Abstract
Recent data in a nonhuman primate model showed that infants postnatally infected with Zika virus (ZIKV) were acutely susceptible to high viremia and neurological damage, suggesting the window of vulnerability extends beyond gestation. In this pilot study, we addressed the susceptibility of two infant rhesus macaques born healthy to dams infected with Zika virus during pregnancy. Passively acquired neutralizing antibody titers dropped below detection limits between 2 and 3 months of age, while binding antibodies remained detectable until viral infection at 5 months. Acute serum viremia was comparatively lower than adults infected with the same Brazilian isolate of ZIKV (n = 11 pregnant females, 4 males, and 4 non-pregnant females). Virus was never detected in cerebrospinal fluid nor in neural tissues at necropsy two weeks after infection. However, viral RNA was detected in lymph nodes, confirming some tissue dissemination. Though protection was not absolute and our study lacks an important comparison with postnatally infected infants born to naïve dams, our data suggest infants born healthy to infected mothers may harbor a modest but important level of protection from postnatally acquired ZIKV for several months after birth, an encouraging result given the potentially severe infection outcomes of this population.
Subject terms: Infectious diseases, Virology
Introduction
Zika virus (ZIKV) emerged in Brazil in 2015 and maternal infection during pregnancy was astutely correlated with an increase in newborns with microcephaly1,2, a profound developmental defect that results in infants with reduced brain size and cognitive capacity. ZIKV was initially discovered in 1947 in the Zika forest of Uganda during surveillance for yellow fever virus3. Soon thereafter, it became clear that human infections with ZIKV in that region were not uncommon4,5 but disease associated with infection appeared to be minor and ZIKV became something of an afterthought. The emergence in Brazil and its association with both major and, more recently, less severe neurological consequences in congenitally-infected newborns6, collectively called “congenital Zika syndrome”, rapidly changed that perception. A profound research effort was subsequently launched to understand mechanisms of pathogenesis7,8, identify cellular receptors9–12 and targets9,12–16, to develop animal models17–23, and to develop and test vaccines24–29 and therapeutics23,30.
Human brain development continues well after birth31 so it stands to reason that the risk of ZIKV associated neurological disease may extend for an unknown period of time after birth. Indeed, a recent study in nonhuman primate infants showed high peak viral loads and dissemination into multiple brain regions at two weeks post-infection and quantifiable neurological defects and cognitive impairment in infants infected in the first few months of life32.
Given the high incidence of ZIKV infection in several South and Central American countries during the height of the ZIKV epidemic, it is likely that a large number of babies without congenital infection or disease sequelae were born to infected mothers. The vulnerability of these infants to newly acquired infection after birth has not been addressed. A recent macaque study showed that fetal infection after subcutaneous inoculation of dams with ZIKV was efficient, with four of four fetuses showing evidence of infection33. Since not all infants exhibit detectable ZIKV disease when born to infected mothers, it remains unclear whether these infants remain uninfected and/or unaffected due to pre-existing passively acquired maternal antibodies, if they mount their own de novo anti-ZIKV immune responses in utero or soon after birth, or if infection can be limited and apathogenic for an unknown reason. Addressing these issues will be key to addressing the susceptibility of newborns to postnatal ZIKV infection in areas with endemic for ZIKV transmission. During this study, we monitored antiviral antibody responses after birth in two infant macaques born to ZIKV infected dams and assessed the level of protection these responses might provide against postnatal infection. Upon infection at five months of age, both infants showed only modest levels of peripheral viremia and no virus detected in neurological tissues. These data suggest that being born to a ZIKV infected mother may confer a small but important level of immunity to postnatal infection.
Results
Infants born healthy with no evidence of viral infection
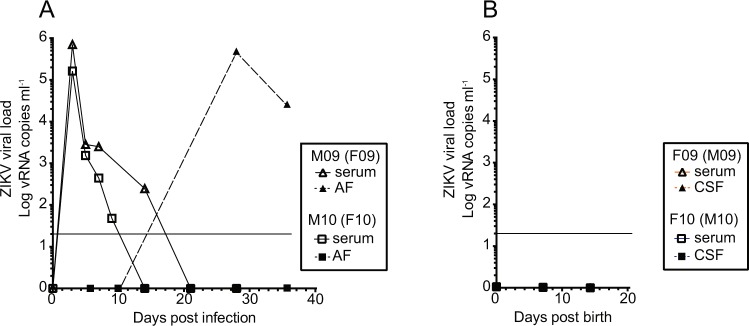
Both infants enrolled in this study were born via caesarean section at full term to dams infected in the third trimester as part of a previous study23. At the time of caesarean section, both dams had cleared serum virus but one dam exhibited a spike of amniotic fluid virus that remained detectable at the time of caesarean section (Fig. 1A). However, at birth, neither infant showed evidence of infection as measured in blood or cerebrospinal fluid (CSF) (Fig. 1B).
Figure 1.
Viral dynamics in the infants in this study and their dams. (A) Blood and amniotic fluid in two female macaques (dams of the infants in this study). Each animal was inoculated with ZIKV during early third trimester and monitored for infection until giving birth via cesarean section at full term (approximately gestational day 155). (B) Blood and CSF viral loads in the infants after birth and before postnatal viral infection. Neither infant had detectable virus in serum or CSF during the first two weeks of life. The limit of detection of approximately 15 copies per milliliter of plasma is shown as a horizontal line in both panels.
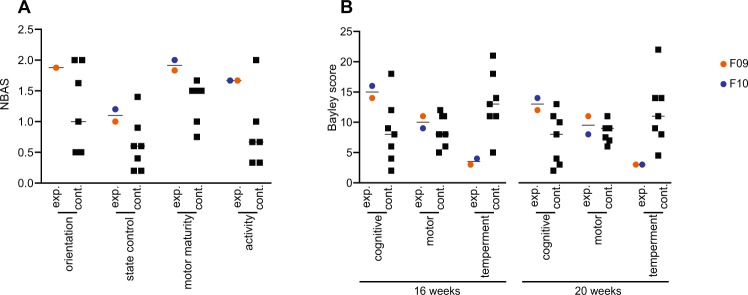
Despite no direct evidence of infection in the infants, we next assessed the possibility that infection had occurred in utero and induced neurological deficits. To do this, we used multiple defined testing parameters to compare the infants with seven control infants raised in the same manner and tested by the same technician. At 15 days of age, both infants showed slightly elevated levels of state control, motor maturity and activity while one infant did not attend to the orientation test (Fig. 2A). At 16 and 20 weeks of age, both infants showed levels of cognitive abilities that were somewhat elevated while motor development was normal. The temperament of both infants was markedly calmer than controls (Fig. 2B). The small sample size negated any meaningful statistical analysis. These behavioral observations were exploratory and were designed to identify any overt abnormalities that might be explored in future studies and none were noted.
Figure 2.
Behavioral assessment of the infants from birth unitl postnatal ZIKV infection. Neurobehavioral scores for F09 and F10 and 7 controls. (A) The infants were assessed for behavioral abnormalities and test scores were measured at 15 days of age, as described in the text. F10 was not attentive during the test of orientation so was not assessed. For each category, scores for individual tests were averaged for analysis. (B) Each infant was likewise assessed for cognitive, motor, and temperament at sixteen and twenty weeks of age. Scores within a category were summed for analysis.
Viral dynamics in the infants
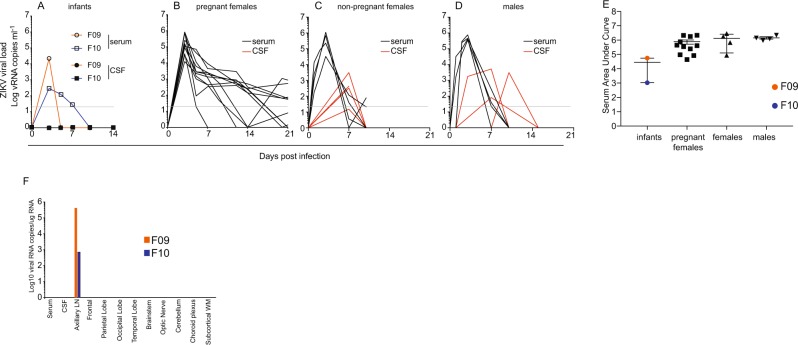
At approximately five months of age (148 days for F10, 155 days for F09), we inoculated both infants with the same dose (104 PFU), via the same subcutaneous route, of the same Brazilian isolate of ZIKV that their dams had been infected with. Peak viral load in infant F09 was approximately 20,000 viral RNA copies per milliliter of plasma, which was completely cleared by day 5 post infection. F09 was the only animal in our studies to clear blood viral RNA prior to day 5. In infant F10, the viral load remained below 1,000 copies per milliliter but remained detectable until day 7 (Fig. 3A). No virus was detected in CSF at any time point in either infant.
Figure 3.
Viral dynamics in the infants after postnatal infection along with adults in other studies. (A) Viral loads (serum and CSF) were assessed at days 0, 3, 5, 7, 10, and at necropsy on day 14 in both infants. Viral loads for eleven pregnant females (B) infected with the same dose and route of the same stock of virus, as well as four adult non-pregnant females (C), and four adult males (D) infected with the same dose and route of a separate stock of the same isolate of ZIKV, which was passaged an additional time in Vero cells. The limit of detection of approximately 15 copies per milliliter of plasma is shown as a horizontal line. Viremia remained detectable beyond 21 days in several pregnant females but these values are cut from panel (B) for clarity. (E) Area under the curve analysis to assess total viremia in our infants relative to other animals in our studies. Data from the pregnant females includes all time points with viremia, including beyond day 21. (F) At necropsy, the presence of ZIKV viral RNA was assessed by qRT PCR from blood, CSF, and several brain sections as well as a lymph node from each animal.
For comparison, viral loads are shown from 11 pregnant females infected with the same stock of the same strain (Rio-U1/2016) of the virus at the same dose (104 pfu) and route (subcutaneous) (Fig. 3B) as well as four non-pregnant females (Fig. 3C) and four adult males (Fig. 3D) infected with the same dose and strain (Rio-U1/2016) of the virus, but that was cultured one additional passage in Vero cells. CSF samples are shown for all groups as well. Virus was not detected in CSF in our pregnant females but we note that CSF was sampled less frequently in the pregnant females due to safety concerns. Area under the curve (AUC) analyses was performed to assess the total viremia in these two infants relative to adults infected in our studies (Fig. 3E). At necropsy, we performed RT PCR for ZIKV RNA on serum, CSF, multiple brain regions (frontal cortex, parietal lobe, occipital lobe, temporal lobe, brain stem, optic nerve, cerebellum, choroid plexus, and subcortical white matter), and axillary lymph nodes and virus was detected only in the axillary lymph in both animals (Fig. 3F).
Neither infant in this study showed signs of potential virus-induced pathology at necropsy. F10 harbored a choroid plexus cyst that resulted in unilateral hydrocephalus in the brain, but such cysts are common, are generally considered of little consequence and are not likely viral in origin.
Antibody responses
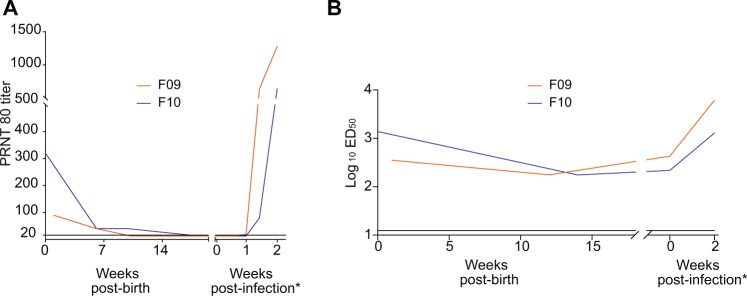
We next examined humoral responses in the infants to see if they might explain the strikingly low viral loads. We used a plaque reduction neutralization test (PRNT) to assess neutralizing antibodies in serum after birth and after infection in both infants. Both showed detectable levels of neutralization at birth, which quickly waned below the limit of detection by 2 to 3 months. Neutralizing antibodies reemerged after infection and continued to rise until euthanasia at 2 weeks post infection (Fig. 4A). To measure binding antibodies, we employed a whole virion ELISA assay using plasma samples collected throughout the infants’ lives both before and after infection. Binding IgG titers decreased between birth and 3–4 months of age, consistent with the expected kinetics of passively-transferred maternal IgG, but remained detectable until viral inoculation at five months, and then rose after infection, similar to the neutralizing antibody titers (Fig. 4B).
Figure 4.
ZIKV-specific binding and neutralizing antibodies. (A) Neutralizing antibodies as assessed using a Plaque reduction neutralization test (PRNT). Shown are PRNT 80% values using plasma from both infants. Plasma samples with PRNT titers of <20 are reported at 20. Each sample was tested in duplicate; the average titer is shown. (B) Anti-ZIKV binding antibodies as assessed using a whole virion ELISA (WVE) test. Each sample was tested in duplicate and the average titer is shown. The lower limit of detection in this assay was determined to be 12.5 (horizontal line). (A,B) The infants were infected at 20 and 21 weeks of age but antibody responses post-infection are shown at time points expressed as weeks post infection, as indicated by the asterisk, for clarity.
Discussion
ZIKV reemerged in 2015 in South America and was quickly correlated with an increase in infants born with profound neurological defects1,2. It’s not entirely clear what fraction of pregnant women that become infected with ZIKV during pregnancy transmit the virus to their developing fetus but consequences to the fetus can range from mild to severe. Additionally, nonhuman primate studies suggest that infants may remain susceptible to ZIKV induced disease even if infected after birth32, but the frequency and disease severity postnatal ZIKV infections in human infants remains to be fully examined34,35.
Given the high incidence of ZIKV in several countries during the height of the outbreak36, the relative abundance of the most competent vector for ZIKV transmission, the mosquito Aedes aegypti, particularly in urban environments (Centers for Disease Control [CDC], 2017), it’s likely that many infants born healthy to infected mothers are themselves exposed to the virus via mosquito bite after birth. It is not clear if passively acquired maternal antibodies against ZIKV can offer some level of protection and for what period of time after birth. It is also not clear if infants exposed to the virus in utero, but born healthy and seemingly uninfected, may themselves have mounted de novo immune responses against the virus in utero. Induction of adaptive immune responses in utero is possible but few examples have been described. For example, functional, malaria-specific T cell responses have been detected in fetuses37. Further, infants are routinely vaccinated against hepatitis B virus within twenty four hours of birth, which has dramatically reduced the frequency of infant infection38. These data suggest a high level of immune competence very early in life. In the context of ZIKV, macaque data suggest that vertical transmission is quite common33 but, to date, there is no data suggesting these infants mount antiviral adaptive immune responses.
Passively acquired maternal antibodies are fairly well described. Their magnitude, transmission efficiency in utero, and decay kinetics after birth have been described in the context of infection with and vaccination against several pathogens39,40. For instance, maternal anti-varicella-zoster virus (VSV) antibodies are readily detectable in infants born to mothers that were infected VSV earlier in life, but decline below the threshold of detection by 4–5 months of age40. These kinetics are similar to the decay rate of binding and neutralizing anti-ZIKV antibodies in our study. Maternal antibodies to dengue virus (DENV) have also been described in newborns41–44 and may facilitate the development of dengue hemorrhagic fever in infants postnatally infected with a heterologous serotype of DENV43,44. Intriguingly, maternal anti-DENV antibodies have been shown to enhance ZIKV associated fetal disease in mice45 while administration of sub-neutralizing doses of the pan-flavivirus specific monoclonal antibody 4G2 to pregnant, immunocompetent, ZIKV infected mice resulted in enhanced neuropathological outcomes in infants46. These published data suggest that maternally acquired heterotypic antibodies may enhance pathology associated with postnatal infection. Since our study used fully homologous virus to infect both the dams and their infants, it is possible our results would have been different had we infected the infants with a heterologous virus.
Here, we report the results of a small pilot study describing results from two infant macaques born to dams infected with ZIKV during the third trimester. One dam had relatively high levels of viral RNA detected in amniotic fluid near full gestation, possibly suggesting fetal infection, but no virus was detected in either infant after birth. A recent study of two infants infected with ZIKV after birth showed significant impairment of cognitive function and reduced reaction to fearful stimuli32, which the authors interpreted as a likely consequence of infection during early infancy. Our behavioral analyses detected no indications that infant development was negatively affected by the maternal infection status. Both infants showed levels of orientation, state control, motor acuity, and activity soon after birth that were largely within normal ranges and at 16 and 20 weeks showed normal cognitive and motor abilities. Both also showed lower than normal temperament, however, this measurement is highly variable in healthy infants and could be a result of the small sample size. Taken together, behavioral data from our infants showed no direct evidence of infection nor negative consequences due to infection of their dams during pregnancy. Our data and that from a published study32 cannot be compared for several reasons. Most significantly, the infants in that study were observed for behavioral abnormalities after direct infection early in life, whereas our analyses were conducted on infants born to infected mothers, but prior to experimental infection at approximately five months of age. Finally, comparisons of viral kinetics and tissue dissemination are also limited due to the use of similar but non-identical viruses and at different doses. We infected our infants with 104 pfu of ZIKV whereas they used 105. It’s not known if such a difference could explain different viral kinetics seen in their study. However, we propose the presence of detectable maternal antibodies in our infants at the time of infection likely limited viral replication in blood and reduced viral dissemination, but we cannot state this with certainty without proper controls.
Both infants in our study harbored detectable levels of anti-ZIKV neutralizing antibodies at birth that declined between one- and four-months post birth. We interpret these data to suggest these antibodies were passively acquired from the dams as opposed to mounted directly by the infants. ZIKV-binding IgG also declined after birth but remained detectable between at five months of age, when we experimentally infected the animals with ZIKV. Upon ZIKV infection, both exhibited low peak serum viremia that was rapidly cleared resulting in no evidence of infection in neurological tissues or CSF, but viral RNA was detected in the only lymph node sampled. Other potentially important tissues, including spleen and additional lymph nodes, were not sampled, so larger conclusions on viral dissemination cannot be drawn. These data contrast with published data on postnatally ZIKV-infected infant macaques32 born to ZIKV naïve dams, but comparisons with this and the previous study are limited due to the use of different viruses and methodologies.
ZIKV binding antibodies, likely maternal in origin, remained detectable from birth until the day of infection, possibly mediating some level of viral control. It is also possible neutralizing maternal antibodies, though undetectable in the PRNT assay at the time of infection, remained at a sufficient level to provide partial protection to the infants. In support of this possibility, infant F10, who retained detectable levels of neutralizing antibodies longer than F09, also had the lowest peak of viremia in the blood and then mounted a weaker and slower de novo antibody response to the virus and had less virus in lymph nodes. Alternatively, maternal antibodies may mediate protection via non-neutralizing functions, such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). Maternal antibodies with ADCC function have been detected in infants born to HIV infected mothers47. Both infants harbored viral RNA in axillary lymph nodes at necropsy, suggesting that even a brief period of serum viremia is sufficient for tissue dissemination, which may result in consequences not tested in our study, including inflammation.
Our data provide an important body of data on the study of developing infants infected postnatally with ZIKV. The results of this small pilot study should be interpreted with caution. Our sample size was small and lacked an important control group of infants born to healthy, ZIKV naïve mothers. Further, we infected the infants with virus that was fully homologous to the strain used to infect their dams so our data cannot be used to infer potential outcomes when infants are infected with heterologous isolates of the virus. Similar studies using heterologous virus could demonstrate differing outcomes.
Taken together, our data suggest that infants born healthy to ZIKV infected mothers harbor maternal antibodies that may provide a modest but important level of protection from ZIKV that dampened acute viral loads and limited tissue dissemination of the virus. This study, along with others, provide a framework for the assessment of the vulnerability of infants exposed postnatally to flaviviruses.
Materials and Methods
Cohort
All macaques used in this study were housed at the Tulane National Primate Research Center (TNPRC), which is fully accredited by AAALAC (Association for the Assessment and Accreditation of Laboratory Animal Care) International, Animal Welfare Assurance No. A3180-01. Animals were cared for in accordance with the NRC Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Tulane University (protocol P0336). Two adult female, purpose bred Indian rhesus macaques, were identified as pregnant and subsequently assigned to the study. These macaques were infected with ZIKV during early third trimester. Infants were delivered via caesarian section at approximately gestational day 155 (full term) and housed in a primate nursery until 5 months of age and then infected with ZIKV subcutaneously with 104 PFU of a Brazilian isolate (Rio-U1/2016 GenBank KU926309), which was passage twice in Vero cells post-virus isolation, which is the same dose we have used in our previous studies23 and similar to those used in several nonhuman primate ZIKV studies, which inoculated with doses ranging from 103 48 to 105 32,49. The animals were euthanized fourteen days later for tissue harvest and pathological assessment.
Viral load measurements
Viral RNA was amplified and quantified based on a previously described assay23. Briefly, RNA was manually extracted from 200 ul fluid samples (CSF or blood serum) unless sample volume was limiting using the High Pure Viral RNA Kit (Roche). RNA was then subjected to reverse transcription and quantitative PCR using primers (Forward 5′ TTG AAG AGG CTG CCA GC 3′; Reverse 5′CCC ACT GAA CCC CAT CTA TTG 3′) and a double-quenched (ZEN/Iowa Black FQ) and fluorescently labeled (FAM) probe (5′TGA GAC CCA GTG ATG GCT TGA TTG C 3′) (all oligos were from Integrated DNA Technologies) on an Applied Biosystems 7900 instrument. For absolute quantification, a standard curve was generated with every run generated using tenfold serial dilutions of a 401 bp in vitro RNA transcript encoding the ZIKV capsid gene starting at approximately 1 × 108 RNA copies per 30 ul reaction. The limit of detection was approximately 15 viral RNA copies/ml. ZIKV-positive and -negative samples were included in every run.
Plaque reduction neutralization test (PRNT) 80 measurements
Neutralizing antibody quantification by plaque reduction neutralization test (PRNT) endpoint 80% PRNT titers were determined in infant macaque plasma, where each sample was tested in duplicate. Plasma samples were heated to 56 °C for 30 minutes to inactivate complement, serially 2-fold diluted starting at 1:10 (1:20 final virus:plasma dilution) in 150 µl Dulbecco’s Modified Eagle Medium (DMEM) with 2% fetal bovine serum, and then incubated for 1 hour at 37 °C with approximately 100 plaque forming units of a 2015 Brazilian ZIKV strain (SPH2015, GenBank accession number: KU321639.1) from a third Vero cell passage. After 1 hour, virus-antibody or virus-only mixtures were overlaid on confluent African Green Monkey Kidney (Vero) cell monolayers and incubated for 1 hour with rocking every 15 minutes. The plaques developed under 0.5% agar overlays in DMEM were counted after 7 days under crystal violet staining. Dilutions of plasma that caused a > 80% reduction in the number of plaques, as compared with negative controls (DMEM only), were considered positive. The reciprocal of the highest dilution of plasma (represented as the mean final virus-serum dilution from both replicates) that inhibited at least 80% of plaques is reported as the antibody titer.
Detection of ZIKV-specific IgG in rhesus plasma
High-binding 96-well ELISA plates (Greiner; Monroe, NC) were coated with 40 ng/well of 4G2 monoclonal antibody, produced in a mouse hybridoma cell line (D1-4G2-4-15,ATCC; Manassas, VA), diluted to 0.8 ng/uL in 0.1M carbonate buffer (pH 9.6) and incubated overnight at 4 °C. Plates were blocked in 1X Tris-buffered saline containing 0.05% Tween-20 and 5% normal goat serum for 1 hour at 37 °C, followed by an incubation with diluted ZIKV (strain PRVABC59, BEI; Manassas, VA) for 1 hour at 37 °C. Optimal virus dilution was determined by whole virion ELISA (WVE) and a 1:5 dilution was used in these assays. Plasma samples were tested at a dilution of 1:12.5-204,800 in serial 4-fold dilutions and incubated for 1 hour at 37 °C, along with a ZIKV-specific monoclonal antibody, H24 (10 ug/mL), isolated from a ZIKV-infected rhesus macaque. Horseradish peroxidase (HRP)-conjugated mouse anti-monkey IgG secondary antibody (Southern BioTech; Birmingham, AL) was used at a 1:4,000 dilution and incubated at 37 °C for 1 hour, followed by the addition of SureBlue Reserve TMB Substrate (KPL; Gaithersburg, MD). Reactions were stopped by Stop Solution (KPL; Gaithersburg, MD) after a 7-minute incubation per plate in the dark. Optical density (OD) was detected at 450 nm on a Victor X Multilabel plate reader (PerkinElmer; Waltham, MA). Binding was considered detectable if the sample OD value at the lowest dilution was greater than that of the Background OD, defined as the OD value of the negative control at the lowest dilution plus 2 x standard deviations (SD). For samples considered positive, their OD values for the serial dilution were entered into Prism v8 (GraphPad Software; San Diego, CA) to determine the 50% effective dilution (ED50). The ED50 was calculated by first transforming the x-axis values, the dilution series 12.5-204,800 4F, into Log10. The transformed data was then analyzed using a sigmoidal dose-response nonlinear regression model. Any sample considered negative was assigned an ED50 of 12.5, the lowest dilution tested, because ED50 cannot be accurately calculated below the lowest dilution tested. Zika-specific IgG binding was reported in Log10 ED50.
Behavioral observations
We employed two age-appropriate behavioral tests that are designed for use in infant nonhuman primates. These neurobehavioral tests were performed to identify any effects of potential prenatal exposure to ZIKV and were modelled upon testing tools used for human infants50,51 and adapted for use in nonhuman primates52. Tests were administered every two weeks, from 14 days of age until euthanasia at 20 (F10) or 21 weeks (F09). Each infant’s scores were compared descriptively against the mean and standard deviation across seven control animals reared in the same fashion and tested by the same behavioral technician. Data for control animals were available at three time points.
Infant neurobehavioral assessment scale (NBAS)
This test is adapted for nonhuman primates from the Brazelton Neonatal Behavioral Assessment Scales used with humans53. This test is valid for infants during the first four weeks of life. The scale consists of a 20-minute battery of tests that assess an infant’s motor functioning, temperament, and interactive skills54–56. In each test, an animal was scored using a 3- to 5-interval scale, depending on the test element. Test elements were categorized for analysis following factor analyses by Schneider et al. (1991)56: Orientation (visual orientation, visual follow, duration of looking, attention), State control (initial state, consolability, struggle during test, predominant state), Motor maturity (head posture prone, head posture supine, labyrinthian righting, speed of responses to tester), and Activity (motor activity, coordination, spontaneous crawl, proportion of test active). For each category, scores for individual tests were averaged for analysis. Study subjects were compared with age-matched controls at 14 days of age.
Bayley test of infant development
This test originally was developed for use with human infants but modified for nonhuman primates ranging in age from 1 month to 1 year-old57. The 10-minute evaluation consists of cognitive, motor, and temperament tests. Briefly, each subject is held on a table by one examiner and another places test items in front of the subject and records their responses. Separate motor and behavioral test variables were collapsed into 3 categories for analysis: Cognition (lift cup, uncover toy, pull string, remove treat from bottle, object orientation, goal directedness), Motor abilities (labyrinthian righting, muscle tonus, fine motor coordination), and Temperament (response to examiner, irritability, activity, response intensity, struggle, consolability). Each test element was scored using a 4–5 interval scale depending on the test element. Scores within a category were summed for analysis. Tests were conducted monthly between 3 months of age through the end of the study. Study subjects were compared age-matched control animals.
Data Availability
All data is available from the corresponding author on request.
Acknowledgements
This work was supported by the Bill and Melinda Gates Foundation (OPP1152818) and the National Institute of Health ORIP/OD P51OD011104 to the Tulane National Primate Research Center and NIH grant P30AI073961 to the Miami Center for AIDS Research (CFAR). Support to the Duke Human Vaccine Institute was provided by the National Institute of Allergy and Infectious Disease (1R21AI132677, 1P01AI132132). The funders had no role in study design.
Author Contributions
N.J.M. planned the studies and wrote the first draft. B.S., A.S., M.D., M.H.G., F.S., P.P.A., K.B. and R.V.B. conducted the experiments. N.J.M., R.P.B., K.B., K.K.A.V.R., A.A.L., M.C.B., R.V.B., S.R.P., L.L.C., A.T.P. and D.M. interpreted the studies. All authors reviewed, edited, and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jacob JA. Researchers Focus on Solving the Zika Riddles. JAMA. 2016;315:1097–1099. doi: 10.1001/jama.2016.1219. [DOI] [PubMed] [Google Scholar]
- 2.Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ 353, i3182, 10.1136/bmj.i3182 (2016). [DOI] [PubMed]
- 3.Dick GW, Horgan ES. Vaccination by scarification with a combined 17D yellow fever and vaccinia vaccine. J Hyg (Lond) 1952;50:376–383. doi: 10.1017/S0022172400019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 5.Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve Isolations of Zika Virus from Aedes (Stegomyia) Africanus (Theobald) Taken in and above a Uganda Forest. Bull World Health Organ. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Brasil P, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuaib W, Stanazai H, Abazid AG, Mattar AA. Re-Emergence of Zika Virus: A Review on Pathogenesis, Clinical Manifestations, Diagnosis, Treatment, and Prevention. The American journal of medicine. 2016;129:879 e877–879 e812. doi: 10.1016/j.amjmed.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Tappe D, et al. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2016;205:269–273. doi: 10.1007/s00430-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, DeLalio LJ, Isakson BE, Wang TT. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circulation research. 2016;119:1183–1189. doi: 10.1161/CIRCRESAHA.116.309866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowakowski TJ, et al. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell stem cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savidis G, et al. Identification of Zika Virus and Dengue Virus Dependency Factors using Functional Genomics. Cell reports. 2016;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Meertens L, et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell reports. 2017;18:324–333. doi: 10.1016/j.celrep.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Miner JJ, Diamond MS. Understanding How Zika Virus Enters and Infects Neural Target Cells. Cell stem cell. 2016;18:559–560. doi: 10.1016/j.stem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen HN, Qian X, Song H, Ming GL. Neural stem cells attacked by Zika virus. Cell Res. 2016;26:753–754. doi: 10.1038/cr.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quicke KM, et al. Zika Virus Infects Human Placental Macrophages. Cell host & microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simoni Michael K., Jurado Kellie Ann, Abrahams Vikki M., Fikrig Erol, Guller Seth. Zika virus infection of Hofbauer cells. American Journal of Reproductive Immunology. 2016;77(2):e12613. doi: 10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman HD, Pierson TC. Zika in the Brain: New Models Shed Light on Viral Infection. Trends Mol Med. 2016;22:639–641. doi: 10.1016/j.molmed.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WW, et al. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell reports. 2016;17:3091–3098. doi: 10.1016/j.celrep.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elong Ngono A, et al. Mapping and Role of the CD8(+) T Cell Response During Primary Zika Virus Infection in Mice. Cell host & microbe. 2017;21:35–46. doi: 10.1016/j.chom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermillion MS, et al. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nature communications. 2017;8:14575. doi: 10.1038/ncomms14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan L, et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science. 2017 doi: 10.1126/science.aam7120. [DOI] [PubMed] [Google Scholar]
- 22.Winkler CW, et al. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J Immunol. 2017;198:3526–3535. doi: 10.4049/jimmunol.1601949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnani DM, et al. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nature communications. 2018;9:1624. doi: 10.1038/s41467-018-04056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierson TC, Graham BS. Zika Virus: Immunity and Vaccine Development. Cell. 2016;167:625–631. doi: 10.1016/j.cell.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shan C, et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nature medicine. 2017;23:763–767. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Camacho C, et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nature communications. 2018;9:2441. doi: 10.1038/s41467-018-04859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett ADT. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. NPJ Vaccines. 2018;3:24. doi: 10.1038/s41541-018-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang H, et al. Recombinant Zika virus envelope protein elicited protective immunity against Zika virus in immunocompetent mice. PloS one. 2018;13:e0194860. doi: 10.1371/journal.pone.0194860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prow NA, et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nature communications. 2018;9:1230. doi: 10.1038/s41467-018-03662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keeffe JR, et al. A Combination of Two Human Monoclonal Antibodies Prevents Zika Virus Escape Mutations in Non-human Primates. Cell reports. 2018;25:1385–1394 e1387. doi: 10.1016/j.celrep.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavigner Maud, Raper Jessica, Kovacs-Balint Zsofia, Gumber Sanjeev, O’Neal Justin T., Bhaumik Siddhartha K., Zhang Xiaodong, Habib Jakob, Mattingly Cameron, McDonald Circe E., Avanzato Victoria, Burke Mark W., Magnani Diogo M., Bailey Varian K., Watkins David I., Vanderford Thomas H., Fair Damien, Earl Eric, Feczko Eric, Styner Martin, Jean Sherrie M., Cohen Joyce K., Silvestri Guido, Johnson R. Paul, O’Connor David H., Wrammert Jens, Suthar Mehul S., Sanchez Mar M., Alvarado Maria C., Chahroudi Ann. Postnatal Zika virus infection is associated with persistent abnormalities in brain structure, function, and behavior in infant macaques. Science Translational Medicine. 2018;10(435):eaao6975. doi: 10.1126/scitranslmed.aao6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen SM, et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS pathogens. 2017;13:e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Read JS, et al. Symptomatic Zika Virus Infection in Infants, Children, and Adolescents Living in Puerto Rico. JAMA Pediatr. 2018;172:686–693. doi: 10.1001/jamapediatrics.2018.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asturias EJ. Uncovering the Spectrum of Postnatal Zika Infection in Children. JAMA Pediatr. 2018;172:624–625. doi: 10.1001/jamapediatrics.2018.0921. [DOI] [PubMed] [Google Scholar]
- 36.Quintana-Domeque C, Carvalho JR, de Oliveira VH. Zika virus incidence, preventive and reproductive behaviors: Correlates from new survey data. Econ Hum Biol. 2018;30:14–23. doi: 10.1016/j.ehb.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Odorizzi Pamela M., Jagannathan Prasanna, McIntyre Tara I., Budker Rachel, Prahl Mary, Auma Ann, Burt Trevor D., Nankya Felistas, Nalubega Mayimuna, Sikyomu Esther, Musinguzi Kenneth, Naluwu Kate, Kakuru Abel, Dorsey Grant, Kamya Moses R., Feeney Margaret E. In utero priming of highly functional effector T cell responses to human malaria. Science Translational Medicine. 2018;10(463):eaat6176. doi: 10.1126/scitranslmed.aat6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Ende C, Marano C, van Ahee A, Bunge EM, De Moerlooze L. The immunogenicity of GSK’s recombinant hepatitis B vaccine in children: a systematic review of 30 years of experience. Expert Rev Vaccines. 2017;16:789–809. doi: 10.1080/14760584.2017.1338569. [DOI] [PubMed] [Google Scholar]
- 39.Leineweber B, Grote V, Schaad UB, Heininger U. Transplacentally acquired immunoglobulin G antibodies against measles, mumps, rubella and varicella-zoster virus in preterm and full term newborns. The Pediatric infectious disease journal. 2004;23:361–363. doi: 10.1097/00006454-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25:6296–6304. doi: 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 41.van Panhuis WG, et al. Decay and persistence of maternal dengue antibodies among infants in Bangkok. Am J Trop Med Hyg. 2011;85:355–362. doi: 10.4269/ajtmh.2011.11-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pengsaa K, Limkittikul K, Yoksan S, Wisetsing P, Sabchareon A. Dengue antibody in Thai children from maternally transferred antibody to acquired infection. The Pediatric infectious disease journal. 2011;30:897–900. doi: 10.1097/INF.0b013e31821f07f6. [DOI] [PubMed] [Google Scholar]
- 43.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 44.Halstead SB, O’Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. The Journal of experimental medicine. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathore APS, Saron WAA, Lim T, Jahan N, St. John AL. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci Adv. 2019;5:eaav3208. doi: 10.1126/sciadv.aav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camargos VN, et al. In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy. EBioMedicine. 2019;44:516–529. doi: 10.1016/j.ebiom.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell host & microbe. 2015;17:500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinot AJ, et al. Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell. 2018;173:1111–1122 e1110. doi: 10.1016/j.cell.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Connor MA, et al. Early cellular innate immune responses drive Zika viral persistence and tissue tropism in pigtail macaques. Nature communications. 2018;9:3371. doi: 10.1038/s41467-018-05826-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albers CA, Grieve AJ. Bayley scales of infant and toddler development, third edition. J Psychoeduc Assess. 2007;25:180–190. doi: 10.1177/0734282906297199. [DOI] [Google Scholar]
- 51.Als H, Tronick E, Lester BM, Brazelton TB. The Brazelton Neonatal Behavioral Assessment Scale (BNBAS) J Abnorm Child Psychol. 1977;5:215–231. doi: 10.1007/BF00913693. [DOI] [PubMed] [Google Scholar]
- 52.Champoux M, Suomi SJ, Schneider ML. Temperament differences between captive Indian and Chinese-Indian hybrid rhesus macaque neonates. Laboratory animal science. 1994;44:351–357. [PubMed] [Google Scholar]
- 53.Brazelton TB. Assessment of the infant at risk. Clin Obstet Gynecol. 1973;16:361–375. doi: 10.1097/00003081-197303000-00020. [DOI] [PubMed] [Google Scholar]
- 54.Schneider ML, Suomi SJ. Neurobehavioral Assessment in Rhesus-Monkey Neonates (Macaca-Mulatta) - Developmental-Changes, Behavioral Stability, and Early Experience. Infant Behav Dev. 1992;15:155–177. doi: 10.1016/0163-6383(92)80021-L. [DOI] [Google Scholar]
- 55.Schneider ML. The Effect of Mild Stress during Pregnancy on Birth-Weight and Neuromotor Maturation in Rhesus-Monkey Infants (Macaca-Mulatta) Infant Behav Dev. 1992;15:389–403. doi: 10.1016/0163-6383(92)80009-J. [DOI] [Google Scholar]
- 56.Schneider ML, Moore CF, Suomi SJ, Champoux M. Laboratory Assessment of Temperament and Environmental Enrichment in Rhesus-Monkey Infants (Macaca-Mulatta) American Journal of Primatology. 1991;25:137–155. doi: 10.1002/ajp.1350250302. [DOI] [PubMed] [Google Scholar]
- 57.Bayley, N. Bayley Scales of Infant Development - Second Edition. The Psychological Corporation (1993).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available from the corresponding author on request.