Abstract
Polyamines have fundamental roles in brain homeostasis as key modulators of cellular excitability. Several studies have suggested alterations in polyamine metabolism in stress related disorders, suicide, depression, and neurodegeneration, making the pharmacological modulation of polyamines a highly appealing therapeutic strategy. Polyamines are small aliphatic molecules that can modulate cationic channels involved in neuronal excitability. Previous indirect evidence has suggested that polyamines can modulate anionic GABAA receptors (GABAARs), which mediate inhibitory signaling and provide a direct route to reduce hyperexcitability. Here, we attempted to characterize the effect that spermine, the polyamine with the strongest reported effect on GABAARs, has on human postmortem native GABAARs. We microtransplanted human synaptic membranes from the dorsolateral prefrontal cortex of four cases with no history of mental or neurological disorders, and directly recorded spermine effects on ionic GABAARs responses on microtransplanted oocytes. We show that in human synapses, inhibition of GABAARs by spermine was better explained by alkalization of the extracellular solution. Additionally, spermine had no effect on the potentiation of GABA-currents by diazepam, indicating that even if diazepam binding is enhanced by spermine, it does not translate to changes in functional activity. Our results clearly demonstrate that while extracellular spermine does not have direct effects on human native synaptic GABAARs, spermine-mediated shifts of pH inhibit GABAARs. Potential spermine-mediated increase of pH in synapses in vivo may therefore participate in increased neuronal activity observed during physiological and pathological states, and during metabolic alterations that increase the release of spermine to the extracellular milieu.
Subject terms: Physiology, Pharmacodynamics
Introduction
Polyamines (putrescine, spermidine, spermine, and agmatine) are positively charged molecules that have fundamental roles in brain homeostasis by modulating neurotransmission, cellular excitability and membrane permeability1,2. Due to their role in neuronal signaling, metabolic alterations that affect intracellular or extracellular concentrations of polyamines have been associated to maladaptive stress, mental disorders, and neurodegeneration3–8. Clinical evidence and animal models have shown that abnormally increased levels of polyamines could lead to self-sustained stress responses of circuits within frontal cortical-limbic structures1,9,10, which is a phenomenon frequently observed in major depressive disorder (MDD) and mood disorders11,12. Moreover, abnormal spermine metabolism has been linked to the neurotoxicity observed in Alzheimer’s disease13,14. Because sustained polyamine stress responses and neurotoxicity are mediated, at least in part, by the interaction between polyamine levels and membrane receptors involved in the control of cellular excitability, a better understanding of polyamine membrane targets is essential for the development of new pharmacological therapeutic interventions in brain disorders targeting polyamine modulation. Most polyamine targets in cellular membranes are proteins that interact with cations (e.g., ion channels permeable to Na+, K+, and Ca2+) and modulate neuronal excitatory drive. Indirect evidence also suggests that ion channels with specific permeability to anions, and consequently modulating inhibitory transmission, could also be modulated by polyamines, particularly spermine15–17. Thus, direct electrophysiological evidence of spermine on GABAA receptors (GABAARs) may indicate a novel polyamine mechanism of action and lead to novel therapeutic and translational opportunities if confirmed.
GABAARs are heteropentameric anionic channels that are essential for inhibitory signaling in the brain18,19, and the homeostatic control of synaptic excitatory to inhibitory balance20. An initial report showed that ion responses mediated by GABAARs, heterologously expressed in Xenopus oocytes using mRNA from rat brain, were potentiated by spermine15. It was later found that spermine modulated the binding of diazepam to GABAARs in synaptoneurosomal preparations, but the effect was abolished after treatment with non-ionic detergents, even though diazepam binding was still preserved17. Later work by Discenna et al., showed that spermine reduced GABA-mediated inhibitory postsynaptic potentials (IPSPs) by 55% in rat hippocampal slices16. The authors attributed these changes to inhibitory effects on presynaptic voltage-gated calcium channels (VGCC), which in turn would reduce the release of GABA stored in synaptic vesicles. Nonetheless, the concentration of polyamines needed to reduce half of VGCC ion responses is very high and unlikely to have physiological effects at the synaptic level (spermine, ≈4.7 mM < spermidine, ≈11 mM < putrescine, ≈90 mM for N-type VGCC;21). The estimated concentration of spermine in synaptic vesicles is in the 1.5–2.8 mM range22, which suggests that additional mechanisms and targets such as extracellular H+, or direct modulation of GABAARs need to be explored. The primary goal of our study was to determine whether extracellular spermine, the polyamine producing the largest effects in previous studies15–17,21, exhibits a modulatory effect on native human synaptic GABAARs. For this, we microtransplanted human receptors from the dorsolateral prefrontal cortex, still embedded in their native membranes and associated with their accessory proteins23–26, and studied the effects of extracellular spermine on GABA-elicited ion currents.
Materials and methods
Oocyte preparation
Frogs were placed in anesthetic solution (0.17% MS-222) for 10–15 min before extracting the ovaries; following procedures in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the IACUC at the University of California, Irvine (IACUC: 1998–1388), and at the University of Texas Medical Branch at Galveston (IACUC:1803024). Oocytes were isolated and defolliculated by carefully stirring them in a solution containing 2 mg/mL collagenase for 2 h at 30 °C. Then, oocytes were transferred to a Petri dish containing Barth’s solution [88 mM NaCl, 1 mM KCl, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3, 5 mM HEPES (pH 7.4)], and placed in a temperature-controlled environment at 16 °C for 24 h. Stage V–VI oocytes were manually separated and placed in a fresh Barth’s solution for injection of synaptoneurosomal enriched membranes.
Microtransplantation of synaptic membranes (MSM)
The subjects for this study consisted of postmortem human dorsolateral prefrontal cortex (DLPFC), from four psychiatrically healthy subjects (Table 1). The brains and samples were collected by the University of California, Irvine Brain Bank (UCIBB) in accordance with the University’s Institutional Review Board after obtaining consent from next of kin. These brains have been characterized using UCIBB psychological autopsy protocol that has been extensively used by our group in past years27–30. Human synaptoneurosomes, harboring GABA receptors, were isolated from ≈50 mg frozen DLPFC from each brain donor using Syn-PER method (Thermo Fisher Scientific). The resultant pellet, enriched in synaptoneurosomes, was suspended in sterile distilled water and sonicated to create small proteoliposomes that can fuse to the oocytes’ extracellular membrane. After the protein concentration was determined by using Qubit protein assay reagent kit (Thermo Fisher Scientific) the membrane preparations were stored at −80 °C until the moment of injection. One day before electrophysiological recordings the synaptic membranes were injected into stage V–VI Xenopus laevis oocytes using protocols previously published for cellular membranes23,31,32. Each oocyte was injected with 50 nL of synaptic proteoliposomes (2 mg/mL protein concentration) and characterized 18–36 h post-injection.
Table 1.
Demographics
| Donor | Age/Gender | PMI (hours) | pH | RIN |
|---|---|---|---|---|
| S1 | 50/M | 29 | 6.6 | 8.3 |
| S2 | 52/M | 18.8 | 6.53 | 9.2 |
| S3 | 64/M | 10.5 | 7.13 | 9.8 |
| S4 | 56/M | 9 | 6.64 | 9.7 |
S subject, M male, age is counted in years and postmortem interval (PMI) in hours. RIN RNA integrity number
Heterologous expression of GluR3
To test and monitor the biological activity of extracellular spermine we expressed GluR3 receptors in oocytes as previously reported33. Briefly, 50 nL of cRNA (1 mg/ml) for human GRIA3 (Clontech, Mountain View, CA) were injected into the equatorial band of defolliculated Xenopus oocytes and Kept in Barth’s solution until the moment of recording 2–4 days after injection.
Electrophysiological recordings
Agonist-elicited ion currents were recorded by the two-electrode voltage clamp (TEVC) method34. Microelectrodes were filled with 3 M KCl and resistance of the microelectrodes ranged from 0.5 to 3.0 MΩ. Piercing and recording took place in a chamber (volume ≈0.1 ml) continuously perfused (5–10 ml/min) with Ringer’s solution [115 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 5 mM Hepes (pH 7.4)] at room temperature (19–21 °C). Oocytes were voltage clamped to −80 mV. Ion currents were recorded and stored with WinEDR version 2.3.8 Strathclyde Electrophysiology Software (John Dempster, Glasgow, United Kingdom). Most drugs were from Sigma (St. Louis. MO). Kainic acid, baclofen and CGP-55845 hydrochloride were from Tocris (Minneapolis. MN). Working solutions were made by diluting aqueous 1 M spermine or ethanolic 10 mM diazepam stocks in Ringer’s solution. Same results were obtained when using spermine, freshly prepared, or from frozen aliquots, from three different lots. The biological activity of spermine was tested by its antagonist effect on GluR3 receptors which, similarly to all Ca2+-permeable AMPA receptors, are sensitive to extracellular polyamines35,36. After addition of spermine the Ringer’s solution pH was fixed at pH 7.4 by the addition of hydrochloric acid21. In some experiments the pH of the extracellular solution was adjusted to 10 or 6 by adding either sodium hydroxide or hydrochloric acid and used immediately.
Data analysis
The EC50, EC25 and the Hill coefficient were determined by fitting the Hill equation in the form I = Imax/(1 + (EC50/[A])n), in which I is the current amplitude, Imax is the maximum current amplitude at the concentration of the agonist [A], EC50 is the agonist concentration that induces 50% of the maximal response, and n is the Hill coefficient. The EC50 and EC25 were estimated for each brain donor (biological replicates). The number of microtransplanted oocytes tested (technical replicates) was determined by analyzing the magnitude of the effect and the dispersion of the variability, and using paired data when possible, similarly to pharmacological analysis of heterologous expression of GABA and AMPA receptors in Xenopus oocytes37,38. The experimental data are shown as the mean ± S.E.M. Statistical differences were determined by two-sided Student t-test and considered significant when p < 0.05 (JMP version 14; SAS Institute, Cary, NC).
Results
Effects of spermine and pH on GABA currents
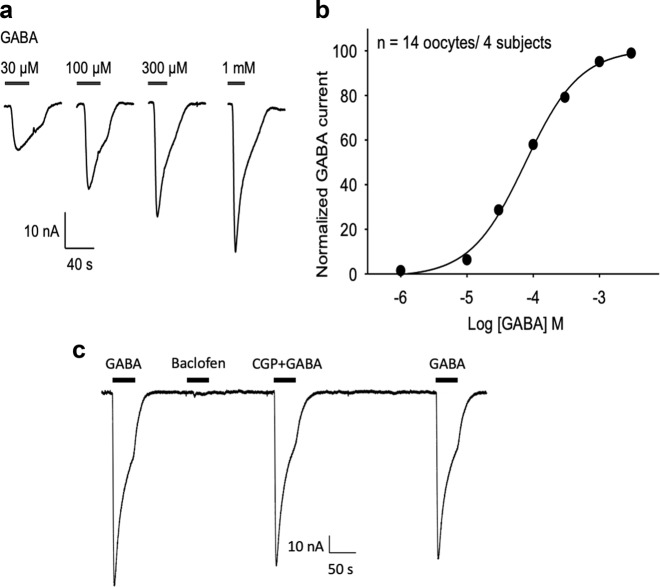
Perfusion of 1 mM GABA to oocytes microtransplanted with human postmortem synaptoneurosomal membranes preparation elicited fast activating ion currents (GABA currents) with a maximum amplitude of 100 nA ± 14 nA (n = 14 oocytes/4 subjects). The amplitude of GABA currents was dependent on the GABA concentration with an EC50 of 78 µM (Fig. 1a, b). In contrast, non-injected oocytes showed no responses to GABA (Fig. 2a), confirming that GABA currents in microtransplanted oocytes were exclusively mediated by human transplanted receptors. To determine the potential contribution of GABA-mediated metabotropic responses present in synaptoneurosomes we used 100 μM baclofen, a specific agonist, and 5 μM CGP-55845, a specific antagonist, for GABAB receptors (Fig. 1c). Baclofen elicited negligible responses in microtransplanted oocytes and CGP-55845 did not affect GABA-elicited currents (97.3 ± 2.1% of control; n = 16 oocytes/4 subjects), indicating that microtransplanted GABAB receptors may be uncoupled to the oocytes’s intracellular signaling, and GABA elicited currents in these oocytes were due to direct activation of GABAARs.
Fig. 1. Reactivation of GABAA receptors from frozen human synaptoneurosomes from the DLPFC.
a Synaptoneurosomal preparations, isolated from the dorsolateral prefrontal cortex of four subjects with no history of psychiatric disorders, were microtransplanted into Xenopus oocytes. Native GABA receptors were activated by perfusing sequentially increasing concentrations of GABA. b Average concentration response curve for GABA, using data from 4 subjects, 3–4 oocytes each. Mean ± s.e.m., error bars are smaller than the symbols. The average EC50 for all subjects pooled was 77.9 ± 4.7 µM, nH = 1 ± 0.03 (mean ± s.e.m.). The individual EC50 for each subject was: S1 = 68.3 ± 4 µM, nH = 1 ± 0.02 (n = 4 oocytes); S2 = 80.1 ± 9 µM, nH = 1 ± 0.01 (n = 3 oocytes); S3 = 96.7 ± 3 µM, nH = 1.1 ± 0.05 (n = 4 oocytes) and S4 = 63 ± 11 µM, nH = 1.2 ± 0.1 (n = 3 oocytes). c Baclofen (100 μM), an agonist of metabotropic GABAB receptors elicit negligible responses in microtransplanted oocytes. CGP-55845 (5 μM) did not significantly affect GABA elicited currents (97.3 ± 2.1% of control; n = 16 oocytes/4 subjects) in microtransplanted oocytes
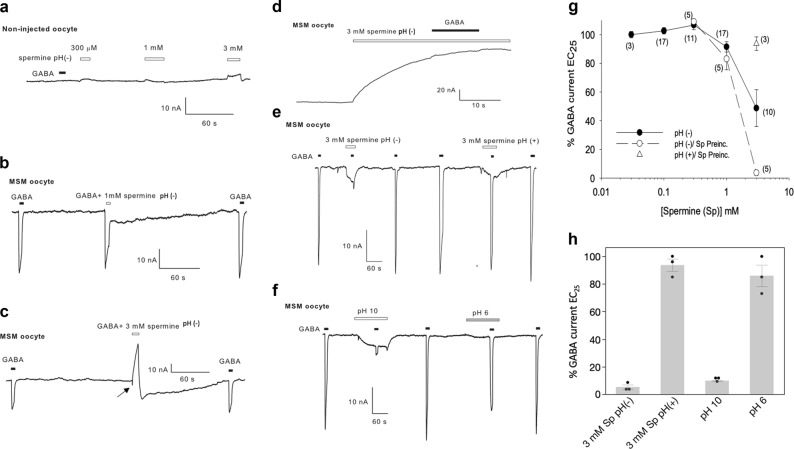
Fig. 2. Spermine and pH effects on native GABA currents.
a Non-transplanted oocytes were unresponsive to extracellular perfusion of GABA (30 μM) but elicited concentration-dependent biphasic responses to perfusion of spermine when the pH of the Ringer’s solution was not readjusted to 7.4 after addition of spermine (pH (−)). b, c. Oocyte microtransplanted with synaptic membranes (MSM) from subject S3 showing responses to 30 μM GABA (EC25 for S3) and to the co-application of 30 μM GABA with different concentrations of spermine pH(−). Notice that an initial inward current (arrow in C), elicited during co-application of GABA and 3 mM spermine pH(−), was cut short by the appearance of a large outward current. d Preincubation with 3 mM spermine pH(−) inhibited the responses to 30 μM GABA. e GABA responses (30 μM) in microtransplanted oocytes were inhibited during perfusion of 3 mM spermine pH(−). However, when the pH was titrated to 7.4 (pH( + )) GABA responses were minimally reduced. f Perfusion of extracellular solution, titrated to pH 10, and to the same oocyte shown in E, elicited a slow inward current and reduced GABA responses to 9.9 ± 0.4% of the control. g Plot summarizing the effects of spermine on GABA’s EC25. Circles indicate the mean ± s.e.m., of GABA currents derived from all subjects when the pH of the Ringer’s solution was not readjusted after addition of spermine (pH (−)). Spermine at concentrations of 100 µM, 1 mM, and 3 mM, changed the pH to 7.56, 8.9, and 10.3, respectively. Black circles represent the effects produced by co-application of spermine and GABA, and white circles the added effects of the preincubation with spermine for at least 10 s, or when the baseline current was stable. The white triangle indicates the effects of 3 mM spermine, preincubated and then co-applied with GABA, in Ringer’s solution with the pH fixed at 7.4 (pH ( + )). The number in parenthesis indicates the number of oocytes tested. h Comparison of the effects produced by high concentration of spermine with and without readjusting the pH, and with solutions without spermine but high pH in subject S3 which had the strongest effect to 3 mM spermine pH(−)
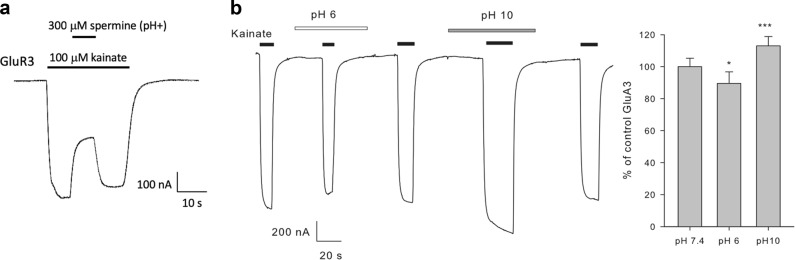
Because the directionality of a putative modulation by polyamines was not known we tested spermine on the EC25 for GABA in each subject (≈30 μM), a concentration that allows the characterization of positive or negative modulation of native GABA receptors. In our first experiments, we initially tested the effects of extracellular spermine without readjusting the pH after spermine addition (pH (−)) (Fig. 2a–d). In the pH (−) condition, the perfusion of spermine by itself at concentrations of 100 µM (pH = 7.56), or lower, did not have effects on transplanted oocytes. Higher concentrations of spermine elicited non-specific slow activating currents in transplanted and non-transplanted oocytes. The non-specific effects were similar to results reported previously with spermine alone15, or alkaline pH39, eliciting inward or biphasic currents15, or in some batches of oocytes that were highly sensitive to alkaline pH, spermine elicited strong outward currents39 (Fig. 2c, d). Whereas co-perfusion of 100 µM spermine in pH (−) solution had only minimal effects on GABA currents (102 ± 2.2% of control; n = 11 oocytes/4 subjects), 1 mM and 3 mM spermine reduced GABA currents to 91 ± 4% and 49 ± 13% of the response elicited by GABA alone (n = 17 and 10 oocytes/4 subjects). Preincubation with 3 mM spermine pH (−) for at least 10 s, to wait for the stabilization of the non-specific current, blocked GABA elicited currents to 4 ± 2% of the control (n = 5 oocytes/the subject with the strongest spermine pH(−) effect) (Fig. 2d, e, g), initially suggesting that the application of spermine at high concentrations had a direct effect on GABA currents. However, further experiments testing pH and spermine in the same oocytes showed that spermine-induced alkalization of Ringer’s pH was responsible for the non-specific current and the negative modulation of GABA responses (Fig. 2f–h). Titration of Ringer’s solution to 7.4 after spermine addition prevented the negative modulation of GABA receptors even at 3 mM spermine, eliciting GABA currents 94 ± 5% of the control (n = 3 oocytes from the subject with the strongest spermine pH(−) effect; S3). In contrast, Ringer’s solution with alkaline pH (pH = 10), which is similar to the change in pH produced by 3 mM spermine, blocked GABA currents to 9.9 ± 0.4% of the control (n = 3 oocytes/subject S3). The blockade by 3 mM spermine pH(−) was not statistically different from the blockade elicited by pH 10 alone (5.3 ± 1.6% vs 9.9 ± 0.4 %; p = 0.139, paired, two-sided Student’s t-test). To confirm that spermine was biologically active at pH 7.4 we tested its effects on heterologously expressed glutamate receptors (GluR3) (Fig. 3); spermine (300 μM) blocked kainate-elicited currents by 51 ± 2.5% (n = 6; mean ± s.e.m.). Interestingly, acidification of the pH reduced the kainate response to 89 ± 7.2% of the control and alkalization increased it to 114 ± 5.8% (n = 5; p < 0.01 paired, two-sided t Student’s test). These results indicate that extracellular spermine is biologically active and, by itself, does not directly modify the amplitude of GABA currents. It is the increase in the pH of the buffered solutions that negatively modulates GABAARs.
Fig. 3. Effects of spermine and pH on homomeric GluR3.
a Ion currents elicited with 100 mM kainate were inhibited by spermine with pH adjusted to 7.36 by 51 ± 2.5% (n = 6). b Perfusion of extracellular solution with pH adjusted to 6 inhibited kainate currents to 89 ± 7.2% of the control and pH adjusted to 10 increased it to 114 ± 5.8 % (n = 5; p < 0.01 paired, two-sided t Student’s test)
Effects of spermine on the modulation by Diazepam
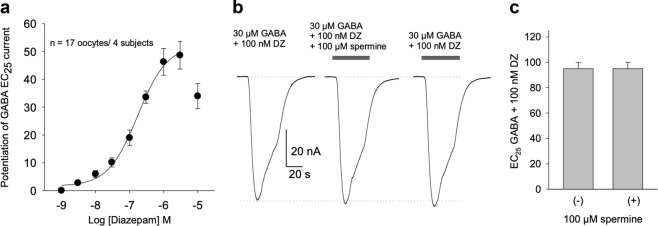
Diazepam positively modulated GABA responses by 49 ± 5% of the control, with an average EC50 of 275 nM (Fig. 4). High concentrations of diazepam (>3μM) reduced the level of potentiation, as has been previously observed for heterologously expressed GABAA receptors40. Because in previous studies 100 µM spermine was the concentration that elicited the largest increase of diazepam binding on GABA17, we used the same concentration to maximize its putative effects of current potentiation. Spermine (pH 7.4) had no effects on the amplitude of GABA currents in presence of 1 µM diazepam (change of current to 107 ± 9.2% of the control, n = 27 oocytes/4 subjects). To avoid the possibility that desensitization of GABA currents could counteract spermine effects, we tested the effect of spermine with a low concentration of diazepam (100 nM) in order to see if the binding for diazepam was enhanced by spermine then the GABA current amplitude should be potentiated proportionally. In these conditions, we did not see spermine effects on the amplitude of GABA currents (n = 6 oocytes/3 subjects). To discard the possibility that spermine did not potentiate GABA currents because the potentiation was already at its maximum level, further potentiation by 1 µM diazepam after spermine was confirmed (n = 6 oocytes/3 subjects).
Fig. 4. Spermine has no effect on diazepam potentiation of GABAARs.
a Average concentration-response curve of diazepam potentiation of GABA currents, using data from 4 subjects, 4–5 oocytes each. Mean ± s.e.m. The current elicited by GABA’s EC25 in each oocyte was defined as zero in the plot. The average EC50 for all subjects was 275 ± 66 nM (mean ± s.e.m; n = 17). The individual EC50 for each subject was: S1 = 253 ± 11 nM (n = 4), S2 = 273 ± 16 nM (n = 4), S3 = 159 ± 55 nM (n = 4) and S4 = 670 ± 121 nM (n = 5). Higher concentrations of diazepam reduced the efficacy of the potentiation. b Representative traces of currents elicited by GABA’s EC25 co-applied with 100 nM diazepam before and in the presence of 100 µM spermine. c The plot shows no change of the normalized maximal current responses elicited by GABA’s EC25 plus 100 nM diazepam and 100 µM spermine (+) compared to those without it (−) (n = 6 oocytes/3 subjects)
Discussion
Polyamines, by modifying the current properties of ionic receptors, play important neuromodulatory roles in health and disease1. Spermine modulation of synaptic GABAARs could have significant consequences on inhibitory neurotransmission and important translational relevance for neuropsychiatric and neurodegenerative disorders. Our results, however, clearly show that spermine has no direct modulatory role on the functional responses of synaptic human GABAARs. Instead, it is the alkalization of the extracellular solution by spermine that can explain some effects previously reported. Spermine effects on GABAARs in Xenopus oocytes was first observed simultaneously with the activation of a non-specific biphasic current15. We were able to replicate the non-specific current seen in Brackley et al., in our own experiments when the pH was not corrected after spermine addition. This effect was similar to the cAMP-mediated K+ outward current elicited by alkaline extracellular solutions and was more evident in oocytes highly sensitive to alkaline treatment39. We also did not find evidence that spermine modifies the amplitude of GABA currents in presence of diazepam, indicating that even if diazepam binding is enhanced by spermine, it does not translate to changes of functional activity. Most cortical synaptic GABAAR subunits are arranged in α1γ2β2α1β2 counterclockwise manner, as seen from the outside of the cell41,42. Accordingly, we have confirmed, by proteomics, the presence of α1, β2, β3 and γ2 subunits in our synaptic preparations43. Diazepam can bind these GABAARs receptors at a high-affinity site at the α1/γ2 interface42, or at transmembrane low-affinity sites at the other subunits interfaces44,45. Because it was previously reported that polyamine-mediated modulation by diazepam binding disappeared after treatment with non-ionic detergents it is possible that lipids were mediating spermine effects17. The activity and pharmacology of GABAARs is affected by the composition and dynamics of surrounding lipids46,47, and intracellular spermine stabilizes the cellular membrane fluidity48; therefore an interaction between increased intracellular spermine and lipids in rat synaptoneurosomal preparations could have mediated the effect on diazepam-binding experiments.
Alkaline pH can also affect benzodiazepine effects, at pH 8.4 flunitrazepam had a slightly higher potentiation of GABA currents in cerebellar granule cells (17%), than at pH 7.449, suggesting more binding of flunitrazepam at higher pH. Besides the pH effect, discrepancies between our results and previous reports could also be due to potential interspecies differences between GABAARs in humans and animal models used in previous studies50,51 (e.g., species-specific posttranslational modifications or interactions with their accessory proteins52,53). The potential effect of spermine on GABAARs has important clinical implications especially in the context of complex neurological and mental disorders with known alterations in GABAergic neurotransmission. Therefore, testing spermine on human receptors with their own posttranslational modifications, accessory proteins, and surrounding human lipids, is a needed step to avoid confounding factors and better dissect the role of polyamines in disorders like suicide and depression1,30. This type of in vitro pharmacological profiling using post-mortem native human receptors has important applications to study normal and diseased conditions but also to characterize, in vitro, new drugs for the pharmacological treatment of neuropsychiatric and neurodegenerative conditions. Because our experiments indicate that spermine does not have a direct functional effect on human GABAARs, the rule of thumb that spermine only affects cationic channels still holds up. It is important to note that our results only apply to cortical human synaptic receptors composed by α1, β2, β3, and γ2 subunits which: (1) are translationally relevant due to their putative modulation by spermine, (2) are the most abundant in the human cortex50, and (3) we have confirmed to be present in our synaptic preparations43. While there is evidence of presynaptic receptors in the spinal cord, hippocampus, cerebellum and layer 4 of the primary visual in cortex54–56, to our knowledge, there is no direct evidence for the presence of presynaptic GABAARs in the frontal, temporal or parietal cortices; therefore, it is highly likely that the majority of microtransplanted receptors are postsynaptic. Our results cannot discard the possibility that spermine might have effects on extrasynaptic receptors, or in any other of the number of isoforms composed by the combination of the 19 genes for GABAARs.
Interestingly, high alkaline pH blocked GABA currents in microtransplanted oocytes, functionally confirming that GABAARs in our synaptoneurosomal preparations are predominantly composed by α1β2γ2 subunits43,57. Although the physiological relevance of GABAergic inhibition by alkaline pH is not completely understood58, it is known that during synaptic transmission a fast and strong acidosis of the synaptic cleft is followed by a long but transient rise of pH59 which likely results from membrane transport and fluxes of H+ and bicarbonate58. Moreover, rises of pH increase neuronal activity and excitability58. Because spermine can be accumulated in synaptic vesicles and be released during depolarization22, it would not be surprising that synaptic release of spermine participates in the transient rise of pH to remove H+-induced inhibition of NMDA receptors60 and inhibit GABAARs to synergistically increase neuronal activity in normal physiological conditions.
In conclusion, our experiments demonstrate that spermine has no direct effect on human native synaptic GABAARs. However, spermine-mediated shifts of pH inhibit GABAARs. If a similar rise in pH is also observed in vivo in the synaptic cleft micro-environment it could participate in the increased neuronal activity observed during alkaline physiological and pathological states, and during metabolic alterations that increase the release of spermine to the extracellular milieu.
Acknowledgements
Some of the authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, HudsonAlpha Institute of Biotechnology, the Universities of California at Davis and at Irvine, to encourage the development of appropriate findings for research and clinical applications. We are also grateful to the donors’ families for the brain donation and David Walsh, Preston Cartagena and Casey Kathleen Burke for characterizing, acquiring, and storing the postmortem human and Dr. Mamdani for her help and comments. The research was further supported by The Brain & Behavior Research Foundation (former NARSAD) Young Investigator Award (A.S.), NIMH research grant R01MH097082 (A.S.), NIA grant R21AG053740 (A.L.), NIMH research grant R01MH085801 (M.P.V.), and NIMH research grant R21MH113177 (A.L., M.P.V.).
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A. Limon, Email: agenor.limon@utmb.edu
A. Sequeira, Email: psequeir@uci.edu
References
- 1.Limon A, Mamdani F, Hjelm BE, Vawter MP, Sequeira A. Targets of polyamine dysregulation in major depression and suicide: activity-dependent feedback, excitability, and neurotransmission. Neurosci. Biobehav. Rev. 2016;66:80–91. doi: 10.1016/j.neubiorev.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skatchkov SN, Woodbury-Farina MA, Eaton M. The role of glia in stress: polyamines and brain disorders. Psychiatr. Clin. North Am. 2014;37:653–678. doi: 10.1016/j.psc.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewandowski NM, et al. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc. Natl Acad. Sci. USA. 2010;107:16970–16975. doi: 10.1073/pnas.1011751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivo M, et al. Polyamines in the basal ganglia of human brain. Influence of aging and degenerative movement disorders. Neurosci. Lett. 2001;304:107–111. doi: 10.1016/S0304-3940(01)01776-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen GG, et al. Evidence of altered polyamine concentrations in cerebral cortex of suicide completers. Neuropsychopharmacology. 2010;35:1477–1484. doi: 10.1038/npp.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiori LM, Turecki G. Implication of the polyamine system in mental disorders. J. Psychiatry Neurosci. 2008;33:102–110. [PMC free article] [PubMed] [Google Scholar]
- 8.Turecki G. The molecular bases of the suicidal brain. Nat. Rev. Neurosci. 2014;15:802–816. doi: 10.1038/nrn3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilad GM, Gilad VH. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cell Mol. Neurobiol. 2003;23:637–649. doi: 10.1023/A:1025036532672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci. Biobehav. Rev. 2010;34:592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Wong ML, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl Acad. Sci. USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol. Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 13.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Guerra GP, Rubin MA, Mello CF. Modulation of learning and memory by natural polyamines. Pharm. Res. 2016;112:99–118. doi: 10.1016/j.phrs.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Brackley P, Goodnow R, Jr., Nakanishi K, Sudan HL, Usherwood PN. Spermine and philanthotoxin potentiate excitatory amino acid responses of Xenopus oocytes injected with rat and chick brain RNA. Neurosci. Lett. 1990;114:51–56. doi: 10.1016/0304-3940(90)90427-B. [DOI] [PubMed] [Google Scholar]
- 16.DiScenna PG, Ferchmin PA, Eterovic VA, Teyler TJ. Spermine depresses NMDA, K/AMPA and GABAA-mediated synaptic transmission in the rat hippocampal slice preparation. Brain Res. 1994;647:353–356. doi: 10.1016/0006-8993(94)91335-8. [DOI] [PubMed] [Google Scholar]
- 17.Gilad GM, Gilad VH, Wyatt RJ. Polyamines modulate the binding of GABAA-benzodiazepine receptor ligands in membranes from the rat forebrain. Neuropharmacology. 1992;31:895–898. doi: 10.1016/0028-3908(92)90127-B. [DOI] [PubMed] [Google Scholar]
- 18.Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update Pharm. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatti R, Haley MS, Swanson OK, Tselha T, Maffei A. Neurophysiology and regulation of the balance between excitation and inhibition in neocortical circuits. Biol. Psychiatry. 2016;81:821–831. doi: 10.1016/j.biopsych.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Harnett MT, Smith SM. Modulation of neuronal voltage-activated calcium and sodium channels by polyamines and pH. Channels. 2007;1:281–290. doi: 10.4161/chan.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuko T, et al. Polyamine transport, accumulation, and release in brain. J. Neurochem. 2003;84:610–617. doi: 10.1046/j.1471-4159.2003.01558.x. [DOI] [PubMed] [Google Scholar]
- 23.Limon A, Reyes-Ruiz JM, Miledi R. Microtransplantation of neurotransmitter receptors from postmortem autistic brains to Xenopus oocytes. Proc. Natl Acad. Sci. USA. 2008;105:10973–10977. doi: 10.1073/pnas.0804386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limon A, Reyes-Ruiz JM, Miledi R. GABAergic drugs and Alzheimer’s disease. Future Med. Chem. 2011;3:149–153. doi: 10.4155/fmc.10.291. [DOI] [PubMed] [Google Scholar]
- 25.Eusebi F, Palma E, Amici M, Miledi R. Microtransplantation of ligand-gated receptor-channels from fresh or frozen nervous tissue into Xenopus oocytes: a potent tool for expanding functional information. Prog. Neurobiol. 2009;88:32–40. doi: 10.1016/j.pneurobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Mazzo F, et al. Reconstitution of synaptic Ion channels from rodent and human brain in Xenopus oocytes: a biochemical and electrophysiological characterization. J. Neurochem. 2016;138:384–396. doi: 10.1111/jnc.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamdani F, et al. Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl. Psychiatry. 2015;5:e636. doi: 10.1038/tp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunney WE, et al. Microarray technology: a review of new strategies to discover candidate vulnerability genes in psychiatric disorders. Am. J. Psychiatry. 2003;160:657–666. doi: 10.1176/appi.ajp.160.4.657. [DOI] [PubMed] [Google Scholar]
- 29.Sequeira A, et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch. Gen. Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Sequeira A, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsal J, Tigyi G, Miledi R. Incorporation of acetylcholine receptors and Cl- channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc. Natl Acad. Sci. USA. 1995;92:5224–5228. doi: 10.1073/pnas.92.11.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl Acad. Sci. USA. 2012;109:10071–10076. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limon A, Reyes-Ruiz JM, Eusebi F, Miledi R. Properties of GluR3 receptors tagged with GFP at the amino or carboxyl terminus. Proc. Natl Acad. Sci. USA. 2007;104:15526–15530. doi: 10.1073/pnas.0706773104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc. R. Soc. Lond. B Biol. Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 35.Herlitze S, et al. Argiotoxin detects molecular differences in AMPA receptor channels. Neuron. 1993;10:1131–1140. doi: 10.1016/0896-6273(93)90061-U. [DOI] [PubMed] [Google Scholar]
- 36.Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc. Natl Acad. Sci. USA. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limon A, Estrada-Mondragon A, Ruiz JM, Miledi R. Dipicrylamine modulates GABArho1 receptors through interactions with residues in the TM4 and Cys-loop domains. Mol. Pharm. 2016;89:446–456. doi: 10.1124/mol.116.103432. [DOI] [PubMed] [Google Scholar]
- 38.Limon A, Reyes-Ruiz JM, Vaswani RG, Chamberlin AR, Miledi R. Kaitocephalin antagonism of glutamate receptors expressed in Xenopus oocytes. ACS Chem. Neurosci. 2010;1:175–181. doi: 10.1021/cn900037c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida S, Yoshimura M, Taniyama K. Activation of a potassium conductance by extracellular alkaline pH in oocytes of Xenopus laevis. Jpn J. Pharm. 2001;87:202–207. doi: 10.1254/jjp.87.202. [DOI] [PubMed] [Google Scholar]
- 40.Sigel E, Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus oocytes. J. Neurosci. 1988;8:289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigel E, Ernst M. The benzodiazepine binding sites of GABAA receptors. Trends Pharm. Sci. 2018;39:659–671. doi: 10.1016/j.tips.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Limon A, et al. Microtransplantation of synaptic membrane reveals functional alterations in the balance of excitatory and inhibitory currents in schizophrenia. Neuropsychopharmacology. 2016;41:S116–S288. doi: 10.1038/npp.2016.240. [DOI] [Google Scholar]
- 44.Maldifassi MC, Baur R, Sigel E. Molecular mode of action of CGS 9895 at alpha1 beta2 gamma2 GABAA receptors. J. Neurochem. 2016;138:722–730. doi: 10.1111/jnc.13711. [DOI] [PubMed] [Google Scholar]
- 45.Masiulis S, et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature. 2019;565:454–459. doi: 10.1038/s41586-018-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sooksawate T, Simmonds MA. Influence of membrane cholesterol on modulation of the GABA(A) receptor by neuroactive steroids and other potentiators. Br. J. Pharm. 2001;134:1303–1311. doi: 10.1038/sj.bjp.0704360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40:178–184. doi: 10.1016/S0028-3908(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 48.Ballas SK, Mohandas N, Marton LJ, Shohet SB. Stabilization of erythrocyte membranes by polyamines. Proc. Natl Acad. Sci. USA. 1983;80:1942–1946. doi: 10.1073/pnas.80.7.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robello M, Baldelli P, Cupello A. Modulation by extracellular pH of the activity of GABAA receptors on rat cerebellum granule cells. Neuroscience. 1994;61:833–837. doi: 10.1016/0306-4522(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 50.Sequeira A, Shen K, Gottlieb A, Limon A. Human brain transcriptome analysis finds region- and subject-specific expression signatures of GABAAR subunits. Commun. Biol. 2019;2:153. doi: 10.1038/s42003-019-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendu SK, Bhandage A, Jin Z, Birnir B. Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes. PLoS ONE. 2012;7:e42959. doi: 10.1371/journal.pone.0042959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J. Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- 53.Keller CA, et al. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J. Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Kloc M, Maher E, Erisir A, Maffei A. Presynaptic GABAA Receptors Modulate Thalamocortical Inputs in Layer 4 of Rat V1. Cereb. Cortex. 2019;29:921–936. doi: 10.1093/cercor/bhx364. [DOI] [PubMed] [Google Scholar]
- 55.Kullmann DM, et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog. Biophys. Mol. Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Draguhn A, Axmacher N, Kolbaev S. Presynaptic ionotropic GABA receptors. Results Probl. Cell Differ. 2008;44:69–85. doi: 10.1007/400_2007_040. [DOI] [PubMed] [Google Scholar]
- 57.Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABA(A) receptor is associated with the receptor subunit composition. J. Physiol. 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinning A, Hubner CA. Minireview: pH and synaptic transmission. FEBS Lett. 2013;587:1923–1928. doi: 10.1016/j.febslet.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 59.Krishtal OA, Osipchuk YV, Shelest TN, Smirnoff SV. Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Res. 1987;436:352–356. doi: 10.1016/0006-8993(87)91678-7. [DOI] [PubMed] [Google Scholar]
- 60.Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]