Short abstract
Background
Methods to improve the clinical efficacy of currently available antibiotics against multidrug resistant bacteria in cystic fibrosis (CF) chronic rhinosinusitis (CRS) are greatly needed. Ivacaftor, a cystic fibrosis transmembrane conductance regulator potentiator, was recently identified as having potentially beneficial off-target effects as a weak inhibitor of bacterial DNA gyrase and topoisomerase IV. The objective of the current study is to evaluate whether ivacaftor enhances the antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa.
Methods
The planktonic growth of the PAO-1 strain of P. aeruginosa was studied in the presence of ciprofloxacin and/or ivacaftor. Effects were measured according to optical density of cultured PAO-1 at 600 nm. For a static PAO-1 biofilm assay, the PAO-1 strain was inoculated and cultured for 72 h in the presence of the drugs. Formed PAO-1 biofilms were quantified by crystal violet staining and imaged with confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM).
Results
PAO-1 growth was significantly reduced in the presence of ivacaftor (8 or 16 µg/mL) and ciprofloxacin (0.02 or 0.05 µg/mL) compared to ciprofloxacin alone (P < .001). Similarly, ivacaftor (8 or 16 µg/mL) showed a significant reduction of PAO-1 biofilms when treated with 0.05 µg/mL of ciprofloxacin. Significant synergism was noted between ciprofloxacin and 16 µg/mL of ivacaftor (P < .0001) in reducing planktonic growth and biofilm formation. Quantitative measurements with crystal violet staining were correlated to CLSM and SEM images.
Conclusion
Ivacaftor enhanced ciprofloxacin’s antimicrobial activity against P. aeruginosa. Further studies evaluating the efficacy of ivacaftor/ciprofloxacin combination for P. aeruginosa for CF CRS are warranted.
Keywords: Pseudomonas aeruginosa, ciprofloxacin, ivacaftor, biofilm, chronic rhinosinusitis, sinusitis, cystic fibrosis transmembrane conductance regulator, cystic fibrosis
Background
Cystic fibrosis (CF) patients have an incidence of chronic rhinosinusitis (CRS) approaching 100% of individuals, and the sinuses serve as a reservoir of recurrent infection for the lower airway.1,2 Chronic pulmonary and sinus infections in CF are particularly resistant to treatment due to bacterial biofilm formation.3 These complex microbial communities are a major contributing factor to medically recalcitrant CF infections. Bacterial biofilms are 3-dimensional aggregates of bacteria encased in secreted exopolysaccharides (slime) and lack the susceptibility to antibiotics that planktonic bacteria demonstrate.4 Bacteria in biofilms are known to be approximately 100 to 1000 times more resistant to the effects of antibiotics than planktonic bacteria and are broadly resistant to antibiotics that attack only dividing cells.5,6 The most frequently observed biofilms in CF CRS are those formed by Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenza, and coagulative-negative staphyloccoci.7,8
Sufficient and prolonged antibiotic courses are required to ensure eradication of microorganisms in CF airway disease. Incomplete treatment with concentrations under the minimal inhibitory concentration (MIC) aggravate the development of antibiotic resistance.9 Methicillin-resistant Staphylococcus aureus and multidrug resistant P. aeruginosa are common clinical isolates of CF patients from sputum and sinus secretions and a cause of antibiotic treatment failures since significantly high doses in a toxic range are needed to achieve biofilm eradication.7,10,11 Agents that enhance the antimicrobial activity of currently available antibiotics represent a valuable and cost-effective means to improve clinical efficacy against multidrug resistant bacterial pathogens.12
Ivacaftor is a cystic fibrosis transmembrane conductance regulator potentiator that enhances Cl− secretion in airway epithelia, including the sinonasal mucosa.13–16 The drug was recently identified to have potentially beneficial off-target effects as a weak inhibitor of bacterial DNA gyrase and topoisomerase IV due to its potential chemical similarity with ciprofloxacin (fluoroquinolones).12,17 Simultaneous delivery of ciprofloxacin and ivacaftor may have synergistic bactericidal effects, while also increasing mucociliary clearance by promoting Cl− transport.17,18 The objective of the current study was to evaluate whether ivacaftor enhances the antimicrobial activity of ciprofloxacin against P. aeruginosa.
Methods
Drugs
A 10 mg/mL aqueous stock solution of ciprofloxacin hydrochloride (CF; GenHunter, Nashville, TN) was diluted to desired concentrations in Luria-Bertani Miller (LB-Miller) broth. Ivacaftor (VX-770; Selleckchem, Houston, TX) was dissolved in dimethyl sulfoxide (DMSO) to create ivacaftor stock solution (10 mg/mL).
Preparation of Pseudomonas aeruginosa PAO-1 Strain
P. aeruginosa PAO-1 strain was obtained as a gift from the University of Pennsylvania (Noam Cohen, MD). The MIC of ciprofloxacin for strain PAO-1 used in this study was 0.75 µg/mL. There was a linear relationship between the colony forming unit (CFU) counts and the optical density (OD) measurement. The OD of cultured PAO-1 was measured at 600 nm using a microplate reader (Synergy HK, BIO-TEK Instruments, Winooski, VT). An OD600 of 0.1 was equal to 3 × 108 CFU/mL, and OD readings between 0.2 and 0.8 are considered reliable.
Planktonic Growth of PAO-1 Strain
PAO-1 strain stock plates were inoculated by transferring bacterial frozen stock (−80°C) to 50 mL of Luria-Bertani (LB)-Miller broth overnight (37°C) and shaken at 200 rpm. Overnight cultures were transferred to 2 LB-Miller agar plates using quadrant streaking method to ensure purity. After overnight growth (37°C), an isolated colony was vortexed in deionized (DI) water and used to create stock agar plates. For experiments, an isolated colony was transferred to 10 mL of LB-Miller broth and incubated (37°C) on a shaker (200 rpm) for 18 to 20 h overnight. Overnight stock was then diluted by a factor of 10 in new LB-Miller broth and left to incubate in identical conditions for 4 h; 100 µl of the PAO-1 culture was inoculated into 400 µl total of LB-Miller broth and cultured for 4 h in a 24-well plate with different concentrations of ciprofloxacin and ivacaftor. Ethanol (50%) in LB-Miller broth inhibits planktonic growth and served as the negative control.
Biofilm Formation of PAO-1
To determine synergistic effects of ciprofloxacin and ivacaftor on PAO-1 biofilm formation, PAO-1 biofilms were induced as previously described.19 The seeding concentration of PAO-1 (1 × 104) was attained by diluting cultures grown to an OD of 0.2 and confirmed with CFU plate counts. The bacteria and drugs were suspended in 2 mL of LB-Miller broth in a 24-well plate for 72 h at 25°C in static condition.
Crystal Violet Staining of Biofilms
For quantifying the PAO-1 biofilms developed on the inner walls and the bottom of the 48-well plate, crystal violet (CV) staining was used as previously described.20 Cultured media were gently aspirated by pipette, the wells washed by submerging the plates under DI water, and then excess liquid blotted 3 times. Biofilms were incubated for 15 min with 2 mL of 0.1% (w/v) CV. After aspirating the excess CV, the plates were washed 3 times. The remaining CV was dissolved in 2 mL of 95% ethanol. Absorbance reading of the dissolved CV was obtained at 590 nm.
Bacterial Biofilm Viability
PAO-1 in LB-Miller media were incubated for 4 h in a 35-mm dish with a coverslip (MatTek, Ashland, MA). PAO-1 biofilms formed on 14-mm glass coverslips were stained with SYTO9 and propidium iodide staining (BacLight™ Live/Dead Bacterial Viability Kit; Molecular Probes, Eugene, OR). The viability of PAO-1 was measured with confocal laser scanning microscopy (CLSM; A1R, Nikon, Tokyo, Japan). Live and dead bacteria were quantified using Image J (http://rsb.info.nih.gov/ij/). Relative proportions of live and dead cells were calculated by counting fluorescence-specific pixels in digital fluorescent images. Five different images were selected for analysis per condition.
Scanning Electron Microscopy
To observe the morphological difference of PAO-1 biofilms in the presence of ciprofloxacin and ivacaftor, wells were prepared for scanning electron microscopy (SEM) in an identical fashion to CV staining except for the addition of a cover slip inserted into the well in an upright position. Coverslips containing PAO-1 biofilms were mounted on aluminum stubs and prepared with Au-Pd sputter coating to promote surface conductivity and reduce charging artifacts. The PAO-1 biofilms were imaged using a Quanta FEG 650 Scanning Electron Microscope (FEI, Hillsboro, OR) at an accelerating voltage of 20 kV.
Statistical Analysis
All experiments were performed at least 4 independent times. Normalized values for relative planktonic growth were expressed as ± standard deviation of the mean. Statistical analyses were conducted with Excel 2016 and GraphPad Prism 6.0 software (La Jolla, CA) using a subgroup 1-way analysis of variance (ANOVA) with post hoc Tukey’s test with significance set at P < .05. For testing interaction between ciprofloxacin and ivacaftor, a 2-way ANOVA was performed with a post hoc Tukey’s test.
Results
Antibacterial Activity of Ciprofloxacin and Ivacaftor Against PAO-1
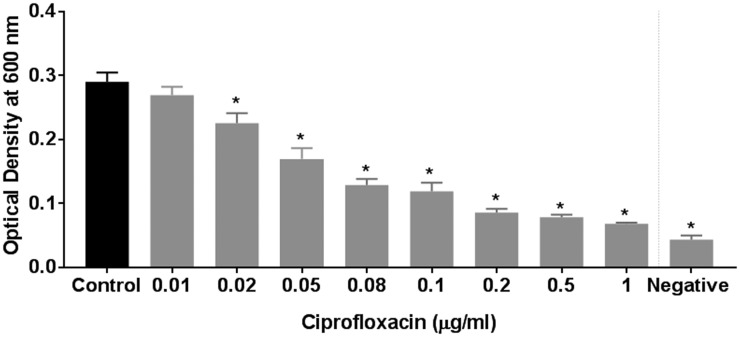
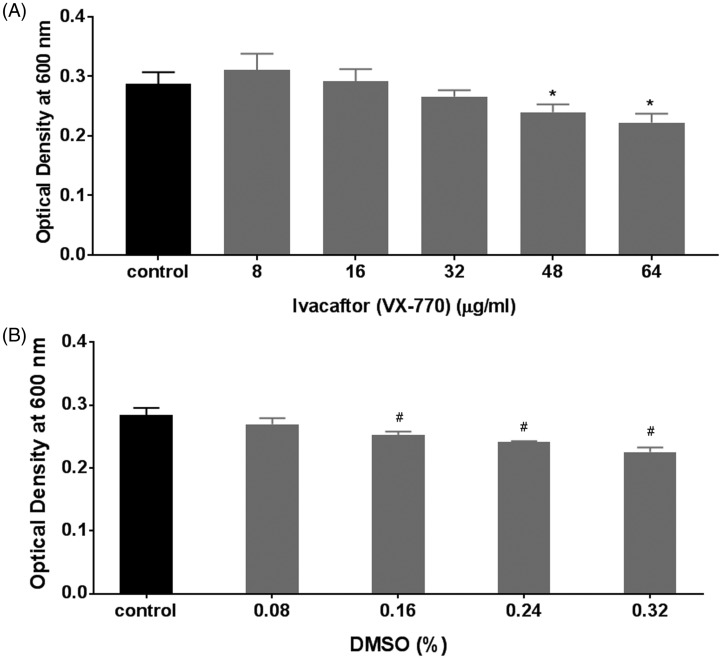
To determine the antibacterial activity of ciprofloxacin against the PAO-1 strain in planktonic growth conditions, escalating concentrations of 0.01, 0.02, 0.04, 0.05, 0.08, 0.1, 0.2, 0.5, and 1 µg/mL were used (Figure 1). Dose-dependent inhibition of planktonic growth was observed with escalating concentrations of ciprofloxacin (range of OD600: 0.07 ± 0.002 (1 µg/mL) to 0.27 ± 0.01 (0.01 µg/mL), 1-way ANOVA with post hoc analysis P < .05). The 3 least subinhibitory concentrations (0.01, 0.02, and 0.05 µg/mL) were selected to study the synergistic effects of ciprofloxacin and ivacaftor. Antibacterial activity of ivacaftor against PAO-1 was evaluated with escalating concentrations (8 µg/mL to 72 µg/mL); 72 µg/mL was the highest concentration dissolvable in LB-Miller broth. Weak dose-dependent antibacterial activity was observed above 48 µg/mL (Figure 2(A)). Antibacterial activity was also measured against vehicle control (DMSO) to rule out solvent antibacterial effects (Figure 2(B)). DMSO carrier did reduce PAO-1 concentrations with escalating doses. To eliminate the contribution of DMSO’s antibacterial effect, the 8 and 16 µg/mL ivacaftor concentrations were selected where the antibacterial effect of the vehicle was negligible.
Figure 1.
Effect of ciprofloxacin on the planktonic growth of PAO-1. Dose-dependent inhibition of planktonic growth was observed with escalating concentrations of ciprofloxacin (P < .05). Asterisk represents significance compared to the control group (P < .05, 1-way ANOVA and Tukey’s test; n = 6).
Figure 2.
A, Effect of ivacaftor (VX-770) on the planktonic growth of PAO-1. Dose-dependent inhibition of planktonic growth was observed with escalating concentrations of ivacaftor above 48 µg/mL. Asterisk represents significance compared to the control group (P < .05, 1-way ANOVA and Tukey’s test; n = 6). B, Effect of corresponding dimethyl sulfoxide (DMSO) % served as dissolving agents of ivacaftor on the planktonic growth of PAO-1: DMSO carrier did reduce PAO-1 concentrations with escalating above 0.16%. Asterisk represents significance compared to the control group (P < .05, 1-way ANOVA and Tukey’s test; n = 4).
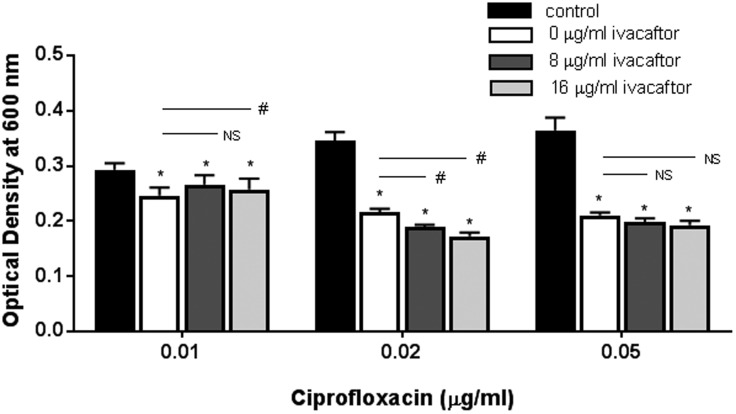
Synergistic Effect on Planktonic Growth of PAO-1 by Ciprofloxacin and Ivacaftor
To evaluate the synergistic effect of ciprofloxacin and ivacaftor, 3 ciprofloxacin concentrations (0.01, 0.02, and 0.05 µg/mL) were combined with ivacaftor at concentrations of 8 or 16 µg/mL (Figure 3). Synergistic antibacterial effect of ciprofloxacin and ivacaftor was noticed at 0.02 µg/mL and 0.05 µg/mL of ciprofloxacin with 8 and 16 µg/mL of ivacaftor although only statistically significant at the 0.02 µg/mL concentration of ciprofloxacin. Ivacaftor induced a dose-dependent reduction in OD measurement when 8 and 16 µg/mL of ivacaftor were combined with 0.02 µg/mL of ciprofloxacin (P < .05). We also found similar synergistic antibacterial activity of ciprofloxacin at the 0.05 µg/mL concentration with 2 different concentrations of ivacaftor (8 µg/mL = 0.198 ± 0.00; 16 µg/mL = 0.192 ± 0.00) compared to ciprofloxacin alone but statistical significance was lacking (P = .21). To understand combined effects, a 2-way ANOVA test was performed to demonstrate any statistically significant synergism between ciprofloxacin and ivacaftor.21 For antibacterial activity, statistically significant synergism was noticed between ciprofloxacin and 16 µg/mL of ivacaftor (P < .0001). However, ciprofloxacin and 8 µg/mL of ivacaftor did not act synergistically (P = .073).
Figure 3.
Enhanced antibacterial activity of ciprofloxacin against Pseudomonas aeruginosa PAO-1 in combination with different ivacaftor concentrations. Antibacterial effects of ciprofloxacin and ivacaftor were noticed at the 0.02 µg/mL and 0.05 µg/mL concentrations of ciprofloxacin with 8 and 16 µg/mL of ivacaftor. Two-way ANOVA demonstrated a statistically significant synergism between ciprofloxacin and 16 µg/mL of ivacaftor (P < .0001). Asterisk represents significance of each ciprofloxacin group compared to its respective control group (P < .05, 1-way ANOVA and Tukey’s test). Three different concentrations of ciprofloxacin were used: 0.01, 0.02, and 0.05 µg/mL. Within the same ciprofloxacin group, a statistical difference with P < .05 among different ivacaftor groups was marked by a #. NS means no statistical significance. All groups consisted of at least 4 experiments.
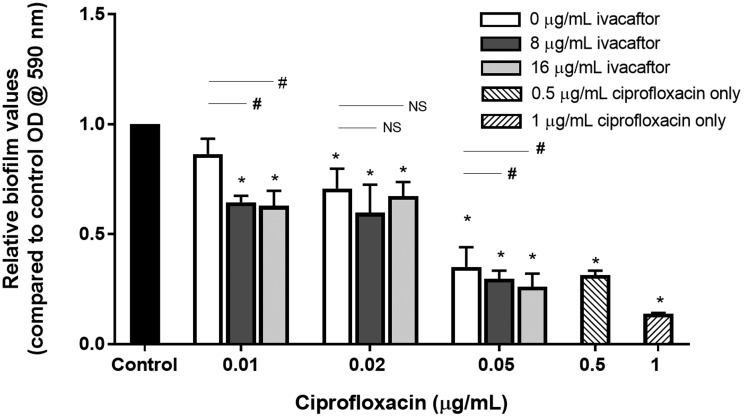
Synergistic Effect on PAO-1 Biofilm Formation by Ciprofloxacin and Ivacaftor
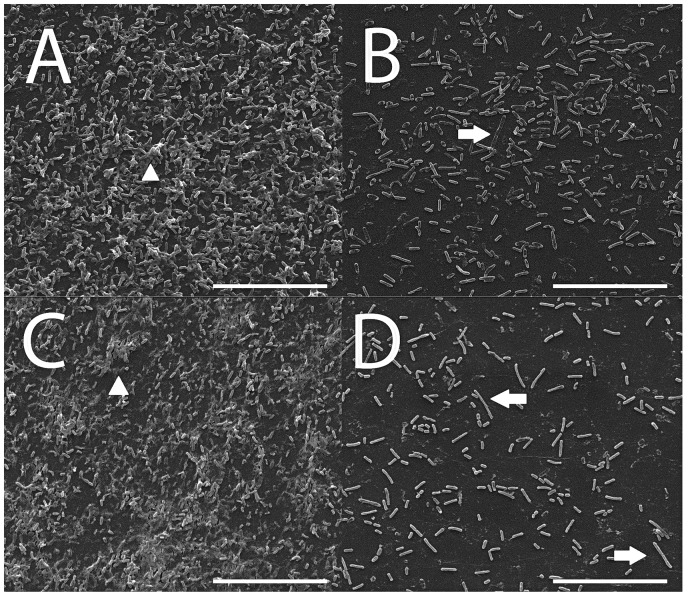
PAO-1 biofilms were grown for 72 h in the presence of different combinations of ciprofloxacin and ivacaftor (Figure 4). There was no inhibitory effect on the formation of biofilms at lower ciprofloxacin concentrations (0.01 µg/mL or 0.02 µg/mL). In contrast, higher concentrations (0.05 µg/mL ciprofloxacin and 8 and 16 µg/mL of ivacaftor) significantly reduced biofilm mass in a synergistic fashion with ivacaftor. Similar to inhibition of PAO-1 planktonic growth, 2-way ANOVA results indicated that statistically significant synergism was noticed between ciprofloxacin (0.05 µg/mL) and 16 µg/mL of ivacaftor (P < .0001) in reducing PAO-1 biofilm formation. Quantitative measurements with CV staining were correlated to CLSM and SEM imaging findings. Bacterial viability was measured in the presence of ciprofloxacin (0.05 µg/mL) and ivacaftor (16 µg/mL) for 4 h in a static condition (Figure 5). Biofilm formation was significantly decreased in the presence of ivacaftor/ciprofloxacin compared to ciprofloxacin or ivacaftor alone (% of dead cells; control = 23 ± 5%; ciprofloxacin = 46 ± 9%; ivacaftor alone = 33 ± 6%; ciprofloxacin + ivacaftor = 80 ± 1%, n = 4 per condition, P < .0001). Ciprofloxacin and ivacaftor mediated inhibition of PAO-1 biofilm mass (at 0.05 µg/mL and 16 µg/mL, respectively) and changes to the morphology of cells are reflected in SEM micrographs (Figure 6). PAO-1 biofilm at maturation phase with rod-shaped bacteria was colonized through secreted extracellular polymeric substances (EPS) in controls (Figure 6(A)). In terms of morphology, samples treated with ciprofloxacin alone (0.05 µg/mL) or ciprofloxacin (0.05 µg/mL) + ivacaftor (16 µg/mL) both demonstrated destruction of EPS with residual filamentous cells due to inhibition of cell division from targeting DNA gyrase.22 However, weakly adherent P. aeruginosa isolates formed only a monolayer when treated with both ciprofloxacin and ivacaftor (Figure 6(D)) compared to moderate and strongly adherent isolates forming clumps of cells in single therapy samples (Figure 6(B) and (C)).
Figure 4.
Combinational effect of ciprofloxacin and ivacaftor on Pseudomonas aeruginosa PAO-1 biofilms; 0.05 µg/mL ciprofloxacin and 8 and 16 µg/mL of ivacaftor significantly reduced biofilm mass in a synergistic fashion with ivacaftor. Statistically significant synergism was noticed between ciprofloxacin and 16 µg/mL of ivacaftor (P < .0001) in reducing PAO-1 biofilm formation. Asterisk represent significance compared to the control group (P < .05, 1-way ANOVA and Tukey’s test). Three different concentrations of ciprofloxacin were used: 0.01, 0.02, and 0.05 µg/mL. Within the same ciprofloxacin group, a statistical difference with a P < .05 among different ivacaftor groups was marked by a #. NS means no statistical significance. All groups consisted of at least 4 experiments.
Figure 5.
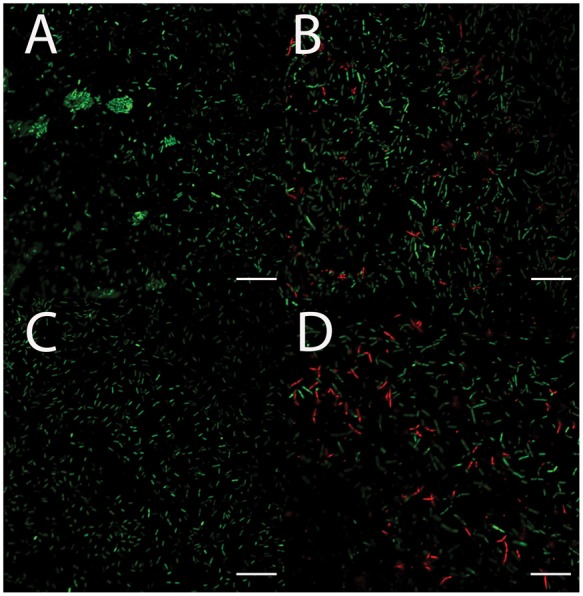
Representative confocal laser scanning microscopy images of Pseudomonas aeruginosa PAO-1 biofilms after 4 h. A, Control. B, Ciprofloxacin 0.05 µg/mL. C, Ivacaftor 16 µg/mL. D, Ciprofloxacin 0.05 µg/mL and Ivacaftor 16 µg/mL; Green—live cells; Red—dead cells. Scale bars are 20 μm.
Figure 6.
Representative scanning electron microscopy images of Pseudomonas aeruginosa PAO-1 biofilms after 72 h. A, Control. B, Ciprofloxacin 0.05 µg/mL. C, Ivacaftor 16 µg/mL. D, Ciprofloxacin 0.05 µg/mL and ivacaftor 16 µg/mL arrows—filamentous PAO-1. Arrow heads—PAO-1 biofilms with extra-cellular polymeric substances. Scale bars are 20 μm.
Discussion
To prevent antimicrobial resistance, combination treatments have been used previously for P. aeruginosa infections.23 Human trials in patients with CF also showed that P. aeruginosa resistance occurred less frequently during combination rather than single therapy.24 In the current study, ivacaftor enhanced the antimicrobial activity of ciprofloxacin against the PAO-1 strain of P. aeruginosa in both planktonic growth and biofilm-forming mass. The synergy between ciprofloxacin and ivacaftor ranging from sub-MIC to MIC concentrations has not been previously elucidated. As the biofilm mass of P. aeruginosa can easily form below the MIC, preventing bacterial biofilms even at sub-MIC levels of antibiotics is a clinically practical strategy to treat chronic CF airway infections.25 Significant reduction of PAO-1 biofilm formation was observed when sub-MIC levels of ciprofloxacin were combined with ivacaftor. This finding suggests that improved treatment of recalcitrant Pseudomonas infections is possible in CRS patients if ivacaftor were coadministered with ciprofloxacin.
Reznikov et al. published positive interactions of ivacaftor with other antibiotics suggestive of additivity or synergy.17 When treated with ivacaftor (1 µg/mL), vancomycin showed better antibacterial action against Staphylococcus aureus than what was observed with vancomycin alone. To achieve antimicrobial properties, a concentration of ivacaftor higher than 1 µg/mL (16 µg/mL) was necessary to achieve synergism based on our experiment. Because the serum concentration of ivacaftor administered orally approximates 1 µg/mL, the best way to achieve synergistic concentrations is through topical delivery.26
Drug delivery systems that provide prolonged mucosal contact time with local absorption and minimal depletion are desired requirements for eradicating biofilms.27 Many drugs used for CF upper and lower airway infections are administered as topical therapy using aerosolized or irrigation methods of delivery.28 In the sinuses, drug-eluting implants can deliver continual drug release with prolonged mucosal contact duration and thus may provide a suitable therapeutic option. We recently developed a novel ciprofloxacin-coated sinus stent which releases antibiotics over a sustained duration of several weeks.29 Combining ivacaftor with ciprofloxacin as topical therapy incorporates several synergistic elements: (1) sustained release of ciprofloxacin and ivacaftor for bactericidal effects and (2) ivacaftor enhancement of sinus mucociliary clearance. The possibility of introducing these 2 antimicrobials together into devices such as drug-eluting sinus stents has potential possibilities and deserves further study.
There are several limitations to this study. Ivacaftor is dissolved in DMSO for in vitro assays, which can be toxic to multiple cell types. The drug has high bioavailability upon ingestion, but different delivery mechanisms and solvents will need to be tested in preclinical studies before any future human trials. Lower concentrations of ivacaftor with negligible toxic effects were used to test synergism. Improved synergism at higher concentrations (>16 µg/mL) is a possibility. In addition, only 1 strain of P. aeruginosa was tested, which is well known for its intrinsic resistance to a variety of antimicrobial agents and toxic compounds.30 Further studies are planned to test against different multidrug resistant strains of P. aeruginosa.
Conclusion
Ciprofloxacin’s antimicrobial activity against the PAO-1 strain of P. aeruginosa was enhanced with ivacaftor. Combination topical therapy represents a novel treatment approach to recalcitrant P. aeruginosa infections in CF airway disease. Translation of these findings to preclinical trials is warranted.
Acknowledgments
The authors would like to show their gratitude to William Stonewall Monroe for SEM images and Joshua S. Richman, MD, PhD, for statistical analysis.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute (1 R01 HL133006-03) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-05, CF Research Center Pilot Award) to B.A.W., and John W. Kirklin Research and Education Foundation Fellowship Award and UAB Faculty Development Research Award to D.Y.C. D.Y.C., MD, receives research grant support from Bionorica Inc. B.A.W., MD, receives grant support from Bionorica Inc. and Cook Medical.
References
- 1.Gentile VG, Isaacson G. Patterns of sinusitis in cystic fibrosis. Laryngoscope. 1996; 106:1005–1009. [DOI] [PubMed] [Google Scholar]
- 2.Henriksson G, Westrin KM, Karpati F, Wikstrom AC, Stierna P, Hjelte L. Nasal polyps in cystic fibrosis: clinical endoscopic study with nasal lavage fluid analysis. Chest. 2002; 121:40–47. [DOI] [PubMed] [Google Scholar]
- 3.Tajudeen BA, Schwartz JS, Palmer JN. Understanding biofilms in chronic sinusitis. Curr Allergy Asthma Rep. 2016; 16:10. [DOI] [PubMed] [Google Scholar]
- 4.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008; 22:235–238. [DOI] [PubMed] [Google Scholar]
- 5.Amorena B, Gracia E, Monzon M, et al. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 1999; 44:43–55. [DOI] [PubMed] [Google Scholar]
- 6.Post JC, Stoodley P, Hall-Stoodley L, Ehrlich GD. The role of biofilms in otolaryngologic infections. Curr Opin Otolaryngol Head Neck Surg. 2004; 12:185–190. [DOI] [PubMed] [Google Scholar]
- 7.Aanaes K. Bacterial sinusitis can be a focus for initial lung colonisation and chronic lung infection in patients with cystic fibrosis. J Cyst Fibros. 2013; 12(Suppl 2):S1–S20. [DOI] [PubMed] [Google Scholar]
- 8.Choi KJ, Cheng TZ, Honeybrook AL, et al. Correlation between sinus and lung cultures in lung transplant patients with cystic fibrosis. Int Forum Allergy Rhinol. 2018; 8:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo). 2011; 64:625–634. [DOI] [PubMed] [Google Scholar]
- 10.Burns JL, Rolain JM. Culture-based diagnostic microbiology in cystic fibrosis: can we simplify the complexity? J Cyst Fibros. 2014; 13:1–9. [DOI] [PubMed] [Google Scholar]
- 11.Moller ME, Alanin MC, Gronhoj C, Aanaes K, Hoiby N, von Buchwald C. Sinus bacteriology in patients with cystic fibrosis or primary ciliary dyskinesia: a systematic review. Am J Rhinol Allergy. 2017; 31:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider EK, Azad MA, Han ML, et al . An “unlikely” pair: the antimicrobial synergy of polymyxin B in combination with the cystic fibrosis transmembrane conductance regulator drugs KALYDECO and ORKAMBI. ACS Infect Dis. 2016; 2:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013; 27:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med. 2014; 20:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe SM, Liu B, Hill A, et al. Optimizing nasal potential difference analysis for CFTR modulator development: assessment of ivacaftor in CF subjects with the G551D-CFTR mutation. PLoS One. 2013; 8:e66955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Blount AC, McNicholas CM, et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PLoS One. 2013; 8:e81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reznikov LR, Abou Alaiwa MH, Dohrn CL, et al. Antibacterial properties of the CFTR potentiator ivacaftor. J Cyst Fibros. 2014; 13:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernarde C, Keravec M, Mounier J, et al. Impact of the CFTR-potentiator ivacaftor on airway microbiota in cystic fibrosis patients carrying a G551D mutation. PLoS One. 2015; 10:e0124124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnanadhas DP, Elango M, Datey A, Chakravortty D. Chronic lung infection by Pseudomonas aeruginosa biofilm is cured by L-Methionine in combination with antibiotic therapy. Sci Rep. 2015; 5:16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol 2005;Chapter 1:Unit 1B 1. [DOI] [PMC free article] [PubMed]
- 21.Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998; 30:723–731. [DOI] [PubMed] [Google Scholar]
- 22.Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014; 22:438–445. [DOI] [PubMed] [Google Scholar]
- 23.Wolter DJ, Lister PD. Mechanisms of beta-lactam resistance among Pseudomonas aeruginosa. Curr Pharm Des. 2013; 19:209–222. [PubMed] [Google Scholar]
- 24.Mouton JW. Combination therapy as a tool to prevent emergence of bacterial resistance. Infection. 1999; 27 Suppl 2:S24–S28. [DOI] [PubMed] [Google Scholar]
- 25.Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci. 2006; 103:19484–19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porsio B, Craparo EF, Mauro N, Giammona G, Cavallaro G. Mucus and cell-penetrating nanoparticles embedded in nano-into-micro formulations for pulmonary delivery of ivacaftor in patients with cystic fibrosis. ACS Appl Mater Interfaces. 2018; 10:165–181. [DOI] [PubMed] [Google Scholar]
- 27.Albu S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des Devel Ther. 2012; 6:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klodzinska SN, Priemel PA, Rades T, Morck Nielsen H. Inhalable antimicrobials for treatment of bacterial biofilm-associated sinusitis in cystic fibrosis patients: challenges and drug delivery approaches. Int J Mol Sci. 2016; 17:E1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho DY, Hoffman K, Skinner D, et al. Tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent in a preclinical model. Int Forum Allergy Rhinol. 2017; 7(4):352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolter DJ, Black JA, Lister PD, Hanson ND. Multiple genotypic changes in hypersusceptible strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients do not always correlate with the phenotype. J Antimicrob Chemother. 2009; 64:294–300. [DOI] [PubMed] [Google Scholar]