Abstract
Ammonia represents a promising liquid fuel for hydrogen storage, but its large-scale application is limited by the need for precious metal ruthenium (Ru) as catalyst. Here we report on highly efficient ammonia decomposition using novel high-entropy alloy (HEA) catalysts made of earth abundant elements. Quinary CoMoFeNiCu nanoparticles are synthesized in a single solid-solution phase with robust control over the Co/Mo atomic ratio, including those ratios considered to be immiscible according to the Co-Mo bimetallic phase diagram. These HEA nanoparticles demonstrate substantially enhanced catalytic activity and stability for ammonia decomposition, with improvement factors achieving >20 versus Ru catalysts. Catalytic activity of HEA nanoparticles is robustly tunable by varying the Co/Mo ratio, allowing for the optimization of surface property to maximize the reactivity under different reaction conditions. Our work highlights the great potential of HEAs for catalyzing chemical transformation and energy conversion reactions.
Subject terms: Heterogeneous catalysis, Hydrogen storage, Nanoparticles, Synthesis and processing
Alloys are important materials for catalysis but are usually limited by miscibility gaps present in their phase diagrams. Here the authors break this limitation by developing high-entropy alloy catalysts made of five earth-abundant elements and demonstrate great catalytic enhancements for ammonia decomposition.
Introduction
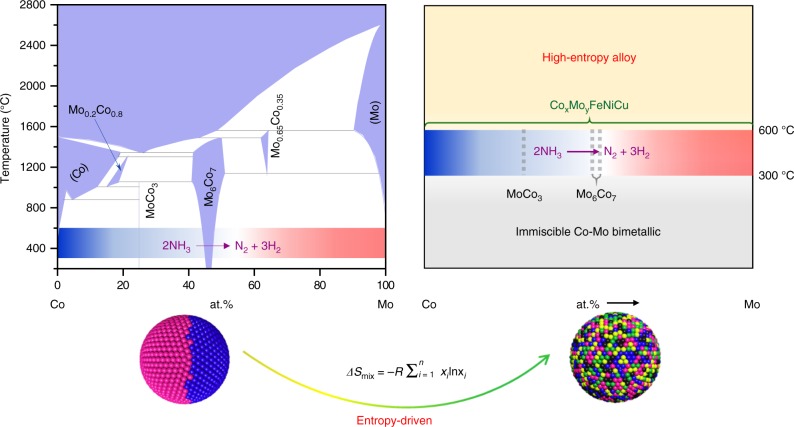
The ammonia (NH3) decomposition reaction has received increasing attention for the potential use of NH3 as a hydrogen storage medium1,2. NH3 can be readily liquefied at a mild pressure of ~8 bar at room temperature, giving rise to an energy density of 4.25 kWh/L. Ruthenium (Ru) has been known as the most active metal for catalyzing the decomposition of ammonia, but its large-scale application is limited due to the scarcity and high cost of this precious metal3–5. Literature efforts have thus turned to alloys of earth-abundant elements5–9. In particular, bimetallic Co-Mo has been shown to be promising for ammonia decomposition, as rationalized by the computational interpolation of adsorption properties on the alloy surfaces with mixed sites9,10. However, the functional tuning and catalytic activity of Co-Mo catalysts are largely constrained by the large miscibility gap present in the phase diagram of this binary alloy (Fig. 1), and the experimental studies have primarily been limited to the catalysts with elemental ratio of Co/Mo around one11–15. How to overcome this hurdle is of great interest for both fundamental understanding of alloy catalysts and pratical improvement of the performance of earth-abundant materials in energy-conversion systems.
Fig. 1.
HEA catalysts breaking the miscibility limitation of conventional binary alloys. The phase diagram of bimetallic Co-Mo (left) is reprinted from Wang et al.51, with permission from Elsevier
Here we report on a new class of high-entropy alloy (HEA) catalysts made of earth-abundant elements for highly efficient decomposition of ammonia. Enabled by a carbothermal shock technique, we have been able to grow HEA nanoparticles with five metals incorporated into a single solid-solution phase. We demonstrate breaking the miscibility limitation in bimetallic Co-Mo alloys by robustly tuning the Co/Mo element ratio in CoMoFeNiCu HEA nanoparticles (Fig. 1). These HEA nanoparticles are subjected to systematic and comprehensive catalytic studies for the NH3 decomposition and compared to bimetallic Co-Mo and monometallic Ru catalysts. A series of characterization techniques including atomically resolved scanning transmission electron microscopy (STEM), X-ray photoemission spectroscopy (XPS), and surface-specific temperature programmed desorption of nitrogen (nitrogen TPD) is employed to evaluate the structure-property relationship of the HEA catalysts. The obtained knowledge is further integrated with the measured reaction kinetics and simulated atomic structures of the HEA catalysts to understand the catalytic enhancement mechanisms.
Results
Synthesis and characterization of HEA nanoparticles
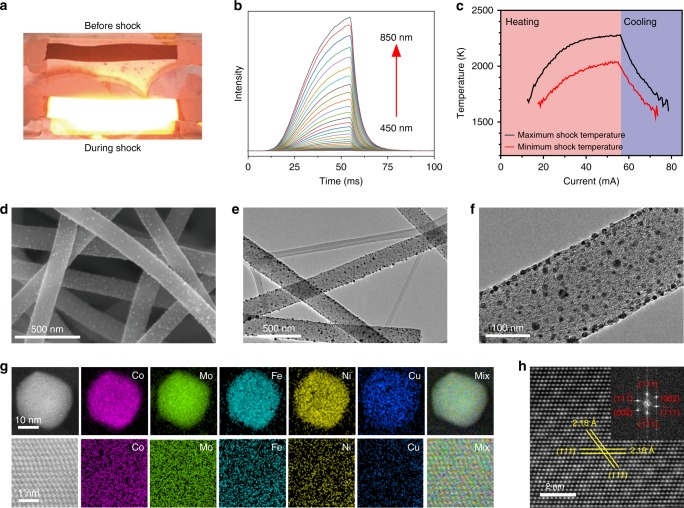
The CoMoFeNiCu HEA nanoparticles were synthesized by employing flash heating and cooling of metal precursors on oxygenated carbon supports, with the temperature going up to 2000–2300 K, the temperature ramping rate on the order of 105 K s−1 and the duration of shocks as short as ~55 ms (Fig. 2a–c)16. Under such conditions, the high temperature induced rapid thermal-decomposition of the precursors, forming small liquid droplets of multimetallic solutions. The subsequent rapid cooling enabled crystallization of these liquid droplets into uniform and homogeneous alloy nanoparticles without being subjected to aggregation/agglomeration, element segregation or phase separation. Five types of HEA nanoparticles with the general composition CoxMoyFe10Ni10Cu10 (x + y = 70) were prepared by using this method, in which the atomic ratio of Co/Mo was controlled to be 15/55, 25/45, 35/35, 45/25, and 55/15 by varying the loading of precursors, the overall loading of metals in each catalyst was controlled at 10 wt% during synthesis. These nanoparticles are denoted as HEA-CoxMoy (e.g., HEA-Co15Mo55 for Co15Mo55Fe10Ni10Cu10) in the following discussion. Bimetallic Co-Mo and monometallic Ru nanoparticles were also synthesized using the same method with similar metal loadings, which served as control in this study.
Fig. 2.
High-entropy alloy CoMoFeCoNi (HEA-CoxMoy) nanoparticles. a Digital images of the samples before and during the thermal shock synthesis. b Intensity of the emitted light from the shock at different wavelengths. c Typical temperature profiles during the thermal shocks as determined from the emission intensities. d Representative SEM and e, f TEM images of the obtained HEA nanoparticles well dispersed on carbon nanofibers (CNFs). g STEM-based elemental maps of the HEA-Co25Mo45 nanoparticles at low- (upper panel) and high- (lower panel) resolutions. h High-resolution HAADF-STEM image with the fast Fourier transform (FFT) pattern indicating an fcc crystal structure
Figure 2d shows representative scanning electron microscopy (SEM) images of the C25Mo45Fe10Ni10Cu10 nanoparticles uniformly dispersed on the carbon nanofibers (see more images in Supplementary Fig. 1). The spacing between neighboring nanoparticles varies from ~10 to ~100 nm. The average particle size is measured to be ~22 nm from the transmission electron microscopy (TEM) images (Fig. 2e, f; also see more images in Supplementary Fig. 2). Low- and high-magnification elemental maps depict homogeneous distribution of all the five elements throughout the C25Mo45Fe10Ni10Cu10 nanoparticles (Fig. 2g and Supplementary Fig. 3), and similar observations were also obtained on the HEA nanoparticles of different compositions (Supplementary Figs. 4–7). In contrast, the bimetallic Co36Mo64 nanoparticles (corresponding to Co/Mo = 25/45) show separated Co- and Mo-rich phases (Supplementary Fig. 8), as this composition falls into the miscibility gap in the corresponding binary phase diagram (Fig. 1). Atomically resolved high-angle annular dark-field scanning TEM (HAADF-STEM) imaging and the corresponding fast Fourier transform analysis reveal a face-centered cubic (fcc) phase for the HEA nanoparticles, with the inter-plane spacing measured to be 2.18 Å for the (111) lattice fringes (Fig. 2h).
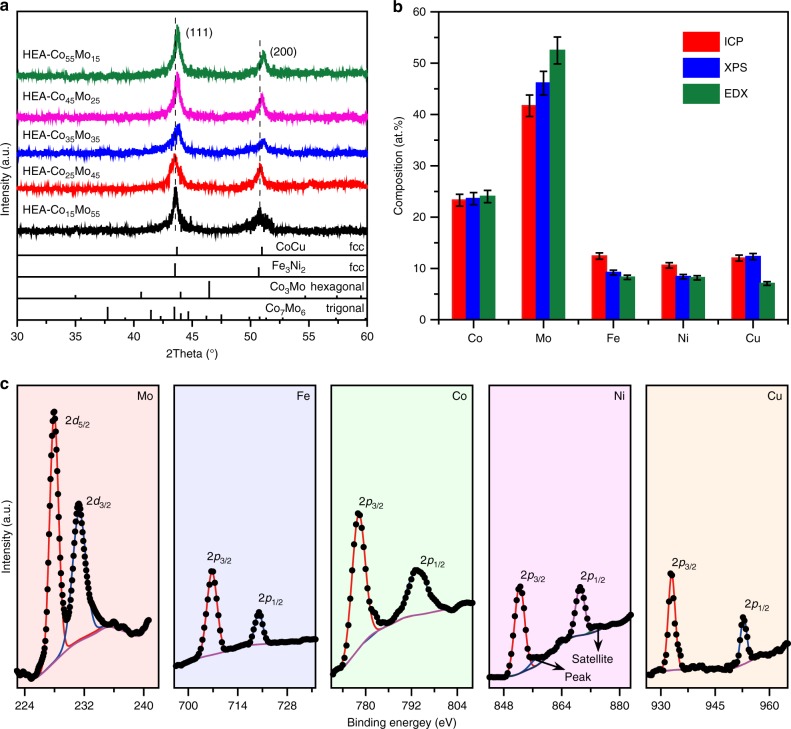
Crystal structure of the HEA nanoparticles was further confirmed by X-ray diffraction (XRD) analysis. In the XRD patterns (Fig. 3a), the HEA nanoparticles typically exhibit two peaks at around 44o and 51o, which can be assigned to the (111) and (200) planes of a fcc crystal, A small upshift of the peak position is discernible as the Co/Mo ratio increases, but merely by ~0.5o for the (111) peak from C15Mo55Fe10Ni10Cu10 to C55Mo15Fe10Ni10Cu10, indicating rather small differences in lattice strain among the HEA nanoparticles of various compositions. It is noticed that only two crystal phases are known for bimetallic Co-Mo alloys, i.e., Co3Mo with a hexagonal structure (P63/mmc) and Co7Mo6 with a trigonal structure (R-3m). The XRD patterns recorded for the HEA-CoxMoy nanoparticles do not match either one of these two phases (Fig. 3a), but are more in line with those of the bimetallic alloys in fcc phases albeit with shifted peak positions (Fig. 3a and Supplementary Fig. 8). This observation is consistent with the features of high-entropy alloys and confirms that the derived nanoparticles are in a single fcc solid-solution phase17–19. The rather low peak intensities can be owing to the much smaller particle sizes than those previously reported HEAs20.
Fig. 3.
Characterization of the HEA-CoxMoy nanoparticles. a XRD patterns of the HEA nanoparticles supported on CNFs in comparison to the bimetallic standards of CoCu (PDF# 50–1452), Fe3Ni2 alloy (PDF# 65–5131), Co3Mo (JCDPS no. 29–0488), and Co7Mo6 (JCDPS no. 29–0489). b Comparison of the composition analyses for the HEA-Co25Mo45 nanoparticles based on three different techniques: ICP-MS, XPS, and EDX, verifying the alloy homogeneity in the HEA nanoparticles. Error bars denote SDs. c XPS spectra collected at the Mo 3d, Fe 2p, Co 2p, Ni 2p, and Cu 2p edges for the HEA-Co25Mo45 nanoparticles
A combination of inductively coupled plasma-mass spectrometer (ICP-MS), X-ray photoemission spectroscopy (XPS) and energy dispersive X-ray spectroscopy (EDX) was employed to analyze the element compositions of the HEA nanoparticles. Among these three techniques, ICP (Table 1) and EDX provide composition information for the bulk of the nanoparticles, and average over the whole catalyst, whereas XPS is more sensitive to the surface region (~1 nm in depth estimated from the beam energy of Al Kα). The three methods generated quite consistent results for the alloy compositions (Fig. 3b), which is consistent with the homogeneous alloy nature as revealed by the STEM-based element mapping and excludes the occurrence of surface segregation or phase separation. The XPS analysis also shows that all the constituting elements of the HEA nanoparticles are in metallic states. Figure 3c presents representative XPS spectra recorded at the 3d edge of Mo and 2p edges of Co, Fe, Ni, and Cu (see more spectra at the other edges in Supplementary Fig. 9). For example, the two peaks associated with the Mo 3d5/2 and 3d3/2 doublet are located at 228.0 and 231.2 eV, respectively, while the Co 2p3/2 and 2p1/2 peaks are at 778.2 and 793.3 eV, respectively. Both of these two sets of binding energies, as well as those for Fe, Ni, and Cu, are in line with the established values for pure metals21. Charge transfer between the metals, or ligand effect22,23, thus may not be significant in the HEA-CoxMoy nanoparticles.
Table 1.
List of HEA-CoxMoy catalysts employed in this study
| Samples | Co | Mo | Fe | Ni | Cu | Metal loadinga | SBETb |
|---|---|---|---|---|---|---|---|
| % | % | % | % | % | wt.% | m2/g | |
| HEA-Co15Mo55 | 14.3 | 51.8 | 12.6 | 11.2 | 10.1 | 8.2 | 150 |
| HEA-Co25Mo45 | 23.3 | 41.7 | 12.4 | 10.6 | 12.0 | 7.8 | 157 |
| HEA-Co35Mo35 | 33.5 | 32.3 | 12.0 | 10.8 | 11.4 | 8.3 | 160 |
| HEA-Co45Mo25 | 42.9 | 23.5 | 11.7 | 11.5 | 10.4 | 8.8 | 148 |
| HEA-Co55Mo15 | 52.8 | 13.2 | 11.9 | 10.8 | 11.3 | 9.3 | 153 |
a The overall weight percentages of metals in each catalyst as determined by ICP-MS analyses
b Estimated from the N2 adsorption isotherms according to the BET theory
Catalytic studies for ammonia decomposition
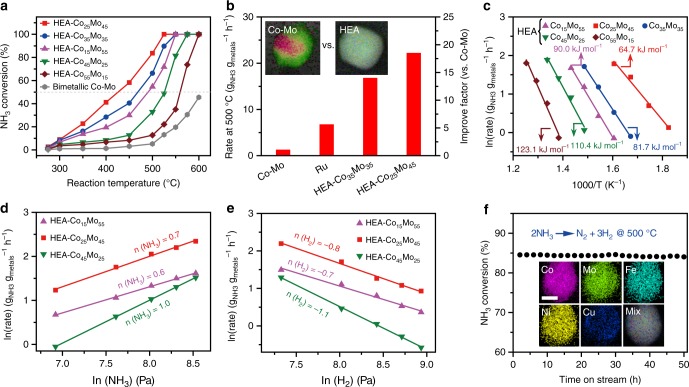
The HEA-CoxMoy nanoparticles supported on carbon nanofibers were directly applied as catalysts for the ammonia decomposition reaction and compared to the bimetallic Co-Mo with the ratio of 25/45 (~20 nm in particle size, Supplementary Fig. 10, see the elemental maps of bimetallic Co-Mo with other ratios in Supplementary Fig. 11) and monometallic Ru (~2–3 nm, Supplementary Fig. 12) catalysts with similar metal loadings and same substrates. The catalytic activity was systematically measured at 250–600 oC using a plug flow reactor and 5 vol% NH3 as the feeding gas. The bare CNF substrate was also measured and confirmed to be inactive for NH3 decomposition (Supplementary Fig. 13). Figure 4a summarizes the measured NH3 conversion as a function of temperature at a gas hourly space velocity (GHSV) of 36 L gcat−1 h−1. For all the HEA-CoxMoy catalysts, the reaction has an onset temperature of ~300 oC, and the NH3 conversion increases with the reaction temperature. Between 300 and 500 oC, the NH3 conversion follows the order HEA-Co25Mo45 > HEA-Co35Mo35 > HEA-Co15Mo55 > HEA-Co45Mo25 > HEA-Co55Mo15, reaching 50% of conversion (T50) at ca. 422, 466, 488, 523, and 558 oC, respectively. The reaction reaches saturation (100% conversion) at ~525 oC on HEA-Co25Mo45, as compared to ~600 oC for HEA-Co55Mo15. Most of the HEA catalysts are much more active than the bimetallic Co-Mo and monometallic Ru, with the latter two giving NH3 conversions of only 46 and 73% at 600 oC (Supplementary Fig. 14). Figure 4b compares the reaction rate measured at 500 oC among the two most active HEA catalysts, bimetallic Co-Mo and monometallic Ru. The HEA-Co35Mo35 and -Co25Mo45 catalysts reach a mass-specific rate of 16.7 and 22.1 gNH3 gmetals−1 h−1 at 500 oC, representing improvement factors of ~14 and ~19 versus Co-Mo, respectively. Moreover, higher mass-specific activities were obtained with the HEA catalysts of reduced particle sizes (Supplementary Fig. 15). Compared to the precious metal Ru, the Co25Mo45Fe10Ni10Cu10 catalyst achieves an improvement factor of ~3 versus Ru. To take the different particle sizes into account, HEA-Co25Mo45 achieved an area-specific reaction rate of 0.74 gNH3 m−2 h−1, representing improvement factors of ~24 versus bimetallic Co-Mo and ~19 versus Ru (Supplementary Fig. 16). The superior performance of the HEA catalysts for NH3 decomposition is further revealed by a comprehensive comparison to the literature results with similar reaction conditions (Table 2).
Fig. 4.
Performance of HEA-CoxMoy catalysts for NH3 (5 vol%) decomposition. a NH3 conversions over different HEA-CoxMoy nanoparticles and bimetallic Co-Mo (Co/Mo = 25/45) depending on the reaction temperature (Space velocity = 36 L gcata−1 h−1). b Comparison of reaction rates measured in the kinetic regime among bimetallic Co-Mo, Ru, and HEA-CoxMoy catalysts (T = 500 oC). Inset: element maps for the bimetallic Co-Mo (Co/Mo = 25/45) and HEA-Co25Mo45 catalysts. c Arrhenius plots for NH3 decomposition on the HEA-CoxMoy catalysts, showing different apparent activation energies (Eapp). d, e Reaction orders of NH3 and H2 determined for the NH3 decomposition reaction at 425 oC on the HEA-CoxMoy catalysts. f Stability test performed at 500 oC for the HEA-Co25Mo45 catalyst. Inset: element map of the catalyst after the stability test; scale bar = 10 nm
Table 2.
Comparison of the catalytic performance for NH3 decomposition of different catalysts
| Catalysts | Metals | NH3 | T | GHSV | Conversion | TOFa | Ref |
|---|---|---|---|---|---|---|---|
| (wt%) | (vol%) | (°C) | (mL gcat−1 h−1) | (%) | (h−1) | ||
| Ru/SiO2 | 10 | 100 | 500 | 30,000 | 64 | 2283 | 2 |
| Ru/Al2O3 | 10 | 100 | 500 | 30,000 | 58 | 2069 | 26 |
| Ru/CNTs | 4.8 | 100 | 500 | 30,000 | 84 | 6241 | 5 |
| Ru/TiO2 | 4.8 | 100 | 500 | 30,000 | 64 | 11,096 | 27 |
| Ru/MgO | 5 | 100 | 500 | 30,000 | 41 | 8189 | 24 |
| Co(en)3MoO4 | 38.8 | 100 | 500 | 36,000 | 12 | 294 | 14 |
| Co3Mo3N | 97 | 50 | 500 | 22,500 | 33 | 409 | 12 |
| CoMo/SiO2 | 5 | 100 | 500 | 36,000 | 14 | 1108 | 47 |
| FeMo/La-Al2O3 | 10 | 100 | 500 | 46,000 | 8 | 1213 | 48 |
| Ni2Mo3N | 97 | 100 | 500 | 21,600 | 29 | 204 | 49 |
| Li2NH-Fe2N | 81.2 | 100 | 500 | 60,000 | 38.1 | 1179 | 50 |
| Li2NH-Mn2N | 81 | 100 | 500 | 60,000 | 59.1 | 2735 | 50 |
| HEA-Co25Mo45 | 7.8 | 5 | 500 | 36,000 | 84 | 1571 | This work |
| HEA-Co35Mo35 | 8.3 | 5 | 500 | 36,000 | 67 | 1128 | This work |
| HEA-Co45Mo25 | 8.8 | 100 | 500 | 36,000 | 64.5 | 19,633 | This work |
| HEA-Co55Mo15 | 9.3 | 100 | 500 | 36,000 | 100 | 25,209 | This work |
a Obtained by normalizing the reported reaction rates with the areal densities of surface atoms estimated from the particle sizes
Kinetic studies were also performed on the HEA catalysts with varying reaction temperature and gas composition. Figure 4c presents the Arrhenius plots of the reaction rate in dependence of temperature. The determined apparent activation energy (Eapp) varies with the Co/Mo ratio, with the trend being consistent with that for the NH3 conversion, namely from the lowest 64.7 kJ mol−1 for the most active HEA-Co25Mo45 to 123.1 kJ mol−1 for the least active HEA-Co55Mo15 catalyst. The activation energy measured here for HEA-Co25Mo45 is comparable to those previously reported for Ru-based catalysts (57.8–83.7 kJ mol−1)24–27. The determined reaction order of NH3 at 425 oC increases with the Co/Mo ratio, varying from 0.6 for HEA-Co15Mo55 to 1.0 for HEA-Co45Mo25 (Fig. 4d). This indicates that the activation of NH3 is more difficult on the Co-rich catalysts. On the contrary, the H2 order (at 425 oC) decreases from −0.7 for HEA-Co15Mo55 to −1.1 for HEA-Co45Mo25, suggesting a less inhibition effect of H2 on the Mo-rich catalysts (Fig. 4e)28. The partial pressure of N2 was found to have little effect on the reaction rate (Supplementary Fig. 17), which is consistent with the previous conclusion drawn from Ru-based catalysts29.
In addition to the enhanced catalytic activities, the HEA-CoxMoy catalysts were further demonstrated to be highly stable under the reaction conditions for NH3 decomposition. Figure 4f presents the NH3 conversion recorded on Co25Mo45Fe10Ni10Cu10 over the course of continuous operation at 500 oC. The degradation in catalytic activity was negligible after ~50 h. The catalyst collected after this prolonged durability test was characterized by element mapping (see the insert in Fig. 4f) and XPS (Supplementary Fig. 18), with nearly no change found in alloy homogeneity or surface composition. A small amount (~1.4 at.%) of N was detected on the used catalyst (Supplementary Fig. 19), but much less than what (e.g., >10% for Mo3Co3N) one would expect for the formation of nitrides throughout the nanoparticles. It is likely that the bulk of the HEA nanoparticles were not subjected to nitridation during the NH3 decomposition reaction. The durable performance of the HEA catalysts is in line with the reported high thermal30 and chemical31,32 stabilities of HEAs.
Adsorption property and catalytic descriptor
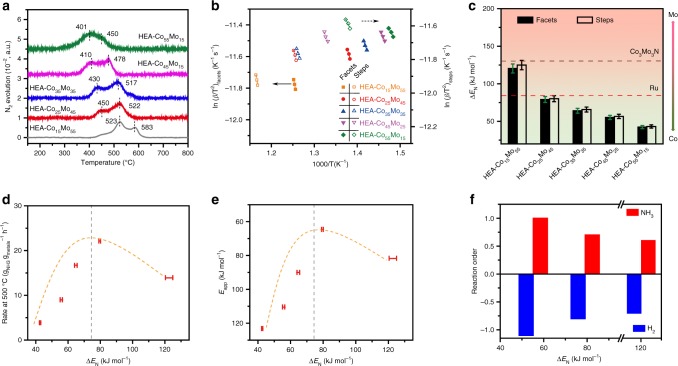
The reaction mechanism of ammonia decomposition has been intensively investigated in both experimental and computational studies1,6,8,25. It is suggested that the recombinative desorption of nitrogen is the rate-determining step on Fe, Co, and Ni, while the kinetics is limited by scission of the first N-H bond on Rh, Ir, Pd, Pt, and Cu catalysts4, although some debates are still present in the literature33,34. Nevertheless, it is generally accepted that the binding strength of nitrogen is a good descriptor for ammonia decomposition catalysts, which largely determines both the stability of adsorbing intermediates (e.g., *N and *NHx) and activation energy of the rate-determining step6,10,12. In order to interpret the observed kinetic performance, we have performed temperature programmed desorption (TPD) of pre-adsorbed atomic nitrogen (2*N → N2) to evaluate the adsorption properties of the HEA catalysts35–38.
Figure 5a shows the nitrogen TPD patterns recorded at a ramping rate of 10 oC min−1. Two distinct desorption peaks are consistently observed on all the HEA-CoxMoy catalysts in the temperature range of 400–600 oC. These features resemble the previously reported results for Ru35,36 and Fe37,38 surfaces, and can be assigned to the recombinative desorption of nitrogen on ordered facets (such as (111) and (100) for the first peak) and undercoordinated sites (steps, edges, defects, etc. for the second peak), with the latter having stronger binding to nitrogen and thereby higher desorption temperatures due to the lower coordination numbers. A clear trend is established for the HEA catalysts of different compositions, with the desorption temperatures (both onset and peak positions) rising at decreasing Co/Mo ratios, suggesting the continuous tuning of nitrogen binding strength by varying the HEA composition. According to the TPD theorem39,40, we have conducted additional measurements at different ramping rates (β = 20 and 30 oC min−1) to establish the plots of ln(β/Tm2) versus 1/Tm (Tm is the peak position), in which the slopes are used to estimate the recombinative desorption energy of nitrogen (ΔEN) (Fig. 5b and Supplementary Fig. 20). Figure 5c summarizes the derived values of ΔEN for the different HEA-CoxMoy catalysts. As expected with the observations from the TPD patterns, the catalysts with higher Co/Mo ratios have lower ΔEN, ranging from ~121–125 kJ mol−1 for HEA-Co15Mo55 to ~42–44 kJ mol−1 for HEA-Co55Mo15. It is noticed that the most active HEA-Co25Mo45 catalyst has a ΔEN value of ~79 kJ/mol, which is very close to the ΔEN (84 kJ mol−1)24 of Ru, explaining the high and comparable catalytic activities of the HEA-CoxMoy to Ru-based catalysts. HEA-Co35Mo35 has the same Co/Mo ratio as Co3Mo3N but binds to nitrogen much less strongly (Fig. 5c). This difference (~65 kJ mol−1) can be ascribed to the presence of other weakly binding metals (Fe, Ni, and Cu) in and on the surface of the HEA catalysts.
Fig. 5.
Surface adsorption properties of HEA-CoxMoy. a Nitrogen TPD profiles recorded with a temperature ramping rate of 10 oC min−1. b, c Estimation of the nitrogen adsorption energies (ΔEN) for the HEA-CoxMoy catalysts by using the plots of Ln(β/T2) ~ 1/T for the nitrogen TPD profiles (β = 10, 20, and 30 oC min−1 represents the ramping rates used for the TPD measurements). d, e Correlations between ΔEN and catalytic activities (d) and Eapp (e) showing a volcano-type behavior for the HEA-CoxMoy catalysts, with HEA-Co25Mo45 being close to the peak position. f Plots of the NH3 and H2 reaction orders (at 425 oC) versus the nitrogen adsorption energies. Error bars denote SDs
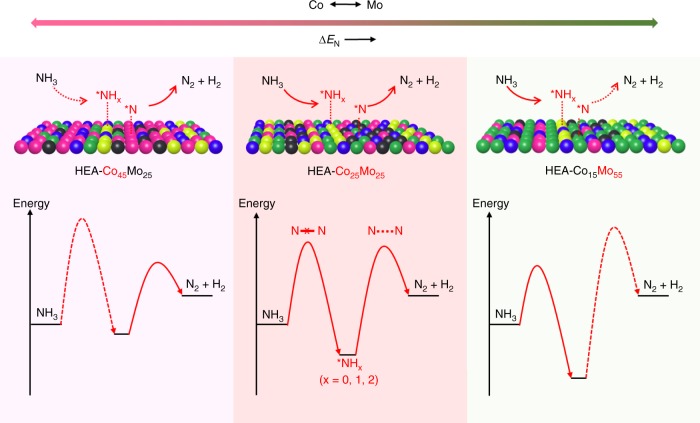
With the estimated nitrogen adsorption energies, we are able to interpret the correlation between composition and catalytic performance of the HEA-CoxMoy catalysts. Figure 5d, e present the plots of NH3 decomposition rate (at 500 oC) and activation energy versus ΔEN. Both plots exhibit a volcano-type behavior with HEA-Co25Mo45 being close to the peak position. According to the Sabatier principle, we can infer that, on the left side of this peak, the catalysts (more Co-rich) bind to N too weakly and thus gives rise to rather higher kinetic barriers for dehydrogenation (NH3 → *NH2 → *NH → *N), whereas on the right side (more Mo-rich) the binding is too strong for N to recombine and desorb from the surface (2*N → N2), and a tradeoff between these two factors gives rise to an optimal, intermediate binding energy for the reaction (Fig. 6). The finding here can be further correlated to the dependence of reaction orders on ΔEN. As shown in Fig. 5f, the catalysts with weaker N binding energy has higher NH3 order, suggesting that the kinetics of NH3 decomposition is more limited by NH3 activation, or probably dehydrogenation of the first N-H bond, on the HEA catalysts of higher Co contents. Since the binding strengths of nitrogenous species (*NHx, x = 0, 1, 2, and 3) likely scale linearly with each other41–43, we can expect that the *NHx species bind less strongly on the more Co-rich HEA catalysts, which is also in line with the stronger hydrogen inhibition effect found for these catalysts (Fig. 5f).
Fig. 6.
Schematic illustration of the rate-limiting factors in NH3 decomposition. The rate-limiting factors are labeled with dash lines in the lower panel. On Co-rich surface (left), the rate is limited by activation or dehydrogenation of NH3; on Mo-rich surface, the rate is limited by the recombinative desorption of *N; Balance for these two steps is reached on an intermediate composition (HEA-Co25Mo45)
From the above discussion, we can attribute the high catalytic activity of NH3 decomposition achieved with the HEA catalysts to the robust tuning of surface adsorption properties, as enabled by the continuous varying of alloy composition (Co/Mo ratio). Noticeably, the optimal value of ΔEN ~ 79 kJ mol−1 identified here is consistent with the value (~74 kJ mol−1) predicted from density functional theory calculations for the given reaction condition, i.e., 5 vol% of NH312. We also notice that, for the optimal design of practical reactors, it is desirable to have cascaded catalyst layers with gradually changing nitrogen binding strengths, so that the surface property can be optimized to match the varying concentration of NH3 along the flow44. We show here that the HEA catalysts can be readily tailored to achieve such a design by simply varying the Co/Mo ratios. For example, HEA-Co55Mo15 have been identified to be the best-performing catalyst for the decomposition of pure NH3, which gives rise to catalytic activity improvement factors of ~118 versus bimetallic Co-Mo and ~40 versus Ru (Fig. 7 and Supplementary Fig. 21). The even more substantial catalytic enhancements found here (than the results presented above for 5 vol% of NH3) can be well understood in such a way: the ΔEN value of HEA-Co55Mo15 (~42 kJ mol−1, according to Fig. 5c) is very close to the optimal (~39 kJ mol−1) predicted for the reaction at 100 vol% of NH312, whereas the values of previous Co3Mo3N and Ru catalysts are quite away from this optimal point.
Fig. 7.
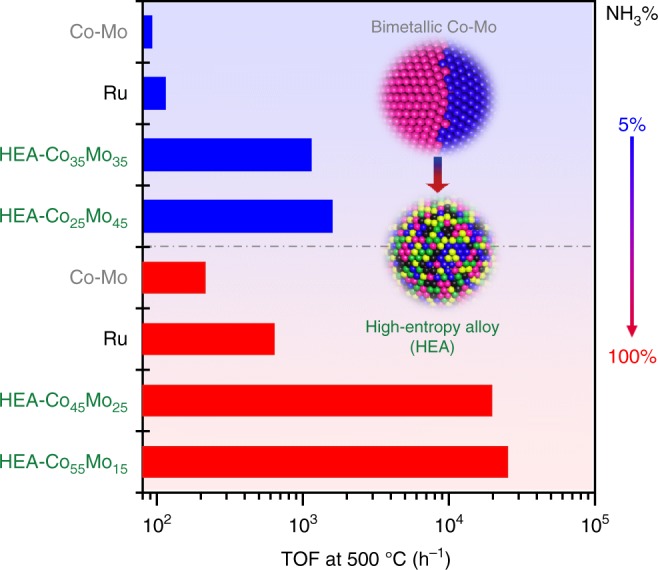
NH3 decomposition at different concentrations. The composition of HEA-CoxMoy catalysts were robustly tuned to optimize the surface properties for different reaction conditions (e.g., 5 vs. 100 vol% NH3)
Atomistic modeling of HEA nanoparticles
The correlations among composition, adsorption property and catalytic performance discussed above point to a scaling relationship between ΔEN and the elemental ratio of Co/Mo (Fig. 5c), which governs the catalytic activity of the HEA catalysts. This relationship can be ascribed to the presence of well mixed Co-Mo sites on the surface with the ratio being consistent with the bulk compositions (Fig. 3b). We have used atomistic models to elucidate this surface site mixing mechanism in the HEA catalysts.
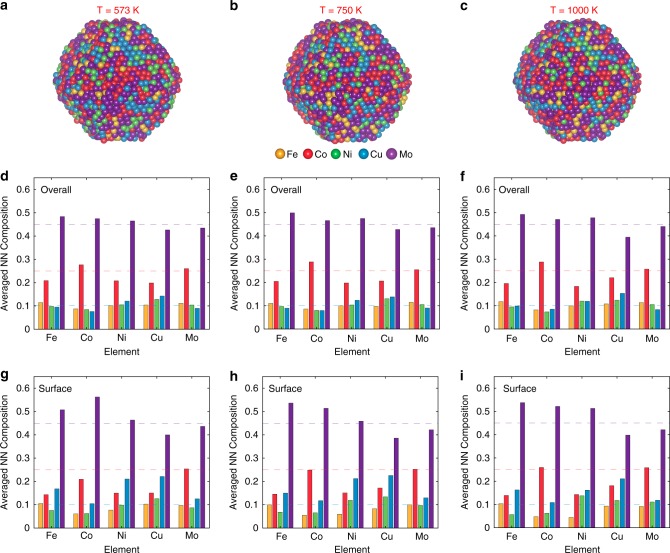
HEA-Co25Mo45 (Co25M45Fe10Ni10Cu10) was chosen as the example and modeled in a cuboctahedral shape with FCC lattice. From the survey of those bulk multi-component alloys to form solid-solution HEAs, it has been found that the formation of HEA most often requires: the parameter gauging the atomic size difference δ ≤ 6.6% and the enthalpy of formation 45,46. In this work, we have calculated that HEA-Co25Mo45 has , , and . Hence, we predict that HEA-Co25Mo45 is likely to form solid-solution HEAs under the ammonia decomposition reaction conditions. The five elements are initially randomly assigned to each lattice site according to the nominal composition. To investigate the phase stability of the HEA nanoparticles, Monte Carlo (MC) simulations are performed at three different temperatures (i.e., 573, 750, and 1000 K). The atomic structures after 10 million MC steps are shown in Fig. 8a–c. All the resulting structures show random distribution of the five elements, and no apparent chemical ordering such as the segregation of single elements or formation of intermetallic phases was observed, confirming the formation of HEA under the given conditions. This finding is further corroborated by statistical analysis of the averaged composition of the nearest-neighbor lattice sites (with a cutoff radius of 3.0 Å) for a given element (Fig. 8d–i). For an ideal solid solution, this averaged composition should be equal to the nominal composition of the HEA nanoparticle, which are represented by the dash lines in Fig. 8d–i. Our results show that the nearest-neighbor (NN) compositions averaged over all the atoms only deviate slightly from the nominal composition throughout the three temperatures investigated, implying the absence of long-range chemical ordering. On the other hand, some degree of short-range ordering is predicted in the modeled nanoparticle: Fe, Co, and Ni atoms exhibit a relatively higher affinity around Mo atom, whereas there is a moderate repulsive interaction between Cu-Mo, Fe-Co, Co-Ni, and Co-Cu atom pairs. This short-range ordering is even prominent for surface atoms, as shown in Fig. 8g–i. Overall, our atomistic modeling reveals that the HEA-CoxMoy nanoparticles have a single solid solution phase with only slight short-range chemical ordering throughout the investigated temperature range. It also confirms the presence of well mixed surface sites on the HEA catalysts and suggests that the experimentally observed scaling relationship between the nanoparticle composition and the surface adsorption property can be understood via the surface site mixing mechanism9,10. It should be noted that our atomistic modeling results are consistent with the prediction based on simple thermodynamic calculations, confirming the tendency of the given quinary composition to form a single solid-solution phase. More importantly, the structural similarity at the different temperatures (from 573 to 1000 K) underlines the high thermal stability of the HEA nanoparticles, consistent with the observation from the catalytic durability studies (see Table 2)47–51.
Fig. 8.
Atomic structure of HEA catalysts. Atomistic model of HEA-Co25Mo45 nanoparticle predicted by using MC simulations at different temperatures: a 573 K; b 750 K; and c 1000 K. The composition of the first-nearest neighbor lattice sites around a specific element type averaged over (d–f) all atoms and (g–i) surface atoms in the corresponding nanoparticle. The dashed lines represent the nominal composition of the nanoparticle (Co0.25Mo0.45Fe0.1Ni0.1Cu0.1)
Discussion
We have developed advanced ammonia decomposition catalysts based on high-entropy alloys made of earth-abundant elements. The HEA CoMoFeNiCu nanoparticles enabled robust tuning of the Co/Mo ratio and breaking the miscibility limitation in conventional bimetallic Co-Mo catalysts. These novel catalysts achieved significantly enhanced catalytic activities and stabilities, with the improvement factors exceeding 20 times as compared to precious metal Ru, and even more so versus conventional Co-Mo catalysts. Their catalytic activities and kinetics were further found to exhibit a volcano-type behavior independent of the Co/Mo ratio, which was successfully interpreted by using the nitrogen adsorption energy as the descriptor and a mixed surface site mechanism derived from atomistic modeling. The robust tunability of alloy composition and surface adsorption property demonstrated on the HEA catalysts indicate great potential for their implementation in practical reactors with the catalytic performance optimized under various reaction conditions.
Methods
Synthesis
8 wt% polyacrylonitrile (PAN, Sigma Aldrich) in dimethylformamide (DMF, Sigma Aldrich) was used to make a polymer nanofiber network via electrospinning (voltage: 15 kV, distance: 10 cm, feed rate: 0.065 ml min−1, collected on a rotating drum at 40 rpm). The derived nanofibers were stabilized in air at 533 K for 6 h and then carbonized at 1173 K for 2 hours in argon using a tube furnace. The CNF films can be further thermally activated at 1023 K for 2 h in CO2 atmosphere to create surface defects for effective particle dispersion.
Typically, the individual metal salts (chloride salts, FeCl3, CoCl2, NiCl2, CuCl2, and MoCl3 from Sigma Aldrich) were dissolved in ethanol with five designed ratios of CoxMoyFe10Ni10Cu10 (x + y = 70, and the atomic ratio of Co/Mo was controlled to be 15/55, 25/45, 35/35, 45/25, and 55/15). The CNF films were suspended over the trench of two glass slides (2 cm gap) and then connected to two copper electrodes by silver paste for subsequent precursor solution loading. Then the salt precursor solution was dipped onto the CNF film with a precursor loading of 5 μmol cm−2. The samples were left to dry at room temperature.
The carbothermal shock process was performed through Joule heating of the precursor-loaded CNF films in an argon-filled glovebox. A Keithley SourceMeter (2425) was used as the electrical power source and the sweep function from Keithley was used to provide the thermal shock, in which the current (temperature), duration (thermal shock time), and cooling speed (ramp rate) of the electrical pulse can be adjusted. We chose a 55-ms electrical pulse to synthesize the high-entropy alloy nanoparticles.
We used a time-resolved pyrometer to measure the emitted light intensity from the sample and estimate the temperature during the thermal shock process. The emitted light was dispersed at a resolution of 6.5 nm mm−1 and then collected by a 32-channel photomultiplier tube array. The full spectrum can be integrated from the 32-channels and then fitted to the blackbody radiation equation to estimate the temperature.
The control sample, bimetallic Co-Mo supported on CNFs (Co/Mo = 15/55, 25/45, 35/35, 45/25, and 55/15), were prepared by the same method as HEA nanoparticles did. Typically, CoCl2 and MoCl3 were dissolved in ethanol with the atomic ratio of Co/Mo was 25/45 and total loading of 10 wt%. Then the salt precursor solution was dipped onto the CNF film with a precursor loading of 5 μmol cm−2. The samples were left to dry at room temperature, and then underwent the carbothermal shock process described as above.
Another control sample, Ru/CNF was prepared by incipient wetness impregnation. Basically, 50 mg carbon nanofibers were dispersed in ethanol, and sonicated for 1 h. Certain amount of RuCl3·xH2O was dissolved in ethanol (Nominal Ru loading is 10 wt%). Then these solutions were mixed together and sonicated for another 30 min. After that, the solvent was removed by using a rotary evaporator. The obtained solid was dried in vacuum and then calcined at 700 oC in argon for 2 h.
Characterization
X-ray diffraction (XRD) patterns were collected on a PANalytical X’Pert3 X-ray diffractometer equipped with a Cu Kα radiation source (λ = 1.5406 Å). Nitrogen adsorption measurements were performed using a Micromeritics ASAP 2010 system with the samples degassed under vacuum at 300 °C for 5 h. Specific surface area (SSA) was calculated using the Brunauer-Emmett-Teller (BET) theory. Inductively coupled plasma-mass spectroscopy (ICP-MS) analysis was carried out using a PerkinElmer Elan DRC II Quadrupole system, for which the solutions were prepared by digesting the catalysts in aqua regia followed by dilution with 2% hydrochloric acid (HCl).
The microstructure and morphology of the HEA catalysts were characterized by using scanning electron microscopy (SEM, Hitachi SU-70 FEG-SEM at 10 kV) and transmission electron microscopy (TEM, JEOL 2100 F FEG TEM/STEM operated at 200 kV) imaging. High-angle annular dark-field (HAADF) STEM images and STEM-EDS mapping were acquired using a JEOL TEM/STEM ARM 200CF (equipped with an Oxford X-max 100TLE windowless X-ray detector) at a 22-mrad probe convergence angle and a 90-mrad inner-detector angle.
X-ray photoelectron spectroscopy (XPS) analysis was performed on a Thermo Fisher Scientific Escalab 250Xi spectrometer with Al Kα radiation as the excitation source. Before measurements, the CoxMoy-HEA catalysts were reduced in H2 at 600 oC for 2 h. The adventitious carbonaceous C 1 s line (284.6 eV) was used to calibrate the binding energy (BE). The XPS spectra were deconvoluted using Gaussian-Lorentzian functions after the Shirley background subtraction, with the integrated peak areas being used to estimate the surface chemical compositions.
The nitrogen TPD experiments were carried out on a Micromeritics AutoChem II chemisorption analyzer equipped with an Omnistar MS detector. Typically, 30 mg of CoxMoy-HEA catalysts were loaded in a U-type sample tube, reduced in H2 (30 mL min−1) at 600 oC for 2 h, and then blown with He (30 mL min−1) for 1 h to remove the hydrogen adsorbed on the catalyst surface. After this, the flowing gas was switched to N2 (30 mL min−1) and kept at 600 oC for 90 min, then cooled down to 100 oC under N2 atmosphere. At 100 oC, N2 was switched to He for removing the residual nitrogen on the catalyst surface. Finally, TPD measurement was conducted under He atmosphere at a ramping rate of 10 oC min−1 and up to 800 oC. Over this process, mass spectrum signals of He (4 amu) and N2 (28 amu) were monitored. For measuring combinative N2 desorption energy, three different ramping rates (10, 20, 30 oC min−1) were used in a similar process.
Catalytic studies
Catalytic decomposition of NH3 was conducted in a fixed-bed flow reactor at atmospheric pressure. Typically, 25 mg of catalyst was loaded into a quartz tube reactor (7 mm i.d.). The catalyst was heated to 600 oC at a rate of 5 oC min−1 under H2 (50 mL min−1) for 2 h and then purged by Helium (50 mL min−1) for 1 h. After this, the catalyst was cooled down to 275 oC under He atmosphere. At 275 oC, the gas flow was switched to 5 or 100 vol% NH3 (balanced by He). The gas hourly space velocity (GHSV) was adjusted to 36 L gcat−1h−1 by controlling the flow rate. The reaction was then carried out at various temperatures, which was increased stepwise from 275 to 600 oC, and steady state was allowed to reach before the product analysis. To determine the conversions of reactant, a FTIR spectrometer (Nicolet 6700, Thermo Scientific) equipped with a long path (5 m) gas cell and a MCT detector (with a resolution of 8 cm−1) was used to analyze NH3 (964 cm−1, 929 cm−1). The NH3 conversion were calculated using , where [NH3]inlet and [NH3]outlet refer to the measured concentrations of NH3 fed into and flowing out of the reactor. N2 and H2 were detected by using a GC-BID equipped with Poropak Q packed column with Helium as the carrier gas.
The measurements of reaction rates and kinetics were carried out at reduced catalyst loadings and the GHSV was adjusted to 100 L gcat−1 h−1, ensuring that the reaction condition was within the kinetic zone (<15% conversion) and mass transfer was not limited. The reaction orders were measured at 425 oC. The TOFs (turnover frequencies) were calculated based on the surface metal atoms. The surface metal atoms of bimetallic Co-Mo and HEA-CoxMoy catalysts were estimated by assuming the nanoparticles as perfect FCC cubo-octahedral particles. From the TEM picture of our particle, we measured the lattice parameter (a) of the FCC lattice to be 3.776 Å. According to the literature52, the number of total atoms (NT) and the number of surface atoms (NS) in a perfect fcc cuboctahedral particle vary, respectively, as a function of and with variable m which is the number of atoms lying on each equivalent edge of the particle. Thus, the diameter of a sphere with a volume equal to NT atoms can be expressed as . Consequently, we estimated that a 21.8 nm particle would contain a total of 403,014 atoms (correspondingly, m = 30) in which 25,232 atoms are on the surface, leading to a surface/volume ratio of about 6.26%. The surface metal atoms of Ru catalysts were obtained by referring the literature26.
Modeling and simulation
The thermodynamic properties of HEA-CoxMoy are also checked by the calculations. Taking HEA-Co25Mo45 as the example, we applied the established procedures to calculate the thermodynamic properties of HEA-Co25Mo45 alloy based on available empirical data of the atomic size of constituents, formation enthalpy of binary alloy combinations, configuration entropy of the ideal solutions. Specifically, we evaluated a parameter (δ) gauging the atomic size difference in the alloys as (Ci is the atomic percentage of the ith component, ri is the atomic radius of the ith component which can be obtained in the reference 53, is the average atomic radius), the enthalpy of mixing of the alloy as ( is the regular solution interaction parameter between the ith and jth elements, is the enthalpy of mixing of binary liquid alloys which are obtained in the reference 54, and the entropy of mixing of the n-element regular solution (R is gas constant).
From the survey of those bulk multi-component alloys to form solid-solution HEAs, it has been found that the formation of HEA most often requires: and 45,46. In this work, we have calculated that HEA-Co25Mo45 has , , and . Hence, we predict that HEA-Co25Mo45 should form solid-solution HEA particles. The atomic interaction energy of HEA-CoxMoy system is evaluated within the framework of the second nearest neighbor Modified Embedded Atom Method (MEAM)55,56. The parameters of the MEAM potentials for pure metal Fe, Co, Ni, Cu, and Mo are taken from references57–59. The cross potentials between different metals are developed by fitting the MEAM predictions of the enthalpy of formation and the lattice constants of binary and quaternary alloys against the predictions from the density functional theory (DFT) calculations. All the DFT calculations are carried out using the plane wave basis set and projector augmented wave method and Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional as implemented in the Vienna Ab-initio Simulation Package (VASP)60–62. The energy cutoff is set to 500 eV for the plane wave expansion, and the Brillouin zone is sampled by a Monkhorst-Pack k-point mesh of 11 × 11 × 11 for the alloy systems63. As presented in Table S1, the MEAM predictions reproduce well the DFT calculation results.
A Monte Carlo (MC) simulation method based on the Metropolis algorithm64 is employed to sample the atomic configurations of HEA-CoxMoy nanoparticle in canonical ensemble65,66. Starting from a random atomic configuration, a series of MC trial steps are attempted by performing either of the following two configuration changes: (1) Small displacement of a randomly selects atom along a randomly selected direction, which accounts for the relaxation and local atomic vibration processes. The magnitude of the small displacement is in the range of (0, 0.02Å). (2) Swapping the position of two atoms with different element types, which represents the ling-range diffusion process in the modeled system.
At given temperature T, the probability (p) to retain the trial configuration is calculated according to Boltzmann distribution with , where ∆E is the total energy change of the alloy system, due to the configuration variation, calculated from the MEAM potentials and kB is the Boltzmann constant.
In each MC simulation, 10 million MC iterations are performed to evaluate the atomic distribution of the nanoparticles, in which the probability of vibrational trial steps (operation 1) is set to 99.9% and the rest steps (0.1%) were assigned to the long-range diffusion operation (operation 2). Thus, we are modeling the equilibrium structures of alloy particles under the situation in which long-range diffusion processes are dynamically slow and limited.
Supplementary information
Acknowledgements
P.X. and C.W. thank the support from the Advanced Research Projects Agency—Energy (ARPA-E), Department of Energy (DOE) and the Petroleum Research Fund, American Chemical Society. The work by Y.Y., T.L. and L.H. was not directly supported by any funding. Y.Y., T. L. and L.H. acknowledge the Maryland Nanocenter, its Surface Analysis Center and AIMLab. R.S.-Y. and Z.H.’s efforts were supported by NSF-DMR Award no. 1809439. Z.L and G.W. gratefully acknowledge the computational resources provided by the University of Pittsburgh Center for Research Computing as well as the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number ACI-1053575.
Author contributions
C.W. and P.X. contributed to the idea and experimental design. P.X. and J.Z. conducted the synthesis of control samples, catalytic studies and materials characterizations. Y.Y., T.L. and L.H. synthesized the HEA and Co-Mo bimetallic nanoparticles. Z.H. and R.S-Y. performed the high-resolution STEM imaging and analysis. Z.L. and G. W. contributed to the computational simulations. All authors contributed to the writing of the manuscript.
Data availability
The data that support the figures in this work and other findings corresponding to this study are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pengfei Xie, Yonggang Yao, Zhennan Huang, Zhenyu Liu.
Contributor Information
Guofeng Wang, Email: guw8@pitt.edu.
Reza Shahbazian-Yassar, Email: rsyassar@uic.edu.
Liangbing Hu, Email: binghu@umd.edu.
Chao Wang, Email: chaowang@jhu.edu.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11848-9.
References
- 1.Schuth F, Palkovits R, Schlogl R, Su DS. Ammonia as a possible element in an energy infrastructure: catalysts for ammonia decomposition. Energy Environ. Sci. 2012;5:6278–6289. doi: 10.1039/C2EE02865D. [DOI] [Google Scholar]
- 2.Choudhary TV, Sivadinarayana C, Goodman DW. Catalytic ammonia decomposition: COx-free hydrogen production for fuel cell applications. Catal. Lett. 2001;72:197–201. doi: 10.1023/A:1009023825549. [DOI] [Google Scholar]
- 3.Yin SF, et al. Investigation on the catalysis of COx-free hydrogen generation from ammonia. J. Catal. 2004;224:384–396. doi: 10.1016/j.jcat.2004.03.008. [DOI] [Google Scholar]
- 4.Ganley JC, Thomas FS, Seebauer EG, Masel RI. A priori catalytic activity correlations: the difficult case of hydrogen production from ammonia. Catal. Lett. 2004;96:117–122. doi: 10.1023/B:CATL.0000030108.50691.d4. [DOI] [Google Scholar]
- 5.Yin SF, Xu BQ, Zhou XP, Au CT. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A Gen. 2004;277:1–9. doi: 10.1016/j.apcata.2004.09.020. [DOI] [Google Scholar]
- 6.Hansgen DA, Vlachos DG, Chen JGG. Using first principles to predict bimetallic catalysts for the ammonia decomposition reaction. Nat. Chem. 2010;2:484–489. doi: 10.1038/nchem.626. [DOI] [PubMed] [Google Scholar]
- 7.Simonsen SB, Chakraborty D, Chorkendorff I, Dahl S. Alloyed Ni-Fe nanoparticles as catalysts for NH3 decomposition. Appl. Catal. A Gen. 2012;447:22–31. doi: 10.1016/j.apcata.2012.08.045. [DOI] [Google Scholar]
- 8.Bell TE, Torrente-Murciano L. H2 production via ammonia decomposition using non-noble metal catalysts: a review. Top. Catal. 2016;59:1438–1457. doi: 10.1007/s11244-016-0653-4. [DOI] [Google Scholar]
- 9.Jacobsen, C. J. H. Novel class of ammonia synthesis catalysts. Chem. Commun. 12, 1057–1058 (2000).
- 10.Jacobsen CJH, et al. Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts. J. Am. Chem. Soc. 2001;123:8404–8405. doi: 10.1021/ja010963d. [DOI] [PubMed] [Google Scholar]
- 11.Boisen A, Dahl S, Jacobsen CJH. Promotion of binary nitride catalysts: Isothermal N-2 adsorption, microkinetic model, and catalytic ammonia synthesis activity. J. Catal. 2002;208:180–186. doi: 10.1006/jcat.2002.3571. [DOI] [Google Scholar]
- 12.Boisen A, Dahl S, Norskov JK, Christensen CH. Why the optimal ammonia synthesis catalyst is not the optimal ammonia decomposition catalyst. J. Catal. 2005;230:309–312. doi: 10.1016/j.jcat.2004.12.013. [DOI] [Google Scholar]
- 13.Podila S, et al. Hydrogen production by ammonia decomposition using high surface area Mo2N and Co3Mo3N catalysts. Catal. Sci. Technol. 2016;6:1496–1506. doi: 10.1039/C5CY00871A. [DOI] [Google Scholar]
- 14.Duan XZ, et al. Understanding Co-Mo catalyzed ammonia decomposition: influence of calcination atmosphere and identification of active phase. Chemcatchem. 2016;8:938–945. doi: 10.1002/cctc.201501275. [DOI] [Google Scholar]
- 15.Srifa A, et al. Hydrogen production by ammonia decomposition over Cs-modified Co3Mo3N catalysts. Appl. Catal. B Environ. 2017;218:1–8. doi: 10.1016/j.apcatb.2017.06.034. [DOI] [Google Scholar]
- 16.Yao YG, et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science. 2018;359:1489–1494. doi: 10.1126/science.aan5412. [DOI] [PubMed] [Google Scholar]
- 17.Yeh JW, et al. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004;6:299–303. doi: 10.1002/adem.200300567. [DOI] [Google Scholar]
- 18.Cantor B, Chang ITH, Knight P, Vincent AJB. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 2004;375:213–218. doi: 10.1016/j.msea.2003.10.257. [DOI] [Google Scholar]
- 19.Zhang Y, Zhou YJ, Lin JP, Chen GL, Liaw PK. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008;10:534–538. doi: 10.1002/adem.200700240. [DOI] [Google Scholar]
- 20.Yusenko KV, et al. First hexagonal close packed high-entropy alloy with outstanding stability under extreme conditions and electrocatalytic activity for methanol oxidation. Scr. Mater. 2017;138:22–27. doi: 10.1016/j.scriptamat.2017.05.022. [DOI] [Google Scholar]
- 21.NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20, National Institute of Standards and Technology, Gaithersburg MD, 20899 (2000), 10.18434/T4T88K, (retrieved 17 October 2018).
- 22.Bligaard T, Norskov JK. Ligand effects in heterogeneous catalysis and electrochemistry. Electrochim. Acta. 2007;52:5512–5516. doi: 10.1016/j.electacta.2007.02.041. [DOI] [Google Scholar]
- 23.Sengar SK, Mehta BR, Gupta G. Charge transfer, lattice distortion, and quantum confinement effects in Pd, Cu, and Pd-Cu nanoparticles; size and alloying induced modifications in binding energy. Appl. Phys. Lett. 2011;98:193115. doi: 10.1063/1.3590272. [DOI] [Google Scholar]
- 24.Ju XH, et al. Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Appl. Catal. B Environ. 2017;211:167–175. doi: 10.1016/j.apcatb.2017.04.043. [DOI] [Google Scholar]
- 25.Mukherjee S, Devaguptapu SV, Sviripa A, Lund CRF, Wu G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 2018;226:162–181. doi: 10.1016/j.apcatb.2017.12.039. [DOI] [Google Scholar]
- 26.Karim AM, et al. Correlating particle size and shape of supported Ru/gamma-Al2O3 catalysts with NH3 decomposition activity. J. Am. Chem. Soc. 2009;131:12230–12239. doi: 10.1021/ja902587k. [DOI] [PubMed] [Google Scholar]
- 27.Yin SF, et al. Carbon nanotubes-supported Ru catalyst for the generation of COx-free hydrogen from ammonia. Catal. Today. 2004;93-5:27–38. doi: 10.1016/j.cattod.2004.05.011. [DOI] [Google Scholar]
- 28.Bradford MCJ, Fanning PE, Vannice MA. Kinetics of NH3 decomposition over well dispersed Ru. J. Catal. 1997;172:479–484. doi: 10.1006/jcat.1997.1877. [DOI] [Google Scholar]
- 29.Prasad V, Karim AM, Arya A, Vlachos DG. Assessment of overall rate expressions and multiscale, microkinetic model uniqueness via experimental data injection: ammonia decomposition on Ru/gamma-Al2O3 for hydrogen production. Ind. Eng. Chem. Res. 2009;48:5255–5265. doi: 10.1021/ie900144x. [DOI] [Google Scholar]
- 30.Pacheco V, et al. Thermal stability of the HfNbTiVZr high-entropy alloy. Inorg. Chem. 2019;58:811–820. doi: 10.1021/acs.inorgchem.8b02957. [DOI] [PubMed] [Google Scholar]
- 31.Hsu YJ, Chiang WC, Wu JK. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005;92:112–117. doi: 10.1016/j.matchemphys.2005.01.001. [DOI] [Google Scholar]
- 32.Chou YL, Yeh JW, Shih HC. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corros. Sci. 2010;52:2571–2581. doi: 10.1016/j.corsci.2010.04.004. [DOI] [Google Scholar]
- 33.Hellman A, et al. Ammonia synthesis and decomposition on a Ru-based catalyst modeled by first-principles. Surf. Sci. 2009;603:1731–1739. doi: 10.1016/j.susc.2008.10.059. [DOI] [Google Scholar]
- 34.Ulissi Z, Prasad V, Vlachos DG. Effect of multiscale model uncertainty on identification of optimal catalyst properties. J. Catal. 2011;281:339–344. doi: 10.1016/j.jcat.2011.05.019. [DOI] [Google Scholar]
- 35.Morgan GA, Kim YK, Yates JT. Electron-stimulated dissociation of molecular N2 adsorbed on Ru(109): A TPD and IRAS investigation. Surf. Sci. 2005;598:1–13. doi: 10.1016/j.susc.2005.07.042. [DOI] [Google Scholar]
- 36.Rosowski F, Hinrichsen O, Muhler M, Ertl G. The temperature-programmed desorption of N2 from a Ru/MgO catalyst used for ammonia synthesis. Catal. Lett. 1996;36:229–235. doi: 10.1007/BF00807624. [DOI] [Google Scholar]
- 37.Ertl G, Lee SB, Weiss M. Kinetics of nitrogen adsorption on Fe(111) Surf. Sci. 1982;114:515–526. doi: 10.1016/0039-6028(82)90702-6. [DOI] [Google Scholar]
- 38.Alstrup I, Chorkendorff I, Ullmann S. The interaction of nitrogen with the (111) surface of iron at low and at elevated pressures. J. Catal. 1997;168:217–234. doi: 10.1006/jcat.1997.1652. [DOI] [Google Scholar]
- 39.Cvetanović RJ, Amenomiya Y. Application of a temperature-programmed desorption technique to catalyst studies. Adv. Catal. 1967;17:103–149. [Google Scholar]
- 40.Guan S, Lin HZ. Effect of an iron oxide precursor on the N-2 desorption performance for an ammonia synthesis catalyst. Ind. Eng. Chem. Res. 2000;39:2891–2895. doi: 10.1021/ie990695g. [DOI] [Google Scholar]
- 41.Fernández EM, et al. Scaling relationships for adsorption energies on transition metal oxide, sulfide, and nitride surfaces. Angew. Chem. Int. Ed. 2008;47:4683–4686. doi: 10.1002/anie.200705739. [DOI] [PubMed] [Google Scholar]
- 42.Lansford, J. L., Mironenko, A. V. & Vlachos, D. G. Scaling relationships and theory for vibrational frequencies of adsorbates on transition metal surfaces. Nat. Commun. 8, 1842 (2017). [DOI] [PMC free article] [PubMed]
- 43.Greeley J. Theoretical heterogeneous catalysis: scaling relationships and computational catalyst design. Annu. Rev. Chem. Biomol. 2016;7:605–635. doi: 10.1146/annurev-chembioeng-080615-034413. [DOI] [PubMed] [Google Scholar]
- 44.Yu L, Abild-Pedersen F. Bond order conservation strategies in catalysis applied to the NH3 decomposition reaction. ACS Catal. 2017;7:864–871. doi: 10.1021/acscatal.6b03129. [DOI] [Google Scholar]
- 45.Yang X, Zhang Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012;132:233–238. doi: 10.1016/j.matchemphys.2011.11.021. [DOI] [Google Scholar]
- 46.Guo S, Hu Q, Ng C, Liu CT. More than entropy in high-entropy alloys: forming solid solutions or amorphous phase. Intermetallics. 2013;41:96–103. doi: 10.1016/j.intermet.2013.05.002. [DOI] [Google Scholar]
- 47.Duan XZ, Qian G, Zhou XG, Chen D, Yuan WK. MCM-41 supported Co-Mo bimetallic catalysts for enhanced hydrogen production by ammonia decomposition. Chem. Eng. J. 2012;207:103–108. doi: 10.1016/j.cej.2012.05.100. [DOI] [Google Scholar]
- 48.Lorenzut B, Montini T, Bevilacqua M, Fornasiero P. FeMo-based catalysts for H-2 production by NH3 decomposition. Appl. Catal. B Environ. 2012;125:409–417. doi: 10.1016/j.apcatb.2012.06.011. [DOI] [Google Scholar]
- 49.Leybo DV, et al. Effects of composition and production route on structure and catalytic activity for ammonia decomposition reaction of ternary Ni-Mo nitride catalysts. Int. J. Hydrog. Energ. 2016;41:3854–3860. doi: 10.1016/j.ijhydene.2015.12.171. [DOI] [Google Scholar]
- 50.Guo JP, et al. Lithium imide synergy with 3d transition-metal nitrides leading to unprecedented catalytic activities for ammonia decomposition. Angew. Chem. Int. Ed. 2015;54:2950–2954. doi: 10.1002/anie.201410773. [DOI] [PubMed] [Google Scholar]
- 51.Wang CP, et al. Experimental investigation and thermodynamic calculation of the phase equilibria in the Co-Mo-W system. Intermetallics. 2009;17:642–650. doi: 10.1016/j.intermet.2009.02.004. [DOI] [Google Scholar]
- 52.Hardeveld RV, Hartog F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 1969;15:189–230. doi: 10.1016/0039-6028(69)90148-4. [DOI] [Google Scholar]
- 53.Kittel, C., “Introduction to Solid State Physics”, 7th edition, John Wiley and Sons, New York 1996.
- 54.Takeuchi A, Inoue A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005;46:2817–2829. doi: 10.2320/matertrans.46.2817. [DOI] [Google Scholar]
- 55.Baskes MI. Modified embedded-atom potentials for cubic materials and impurities. Phys. Rev. B. 1992;46:2727–2742. doi: 10.1103/PhysRevB.46.2727. [DOI] [PubMed] [Google Scholar]
- 56.Lee BJ, Baskes MI. Second nearest-neighbor modified embedded-atom-method potential. Phys. Rev. B. 2000;62:8564–8567. doi: 10.1103/PhysRevB.62.8564. [DOI] [Google Scholar]
- 57.Baskes MI, Johnson RA. Modified embedded atom potentials for Hcp metals. Model Simul. Mater. Sci. Eng. 1994;2:147–163. doi: 10.1088/0965-0393/2/1/011. [DOI] [Google Scholar]
- 58.Baskes MI. Determination of modified embedded atom method parameters for nickel. Mater. Chem. Phys. 1997;50:152–158. doi: 10.1016/S0254-0584(97)80252-0. [DOI] [Google Scholar]
- 59.Lee, B. J., Baskes, M. I., Kim, H. & Cho, Y. K. Second nearest-neighbor modified embedded atom method potentials for bcc transition metals. Phys. Rev. B64, 184102 (2001).
- 60.Kresse G, Hafner J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B. 1994;49:14251–14269. doi: 10.1103/PhysRevB.49.14251. [DOI] [PubMed] [Google Scholar]
- 61.Kresse G, Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 62.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758–1775. doi: 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- 63.Monkhorst HJ, Pack JD. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976;13:5188–5192. doi: 10.1103/PhysRevB.13.5188. [DOI] [Google Scholar]
- 64.Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. J. Chem. Phys. 1953;21:1087–1092. doi: 10.1063/1.1699114. [DOI] [Google Scholar]
- 65.He, Q. F., Ye, Y. F. & Yang, Y. The configurational entropy of mixing of metastable random solid solution in complex multicomponent alloys. J. Appl. Phys. 120, 154902 (2016).
- 66.Wang, G. F., Van Hove, M. A., Ross, P. N. & Baskes, M. I. Monte Carlo simulations of segregation in Pt-Ni catalyst nanoparticles. J. Chem. Phys. 122, 24706 (2005). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the figures in this work and other findings corresponding to this study are available from the corresponding authors upon reasonable request.