Abstract
Chronic graft-vs.-host disease (cGVHD) is a complication of allogeneic hematopoietic stem cell transplantation (alloHSCT). Oral cGVHD is manifested by mucosal, salivary, and/or sclerotic changes that have been linked to pain and poor quality of life. Our aim was to describe the demographic, clinical, and laboratory markers of oral cGVHD in alloHSCT patients (N = 187) enrolled in a cGVHD cross-sectional study at the NIH (#NCT00331968). We propose a meaningful and reproducible measure of disease defined by a cut-off point reflecting clinical minimally detectable change (0-2 = no oral cGVHD, 3-15 = oral cGVHD) on the 15-point NIH cGVHD clinician assessment scale. Forty-four patients had oral cGVHD. Oral cGVHD was associated with a quiescent or de novo type of cGVHD onset (p = 0.05), higher cGVHD severity (p = 0.033), lower albumin (p = 0.0008), higher total complement (p = 0.012), greater bother from foods or oral ulcers and greater mouth pain, and sensitivity (p < 0.0001). Multivariable logistic regression modeling with albumin, mouth pain, and total complement was 74.3% predictive of oral cGVHD and 80.2% predictive of non-oral cGVHD. We propose the use of >2 points on the NIH scale as a reproducible definition of clinically significant oral cGVHD, which may be useful in clinical settings or as eligibility criterion or as an endpoint in clinical trials.
Keywords: autoimmunity, oral medicine, inflammation, stem cell(s), oral diagnosis, clinical practice guidelines
Introduction
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is primarily utilized for high-risk or relapsed hematologic malignancies (Copelan, 2006). Chronic graft-vs.-host disease (cGVHD) is a late alloimmune and autoimmune complication of alloHSCT and is the leading cause of late-transplant-associated morbidity and mortality in long-term survivors (Lee et al., 2003; Pavletic et al., 2006a). Although cGVHD can affect multiple organs, oral cavity involvement has been reported in 50% to 80% of cGVHD patients (Baird and Pavletic, 2006; Schubert and Correa, 2008).
Contemporary standardized criteria for the diagnosis and staging of cGVHD have aided in the classification of oral cGVHD (Filipovich et al., 2005); however, these criteria are based on expert opinions, and a definition of clinically significant oral cGVHD based on empirical data is still lacking. Oral manifestations of cGVHD resemble those of several autoimmune conditions, including lichen planus, Sjögren’s syndrome, and scleroderma. Diagnostic manifestations of oral cGVHD include lichen-planus-like changes, hyperkeratosis or leukoplakia, and sclerotic restriction of mouth opening. Confirmatory biopsy is recommended for diagnosis in the presence of distinctive features of oral cGVHD, which include xerostomia, mucoceles, mucosal atrophy, pseudomembrane, and ulcerations (Filipovich et al., 2005).
Oral cGVHD has a negative impact on oral health, functional capacity, and quality of life. Oral cGVHD patients experience diminished oral-cavity-specific quality of life (Imanguli et al., 2008), taste alteration, and increased levels of oral-related pain and dryness (Fall-Dickson et al., 2010). Patients with salivary gland atrophy or dysfunction may have difficulty swallowing, increased risk for dental caries lesions secondary to changes in salivary composition, and frequent oral co-infections due to diminished salivary defenses (Meier et al., 2011).
One obstacle in advancing therapeutic trials in oral cGVHD is the absence of a standard definition of clinically significant disease. This current work aims to present a clinically meaningful and easily reproducible definition of oral cGVHD using the new NIH oral-specific cGVHD response scale while accounting for observer variability (Pavletic et al., 2006b), and to test this definition in a comprehensive analysis of the clinical presentation, laboratory markers, and burden of disease of oral cGVHD in a large cohort of cGVHD patients.
Materials & Methods
Study Population
Post-alloHSCT patients (207 adults, 10 pediatric) with cGVHD, referred by their primary transplant physician for evaluation of their cGVHD at various stages of progression, were enrolled in a single-visit cross-sectional study of cGVHD at the NIH Clinical Center in Bethesda, Maryland, from 2004 to 2011 (clinicaltrials.gov identifier: NCT00331968). All patients with inconclusive cGVHD (N = 9), late acute GVHD manifestation (N = 2), or no NIH Oral cGVHD score were excluded from the study, thus leaving 187 patients (180 adult, 7 pediatric) with cGVHD and NIH 15-point oral cGVHD scores available. All patients underwent comprehensive evaluation by an interdisciplinary team of clinicians, including trained bone marrow transplant nurse practitioners and physicians experienced with cGVHD and with using the NIH cGVHD activity assessment scale. The acquisition of human subject data complied with the Declaration of Helsinki of the World Medical Association. This research project was approved by the NCI Institutional Review Board (IRB).
Measures
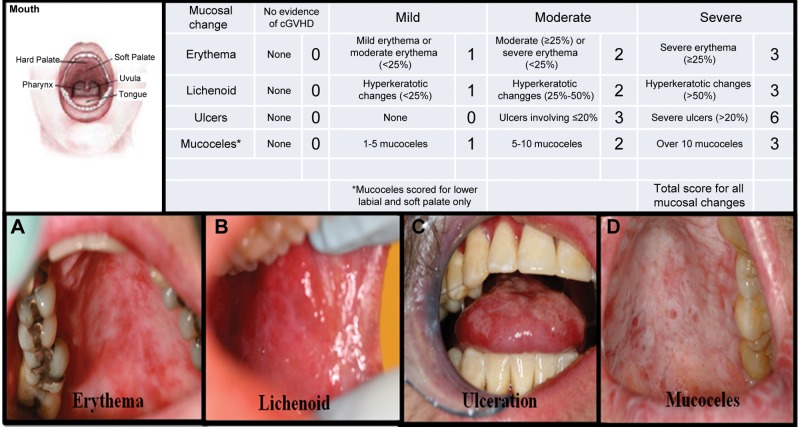
Oral cGVHD activity was defined according to an NIH-clinician-administered 15-point oral cGVHD activity assessment (NIH Oral cGVHD Scale) for the total mucosal changes in patients with cGVHD (Pavletic et al., 2006b) (Fig. 1). The NIH Oral cGVHD scale is a simplification of a 273-point oral mucositis rating scale (Schubert et al., 1992) (Appendix Fig. 1). The NIH Oral cGVHD Scale is limited to the evaluation of erythema, lichenoid lesions, ulcers, and mucoceles. Mucosal erythema was scored by color intensity and extent of involvement, lichenoid and ulceration by the extent of involved, and mucoceles by total number observed (Pavletic et al., 2006b). The NIH Oral cGVHD Scale has been partially validated with an oral pain numerical rating scale (NRS) and has demonstrated construct validity and internal consistency reliability (Elad et al., 2010).
Figure 1.
Clinical presentation of oral mucosal changes assessed through the NIH 15-point Oral cGVHD scale. (A) Erythema of the hard and soft palate. (B) Lichenoid hyperkeratotic changes of the buccal mucosa. (C) Ulcerations of the dorsal tongue. (D) Mucoceles of the soft palate. Response Criteria Appendix A from American Society of Bone Marrow Transplant Web site: (http://asbmt.affiniscape.com/associations/11741/files/ResponseCriteriaAPPENDIXAFormA.pdf). 169 x 98 mm (150 x 150 DPI).
Individuals with a total oral mucosal score of 3 or more on the NIH Oral cGVHD Scale were categorized as “oral cGVHD”, while those with a score of 0 to 2 were categorized “no oral cGVHD”. The choice of this cut-off point for defining clinically detectable oral cGVHD was based on the findings that minimal detectable change falls in the range of 2.1 to 2.9 on this NIH Oral cGVHD Scale (Mitchell et al., 2011). Based on this definition (scores: 3-15), individuals categorized as having “oral cGVHD” were compared with those with “non-oral cGVHD” (Appendix Table 1).
Statistical Analysis
We performed univariate analyses to screen for factors to be evaluated in a multivariable logistic model. Dichotomous parameters were compared between patients with and without oral cGVHD by Fisher’s exact test. Categorical parameters were compared by Mehta’s modification of Fisher’s exact test (Mehta and Patel, 1983). Ordered categorical parameters were compared by a Cochran-Armitage test for trend (Agresti, 1990). Continuous parameters were compared between groups with a form of an exact Wilcoxon rank-sum test. All p-values from univariate analyses were two-tailed and are presented without adjustment for multiple comparison. In the context of an exploratory analysis, only p-values < 0.01 were considered potentially statistically significant with respect to the individual univariate results.
Factors associated with oral cGVHD with a univariate p-value < 0.05 were included in a multivariable logistic regression analysis model. The final model was determined by backward selection. Since the same patients who developed the model were used in its evaluation, independent confirmation would be necessary to validate the model.
The probability of survival from date of entry into the natural history study was determined by the Kaplan-Meier method, with the statistical significance of the difference between survival curves according to having or not having oral cGVHD not determined by the log-rank test.
Results
Descriptive Statistics
Patient Demographic, Transplant, and cGVHD Characteristics
In total, 187 patients were included in this analysis. The median age at enrollment was 46 yrs (range, 4-70 yrs), and 45% were female. Median time from transplant to study enrollment was 3.1 yrs (range, 0-5.7 yrs). Median time from transplant to cGVHD diagnosis was 7.1 mos (range, 0-5.6 yrs). The majority of patients had undergone myeloablative conditioning, received transplants from HLA-matched related donors, and received peripheral blood stem cell grafts (Table 1). Most patients had acute GVHD prior to cGVHD and had progressive-type onset compared with quiescent or de novo types. The majority of patients received moderate or high-intensity immunosuppression and were categorized as having active cGVHD based on the therapeutic intent (increase or decrease immunosuppression) at the time of visit. Mouth involvement was present in 44 (23.5%) patients, as defined by scores of 3 or above on the NIH oral cGVHD 15-point scale (median score 5, range 3-13) (Appendix Fig. 2). Patients presented with erythema (91, 54%), lichenoid (90, 54%), ulcerations (23, 4%), and mucoceles (12, 7%) to various degrees (Appendix Fig. 3). Many patients reported oral dryness (98, 66.7%), pain (68, 45.6%), and sensitivity (87, 59.3%). The majority of patients had severe forms of cGVHD, based on the NIH global scoring (Table 1).
Table 1.
Patient and cGVHD Characteristics at Study Enrollment
| Characteristics | n (%) or (range) |
|---|---|
| Total number of patients | 187 |
| Total number of oral cGVHD patients | 44 (23.5%) |
| Age (median, range, in yrs) | 45.6 (3.7-69.8) |
| Gender | |
| Male | 103 (55%) |
| Female | 85 (45%) |
| Disease | |
| ALL/AML/MDS | 79 (46%) |
| Lymphoma/CML/MM | 71 (41%) |
| CLL | 12 (7%) |
| Aplastic Anemia/PNH | 6 (4%) |
| Other non-malignant | 3 (2%) |
| Conditioning regimen | |
| Myeloablative | 106 (57%) |
| Total Body Irradiation (TBI) | 72 (39%) |
| Donor relationship | |
| Unrelated | 72 (39%) |
| Related | 113 (61%) |
| Cell source | |
| Bone marrow | 35 (19%) |
| Peripheral blood | 146 (79%) |
| Cord blood | 4 (2%) |
| HLA match | |
| Yes | 148 (82%) |
| No | 32 (18%) |
| cGVHD onset type | |
| Progressive | 70 (38.5%) |
| Quiescent | 52 (28.6%) |
| De novo | 60 (33.45) |
| Number of organs involved | 5 (1-8) |
| Eye | 148 (80.4%) |
| Skin | 145 (78.8%) |
| Lung | 141 (77%) |
| Joint or fascia | 115 (62.5%) |
| Liver | 96 (52.5%) |
| Gastrointestinal tract | 84 (45.6%) |
| Genital | 42 (50%) |
| Activity by therapeutic intent | |
| Active | 79 (53.4%) |
| Not active | 69 (46.6%) |
| Intensity of immunosuppression a | |
| None/mild | 46 (25.1%) |
| Moderate | 62 (33.9%) |
| Severe | 75 (41.0%) |
| NIH average score | 1.03 (0-2.33) |
| Median number of mos from transplant to enrollment | 50.6 (4-258.2) |
For all values in this Table, continuous variables are shown as median values with ranges, and categorical variables are shown as frequencies with percentages.
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; PNH, paroxysmal nocturnal hemoglobinuria; M, male; F, female; HLA, human leukocyte antigen.
Intensity of immunosuppression: Mild, single agent prednisone < 0.5; Moderate, prednisone < 0.5 mg/kg/day and/or any single agent/modality; High, 2 or more agents/modalities ± prednisone < 0.5 mg/kg/day.
Differences between Oral cGVHD and Non-oral cGVHD
Patient Demographic, Transplant, and cGVHD Characteristics
Univariate analysis revealed that quiescent and de novo cGVHD onset, as compared with progressive onset, was associated with oral cGVHD (p = 0.0495) (Table 2, Appendix Fig. 4). Age, gender, time from transplant to cGVHD diagnosis or enrollment, and number of lines of prior systemic therapy for cGVHD were not associated with oral cGVHD. Underlying disease diagnosis for which transplant was performed, donor to recipient gender-matching, stem cell source (peripheral blood, bone marrow, umbilical cord blood), cGVHD classification at time of evaluation (classic, overlap, late acute), intensity of immunosuppression, NIH organ-specific scores, conditioning intensity prior to transplantation, total body irradiation (TBI), relationship of donor to recipient, HLA match, history of acute GVHD, therapeutic intent at the time of the visit, and global NIH scores were also not associated with oral cGVHD (data not shown).
Table 2.
Variables Significantly Associated with Oral cGVHD in Univariate Analysis and Multivariable Regression Model of Factors Highly Predictive of Oral cGVHD
| Univariate Analysis | Non-oral cGVHD (N = 147) | Oral cGVHD (N = 44) | p-value | |
|---|---|---|---|---|
| Patient, transplant, and cGVHD characteristics | ||||
| Quiescent or de novo cGVHD onset vs. progressive onset | 79/138 (57.2%) | 33/44 (75%) | 0.0495 | |
| NIH average score | 1.02 (0.03) | 1.21 (0.07) | 0.033 | |
| Laboratory parameters | ||||
| Albumin | 3.7 (0.04) | 3.43 (0.07) | 0.0008 | |
| Total complement | 132.05 (3.35) | 148.25 (5.99) | 0.012 | |
| Patient-reported measures | ||||
| Bother by avoidance of certain foods due to mouth pain (yes vs. no) | 50/117 (42.7%) | 30/36 (83.3%) | < 0.0001 | |
| Bother by ulcers in mouth (yes vs. no) | 37/117 (31.6%) | 24/36 (66.7%) | < 0.0001 | |
| Mouth pain | 1.1 (0.21) | 3.83 (0.56) | < 0.0001 | |
| Mouth sensitivity | 2.12 (0.28) | 4.17 (0.53) | < 0.0001 | |
| Multivariable logistical regression analysis | ||||
| Variable | Estimate | Standard Error | Chi-square | p-value |
| Albumin | −1.15 | 0.25 | 20.47 | <0.0001 |
| Total complement | 0.016 | 0.0056 | 8.02 | 0.0046 |
| Mouth pain | 0.35 | 0.080 | 19.58 | <0.0001 |
For each group (oral and non-oral cGVHD), continuous variables are shown as means (standard error of the mean). Categorical variables are shown as proportions (percentages) for each group.
cGVHD indicates chronic graft vs. host disease, and NIH indicates National Institutes of Health.
The final model was determined by backward selection. In the context of an exploratory analysis, only p-values < 0.01 could be considered potentially statistically significant with respect to the individual univariate results.
cGVHD indicated chronic graft vs. host disease.
Laboratory Parameters
Patients with oral cGVHD had lower median serum albumin levels (oral cGVHD = 3.43, non-oral cGVHD = 3.7, p = 0.0008) and higher median total complement levels (oral cGVHD = 148, non-oral cGVHD = 132, p = 0.012) as compared with patients without oral cGVHD (Table 2). Other laboratory values were not found to be significantly associated with oral cGVHD (Appendix Table 2).
Patient Self-reported Symptom Measures
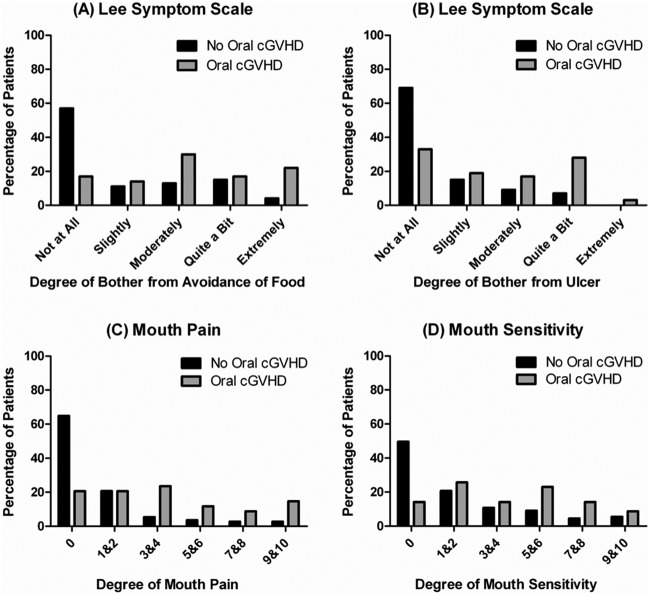
As compared with non-oral cGVHD patients, those with oral cGVHD reported having higher Lee Symptoms Scale scores for degree of bother from avoidance of foods (p < 0.0001), higher Lee Symptoms Scale scores for degree of bother from ulcerations (p < 0.0001), greater self-reported mouth pain (p < 0.0001), and mouth sensitivity (p < 0.0001, Fig. 2).
Figure 2.
Patient self-reported symptoms and oral cGVHD status. A comparison of the distribution of self-reported symptoms between patients with and without oral cGVHD (N = 187). A comparison of oral cGVHD status and the distribution of scores from (A) Lee Symptoms Scale on the degree of bother from avoidance of foods, (B) Lee Symptoms Scale on the degree of bother from ulcerations, (C) self-reported mouth pain, and (D) self-reported mouth sensitivity. 165 x 153 mm (150 x 150 DPI).
Survival Analysis (univariate)
The median follow-up time for survivors was 31.6 mos (range, 4-71 mos). The 3-year post-enrollment survival probability for patients with non-oral cGVHD was 82.5% (95% CI: 74.2 to 88.5%) and 73.4% (95% CI: 56.2 to 85.6%) for patients with oral cGVHD (p = 0.22 for overall comparison of curves) (Appendix Fig. 5).
Predictive Model for Determining Oral cGVHD Activity
Results of the univariate analysis revealed that cGVHD onset type, Lee Symptoms Scale scores for degree of bother from avoidance of foods and from ulcerations, NIH average score, albumin levels, total complement levels, mouth pain, and mouth sensitivity were possible candidates for determining association with oral cGVHD. The final multivariable logistic regression model based on 136 patients with complete data on all included parameters, determined by backward selection, indicated that albumin levels (p < 0.0001), total complement levels (p = 0.0046), and mouth pain (p < 0.0001) were significantly jointly associated with oral cGVHD (Table 2).
The final model was converted into a classification equation for predicting oral cGVHD activity. The classification equation for predicting oral cGVHD activity was if [(1.1516*albumin) – (0.0158*total complement) – (0.3530*mouth pain)] ≤ 1.04597. The classification equation for predicting no oral cGVHD activity was if [(1.1516*albumin) – (0.0158* total complement) – (0.3530*mouth pain)] > 1.04597. This final model was then applied to the original data from which it was developed to determine its predictive accuracy. The model predicted the correct classification of 80.2% of those without oral GVHD and 74.3% of those with oral GVHD when the model was applied in the same cohort of 136 patients used to develop the model.
Discussion
The low oral cGVHD prevalence of 23.5% identified in the study population can be attributed to the use of a conservative definition of clinically significant disease, which excluded NIH Oral cGVHD Scale total scores of 1 and 2. This definition is based on previous research which identified minimal clinically detectable change of 2.1 to 2.9 points on this scale (Mitchell et al., 2011). The definition used in this study is founded on evidence-based measures and should ensure reliability and accuracy of diagnosing oral cGVHD in clinical practice and research.
This study revealed that patients with oral cGVHD were more likely to have quiescent (new onset after resolution of acute GVHD) and de novo (no history of prior acute GVHD) onset of cGVHD, rather than progressive onset (from ongoing acute GVHD). This may explain the previously described association between oral cGVHD and better survival, since the progressive type of onset is a well-known poor prognostic factor for survival in cGVHD across many studies (Akpek et al., 2003; Perez-Simon et al., 2006; Arora et al., 2007). Survival analysis revealed no statistically significant difference in the survival probabilities between patients with or without oral cGVHD; 3-year probabilities were 73.4% and 82.5%, respectively. Lack of significant impact of oral cGVHD on survival could be a consequence of this referral-based cohort of patients, which is enriched for patients with severe cGVHD manifestations (including skin sclerosis and lung) at later times post-transplant.
Patients with mucosal lesions may experience pain that significantly impedes their ability to eat, communicate, and maintain proper oral hygiene (Imanguli et al., 2008). Oral cGVHD has also been associated with altered taste and intolerance for spicy and acidic foods (Schubert and Correa, 2008). Oral dryness is consistently associated with poor quality of life (Fall-Dickson et al., 2010). In this study, patients with oral cGVHD reported having higher Lee Symptoms Scale bother scores from avoidance of foods as well as from ulcerations and also reported having greater mouth pain and sensitivity as compared with patients without oral cGVHD. The lack of association between oral cGVHD and oral dryness observed in our study indicates that this scale does not adequately measure the salivary component of oral cGVHD. Salivary function-specific instruments, including oral dryness scales and salivary flow tests, should be used in conjunction with the NIH oral cGVHD Scale. Nonetheless, the impact of oral cGVHD on symptoms and quality of life highlights the importance of accurate diagnosis and subsequent disease management.
The pathogenesis of oral cGVHD remains poorly understood (Pavletic and Baird, 2006). Oral cGVHD is characterized by lymphocytic infiltration of the mucosa and salivary glands with variable apoptosis of epithelial cells (Shulman et al., 2006). This study confirmed reports that lower albumin levels and higher total complement, well-described negative and positive acute-phase reactants, were associated with oral cGVHD, indicating ongoing tissue inflammation (Gabay and Kushner, 1999). This was further validated by Grkovic et al., who showed elevated serum total complement and lower albumin correlate with active systemic cGVHD (Grkovic et al., 2012). This association between systemic and oral cGVHD supports the proposed definition of clinically significant oral cGVHD.
Limitations of our study include the cross-sectional design that prevents the longitudinal assessment of oral cGVHD. Limited representation of pediatric patients in our study population may have limited the generalizability of our results. Future studies should emphasize the accrual of pediatric patients. These results are based on an instrument that measures mucosal changes and does not assess salivary or sclerotic involvement. Laboratory markers were based on peripheral blood samples, not target tissue or saliva. Finally, the study cohort derived from referrals to the NIH, which includes more severely affected patients with therapy-refractory moderate to severe cGVHD. Future prospective multidisciplinary studies are needed to better examine the complexity of oral cGVHD in newly diagnosed patients. Major challenges in conducting prospective clinical trials in this patient population include late post-transplant onset and a chronic course mandating a lengthy period of data collection and follow-up, which are logistically demanding.
In summary, this study provides an NIH Oral cGVHD Scale-based practical definition of clinically relevant oral cGVHD that accounts for observer variability. This definition is based on total mucosal changes, and future studies should evaluate each component of the scale relative to oral GVHD-specific outcomes. Analysis of factors associated with prediction of oral cGVHD according to this definition revealed that 74% of those with oral cGVHD could be predicted on the basis of three key parameters (albumin, total complement, and mouth pain), as could 80% of those without oral cGVHD. This finding should be validated independently to confirm the associations identified. This definition of oral cGVHD by the NIH 15-point scale provides a reproducible measure of clinically meaningful disease for use in clinical settings and as an endpoint in preventive and therapeutic trials.
Footnotes
This study was supported by the Clinical Research Training Program, supported by Pfizer Inc. and the NIH Intramural Research Program of the National Cancer Institute.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Agresti A. (1990). Categorical data analysis. New York: John Wiley & Sons. [Google Scholar]
- Akpek G, Lee SJ, Flowers ME, Pavletic SZ, Arora M, Lee S, et al. (2003). Performance of a new clinical grading system for chronic graft-vs.-host disease: a multicenter study. Blood 102:802-809. [DOI] [PubMed] [Google Scholar]
- Arora M, Nagaraj S, Wagner JE, Barker JN, Brunstein CG, Burns LJ, et al. (2007). Chronic graft-vs.-host disease (cGVHD) following unrelated donor hematopoietic stem cell transplantation (HSCT): higher response rate in recipients of unrelated donor (URD) umbilical cord blood (UCB). Biol Blood Marrow Transplant 13:1145-1152. [DOI] [PubMed] [Google Scholar]
- Baird K, Pavletic SZ. (2006). Chronic graft vs. host disease. Curr Opin Hematol 13:426-435. [DOI] [PubMed] [Google Scholar]
- Copelan EA. (2006). Hematopoietic stem-cell transplantation. N Engl J Med 354:1813-1826. [DOI] [PubMed] [Google Scholar]
- Elad S, Zeevi I, Or R, Resnick IB, Dray L, Shapira MY. (2010). Validation of the National Institutes of Health (NIH) scale for oral chronic graft-vs.-host disease (cGVHD). Biol Blood Marrow Transplant 16:62-69. [DOI] [PubMed] [Google Scholar]
- Fall-Dickson JM, Mitchell SA, Marden S, Ramsay ES, Guadagnini JP, Wu TX, et al. (2010). Oral symptom intensity, health-related quality of life, and correlative salivary cytokines in adult survivors of hematopoietic stem cell transplantation with oral chronic graft-vs.-host disease. Biol of Blood and Marrow Transplant 11:948-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. (2005). National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-vs.-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11:945-956. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. (1999). Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448-454. [DOI] [PubMed] [Google Scholar]
- Grkovic L, Baird K, Steinberg SM, Williams KM, Pulanic D, Cowen EW, et al. (2012). Clinical laboratory markers of inflammation as determinants of chronic graft-vs.-host disease activity and NIH global severity. Leukemia 26:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanguli MM, Alevizos I, Brown R, Pavletic SZ, Atkinson JC. (2008). Oral graft-vs.-host disease. Oral Dis 14:396-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Vogelsang G, Flowers ME. (2003). Chronic graft-vs.-host disease. Biol Blood Marrow Transplant 9:215-233. [DOI] [PubMed] [Google Scholar]
- Mehta CR, Patel NR. (1983). A network algorithm for performing Fisher‘s exact test in r x c contingency tables. J Am Stat Assoc 78:427-434. [Google Scholar]
- Meier JK, Wolff D, Pavletic S, Greinix H, Gosau M, Bertz H, et al. (2011). Oral chronic graft-vs.-host disease: report from the International Consensus Conference on clinical practice in cGVHD. Clin Oral Investig 15:127-139. [DOI] [PubMed] [Google Scholar]
- Mitchell SA, Jacobsohn D, Thormann Powers KE, Carpenter PA, Flowers ME, Cowen EW, et al. (2011). A multicenter pilot evaluation of the National Institutes of Health chronic graft-vs.-host disease (cGVHD) therapeutic response measures: feasibility, interrater reliability, and minimum detectable change. Biol Blood Marrow Transplant 17:1619-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavletic SZ, Baird K. (2006). Chronic graft vs. host disease. Curr Opin Hematol 13:426-435. [DOI] [PubMed] [Google Scholar]
- Pavletic SZ, Lee SJ, Socie G, Vogelsang G. (2006a). Chronic graft-vs.-host disease: implications of the National Institutes of Health consensus development project on criteria for clinical trials. Bone Marrow Transplant 38:645-651. [DOI] [PubMed] [Google Scholar]
- Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. (2006b). Measuring therapeutic response in chronic graft-vs.-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-vs.-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 12:252-266. [DOI] [PubMed] [Google Scholar]
- Perez-Simon JA, Sanchez-Abarca I, Diez-Campelo M, Caballero D, San Miguel J. (2006). Chronic graft-vs.-host disease: pathogenesis and clinical management. Drugs 66:1041-1057. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Correa ME. (2008). Oral graft-vs.-host disease. Dent Clin North Am 52:79-109, viii-ix. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK. (1992). Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer 69:2469-2477. [DOI] [PubMed] [Google Scholar]
- Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. (2006). Histopathologic diagnosis of chronic graft-vs.-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-vs.-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 12:31-47. [DOI] [PubMed] [Google Scholar]