Abstract
Streptococcus mutans resides in the oral polymicrobial biofilm and is a major contributor to the development of dental caries. Interestingly, high salivary nitrite concentrations have been associated with a decreased prevalence of dental caries. Moreover, the combination of hydrogen peroxide–producing oral commensal streptococci and nitrite has been shown to mediate the generation of reactive nitrogen species, which have antimicrobial activity. The goal of this study was to examine whether nitrite affects S. mutans virulence during polymicrobial infections with the commensal Streptococcus parasanguinis. Here, we report that the combination of S. parasanguinis and nitrite inhibited S. mutans growth and biofilm formation in vitro. Glucan production, which is critical for S. mutans biofilm formation, was also inhibited in 2-species biofilms with S. parasanguinis containing nitrite as compared with biofilms that contained no nitrite. In the in vivo caries model, enamel and dentin carious lesions were significantly reduced in rats that were colonized with S. parasanguinis prior to infection with S. mutans and received nitrite in the drinking water, as compared with animals that had a single S. mutans infection or were co-colonized with both bacteria and received no nitrite. Last, we report that S. mutans LiaS, a sensor kinase of the LiaFSR 3-component system, mediates resistance to nitrosative stress. In summary, our data demonstrate that commensal streptococci and nitrite provide protection against S. mutans pathogenesis. Modulating nitrite concentrations in the oral cavity could be a useful strategy to combat the prevalence of dental caries.
Keywords: Streptococcus mutans, probiotics, nitric oxide, antimicrobials, biofilms, bacterial virulence
Introduction
Oral microbes regulate homeostatic conditions in the mouth. Host, dietary, and bacterial-derived metabolites influence polymicrobial interactions that affect disease outcomes. Due to the complexity of the oral cavity, nutrient shifts can trigger an imbalance of microbes that promote disease. For example, sucrose enhances the pathogenesis of bacteria that cause dental caries (Moye et al. 2014). Alternatively, commensals can maintain homeostasis through hydrogen peroxide (H2O2) production or by reducing nitrate to nitric oxide, which has antimicrobial activity and regulates systemic homeostasis (Kreth et al. 2008; Hyde et al. 2014; Qu et al. 2016).
Streptococcus mutans is a member of the oral biofilm that contributes to caries development (Koo et al. 2013). Interestingly, elevated salivary nitrate and nitrite concentrations have been associated with reduced caries prevalence (Doel et al. 2004). Yet, mechanisms that govern how nitrite protects against dental caries are poorly understood. H2O2 produced by oral bacteria has been shown to interact with nitrite in a manner that inhibits pathogenic bacteria (Scoffield and Wu 2015, 2016), but whether this mechanism reduces caries formation is unknown. However, both nitrite and H2O2 are precursors for reactive nitrogen species (RNS) that are bactericidal (Scoffield and Wu 2015).
Due to fluxes in salivary nitrite concentrations and the ability of some oral bacteria to convert nitrite to RNS (Hyde et al. 2014; Qu et al. 2016), it is likely that S. mutans encounters RNS within oral biofilms. Therefore, we sought to understand the role of nitrite in modulating S. mutans pathogenesis during coinfection with the commensal Streptococcus parasanguinis. Here, we report that S. parasanguinis and nitrite restricted the growth and biofilm of S. mutans in vitro and hindered S. mutans pathogenesis in a rat caries model. Moreover, we identified a S. mutans histidine kinase, LiaS, that mediates resistance to S. parasanguinis and nitrite activity. Our study highlights the importance of nitrite in structuring the composition and function of the oral biofilm and provides new insight for the development of strategies that can promote oral health.
Materials and Methods
Bacterial Strains and Culture Conditions
S. mutans UA159, S. parasanguinis FW213, and derivatives were grown in Todd-Hewitt broth in an atmosphere of 5% CO2 at 37 °C. Sodium nitrite and sodium nitrate were used at a concentration of 2 mM and H2O2 and peroxynitrite (Cayman Chemical) at 1 mM and 40 µM, respectively. The S. mutans liaS mutant (10 µg/mL, erythromycin) was kindly provided by Dr. Sara Palmer (Ohio State University). To complement the liaS mutation, the liaS coding region was polymerase chain reaction (PCR) amplified from UA159, cloned into the SalI and KpnI sites of the streptococcus and E. coli multicopy shuttle vector pVPT-gfp (Zhou et al. 2008), and transformed into the liaS mutant. Complementation was verified by PCR analysis.
Competition Assays
A 10-μL subculture of S. parasanguinis was inoculated onto a Todd-Hewitt agar plate (±2mM nitrite). After overnight incubation, a 10-μL subculture of S. mutans was inoculated next to S. parasanguinis and incubated overnight in an atmosphere of 5% CO2 at 37 °C. Inhibition of S. mutans was assessed by the zone of inhibition at the intersection with S. parasanguinis.
Biofilm Assays
Biofilms were measured with crystal violet (O’Toole 2011). S. mutans and S. parasanguinis were subcultured to an OD470 of 0.5 and inoculated at a 1:1,000 dilution in tryptic soy broth with 0.5% yeast extract containing 1% sucrose, either separately or mixed for mono- or dual-species biofilms. Two hundred microliters of each was added to sterile 96-well plates (Nunc) and incubated for 16 h. Biofilms were stained with 0.1% crystal violet, dissolved with 30% acetic acid, and measured at 562 nm. To quantify each bacterial species, biofilms were washed with phosphate-buffered saline (PBS), scraped from the bottom of each well, vortexed, serially diluted, and plated on blood agar. Colony-forming units (CFUs) were counted after 24 h. Each assay was performed in triplicate and repeated 3 times.
Confocal Laser Scanning Microscopy
S. mutans labeled with green fluorescent protein and S. parasanguinis harboring an mCherry plasmid were used for microscopy. Prior to incubation, 1μM dextran–conjugated Cascade Blue (Molecular Probes, Invitrogen) was added to biofilms to monitor glucan production. Biofilms were grown in an 8-well slide (ibidi) as previously described (Scoffield et al. 2017) and examined with a Nikon A1+ confocal laser scanning microscope (CLSM; Nikon Instruments Inc.).
Rat Caries Model
The caries model was performed as previously described (Garcia et al. 2017). Briefly, 17-d-old Fischer 344 pups were randomly assigned into 4 groups of 5 animals each. Each group was either singly infected with S. mutans or colonized with S. mutans and S. parasanguinis. Each group received plain drinking water or water that contained 2mM nitrite. Groups colonized with S. parasanguinis were swabbed with an overnight culture 24 h prior to being infected with S. mutans. Animals were fed a caries-promoting diet (Teklad Diet 305) containing 5% sucrose (Harlan Laboratories, Inc.). Colonization of S. parasanguinis and S. mutans were confirmed by plating oral swab samples. After 60 d, animals were sacrificed, mandibles defleshed, and the right mandible placed in PBS and sonicated. Plaque samples were diluted in PBS and plated on mitis-salivarius and blood agar plates to enumerate and differentiate S. mutans and S. parasanguinis CFUs. Mandibles were cleaned, stained overnight with 0.4% murexide, dried, sectioned, and scored for caries by the method of Keyes (1958), which measures the number of caries lesions on the tooth surface. Animal use was approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (20563).
Microbiome Analysis
The V4 region of the 16S rRNA gene was amplified and sequenced on an Illumina Miseq as previously discussed (Kumar et al. 2014). Sequences were analyzed with the QIIME 1.7 suite (Quantitative Insight into Microbial Ecology; Caporaso et al. 2010) and a QIIME wrapper called QWRAP (Kumar et al. 2014) and assigned to operational taxonomic units (OTUs) with uclust at 97% similarity. OTUs were assigned taxonomic groups with the Ribosomal Database Project classifier (Wang et al. 2007) and the May 2013 of the Greengenes 16S rRNA sequence database (DeSantis et al. 2006). To determine the differences in taxonomic frequency underlying differences in clustering, OTUs were grouped by genera and tested for significant differences in frequency among groups with the Kruskal-Wallis test (P < 0.05 after false discovery rate correction). Additional details are in the Appendix Materials and Methods.
Transposon Mutagenesis of the S. mutans Genome
Genomic DNA was isolated with the Gentra Puregene Yeast/Bact. Kit (Qiagen). Briefly, 1 µg of DNA was incubated in the presence of HimarC9 transposase and 1 µg of plasmid magellan6 as a donor of the Himar1 Mariner transposon (van Opijnen et al. 2015); however, the original spectinomycin cassette was replaced with an erythromycin cassette (Chen et al. 2002). After repair of the transposition products with T4 DNA polymerase and E. coli DNA ligase, 1 µg of mutagenized DNA was used for transformation in S. mutans. The transposon library was screened for mutants that conferred resistance to peroxynitrite and S. parasanguinis and nitrite-mediated inhibition. Transposon insertion mutants were mapped as described (van Opijnen et al. 2015). Additional details are provided in the Appendix Materials and Methods.
Quantitative Real-time PCR
RNA was extracted from S. mutans with the Direct-zol kit (Zymo Research). Additional details are provided in the Appendix Materials and Methods.
Results
Nitrite Promotes Inhibition of S. mutans by S. parasanguinis
Elevated levels of nitrogenous intermediates are associated with a reduced prevalence of tooth decay (Doel et al. 2004; Hohensinn et al. 2016) and work synergistically with oral commensals to attenuate bacterial pathogenesis (Scoffield and Wu 2015). We hypothesized that the activity elicited by nitrite and commensal bacteria contribute to reduced caries. To test this hypothesis, we performed a plate inhibition assay (±2mM nitrite) with the oral commensal S. parasanguinis and S. mutans. S. mutans was uninhibited by S. parasanguinis when grown on media that contained no nitrite but was inhibited by S. parasanguinis in the presence of nitrite (Fig. 1A). Next, we tested whether S. mutans biofilm formation is disturbed by nitrite and S. parasanguinis activity. Single-species S. mutans formed robust biofilms with or without nitrite as compared with single-species S. parasanguinis biofilms (Fig. 1B). Interestingly, dual-species biofilms of S. mutans and S. parasanguinis produced a smaller biofilm than a single-species S. mutans biofilm, and the presence of nitrite further reduced the dual-species biofilm (Fig. 1B). CFU quantification and CLSM revealed an increase in S. parasanguinis cell number in the 2-species biofilms (± nitrite) as compared with a single-species biofilm. In contrast, S. mutans cell number was reduced in the 2-species biofilm as compared with a single-species S. mutans biofilm, and nitrite further suppressed the coexistence of S. mutans in the dual biofilm. CLSM showed that S. mutans single-species biofilms containing nitrite consisted of fewer large bacterial aggregates when compared with biofilms that contained no nitrite (Fig. 1D). In addition, S. mutans biofilms that contained nitrite produced less glucan (blue), an exopolysaccharide component of the S. mutans biofilm matrix (Koo et al. 2010). Similarly, S. mutans produced less glucan in the dual-species biofilm than in the single-species biofilm, and nitrite promoted the reduction of glucan in the dual-species biofilm as compared with the dual-species biofilm containing no nitrite (Fig. 1D). Taken together, these data demonstrate that nitrite mediates the inhibition of S. mutans during coculture with S. parasanguinis.
Figure 1.
Nitrite promotes the inhibition of Streptococcus mutans growth and biofilm formation by Streptococcus parasanguinis. (A) Plate inhibition assay of Sp (S. parasanguinis) and Smu (S. mutans) on Todd-Hewitt agar ±2mM nitrite. Sp was plated 24 h prior to spotting Smu. (B) Crystal violet and (C) CFU quantification of biofilm biomass of single- and dual-species Sp and Smu biofilms. Values are presented as mean ± SD. (D) Confocal laser scanning microscopy of labeled biofilms: Sp-mCherry (red), Smu-GFP (green), and Smu glucan (blue). All biofilms were grown for 16 h. Data are representative of three experiments performed in triplicate. Scale bar: 20 µM. *P < 0.05. CFU, colony-forming units; ns, not significant.
Dietary Nitrite and S. parasanguinis Reduce S. mutans Pathogenesis in a Rat Caries Model
Our in vitro data showed that nitrite inhibited S. mutans when co-colonized with S. parasanguinis. As a result of these findings, we hypothesized that nitrite attenuates S. mutans virulence in vivo during coinfection with S. parasanguinis. We colonized the rat oral cavity with S. parasanguinis (for dual infections only) 24 h prior to infection with S. mutans. Rats received plain drinking water or water that contained 2mM nitrite. Single infections with S. mutans resulted in fewer carious lesions in the enamel and dentin (slight, moderate, and extensive) buccal surfaces in animals that received nitrite as compared with animals that received no nitrite (Fig. 2A, B). Colonization with S. mutans and S. parasanguinis and nitrite supplementation resulted in a significant reduction in enamel and dentin buccal caries as compared with dual infections where nitrite was not given. A reduction in carious lesions during co-colonization with both species and nitrite supplementation was also consistent in the sulcal (Fig. 2C, D) and proximal (Fig. 2E, F) enamel and dentin surfaces. We did not detect any slight lesions in the dentin proximal surface in animals colonized with both bacteria that received nitrite (Fig. 2F), although this was not statistically significant. There were no moderate and extensive proximal carious lesions detected in any treatment group. These findings suggest that in an animal model, nitrite is effective only in the presence of S. parasanguinis. Microbiome analysis of plaque samples recovered from the animal study revealed small shifts in the microbiota at the genus level at the end of the study. Bifidobacterium abundance was significantly (P < 0.001) enriched in rats that were singly infected with S. mutans and received nitrite as compared with rats that did not receive nitrite (Appendix Fig. 1A). Similar to a single infection, colonization of S. mutans produced a shift in Tissierella abundance, which persisted until the end of the study in animals co-colonized with S. parasanguinis (Appendix Fig. 1B). Also, Clostridium was enriched in animals that were co-colonized with S. mutans and S. parasanguinis and received nitrite-supplemented drinking water as compared with animals that received plain drinking water (Appendix Fig. 1B). Quantification of S. mutans and S. parasanguinis recovered from plaque samples showed no significant difference in bacterial colonization (Appendix Fig. 1C, D). Overall, quantification of S. mutans and S. parasanguinis demonstrated that nitrite and S. parasanguinis interfere with S. mutans pathogenesis but do not affect S. mutans colonization in the rat oral cavity.
Figure 2.
Dietary nitrite reduces the pathogenesis of Streptococcus mutans during coinfection with Streptococcus parasanguinis in a rat caries model of infection. (A) Buccal enamel caries score. (B) Slight, moderate, and extensive dentin caries scores for buccal surface. (C) Sulcal enamel caries scores. (D) Slight, moderate, and extensive dentin caries scores for sulcal surface. (E) Proximal enamel caries scores. (F) Slight dentin scores for proximal surface. n = 5 per group. Differences among groups were compared by analysis of variance. Values are presented as mean ± SD. *P < 0.05. **P < 0.01. ***P < .001. ND, not detected; ns, not significant.
Loss of LiaS Mediates Resistance to Nitrosative Stress
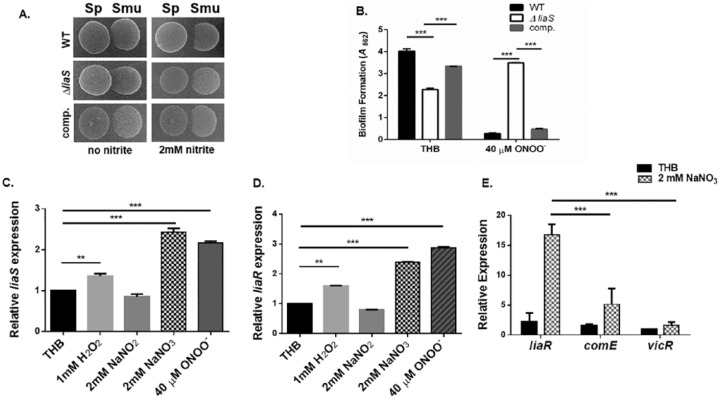
Previous reports have shown that acidified (pH, 5) nitrite and H2O2 generate the production of peroxynitrite, an RNS with potent antimicrobial activity (Kono et al. 1994; Heaselgrave et al. 2010). Similarly, H2O2-producing S. parasanguinis and nitrite presumably generate peroxynitrite (Scoffield and Wu 2015). S. mutans biofilms cocultured with a S. parasanguinis ∆ poxL mutant (deficient for H2O2 production) were more robust in the presence of nitrite when compared with S. mutans biofilms cultured with wild-type S. parasanguinis (Appendix Fig. 2). This result suggests that the inhibition of S. mutans by S. parasanguinis is H2O2 and nitrite dependent and may involve the production of peroxynitrite, particularly since cocultures of S. parasanguinis and S. mutans reach a pH of 4.72 in the presence of nitrite (Appendix Fig. 3). Although H2O2 can inhibit S. mutans to some degree, the addition of nitrite further enhanced the inhibition of S. mutans, and this phenotype can be mediated by S. parasanguinis or exogenous H2O2 (Appendix Fig. 4). To better understand how nitrosative stress mediated by S. parasanguinis and nitrite activity affects S. mutans physiology, we explored mechanisms of resistance by mutagenizing the S. mutans genome with a transposon and screened for mutants resistant to S. parasanguinis/nitrite and peroxynitrite activity. One transposon-resistant mutant mapped to the liaS gene, a histidine kinase of the LiaFSR 3-component system. Basic Local Alignment Search Tool (BLAST) analysis revealed that LiaS has homology to the NarX/NarL nitrate/nitrite sensing system in Escherichia coli (Lee et al. 1999). Given the homology of LiaS to other bacterial nitrate/nitrite sensors, we characterized the role of LiaS in regard to the ability of S. mutans to resist nitrosative stress and sense nitrogenous intermediates.
With the plate inhibition assay, the liaS mutant displayed more resistance to S. parasanguinis and nitrite-mediated activity, and its biofilm was more resistant to peroxynitrite when compared with wild-type S. mutans and the complemented strain (Fig. 3A, B). It is important to note that the liaS mutant has a slight growth advantage in plain Todd-Hewitt broth (±2mM nitrite) as compared with wild-type S. mutans (Appendix Fig. 5 and Table 3). Due to the involvement of LiaS in nitrosative stress resistance, we questioned whether the expression of liaS and liaR (response regulator) is upregulated by nitrogenous and oxidative intermediates since the inhibition of S. mutans is both H2O2 and nitrite dependent. Ironically, liaS and liaR expression was significantly (>2-fold) upregulated in the presence of nitrate and peroxynitrite but not nitrite and was slightly induced by H2O2 (Fig. 3C, D). In addition, genes (SMU.753, SMU.1727, and SMU.2084) that have been found to be directly regulated by LiaR (Shankar et al. 2015) were also significantly more upregulated by the addition of nitrate instead of nitrite (Appendix Fig. 6). Next, we compared the expression of liaR with the expression of 2 global S. mutans response regulators, comE and vicR (Kreth et al. 2006; Tremblay et al. 2009; Peng et al. 2016), in response to nitrate to evaluate whether the LiaFSR systems displays more specificity to nitrate as compared with other response regulators. Strikingly, liaR was expressed approximately 7- and 10-fold more than comE and vicR (Fig. 3E); however, more studies are required to definitively determine if LiaFSR is indeed a nitrate-sensing regulatory system. In summary, loss of LiaS mediates resistance to S. parasanguinis and nitrite-mediated nitrosative stress.
Figure 3.
Loss of LiaS mediates resistance to Streptococcus parasanguinis and peroxynitrite-mediated nitrosative stress. (A) Plate inhibition assay of S. parasanguinis (Sp) and wild-type Streptococcus mutans, the liaS mutant, and complemented strains. (B) Biofilm formation of wild-type S. mutans, the liaS mutant, and complemented strains. RT-qPCR of (C) liaS and (D) liaR in response to hydrogen peroxide, nitrite, nitrate, and peroxynitrite. (E) RT-qPCR of S. mutans response regulators liaR, comE, and vicR in response to nitrate. Values are presented as mean ± SD. **P < 0.01. ***P < 0.001. comp, complemented strains; RT-qPCR, quantitative reverse transcription polymerase chain reaction; THB, Todd-Hewitt broth; WT, wild type.
Discussion
The oral cavity is a dynamic environment that is frequently perturbed by nutritional fluxes. The nitrate-nitrite-nitric oxide pathway, which is regulated by oral commensals, maintains systemic homeostasis (Hyde et al. 2014; Ambe et al. 2016). Nitrite derived from this pathway or diet, in addition to H2O2 produced by oral commensals, provides precursors for antimicrobial RNS (Scoffield and Wu 2015). Unfortunately, the role of RNS in the context of oral polymicrobial infections is poorly understood. In this study, we investigated how nitrite affects S. mutans during coinfection with S. parasanguinis. We report that nitrite and S. parasanguinis interfere with the growth and biofilm of S. mutans in vitro and attenuate S. mutans pathogenesis in a rat caries model of infection. Furthermore, we identified a histidine kinase (LiaS) that is upregulated in the presence of nitrate and peroxynitrite and mediates resistance to nitrosative stress. Our study highlights the significance of oral commensals in maintaining oral health through environmental cues involving nitrite availability.
Elevated nitrite and nitrate concentrations are associated with a decreased prevalence of dental caries (Hohensinn et al. 2016). Our data suggest that oral commensal bacteria and nitrite work in concert to provide protection against S. mutans. Intermediates of the nitrate-nitrite-nitric oxide pathway participate in varying roles in host defense against bacterial pathogens. For example, nitrite and H2O2-producing commensal streptococci protect Drosophila melanogaster from killing by Pseudomonas aeruginosa (Scoffield and Wu 2015). In addition, nitric oxide inhibits cell viability of periodontal pathogens Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis (Backlund et al. 2014), displays antibiofilm properties against S. mutans and P. aeruginosa, and prevents the establishment of Candida albicans biofilms in a rodent catheter model (Barraud et al. 2006; Ahmadi et al. 2016; Backlund et al. 2016). Circulation of nitrogenous intermediates could be beneficial in restricting pathogens while promoting commensals, particularly since S. parasanguinis was not inhibited by nitrite in our study. Additionally, our microbiome data suggest that nitrite supports the enrichment of Bifidobacteria, which has been used as a probiotic to reduce salivary levels of S. mutans (Cagetti et al. 2013; Ashwin et al. 2015). Although nitrite and S. parasanguinis did not impair S. mutans colonization in vivo, S. mutans pathogenesis was inhibited, and S. parasanguinis levels remained abundant. In contrast, S. mutans displaces colonization of a similar oral commensal, Streptococcus gordonii, in the rat caries model (Tanzer et al. 2012). Comparatively, our study signifies the unique efficacy of nitrite and S. parasanguinis in oral health maintenance and warrants further investigation of how nitrite not only structures oral polymicrobial communities but alters their function in a manner that promotes human health.
Mechanisms of nitrosative stress resistance in oral bacteria are poorly understood. A transposon mutant that mapped to the liaS gene conferred resistance to nitrite/S. parasanguinis and peroxynitrite inhibition. LiaS displays homology to the NarL/NarX 2-component system of E. coli (Lee et al. 1999), and expression of liaS (histidine kinase) and liaR (response regulator) was upregulated in response to nitrate and peroxynitrite. The LiaFSR system modulates envelope stress, mutacin production, and biofilm formation (Chong et al. 2008; Suntharalingam et al. 2009; Zhang and Biswas 2009; Shankar et al. 2015). It is unclear how loss of LiaS mediates resistance to nitrosative stress. One possible explanation is that deletion of liaS promotes a nitrite-dependent growth advantage or alters cell wall permeability in a manner that restricts the activity of toxic molecules. A phenotypic microarray analysis showed that the liaS mutant displays a growth advantage on nitrite and has increased tolerance to antibiotics that inhibit DNA synthesis and target cell wall biosynthesis (Zhang and Biswas 2009). Nevertheless, the role of LiaS in S. mutans nitrosative stress resistance requires further investigation.
In summary, our study highlights the protective effect of nitrite during oral polymicrobial infections and demonstrates how an oral commensal works synergistically with nitrite to promote health. Few studies have examined the contribution of nitrogenous intermediates on bacterial competition within multispecies infections. By exploiting nitrite/nitrate availability, oral commensals could restrict the virulence of oral pathogens. Understanding antagonistic interactions that occur during polymicrobial infections could lead to the development of novel therapeutics.
Author Contributions
J. Scoffield, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S. Michalek, contributed to design, data acquisition, analysis, and interpretation, critically revised the manuscript; G. Harber, contributed to data acquisition, critically revised the manuscript; P. Eipers, C. Morrow, contributed to data acquisition and analysis, critically revised the manuscript; H. Wu, contributed to conception, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519855348 for Dietary Nitrite Drives Disease Outcomes in Oral Polymicrobial Infections by J. Scoffield, S. Michalek, G. Harber, P. Eipers, C. Morrow and H. Wu in Journal of Dental Research
Acknowledgments
The authors thank Dr. Sara Palmer from Ohio State University for gifting the liaS mutant.
Footnotes
A supplemental appendix to this article is available online.
This work was supported by the National Institutes of Health / National Institute of Dental and Craniofacial Research (grant K99 DE025913 to J.S.). The following are acknowledged for their support of the Microbiome Resource at the University of Alabama at Birmingham: Comprehensive Cancer Center (P30AR050948), Center for Clinical Translational Science (UL1TR001417), University Wide Institutional Core, Heflin Center for Genomic Sciences, and Microbiome Center. H. W. is supported by NIH/NIDCR grants R01 DE022350 and R01 DE017954.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahmadi MS, Lee HH, Sanchez DA, Friedman AJ, Tar MT, Davies KP, Nosanchuk JD, Martinez LR. 2016. Sustained nitric oxide-releasing nanoparticles induce cell death in Candida albicans yeast and hyphal cells, preventing biofilm formation in vitro and in a rodent central venous catheter model. Antimicrob Agents Chemother. 60(4):2185–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambe K, Watanabe H, Takahashi S, Nakagawa T, Sasaki J. 2016. Production and physiological role of NO in the oral cavity. Jpn Dent Sci Rev. 52(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwin D, Ke V, Taranath M, Ramagoni NK, Nara A, Sarpangala M. 2015. Effect of probiotic containing ice-cream on salivary mutans streptococci (SMS) levels in children of 6–12 years of age: a randomized controlled double blind study with six-months follow up. J Clin Diagn Res. 9(2):ZC06-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund CJ, Sergesketter AR, Offenbacher S, Schoenfisch MH. 2014. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J Dent Res. 93(11):1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund CJ, Worley BV, Schoenfisch MH. 2016. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 29:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS. 2006. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol. 188(21):7344–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingstrom P, Campus G. 2013. The use of probiotic strains in caries prevention: a systematic review. Nutrients. 5(7):2530–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wu H, Fives-Taylor PM. 2002. Construction of a novel transposon mutagenesis system useful in the isolation of Streptococcus parasanguis mutants defective in Fap1 glycosylation. Infect Immun. 70(12):6534–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P, Drake L, Biswas I. 2008. LiaS regulates virulence factor expression in Streptococcus mutans. Infect Immun. 76(7):3093–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16s rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 72(7):5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doel JJ, Hector MP, Amirtham CV, Al-Anzan LA, Benjamin N, Allaker RP. 2004. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur J Oral Sci. 112(5):424–428. [DOI] [PubMed] [Google Scholar]
- Garcia SS, Blackledge MS, Michalek S, Su L, Ptacek T, Eipers P, Morrow C, Lefkowitz EJ, Melander C, Wu H. 2017. Targeting of Streptococcus mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. J Dent Res. 96(7):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaselgrave W, Andrew PW, Kilvington S. 2010. Acidified nitrite enhances hydrogen peroxide disinfection of Acanthamoeba, bacteria and fungi. J Antimicrob Chemother. 65(6):1207–1214. [DOI] [PubMed] [Google Scholar]
- Hohensinn B, Haselgrubler R, Muller U, Stadlbauer V, Lanzerstorfer P, Lirk G, Hoglinger O, Weghuber J. 2016. Sustaining elevated levels of nitrite in the oral cavity through consumption of nitrate-rich beetroot juice in young healthy adults reduces salivary pH. Nitric Oxide. 60:10–15. [DOI] [PubMed] [Google Scholar]
- Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, et al. 2014. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. 9(3):e88645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes PH. 1958. Dental caries in the molar teeth of rats: II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 37(6):1088–1099. [DOI] [PubMed] [Google Scholar]
- Kono Y, Shibata H, Adachi K, Tanaka K. 1994. Lactate-dependent killing of Escherichia coli by nitrite plus hydrogen-peroxide: a possible role of nitrogen dioxide. Arch Biochem Biophys. 311(1): 153–159. [DOI] [PubMed] [Google Scholar]
- Koo H, Falsetta ML, Klein MI. 2013. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 92(12):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Xiao J, Klein MI, Jeon JG. 2010. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 192(12):3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Zhu L, Shi W, Qi F. 2006. Cell density- and comE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS Microbiol Lett. 265(1):11–17. [DOI] [PubMed] [Google Scholar]
- Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 190(13):4632–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Eipers P, Little RB, Crowley M, Crossman DK, Lefkowitz EJ, Morrow CD. 2014. Getting started with microbiome analysis: sample acquisition to bioinformatics. Curr Protoc Hum Genet. 82:18.8.1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AI, Delgado A, Gunsalus RP. 1999. Signal-dependent phosphorylation of the membrane-bound NarX two-component sensor-transmitter protein of Escherichia coli: nitrate elicits a superior anion ligand response compared to nitrite. J Bacteriol. 181(17):5309–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye ZD, Zeng L, Burne RA. 2014. Fueling the caries process: carbohydrate metabolism and gene regulation by Streptococcus mutans. J Oral Microbiol. 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA. 2011. Microtiter dish biofilm formation assay. J Vis Exp. 47:2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Zhang Y, Bai G, Zhou X, Wu H. 2016. Cyclic di-AMP mediates biofilm formation. Mol Microbiol. 99(5):945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ, Wang SL. 2016. From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J Dent Res. 95(13):1452–1456. [DOI] [PubMed] [Google Scholar]
- Scoffield JA, Duan D, Zhu F, Wu H. 2017. A commensal streptococcus hijacks a Pseudomonas aeruginosa exopolysaccharide to promote biofilm formation. PLoS Pathog. 134:e1006300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffield JA, Wu H. 2015. Oral streptococci and nitrite-mediated interference of Pseudomonas aeruginosa. Infect Immun. 83(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffield JA, Wu H. 2016. Nitrite reductase is critical for Pseudomonas aeruginosa survival during co-infection with the oral commensal Streptococcus parasanguinis. Microbiology. 162(2):376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar M, Mohapatra SS, Biswas S, Biswas I. 2015. Gene regulation by the LiaSR two-component system in Streptococcus mutans. PLoS One. 10(5):e0128083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam P, Senadheera MD, Mair RW, Levesque CM, Cvitkovitch DG. 2009. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J Bacteriol. 191(9):2973–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Thompson A, Sharma K, Vickerman MM, Haase EM, Scannapieco FA. 2012. Streptococcus mutans out-competes Streptococcus gordonii in vivo. J Dent Res. 91(5):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay YD, Lo H, Li YH, Halperin SA, Lee SF. 2009. Expression of the Streptococcus mutans essential two-component regulatory system VicRK is pH and growth-phase dependent and controlled by the LiaFSR three-component regulatory system. Microbiology. 155(Pt 9):2856–2865. [DOI] [PubMed] [Google Scholar]
- van Opijnen T, Lazinski DW, Camilli A. 2015. Genome-wide fitness and genetic interactions determined by Tn-Seq, a high-throughput massively parallel sequencing method for microorganisms. Curr Protoc Microbiol. 36:1E.3.1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 73(16):5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Biswas I. 2009. A phenotypic microarray analysis of a Streptococcus mutans liaS mutant. Microbiology. 155(Pt 1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Fives-Taylor P, Wu H. 2008. The utility of affinity-tags for detection of a streptococcal protein from a variety of streptococcal species. J Microbiol Methods. 72(3):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519855348 for Dietary Nitrite Drives Disease Outcomes in Oral Polymicrobial Infections by J. Scoffield, S. Michalek, G. Harber, P. Eipers, C. Morrow and H. Wu in Journal of Dental Research