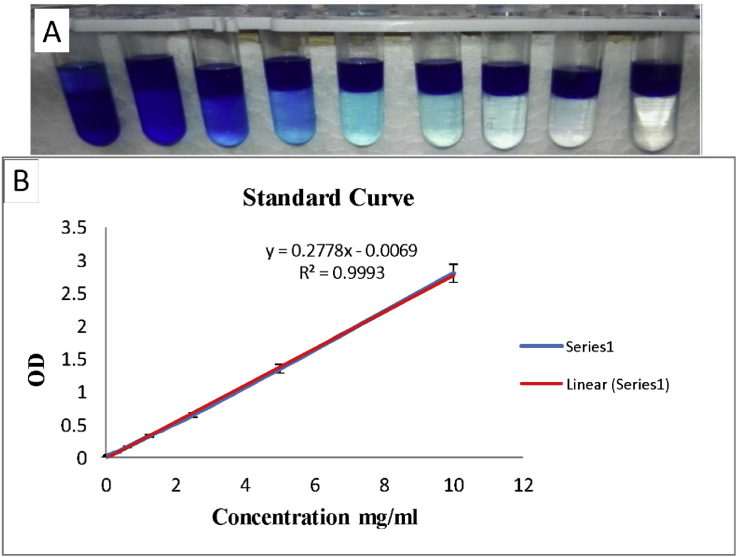
The concentration of SDS in effluents was calculated via linear regression of the respective standard curve. To obtain a standard curve, 0.0125gr MB powder was dissolved in 100 ml distilled water. SDS was prepared at different concentrations (10, 5, 2.5, 1.25, 0.625, 0.312, 0.156, 0.0781, 0 mg/ml) (Fig. 2A). One volume of each concentration was mixed with 100 volumes of MB and vortexed for 1 min. Then, two volumes of chloroform were added to the mixture and vortexed for 1 min and incubated 30 min at room temperature. Finally, one volume of the underlying phase was mixed with nine volumes of chloroform and optical absorbance (OD) was measured at 650 nm by spectrophotometer. A standard curve was drawn by OD/concentration (Fig. 2B). The SDS amount in each sample from the effluent was determined using this standard curve.
Fig. 2.
A) Diluted samples to prepare of standard curve, the SDS concentartion from left to right in each vial (10, 5, 2.5, 1.25, 0.625, 0.312, 0.156, 0.0781, 0 mg/ml). B) The standard SDS curve was drawn by excel software 2013 and used to determine the concentration of residual SDS in decellularized tissue and effluents. For this purpose, the absorption (OD) of each sample in 650 nm was used.
2.7. Assessment of residual SDS in the tissue
For assessment of residual SDS in the tissue, 1 gr of the tissue was scaled and then frozen in liquid nitrogen then homogenized and mixed with 5 ml of water.
Further, 2.5 ml of this water was mixed with 7.5 ml absolute ethanol for sedimentation of the suspended matter. The supernatant was filtered by a 0.22 μm filter. 2 ml of the filtered solution was mixed with 2 ml of MB and vortexed for 1 min. Also, 4 ml of chloroform was added to the solution and vortexed for 1 min and incubated for 30 min at room temperature. Further, one volume of the underlying phase was mixed with nine volumes of chloroform, with optical absorbance (OD) of each sample measured at 650 nm by spectrophotometer. The SDS amount in each sample from tissues was determined using the standard curve (Fig. 2B).
2.8. Assessment of cytotoxicity with MTT assay
The basis of this test is the breakdown of tetrazolium salt by mitochondrial succinate dehydrogenase of living cells, which breaks down the tetrazolium and transforms it into insoluble violet crystals. To perform this test, we extracted cells by flashing method, according to the study we did before. In this study, we performed extraction and characterization according to our earlier study [20, 21]. Further, 105 bone marrow stem cells (BMSCs) of the rat wistar femur were separately dispersed into a 24-well plate and 900 μl of the cell culture medium (DMEM (Sigma) containing 10 % FBS (Gibco) + 1% penicillin-streptomycin) was added. 24 hours, the plate was incubated at 37 °C with 5% CO2 incubator (Memmert). Then, the samples of pericardia put inside of wells that were containing the cells. The toxicity test was conducted at 48 and 72 h. A solution of 5 mg/ml MTT was prepared. 100 μl of MTT was added to each well and placed in an incubator at 37 °C for 4 h (the final concentration of MTT in each well should be 0.5 mg/ml). For the extraction of formazan deposits, 200 μl DMSO was added to each well. After 30 min, each well was transferred to a 96-well plate and read from 570 to 630 nm using the ELISA Reader (Stat fax-2100, USA). The cell survival percentage was calculated using its formula for each concentration [22, 23].
2.9. Histological examination
Hematoxylin and eosin staining (H&E) was used for morphological assessment of the pericardia after decellularization and determining the efficacy of washing with the VW method. Via this method, the pericardium collagen fibers turned pink and fibroblast cells were visible. All the samples were fixed in paraformaldehyde 4% (Merck CAS 30525-89-4). After tissue processing and blockage in paraffin, the tissues were cut in 5 μm thickness by a microtome (Leitz 1512) and stained with H&E.
2.10. Tensile test
The samples were cut in length of 50 mm and width of 150 mm, tissue thickness was 0.8 mm. Then the samples were placed in the clamp of the device (santam st-1, Iran) and after fixing the clamp the length of the gauge was 30 mm and the tensile test was evaluated at a speed of 10 mm/min. The test continued until the samples had been torn. The load cell has an output of 1000 N [24].
2.11. Statistical analysis
Each experiment was performed 3 times and the mean results were evaluated. One-way ANOVA and Tukey's post-hoc test was used for data analysis. The data were analyzed by SPSS software version 18.0 (Inc, Chicago, IL, USA). Mean differences were considered significant at P ≤ 0.05.
3. Results
3.1. The amount of SDS in collected effluent
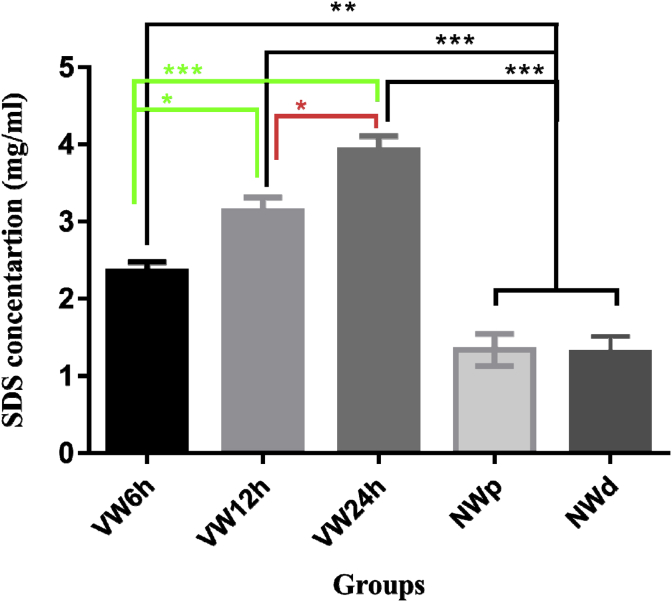
The comparison between all groups and NW (NWd and NWp) groups showed a significant difference. The significance between NW (NWd and NWp) groups and VW12h plus VW24h was P ≤ 0.001 and that between NW groups and VW6h P ≤ 0.01. So, the SDS removal in NW (NWd and NWp) groups was lower than in the others. SDS amount in the effluent of VW groups was higher than that in the NW (NWd and NWp) groups. There was also a difference in significance between the VW groups, the significance between VW6h and VW12h was P ≤ 0.05 and that between VW6h and VW24h was P ≤ 0.001. Also, that of the VW12h and VW24h was P ≤ 0. 05. The results revealed that the SDS removal in the VW6h was almost twice higher than NW (NWd and NWp) groups. SDS removal was greater than VW6h and VW12h in the VW24h. There was no significant difference between the NWd and NWp groups (Fig. 3).
Fig. 3.
Detection of SDS in collected effluents in VW and NW groups demonstrated that the difference in the mean of data between all groups and in comparison to the control groups was significant. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
3.2. The residual SDS in decellularized tissue
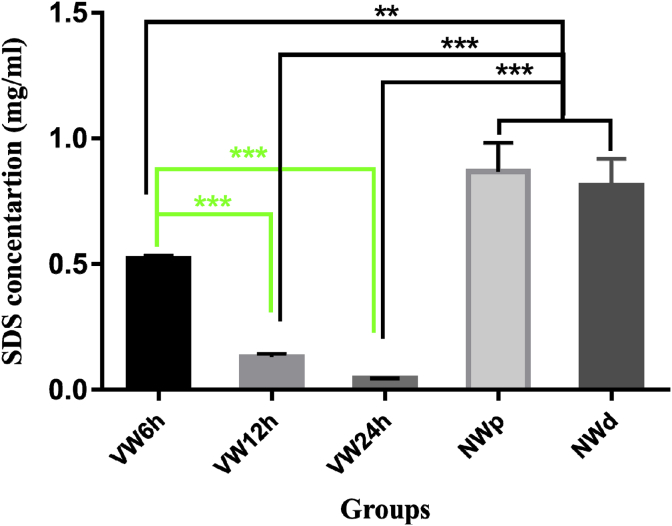
The significance between NW (NWd and NWp) groups with VW12h and VW24h were P ≤ 0.001 and with VW6h was P ≤ 0.01. The residual SDS in the tissue samples of VW groups was less than that in the NW groups. The amount of SDS in the NW (NWd and NWp) tissue samples was twofold higher than VW6h. Also, the SDS amount in the VW6h was fourfold higher than VW12h. The difference between the NW (NWd and NWp) groups and the VW24h group is eightfold. There was no significant difference between the NWd and NWp groups (Fig. 4).
Fig. 4.
Detection of SDS in tissue in VW and NW groups demonstrated that the difference in the mean of data between all groups and in comparison with the NW groups was significant. **P ≤ 0.01, ***P ≤ 0.001.
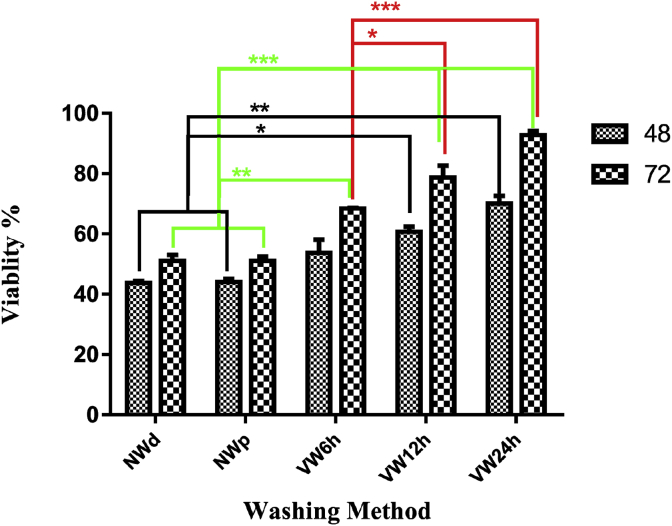
3.3. Evaluation of scaffolds toxicity by MTT assay
Comparison of the mean survival of BMSCs by MTT in 48 and 72 h showed that with increase in the duration of tissue washing, the survival of cells increased in VW groups significantly in comparison to the NW (NWd and NWp) groups. There were no significant differences within 48 h of NW (NWd and NWp) with VW6h and the significance NW (NWd and NWp) with VW12 h P ≤ 0.05 and with VW24h significance P ≤ 0.01. during 72 h the significance was for NW (NWd and NWp) with VW6h P ≤ 0.01 and NW (NWd and NWp) with WV12h and VW24 h P ≤ 0.001 (Fig. 5).
Fig. 5.
The viablity of BMSCs with MTT Assay for decellularized tissue after 48 and 72 h. There was a significant difference between different groups and compared to control groups. *P ≤ 0. 05, **P ≤ 0.01, ***P ≤ 0.001.
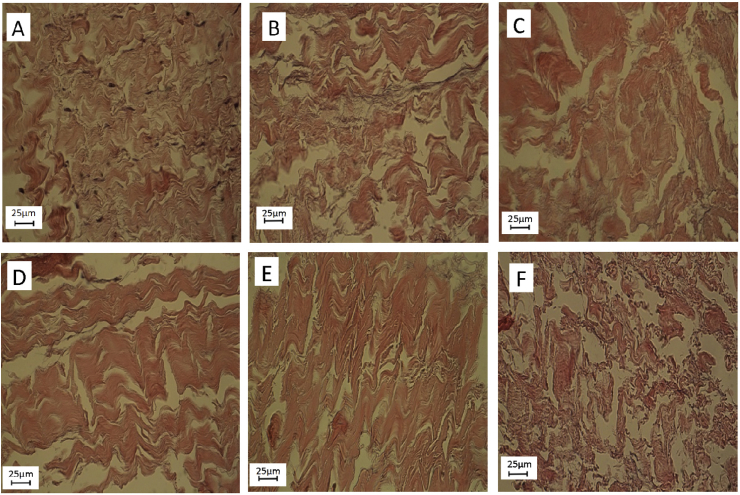
3.4. The effect of VW on the morphology of decellularized tissue
Native tissue showed (Fig. 6A) irregular connective tissue and bundles of collagen fibers. Fibroblast cells and connective tissue cells were also intact. NW (NWd (Fig. 6C) and NWp (Fig. 6B)) decellularized tissues showed that the cells have been completely removed and the collagen bundles have been retained. VW6h (Fig. 6D) showed that cells were completely removed and collagen bundles can be fully seen inside the tissue, but in some places, the bundles were broken apart. VW12h (Fig. 6E) indicated that the collagen bundles began to disrupt but there were many collagen bundles which still maintained their contact with each other. VW24h (Fig. 6F) samples showed that the collagen bundles were not so obvious and there was no considerable bundle in the microstructure, so the collagen structure collapsed and disrupted unfavorably. Meanwhile if we compare the H&E structure of VW24h with the other groups, the results revealed that collagen structure was broken and collapsed. VW24h not only removed a lot of SDS from decellularized tissue but also destroyed the structure of the collagen fibers. In return, the VW6h did not had considerable structural changes compared to NW (NWd and NWp) samples but could remove two folds of SDS from the decellularized tissue. The results with H&E staining indicated that all cells were removed by the SDS decellularization method in all groups (Fig 6 B, C, D, E, F). The morphological inspection in VW6h and VW12h showed that not only the cells were removed, but also the collagen fibers were consistent and similar to normal tissue.
Fig. 6.
Changes and differences of the decellularized tissue in compare to Native tissue.
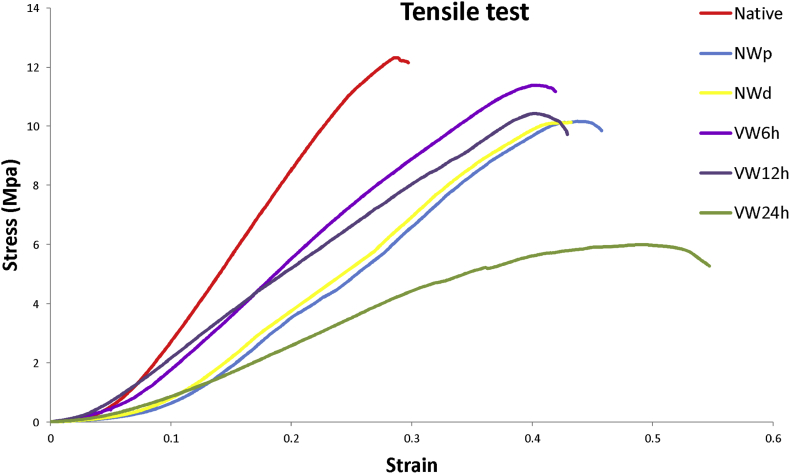
3.5. The effect of VW on tensile test of decellularized tissue
Comparison of the tensile test in different groups showed that the native group had the highest module and the lowest amount was the VW24h sample which was reported with P ≤ 0.001 significance for all groups. The VW12h group did not show any significance with the NW(NWp and NWd) groups but it was reported that VW6h and VW24h were significant compared with the NW(NWp and NWd) groups. The highest stress and moduluse after the native tissue associated with the groups VW6h, VW12h, NW(NWp and NWd) and VW24h respectively (table 1- Fig. 7).
Table 1.
Comparison of different tensile parameters of the decellularized tissue.
| Modulus (Mpa) | Yield Stress (Mpa) | Elastic strain | Ultimate tensile strength (Mpa) | Yield strain | |
|---|---|---|---|---|---|
| Native | 42.57 ± 2.06 | 11.9 ± .39 | .28 ± .008 | 12.29 ± .24 | .29 ± .02 |
| NWp | 24.9 ± .54 | 9.6 ± .27 | .36 ± .05 | 9.89 ± .18 | .41 ± .01 |
| NWd | 25.1 ± .32 | 9.1 ± .12 | .39 ± .04 | 10.1 ± .23 | .42 ± .08 |
| VW6h | 31.57 ± 4.23 | 9.5 ± 1.8 | .26 ± .05 | 9.86 ± 1.8 | .32 ± .08 |
| VW12h | 25.50 ± .57 | 8.8 ± 1.1 | .34 ± .03 | 9.24 ± 1.33 | .36 ± .03 |
| VW24h | 13.3 ± 1.80 | 4.02 ± 1.11 | .3 ± .09 | 4.1 ± 1.2 | .34 ± .11 |
Fig. 7.
Stress-strain results for decellularized tissue in groups VW, NW and native.
4. Discussion
The aim of this research was to use of VW as an optimal method for removal SDS detergents from the pericardium after decellularization. VW for the first time was used in this research for SDS removal. We constructed an apparatus for this objective. Although different pressures have been used in various studies for tissue decellularization [25, 26], we chose The -100 mmHg vacuum because as normal body pressure in resting position is about 100–120 mmHg [27]. So, this vacuum would not cause high damage to the macro and microstructure of pericardium. To apply uniform vacuum on the pericardium, the 60mm stainless steel ring and filtration Buchner Funnel with Uniform holes were used. Several studies on the use of pressure decellularization have been reported so far [25, 26, 28], but no research had been conducted about use of this method for washing and SDS removal. The first finding of this research revealed the vacuum method could remove most of the SDS within the first 6 h (VW6h). Over time, although a large amount of SDS was removed, this amount was less than the amount of SDS removed within the first 6 h. The reason is that at the first steps, the concentration of the SDS was high in scaffold and was reduced during the washing process. Subsequently, the amount of SDS remaining in the VW6h group was maximum in the scaffold, which decreased in the VW12h and VW24h groups after washing. Hence, the way of decellularization and the time of washing are critical in the decellularization process, where the amount of detergent in the tissue was measured in the study [17]. The role of the vacuum application in the removal of SDS was found by comparing the results of the NW (NWd and NWp). We took 24 h for washing for the normal washing group NW (NWd and NWp) without vacuum.
Therefore, what we have innovated is the Vacuum Method for this purpose, tissue Spread on the filtration Buchner funnel then connected to the glass filtration flask and -100 mmHg vacuum was applied with a suction pump. After applying suction and washing at different times, the SDS content in effluent of the VW6h was 2.35 mg/ml, while NWp was 1.32 mg/ml and NWd was 1.28 mg/ml, which had an upper concentrate of SDS.
According to the results, the amount of SDS extracted from the tissue dissolved in the distilled water of VW6h had a higher SDS concentration in washing water compared to NWd and NWp. Also with lengthening the vacuum hour at VW12h, VW24h, much of SDS was removed from the tissue. In the study the SDS toxicity of the remaining part in the heart valve was studied during different washing hours. It was observed that by increasing the time and rate of washing, the amount of SDS remaining in the solvent (PBS) decreased and the toxicity of SDS in human endothelial cells diminished, while the cell survival increased [15]. In another study on the presence of SDS and the toxicity of this matter, the residual SDS in the tissue showed reduced cell growth and limited cell population. Taking into account the relationship between the residual SDS in decellularized tissue and its cytotoxicity, this method reduced both tissue toxicity and washing time. From various studies, it can be concluded that higher amounts of detergent, including SDS, in the decellularized tissue cause cellular toxicity and diminished survival [14, 16] Therefore, the vacuum method proposed here could reduce the toxicity of the prepared tissue in 24 h and cause increased survival and metabolic properties of the cell. The novelty of this study was removing much of SDS residual from inside of the tissue. Accordingly, using new methods, we saved a great deal of time and many resources. In another study, concerning decellularization of bovine pericardium by SDS, after 72 h of routine washing, the H & E histology examination showed a number of cells in some areas [1]. However, in our study after 24 h, H & E method did not show cellularity, perhaps because use of the vacuum method is possible to remove the residual body from the tissue. In a study, alkalines and EDTA were used as a decellularization substance for decellularization of pericardial tissue. Also, glutaraldehyde was used as a crosslinker of collagen bundles. They found that both the collagen bundles and the native tissue were preserved [29]. However, our study histology results indicated that after 24 h of vacuum washing, the collagen structure was dispersed. Thus, possibly if we had used crosslinker before washing, the structure of the collagen would have been preserved.
The side effects of SDS include disorder in cell attachment, homing, differentiation, seeding, etc. A study showed that the SDS reduces the electrical resistance of epithelium in the intestine and increases the membrane permeability at the apex of cells where calcium accumulates in cells. Also, it was seen the SDS made ulcers in the intestinal epithelium because of actin degradation and terminal web disruption in the basal layer. Also, damage to the tight junction decreased cell to cell and cell to matrix connections while increasing the absorption of peptides and medications in the intestine [30]. So, this kind of studies suggests that the electrical decrease on the cell membrane causes a disturbance in the electrical potential difference of the cell membrane where the electrical currents on the cell membrane rise and there will certainly be ion pump disorders in the cell membrane. In other words, electrical disorders in the cell membrane cause disturbances in the function of the ionic pumps. Therefore, as mentioned in the upper paragraph, the SDS caused calcium elevation in cells so that the SDS could induce apoptosis in cells. It was also observed that the existence of SDS in decellularized tissue decreases actin and cell to cell connections along with physical connections of cells to the matrix. So, cell to cell as well as cell to scaffold signaling, will be an obstacle in the process of tissue engineering after cell seeding. There are many problems ahead of use of detergents (such as SDS) in tissue engineering and we have to resolve them. So, creating new ways and introducing them to the removal of detergent from inside of the scaffolds in tissue engineering could be helpful to this branch of science. Meanwhile, pericardial scaffolds have proved to be high practical in regenerative medicine and in many products introduced in the medical market today. Thus, the tissue engineering branch could conduct extensive studies and research on this part of the scaffolds. Accordingly, we tried to provide a new method to remove SDS from inside of the pericardium as a flat tissue after the decellularization process and focused on a new removal detergent system for decellularized scaffolds. In a study of decellularized neural tissue, the results showed that parameters of tissue strength in decellularized tissue were reduced compared to normal nerve tissue by the use of triton x 100 and sodium deoxyculate [31]. In another study performed on decellularized tissue by the Triton X 100, the parameters of tissue strength showed a significant decrease compared to normal tissue [24]. Tissue strength parameters reported a decrease in mechanical strength relative to the native tissue, according to various studies in the field of decellularized tissue. However, in our study, considering to the use of the vacuum method (VW12h) after decellularization of the tissue, there was no significant difference in the tissue strength between the NW groups which was used in the most article with VW12h, so the use of vacuum did not affect tissue resistance within WV12h in compare to the NW groups. The application of the vacuum VW6h showed a significant difference with the NW groups after decellularization and the strength of VW6h group was so close to the native tissue. Thus, the use of the vacuum in 6 h after pericardial tissue decellularization can help to maintain tissue strength. But the vacuum method after 12 h (VW12h) not only removed most of the SDS from the decellularized tissue, but also the tissue matrix compared to the VW24h, showed a better structure. The results suggested that this method could remove the detergent from inside of scaffolds and will help to remove the detergent in both flat tissues and other tissues.
5. Conclusion
Since the removal of SDS from the tissue in the VW12h group was more than the NW(NWd and NWd) and VW6h groups also cell survival was not significantly different from the VW24h group so the VW12h group can therefore be selected as the optimal group. However, the overall morphological structure of the tissue in this sample was better than in VW24h. According to the results, we concluded that the vacuum washing could be an optimal and suitable method to remove detergents from decellularized pericardium as a flat tissue. With this method, we can save time as well as some materials. Further, the time of tissue manufacturing and preparation could be significantly reduced and cell viability increased in scaffolds with SDS reduction. The use of vaccume washing method can be used as a supplement to wash and remove SDS and requires further examination and study for different tissues.
Declarations
Author contribution statement
Morteza Alizadeh, Akram Alizadeh: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Leila Rezakhani, Mostafa Soleimannejad, Esmaeel Sharifi, Maryam Anjomshoa: Analyzed and interpreted the data.
Funding statement
This work was supported by Shahrekord University of Medical Sciences (No. 2818).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Li N., Li Y., Gong D., Xia C., Liu X., Xu Z. Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact. Cardiovasc. Thorac. Surg. 2018;26(5):768–776. doi: 10.1093/icvts/ivx416. [DOI] [PubMed] [Google Scholar]
- 2.Ayala P., Dai E., Hawes M., Liu L., Chaudhuri O., Haller C.A. Evaluation of a bioengineered construct for tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(6):2345–2354. doi: 10.1002/jbm.b.34042. [DOI] [PubMed] [Google Scholar]
- 3.Smart N.J., Marshall M., Daniels I.R. Biological meshes: a review of their use in abdominal wall hernia repairs. The Surgeon. 2012;10(3):159–171. doi: 10.1016/j.surge.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Wiegmann B., Zardo P., Dickgreber N., Länger F., Fegbeutel C., Haverich A. Biological materials in chest wall reconstruction: initial experience with the Peri-Guard Repair Patch®. Eur. J. Cardiothorac. Surg. 2010;37(3):602–605. doi: 10.1016/j.ejcts.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Khan M.A.A., Chipp E., Hardwicke J., Srinivasan K., Shaw S., Rayatt S. The use of Dermal Regeneration Template (Integra®) for reconstruction of a large full-thickness scalp and calvarial defect with exposed dura. J. Plast. Reconstr. Aesthet. Surg. 2010;63(12):2168–2171. doi: 10.1016/j.bjps.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert T.W., Sellaro T.L., Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Hülsmann J., Grün K., El Amouri S., Barth M., Hornung K., Holzfuß C. Transplantation material bovine pericardium: biomechanical and immunogenic characteristics after decellularization vs. glutaraldehyde-fixing. Xenotransplantation. 2012;19(5):286–297. doi: 10.1111/j.1399-3089.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 8.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon A.M., Curnow P., Booth P.J. Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta Biomembr. 2004;1666(1-2):105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Gilpin A., Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaner P.J., Martin N.D., Tulenko T.N., Shapiro I.M., Tarola N.A., Leichter R.F. Decellularized vein as a potential scaffold for vascular tissue engineering. J. Vasc. Surg. 2004;40(1):146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.-L., Guzzardi M.A., Shulman C. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010;16(7):814. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayama K.H., Batchelder C.A., Lee C.I., Tarantal A.F. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng. A. 2010;16(7):2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratzer P.F., Harrison R.D., Woods T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng. 2006;12(10):2975–2983. doi: 10.1089/ten.2006.12.2975. [DOI] [PubMed] [Google Scholar]
- 15.Cebotari S., Tudorache I., Jaekel T., Hilfiker A., Dorfman S., Ternes W. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif. Organs. 2010;34(3):206–210. doi: 10.1111/j.1525-1594.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 16.Syed O., Walters N.J., Day R.M., Kim H.-W., Knowles J.C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014;10(12):5043–5054. doi: 10.1016/j.actbio.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Zvarova B., Uhl F.E., Uriarte J.J., Borg Z.D., Coffey A.L., Bonenfant N.R. Residual detergent detection method for nondestructive cytocompatibility evaluation of decellularized whole lung scaffolds. Tissue Eng. C Methods. 2016;22(5):418–428. doi: 10.1089/ten.tec.2015.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White L.J., Taylor A.J., Faulk D.M., Keane T.J., Saldin L.T., Reing J.E. The impact of detergents on the tissue decellularization process: a ToF-SIMS study. Acta Biomater. 2017;50:207–219. doi: 10.1016/j.actbio.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathapati S., Galla S., Sankaranarayanan K., Verma R.S., Cherian K.M., Guhathakurta S. Qualitative and quantitative detection of sodium deoxycholic acid in decellularized tissue. Indian J. Thorac. Cardiovasc. Surg. 2010;26(2):129–131. [Google Scholar]
- 20.Alizadeh A., Altarihi T., Dashtnavard H. The Influence of lithium chloride on induction of bone marrow stromal cells into neuronal phenotype. Daneshvar Med. 2009;16(79):51–56. [Google Scholar]
- 21.Eftekharzadeh M., Nobakht M., Alizadeh A., Soleimani M., Hajghasem M., Shargh B.K. The effect of intrathecal delivery of bone marrow stromal cells on hippocampal neurons in rat model of Alzheimer’s disease. Iran. J. Basic Med. Sci. 2015;18(5):520. [PMC free article] [PubMed] [Google Scholar]
- 22.Rezakhani L., Khazaei M.R., Ghanbari A., Khazaei M. Crab shell extract induces prostate cancer cell line (LNcap) apoptosis and decreases nitric oxide secretion. Cell J. (Yakhteh) 2017;19(2):231. doi: 10.22074/cellj.2016.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashtbar M., Hadjati J., Ai J., Jahanzad I., Azami M., Shirian S. Characterization of decellularized ovine small intestine submucosal layer as extracellular matrix-based scaffold for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(3):933–944. doi: 10.1002/jbm.b.33899. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza-Novelo B., Avila E.E., Cauich-Rodríguez J.V., Jorge-Herrero E., Rojo F.J., Guinea G.V. Decellularization of pericardial tissue and its impact on tensile viscoelasticity and glycosaminoglycan content. Acta Biomater. 2011;7(3):1241–1248. doi: 10.1016/j.actbio.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Funamoto S., Nam K., Kimura T., Murakoshi A., Hashimoto Y., Niwaya K. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials. 2010;31(13):3590–3595. doi: 10.1016/j.biomaterials.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 26.Conklin B., Richter E., Kreutziger K., Zhong D.-S., Chen C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002;24(3):173–183. doi: 10.1016/s1350-4533(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 27.Pickering T.G., Harshfield G.A., Kleinert H.D., Blank S., Laragh J.H. Blood pressure during normal daily activities, sleep and exercise. Jama. 1982;247(7):992–996. [PubMed] [Google Scholar]
- 28.Montoya C.V., McFetridge P.S. Preparation of ex vivo–based biomaterials using convective flow decellularization. Tissue Eng. C Methods. 2009;15(2):191–200. doi: 10.1089/ten.tec.2008.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa J.N.L., Pomerantzeff P.M.A., Braile D.M., Ramirez V.A., Goissis G., Stolf N.A.G. Comparison between the decellularized bovine pericardium and the conventional bovine pericardium used in the manufacture of cardiac bioprostheses. Braz. J. Cardiovasc. Surg. 2005;20(1):14–22. [Google Scholar]
- 30.Anderberg E.K., Artursson P. Epithelial transport of drugs in cell culture. VIII: effects of sodium dodecyl sulfate on cell membrane and tight junction permeability in human intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 1993;82(4):392–398. doi: 10.1002/jps.2600820412. [DOI] [PubMed] [Google Scholar]
- 31.Mohammad-Bagher G., Arash A., Morteza B.-R., Naser M.-S., Ali M. Synergistic effects of acetyl-l-carnitine and adipose-derived stromal cells on improving regenerative capacity of acellular nerve allograft in sciatic nerve defect. J. Pharmacol. Exp. Ther. 2019;368(3):490–502. doi: 10.1124/jpet.118.254540. [DOI] [PubMed] [Google Scholar]