Abstract
A low frost-point humidity generator has been developed at NBS to provide a capability for calibration, testing and research at very low levels of water vapor content in such gases as atmospheric air, carbon dioxide and nitrogen. The generator produces frost points from −30 to −100 °C at ambient pressures from 500 to 200,000 pascals (0.005 to 2 atm.). This is equivalent to mixing ratios of 4 × 10−6 to 51 grams of water vapor per kilogram of dry air and to vapor pressures of 1.4 × 10 −3 to 38 pascals. The generated test gas can be fed to a test chamber with independent temperature control between +25 and −100 °C. The uncertainty of the frost point in the test chamber is estimated not to exceed 0.05 deg C. Intercomparisons with a frost-point hygrometer as well as two gravimetric checks gave results which in all cases agreed to within 0.2 deg C in frost-point temperature.
Keywords: Calibration, frost-point, generator, humidity, water vapor
1. Introduction
Measurements of trace quantities of water vapor are being made with increasing frequency in the sciences and in industrial and technological areas. For example, there has been a continual effort to determine the humidity of the stratosphere and the mesosphere; the measurement of the moisture content of planetary atmospheres, particularly that of Mars, continues to challenge astronomers and space scientists; processes in the chemical industry, such as butane isomerization, are affected by very low levels of humidity; and the water vapor content of helium in high temperature nuclear reactors requires close control. The sensors and instruments used for these and similar measurements are often empirical and require calibration. Even instruments based on fundamental principles are susceptible to systematic errors that may go undetected without test, calibration, or evaluation. It is therefore desirable, if not essential, that there be some method for the direct calibration of these devices, or for the calibration of transfer standards that, in turn, can be used to check these devices. To fulfill this need, the National Bureau of Standards has recently developed and put into operation a new calibration facility. The purpose of this paper is to describe this facility. With it, calibrations can be performed over a range of frost points from −30 to −100 °C, at ambient pressures from 500 to 200,000 pascals,1 and at ambient temperatures from −100 to +25 °C.
2. Principle of Operation
This humidity calibration facility is a generator, which produces or generates a stream of gas of known moisture content. This stream is fed directly to the test instrument or, alternatively, to a test chamber into which the test instrument or sensor is placed. The test chamber is independently temperature-regulated.
A stream of dry gas first flows through a heat exchanger and then through a saturator that are both immersed in a constant-temperature liquid bath. The saturator is a long metal tube formed into a helical coil, the interior surface of which (about 1200 cm2) is coated with a film of ice approximately one mm in thickness. During its traverse through the saturator no part of the test gas is farther than 4 mm from the ice surface. At a flow rate of 2 liters per minute the mean transit time through the saturator is about 7 s.
The heat exchanger brings the gas stream to bath temperature. Because the mass of water vapor required to saturate the gas stream is very small, the temperatures of the gas and inner ice surface are not perturbed significantly by the sublimation process. Furthermore, the saturator coil being of sufficient length, saturation is completed in the entrance portion of the coil. The remainder of the coil then serves solely as a final heat exchanger, since there is no significant pressure drop. The gas temperature at the exit end of the saturator is, therefore, the same or very close to the saturation temperature, that is, it is the value of the frost point of the gas stream. The pressure within the saturator can be set and regulated at any desired value from 500 to 200,000 pascals.
3. Description
The generator is schematically depicted in figure 1. It is constructed exclusively of stainless steel. The inner coil S is the saturator. It consists of a 16-foot length of ⅜-in tubing of 0.035-in wall thickness wound into a helix 5½ in in o.d. and 8 in long. The outer coil, E, is a heat exchanger likewise constructed from a 16-foot length of similar tubing wound into a 10-in o.d. helix 4½ in long. A vertical tube, F, of similar tubing is connected to the exit of the heat exchanger and to the entrance of the saturator.
Figure 1.
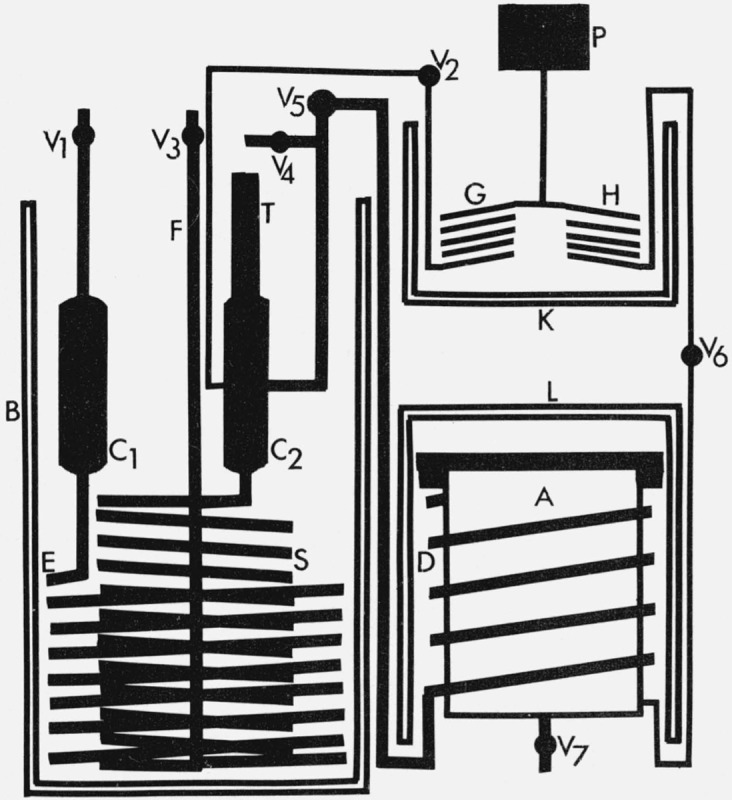
- B Generator Dewar bath
- C1 Heat exchanger cylinder
- E Heat exchanger coil
- F Coating exit tube
- C2 Saturator cylinder
- S Saturator coil
- T Saturator temperature well
- A Test chamber
- D Test chamber heat exchanger coil
- L Test chamber Dewar cylinder
- P Pressure gage
- G Saturator pressure trap coil
- H Test chamber pressure trap coil
- K Pressure trap bath
- V1 Generator inlet valve
- V2 Generator pressure valve
- V3 Coating exit valve
- V4 Coating inlet valve
- V5 Generator exit valve
- V6 Test chamber pressure valve
- V7 Test chamber exit valve
Upstream of the heat exchanger is a 75-cm3 collector cylinder, C1, with an outside diameter of 1½ in and a length of 4½ in. The collector cylinder provides a surface for the freezing out of moisture in the entrance gas which might tend to block the heat exchanger. Downstream of the saturator is a similar saturator cylinder, C2, with an exit port located 1½ in below its top. At this same elevation and diametrically opposite to the exit port is a pressure tap to which is attached tubing of ⅛-in o.d. and 0.035-in wall thickness. This tubing leads to a sensitive quartz helix pressure gage, P. A temperature well -in o.d. and 0.035-in thick extends 2 in into the cylinder, C2, from the top. A bakelite extension tube, T, ½-in o.d. is connected to the well.
There are five valves, V1, V2, V3, V4, V5, associated with the generator. All valves are of the welded bellows type. The lines controlled by these valves are shown in figure 1. Between valve V2 and the pressure gage, P, is a coil, G, of ¼-in o.d. tubing located within a glass Dewar K. With the exception of two pipe fittings in each of the cylinders, all generator connections are either welds or cone compression fittings.
Connected to the generator through valve V5 is a test chamber, A. The chamber is constructed from a stainless steel cylinder 6-in o.d., 6-in long and ¼-in thick. A ¼-in thick stainless steel plate is welded to the bottom and an 8-in vacuum flange is welded to the top. The top of the chamber consists of an 8-in blank vacuum flange and is sealed to the chamber proper with a gold plated copper gasket. Welded into the wall of the test chamber are 12 pass-through electrical connections, six thermocouple wire connectors, a ⅜-in inlet tubing connection near the top, a ⅜-in outlet tubing connection at the bottom and a ¼-in pressure tap connection near the bottom. Valves V5, V7, and V6 respectively control the lines to each of these connections. Surrounding the test chamber is a four-turn helix of ⅜-in o.d. tubing which connects to the inlet and to valve V5. Between valve V6 and pressure gage P is a helical coil of ¼-in o.d. tubing, H, within Dewar K. The entire inner surface of the test chamber including the cover is gold plated. The gold plating is used to reduce the amount of water vapor adsorbed on the walls of the test chamber and thereby reduce equilibration time.
The test chamber rests on expanded polystyrene and is covered with a vacuum-jacketed glass bell jar. All wires and tubing enter the test chamber area through individual holes in the styrofoam insulation. A spiral of ¼-in o.d. copper tubing is located above the test chamber under the bell jar. This spiral is self-supported by a vertical section which extends through the styrofoam insulation. When the test chamber temperature is to be lowered, liquid nitrogen is forced through the spiral and evaporates into the space around the test chamber. The flow of liquid nitrogen is controlled by a solenoid valve which is actuated by an on-off temperature controller operating in conjunction with a thermistor in the space surrounding the test chamber.
The generator can be maintained at any desired temperature by a liquid bath. The generator is suspended from a metal frame above the bath. The bath sits on the platform of a hydraulic lift which can raise the bath until the generator is immersed so that the liquid level is about four inches above the top of the saturator cylinder C2.
The bath is a 50-liter stainless steel Dewar cylinder, measuring 13 in i.d. and 23 in deep. Along the inner wall of the bath is a double helix of ⅜-in stainless steel tubing through which liquid nitrogen is fed for cooling the bath liquid. Between this tubing and the inner wall of the bath are two 8-foot, flexible rod, stainless steel heaters, capable of supplying 2,000 W of heating power.
The bath is filled with a fluid consisting of: methylene chloride 25.3 percent; chloroform 14.5 percent, ethyl bromide 33.4 percent, transdichloroethylene 10.4 percent, and trichloroethylene 16.4 percent by weight. This fluid remains liquid down to −130 °C and is nonflammable [1].1
Suspended from the rack (which supports the generator) are a stirrer and a nickel resistance thermometer which are immersed in the bath liquid when the bath is raised. This resistance thermometer actuates a proportional controller which controls both the heaters and the liquid nitrogen flow and thereby regulates bath temperature. The frost-point temperature is measured by a platinum resistance thermometer located in the temperature well of the saturator cylinder, C2.
The generator is supplied with dry test gas which is cooled to the desired frost-point temperature before it is brought into contact with the ice, avoiding the possibility of condensate being carried through by the gas stream.
If saturation is achieved at the point of temperature and pressure measurement, the accuracy of the generator is dependent on the accuracy of temperature and pressure measurement and the absence of moisture sources or sinks downstream of this point.
4. Operational Procedure
The method of obtaining an ice surface in this generator forms the most important part of the operational procedure and will be described in detail. The method consists of the condensation of water vapor on the inner wall of the saturator tube at −100 °C. At this low temperature the condensate is in the form of ice. In order to obtain a uniform coating of ice, the entrance end of the saturator tube is first cooled, condensation occurring at the end. The cooling is then progressively moved along the saturator tube at a constant velocity providing a continuously new condensation surface until the entire tube is coated with ice.
Prior to coating, the interior of the entire generator is dried thoroughly by passing dry gas through the generator for long periods of time (weeks) at room temperature or for shorter periods of time with the generator in an air oven operating near 100 °C. During this period the bath is empty and remains below the generator. The bath is then filled with the cryogenic fluid, and a stream of moistened gas, at atmospheric pressure, is introduced into the saturator, S. Concurrently a stream of dry gas is introduced into the heat exchanger, E, in order to prevent any deposition of ice on its walls. The two streams join at the tee and emerge through vertical tube F.
The coating operation commences with a vacuum being applied to the outlet of valve V3 and dry gas being fed into valve V1. Valve V1 is adjusted to provide a dry gas inlet flow of between one and two liters per minute. Valves V2 and V5 are closed during this operation. Valve V4 is adjusted to allow an inlet flow of moist gas at approximately 20 liters per minute. Both the dry gas and the moist gas exit through valve V3.
Atmospheric air, or any gas that does not react with water, is moistened so that the partial pressure of the water vapor is in excess of 1000 Pa and is heated to a temperature in excess of 100 °C. This gas enters the saturator cylinder C2 through valve V4.
The bath is raised under manual control to bring the surface of the liquid to the bottom of the saturator and then an automatic mechanism, which raises the bath at a constant rate of in per hour, is activated. The flow of moist gas and the elevation of the bath are continued until the liquid surface of the bath reaches the top of the saturator uppermost coil while the bath fluid is maintained at −100 °C. This process requires more than 7 h. The bath is then raised quickly until the lower half of saturator cylinder C2 is immersed in the bath fluid. The bath liquid is now automatically maintained at this level. The gas flow is continued for an additional 15 to 30 min, then stopped.
The next step is to dry out the exit tubing from cylinder C2. Valves V3 and V4 are closed and the vacuum source is removed from the exit of valve V3. Heated dry air of approximately 100 °C is fed into the exit of the test chamber, A, and valve V5 is opened. When the generator is above atmospheric pressure, valve V3 is opened and the gas is allowed to exhaust into the room. The flow through valve V5 is adjusted to approximately two liters per minute valve V4 is cracked such that there is a small flow (a few cm3 per min) exiting valve V4 into the room. During this time the dry gas flow continues to flow into valve V1. This condition is maintained for 12 to 16 h, after which time valves V3 and V4 are closed and the source of dry gas is removed from the exit of the test chamber. Although the above procedure dries out the exit tubing it removes a negligible amount of the ice in the saturator, due to the fact that the saturator is maintained at −100 °C.
The bath is now raised another 5½ in bringing the surface of the liquid at least 3 in above the saturator cylinder, C2. The bath K is filled with dry ice, causing coils G and H to become vapor traps, thereby minimizing any vapor diffusion between the pressure gage and the generator. The generator is now ready to produce atmospheres of known frost point, pressure and temperature. The generator bath temperature is set to the desired value and valve V2 is opened. For operation below ambient pressure a vacuum source is connected to the test chamber outlet valve V7 and by adjustment of this valve, the pressure in the saturator is set and controlled at the desired value. If temperatures other than ambient are desired in the test chamber, the vacuum jacket bell jar, L, is placed over the test chamber, the liquid nitrogen flow started and the test chamber temperature controller set.
After equilibrium at test conditions has been established, the frost-point temperature in the saturator is determined by measurement with the resistance thermometer in the temperature well T. Freon 11 is used in this well as a heat transfer fluid. Readings of the pressure in the saturator are performed with valve V2 open and valve V6 closed and in the test chamber with valve V6 open and valve V2 closed. These pressure readings provide the data for calculating the difference in frost-point temperature in the test chamber from that measured in the saturator. These measurements also permit the mixing ratio to be computed. Measurement of the test chamber temperature by means of thermocouples provides the additional data for conversion of frost point to any of the common humidity units such as relative humidity. Fixed conditions are maintained for at least 16 h before measurements are attempted on instruments under test or calibration. Sixteen hours was selected to permit a change in conditions to be made everyday and yet allow adequate time for equilibration. The time necessary to achieve equilibration in the test chamber depends on the vapor pressure and temperature as well as flow rate. We estimate that 16 hours will be adequate for all operational conditions.
5. Validation
Reference [3] gives a theoretical analysis of the factors involved in obtaining saturation. Under the operating condition of a 2 liters per minute flow rate, the length of saturator necessary to reduce the degree of unsaturation by a factor of 1/e is 29 cm based on a diffusion constant of 0.1 cm/s which corresponds to the diffusion constant of water vapor into air at atmospheric pressure and −80 °C. The degree of unsaturation for the equipment at 2 liters per minute flow rate is about e−17. At 10 liters per minute, saturation would be 97 percent and at 5 liters per minute, saturation would be 99.9 percent.
A more important consideration at 2 liters per minute is the pressure drop which amounts to approximately 0.025 mb within the saturator. As is shown in reference [3], the pressure drop has a limiting effect on the degree of saturation achievable, independent of the length of the saturator tube. At 2 liters per minute flow and atmospheric pressure, this limit is 99.9988 percent. At 10 liters per minute and atmospheric pressure, this limit would be approximately 99.9935 percent of the saturation at 0 pressure drop.
At reduced pressures, the diffusion constant is greater and the length required for the unsaturation to be reduced by a factor of 1/e is correspondingly less. But the pressure drop through the saturator is relatively independent of the absolute pressure and therefore the relative pressure drop (ΔP/P) is approximately inversely proportional to the absolute pressure. Since the length requirements at reduced pressure are also proportionately reduced this leads to the result that the pressure drop effect is essentially independent of absolute pressure. It may be of interest to note that a 0.1 percent error in saturation has about a 0.009 °C effect on the frost point at −30 °C and decreases to a 0.005 °C effect at a frost point of −100 °C. It is therefore concluded that errors in the degree of saturation are so small as to require no consideration at the operating flow rate of 2 liters per minute.
Estimates of uncertainty have been made based on the accuracies with which the temperature and pressure are measured and the uncertainties in the knowledge of the vapor pressure and the nonideal behavior of the gases.
The accuracy of an individual temperature measurement is ±0.01 °C but short term fluctuations of ±0.02 °C exist in operation. The determination of the frost point temperature might therefore be in error by as much as ±0.03 °C.
The accuracy of the pressure measurement is estimated at ±0.2 percent. The estimated uncertainty in the vapor pressure equation varies with temperature. At −30 °C it is estimated to be ±0.07 percent or ±0.007 °C in frost point and at −100 °C it is estimated to be ±0.54 percent or ±0.026 °C in frost point.
At 1 atm, it is estimated that the uncertainty in moisture content due to uncertainty of the nonideal behavior of moist air varies from ±0.3 percent at −30 °C to ±6 percent at −100 °C [2]. This uncertainty is proportional to pressure. At 5000 Pa, the uncertainty would range from ±0.015 percent at −30 °C to ±0.3 percent at −100 °C.
With regard to frost-point accuracy, only the temperature and pressure measurements are of consequence. With a ±0.2 percent uncertainty in the ratio of the saturation pressure to test pressure, the maximum uncertainty in frost point due to the pressure uncertainty would occur at −30°C frost-point and be ±0.02 °C.
It is, therefore, estimated that the frost-point uncertainty due to both systematic and random errors does not exceed 0.05 °C.
When the moisture content, say mixing ratio, is desired, the uncertainty in the value of vapor pressure and in the correction for nonideal behavior of gases must be added to the uncertainty in frost point. Since the independence of these uncertainties is not established, we take the conservative approach and sum these uncertainties. These errors summed produce an estimated uncertainty in moisture content at 1 atm that varies from 0.9 percent at a frost point −30 °C to 7.4 percent at a frost point of −100 °C. Table 1 gives the estimated total uncertainty in moisture content of a test gas at atmospheric pressure and frost points from −30 to −100 °C. At lower absolute pressures, the uncertainties would be less.
Table 1.
Uncertainties in moisture content
| Frost-point temperature °C | Estimated maximum uncertainties in moisture content due to estimated uncertainties in: | ||||
|---|---|---|---|---|---|
| Temperature measurement percent | Pressure measurement percent | Vapor pressure equation percent | Nonideal gas correction percent | Total percent | |
| −30 | 0.31 | 0.2 | 0.07 | 0.30 | 0.88 |
| −35 | .32 | .2 | .08 | .45 | 1.05 |
| −40 | .34 | .2 | .10 | .60 | 1.24 |
| −45 | .36 | .2 | .12 | .80 | 1.48 |
| −50 | .37 | .2 | .14 | 1.00 | 1.71 |
| −55 | .39 | .2 | .16 | 1.50 | 2.25 |
| −60 | .41 | .2 | .19 | 2.00 | 2.80 |
| −65 | .43 | .2 | .22 | 2.50 | 3.35 |
| −70 | .45 | .2 | .25 | 3.00 | 3.90 |
| −75 | .47 | .2 | .29 | 3.50 | 4.46 |
| −80 | .49 | .2 | .33 | 4.00 | 5.02 |
| −85 | .52 | .2 | .38 | 4.50 | 5.60 |
| −90 | .55 | .2 | .43 | 5.00 | 6.18 |
| −95 | .58 | .2 | .48 | 5.50 | 6.76 |
| −100 | .61 | .2 | .54 | 6.00 | 7.35 |
In order to insure that this generator performs as predicted, it was considered essential that either the generator be checked or its performance verified by some independent means. Therefore, an intercomparison with a precision frost-point hygrometer was made over the frost point range of −30 to −78 °C. The results are given in table 2. This intercomparison is considered to be a nominal confirmation of performance and not a calibration.
Table 2.
Comparison of low frost-point generator with frost-point hygrometer at atmospheric pressure
| Frost point | Mean difference (hygrometer-generator) | Standard deviation | Number of measurements |
|---|---|---|---|
| °C | °C | °C | °C |
| −30 | −0.046 | 0.042 | 6 |
| −50 | −.047 | .045 | 13 |
| −58 | −.048 | .058 | 6 |
| −69 | +.023 | .128 | 9 |
| −78 | −.1l6 | .413 | 2 |
Due to operating limitations on the frost-point hygrometer, comparisons could be made only at atmospheric pressure. It was felt that some verification of performance at reduced pressure was desirable in order to insure proper performance at reduced pressure.
This verification was obtained by making gravimetric moisture measurements at frost points of −49 and −55 °C.
Three absorption tubes arranged in series containing pelletized P2O5 were connected downstream of the test chamber. A vacuum pump was connected to the outlet of the absorption tubes. These tubes were weighed before and after the run in order to determine the total moisture produced by the generator during the run. The test gas was nitrogen supplied by a cylinder and dried. The nitrogen cylinder was weighed before and after the run to determine the amount of dry gas used during the run. The ratio of the mass of moisture to the mass of dry gas is the mixing ratio.
Although the volume flow in the generator was approximately 1.5 liters per minute throughout the run, the volume flow and pressure in the absorption tubes was not known. For this reason, we have no rigorous method of estimating the amount of moisture that may have passed through the absorption tubes without being absorbed nor do we have experience at these conditions which would dictate the correction to be applied due to third tube pick up.
Lacking data for making these corrections, the gravimetric mixing ratio was calculated without correction and the results are given in table 3.
Table 3.
Gravimetric comparisons
| Run A | Run B | ||
|---|---|---|---|
| Mean saturator temperature | −49.47 °C | −55.04 °C | |
| Mean saturator pressure | 4092. Pa | 4630. Pa | |
| Mean mixing ratio | 0.71914 g/Kg | 0.28989 g/Kg | |
| Uncertainties | Mixing ratio | ||
| ± 0.03 °C | |||
| Percent | |||
| ± 0.02 | ± 0.00058 g/Kg | ||
| ± 0.14 | |||
| ± 0.16 | ± 0.00046 g/Kg | ||
| ± 0.05 | |||
| ± 0.08 | ± 0.00023 g/Kg | ||
| Missing data | ± 0.000l5 g/Kg | ||
| Total | |||
| Gravimetric values | |||
| Weight of collected water | 0.7358 ± 0.00044 g | 0.36775 ± 0.00044 g | |
| Weight of expended nitrogen | 1016.83 ± 0.34 g | 1299.78 ± 0.34 g | |
| Additional uncertainties | |||
| Correction for 3d tube pickup | − 0.00501 g | − 0.00627 g | |
| Weight of uncollected water | + 0.0049 g | + 0.0050 g | |
| Comparison | |||
| Generated mixing ratio | |||
| Gravimetrically measured Mixing ratio | |||
| • Equivalent generated frost-point | − 48.784 ± 0.034°C | − 55.028 ± 0.034°C | |
| Gravimetrically determined Frost-point | |||
The −49 °C frost-point run took 6 days and 21 h during which time temperature and pressure data were recorded every 10 min except during malfunctions in the data acquisition system. Such lack of data amounted to 8 percent of the total; however from the trends of the recorded data the values for the missing data were extracted and used in the computation. The −55 °C frost-point run took 8 days and 18 h, during which time 6 percent of the data was missing for which estimated values were used. An uncertainty equal to twice the estimated uncertainty of an individual determination has been assigned to the periods during which data was not collected.
The intercomparison with the frost-point hygrometer gave a maximum difference of 0.12 °C at −78 °C frost point. All other points were in agreement to better than 0.05 °C. The generated frost point at reduced pressure agreed with the gravimetric determinations to within 0.05 and 0.19 °C. The 0.05 °C is well within the estimated band of total uncertainty whereas the 0.19 °C exceeds the estimated band of total uncertainty by 0.01 °C. In all intercomparisons, agreement was better than 0.2 °C in frost point.
6. Discussion and Conclusions
The generator has been operated successfully to produce a wide range of humidities. Although humidties higher than −30 °C in frost point can be established, the ice film in the saturator will not last long enough for most tests. Hence a −30 °C frost point is considered a practical upper limit. Frost points as low as −100 °C have been generated. It is possible to operate the liquid bath, in which the saturator is immersed, at temperature as low as −130 °C and to thereby produce equivalent frost points. However, it is likely that the time required to reach a steady state condition would be excessively long so as to make this impractical.
Any gas can be used as the carrier. Air, nitrogen, and carbon dioxide have been employed without any detectable change in performance. The generator has also been operated at total pressures as low as 500 Pa, again without any observable change in performance.
Two techniques were used to evaluate the performance of the generator: direct measurement of the frost point with a precision frost-point hygrometer and gravimetric measurement of the moisture content with a solid desiccant.
The excellent agreement achieved between the low frost-point generator and the frost-point hygrometer indicates that the generator behaves in a predictable fashion in accordance with the laws of physics, at least at atmospheric pressure in the range of frost points from −30 to −78 °C. There is no reason to suspect any significant departure at lower frost points from predicted behavior. The gravimetric measurements were intended to disclose whether operation of the generator at reduced pressures introduced any unforeseen degradation in performance. It was difficult to design a suitable test for checking both at low frost point and at low absolute pressure. The gravimetric method was chosen because it has great inherent accuracy. Unfortunately at low moisture levels the sampling time is inordinately long and the experiment becomes as much a test of the durability of apparatus and equipment as it is of the humidity. Thus only two runs were made. The agreement achieved, particularly under the circumstances of the test procedure, was again excellent. There does not appear to be any reason for questioning the predicted behavior of the generator at reduced pressures – at least down to 50 mb. There appears to be no reason why the generator would not perform equally well down to 5 mb, the lower operational limit.
Acknowledgments
This work was supported by the Viking Project Office of NASA.
Footnotes
1 Pascal = 1 newton/m2 = 10−5 bar = 10−2 mb = 7.50062 × 10−3 mm Hg.
Figures in brackets indicate the literature references at the end of this paper.
7. References
- [1].Kanolt C. W., Bureau of Standards Sci. Papers 520, Vol. 20, p. 619 (1926). [Google Scholar]
- [2].Wexler Arnold, Calibration of Humidity Measuring Instruments at the National Bureau of Standards, in Analysis Instrumentation Vol. 6, p. 161 (Plenum Press, 1969). [Google Scholar]
- [3].Johnson Daniel P., Note on diffusion of vapor into flowing gas, to be published. [DOI] [PMC free article] [PubMed]


