Abstract
Objectives
Kasai portoenterostomy (KPE) is the primary treatment for biliary atresia (BA) with subsequent liver transplantation in failed cases. The aim of this work was to study the outcome of KPE in children with BA and identify the factors predicting a successful KPE.
Methods
Children diagnosed with BA and undergoing KPE between January 2010 and January 2018 were included in the study. A successful KPE was defined as decrease in bilirubin to less than 2 mg/dL at 6 months after KPE. Factors affecting the outcome (successful KPE and survival with native liver [SNL] at 2 years) were evaluated by logistic regression.
Results
A total of 79 children with post-KPE BA were included. Successful KPE was achieved in 29 (36.7%) of 79 children undergoing KPE. The data for survival with native liver at 2 years were available for 61 children as 9 were lost to follow up before 2 years and another 9 were aged less than 2 years at the time of analysis. Twenty-seven (44.3%) of these 61 survived with their native liver at 2 years. On logistic regression analysis, lower age at KPE, use of postoperative steroids and absence of cholangitis were significant predictors of a successful KPE. A successful KPE at 6 months was the lone independent predictor of SNL at 2 years in these children.
Conclusion
Early age at KPE, use of postoperative steroid and prevention of cholangitis can result in successful KPE. Those with successful KPE are likely to survive with their native liver at 2 years.
Keywords: children, cholangitis, steroids, successful Kasai
Abbreviations: BA, Biliary Atresia; CI, Confidence Interval; INR, International Normalized Ratio; KPE, Kasai Portoenterostomy; LT, Liver Transplantation; RCT, Randomized Controlled Trial; SD, Standard Deviation; SNL, Survival with Native Liver; UDCA, Ursodeoxycholic Acid
Biliary atresia (BA) is a progressive obliterative cholangiopathy of the intrahepatic and extrahepatic bile ducts with a fatal outcome if left untreated.1 The Kasai portoenterostomy (KPE) is the primary treatment with subsequent liver transplantation (LT) in cases in whom it is unsuccessful. The principle of KPE in BA is to remove the atretic extrahepatic ducts early at a time when intrahepatic ducts are still patent so as to establish bile flow to the intestine at an early stage and prevent further progression of the disease. The outcome of KPE is evaluated by the clearance of jaundice (serum bilirubin < 2 mg/dL) within 6 months of KPE2, 3, 4, 5 and the survival with native liver (SNL). SNL after KPE has been observed in the range of 32–59% at 5 years and between 27 and 52% at 10 years, whereas the overall survival was 42–89% at 5 years and 40–68% at 10 years.2, 3, 4, 5, 6 Factors known to influence the success of KPE include early age at surgery,2, 7, 8 experience of the surgical centre,5, 9 presence of associated abnormalities7, 10, 11 and anatomic and histological appearance of the extrahepatic biliary tree.12, 13, 14, 15 Steroids, by virtue of their antiinflammatory and choleretic roles, can favourably influence the outcome of BA.16 Repeated episodes of cholangitis can adversely affect the outcome of BA after KPE.17 However, there is scarcity of Indian data on outcomes of patients with BA and the factors determining the success of KPE.18, 19, 20 So, the present study was undertaken to study the outcome of KPE in Indian children and the factors influencing the outcome after KPE.
Material and methods
The study was conducted at a public sector, tertiary care liver institute. It was a retrospective study, and approval was obtained from the institutional ethics committee (IEC no: IEC/2016/45/MA/06). All children aged less than 1 year diagnosed with BA between January 2010 and January 2018 were included in the study. Demographic, clinical, laboratory, radiological and surgical information was collected from the patient's records in the hospital information system and entered in a pretested proforma. Operative cholangiography was the gold standard for diagnosis of BA in those operated. Among those who were not operated, diagnosis of BA was based on characteristic histopathological features of bile ductular proliferation, portal expansion with loose oedematous fibrosis and ductular bile plugs,21, 22 with or without ultrasonographic features,23, 24 in addition to supportive history and examination. Baseline liver function tests, haemogram and international normalized ratio (INR) were recorded. The degree of fibrosis was assessed on liver biopsy based on METAVIR staging system, and F3/F4 were labelled as advanced fibrosis.25 All patients undergoing KPE underwent a wedge liver biopsy intraoperatively. The duct size was determined from remnant hilar tissue specimen sent at surgery. The presence of ductal plate malformation was also reviewed from preoperative liver biopsy specimens. Liver stiffness was measured by transient elastography (TE) (FibroScan touch 502, Echosens, E300M001.5, Version 5), and the value was expressed in kilopascals (kPa). The use of steroids and prophylactic antibiotics in the postoperative period was recorded. In the group operated at our centre from 2014 onwards, postoperative steroids were started on day 7 if no evidence of infection was found. Prednisolone was given for 2 weeks at a dose of 2 mg/kg/day, followed by gradual tapering over the next 4 weeks. All children operated or followed up at our centre received prophylactic cyclical antibiotics for a period of at least one year after surgery/last episode of cholangitis, whichever was later. The antibiotics were alternated cyclically at an interval of 3 weeks. The antibiotics used were cefixime at a dose of 4 mg/kg/day and ofloxacin at a dose of 7.5 mg/kg/day. Cholangitis was defined as the presence of any three of the following four: fever (absence of other causes), increasing jaundice, acholic stools and leucocytosis. All patients received ursodeoxycholic acid (UDCA), fat-soluble vitamins, multivitamin supplements and nutritional rehabilitation. Those patients who could not be operated because of late presentation with advanced liver disease were listed for primary liver transplantation. Successful KPE was defined as a decrease in the total serum bilirubin to less than 2 mg/dL within first 6 months after KPE.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and categorical variables as proportions. Univariate analysis for successful KPE and SNL at 2 years was performed using independent samples t-test for continuous variables and chi-square test for categorical variables. Logistic regression analysis was used to identify the independent predictors of successful KPE and SNL at 2 years. P value < 0.05 was taken as statistically significant. Kaplan–Meier survival curves were made for factors affecting SNL at 2 years. All analyses were performed using SPSS v 22.0 (SPSS, Chicago, IL).
Results
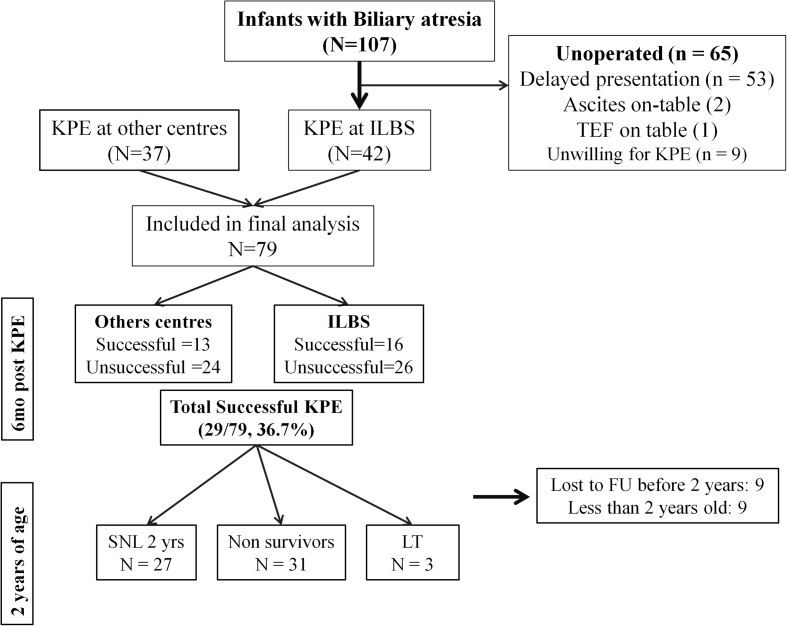
A total of 107 new patients with BA presented to this hospital, of which 42 were operated and 65 could not be operated because of various reasons: 55 children had histological cirrhosis and/or decompensation, 1 who had tracheoesophageal fistula was referred to another hospital and in 9 patients, consent was not given for KPE. The 55 patients with advanced liver disease were offered primary liver transplant. An additional 37 cases of post-KPE BA referred to our hospital within 1 month of their surgery were also included. Hence, a total of 79 cases of post-KPE BA were included in the final analysis [Figure 1]. The baseline demographic, clinical and laboratory profile of patients operated at our centre and other centres is depicted in Table 1. Nine patients (11.4%) were operated at an age less than 60 days; 40 (50.6%), between 60 and 90 days; 20 (25.3%), at an age of 90–120 days and 10 (12.7%), beyond 120 days of life. The youngest child operated was 49 days old operated at the Institute of Liver and Biliary Sciences, and the oldest child operated aged 210 days at another centre. Fifty-six of 79 (70.9%) children experienced at least 1 episode of cholangitis after KPE within 2 years of age. Twenty-six patients received steroids after KPE.
Figure 1.
Patient selection and inclusion and exclusion criteria. FU, follow-up; ILBS, Institute of Liver and Biliary Sciences; KPE, Kasai portoenterostomy; LT, liver transplantation; SNL, survival with native liver; TEF, Tracheo-esophageal fistula.
Table 1.
Baseline Demographic and Laboratory Profile of Post-Kasai Portoenterostomy Patients.
| Parameters | Operated at our centre (n = 42) | Operated at other centres (n = 37) | P value |
|---|---|---|---|
| Age at presentation (days) | 83.2 ± 29.6 | 75.2 ± 27.4 | 0.195 |
| Age at KPE (days) | 89.9 ± 30 | 89.8 ± 30 | 0.958 |
| Gender, male/female | 27/15 | 23/14 | |
| Total bilirubin (mg/dl) | 11.4 ± 3.7 | 9.6 ± 3 | 0.04 |
| Aspartate aminotransferase (IU/ml) | 322.8 ± 209.9 | 306.9 ± 355.6 | 0.862 |
| Alanine aminotransferase (IU/ml) | 223.7 ± 184.8 | 217.4 ± 164.4 | 0.949 |
| Alkaline phosphatase (IU/ml) | 588.7 ± 268.4 | 839.9 ± 509.7 | 0.029 |
| Gamma-glutamyl transferase (IU/ml) | 599.3 ± 507.2 | 663.8 ± 517 | 0.480 |
| Serum albumin (G/dl) | 3.4 ± 0.4 | 3.3 ± 0.5 | 0.204 |
| Transient elastography (kPa) | 23.6 ± 19.5 | NA | |
| Imaging findings n (%) | |||
| Nonvisualization of CBD | 42 (100) | ||
| Nonvisualization of GB | 30 (71.4) | ||
| Pseudo gall bladder | 12 (28.6) | ||
| Triangular cord sign | 8 (19) | NA | |
| Splenic malformation | 3 (7.1) | ||
| Cholangitis after KPE | 26 (61.9) | 30 (81.1) | 0.083 |
| Use of steroids after KPE | 18 (42.9) | 8 (21.6) | 0.06 |
| Decrease in bilirubin to < 2 mg/dl within 6 months of KPE | 16 (38.1) | 13 (35.1) | 0.819 |
CBD, common bile duct; GB, gall bladder; ILBS, Institute of Liver and Biliary Sciences; kPa, kilo-Pascals; KPE, Kasai portoenterostomy.
Successful KPE at 6 months
Of the 79 patients who underwent KPE, 29 (36.7%) patients cleared their jaundice within 6 months of KPE. In children operated at < 60 days, 55.6% cleared their jaundice by 6 months, whereas in those operated between ages 61–90 and 91–120 days, 42.5% and 35% of the patients cleared their jaundice, respectively. All infants operated beyond 120 days had unsuccessful KPE. On univariate analysis of children with successful vs unsuccessful KPE, lower age of presentation (mean difference = 16 days, 95% confidence interval [CI] = 5–27, P = 0.006), lower age at the time of KPE (mean difference = 17 days, 95% CI = 6–29, P = 0.003), absence of advanced fibrosis (odds ratio [OR] = 0.36, 95% CI = 0.13–0.96, P = 0.046), use of steroids (OR = 5.3, 95% CI = 1.9–14.8, P = 0.001) and absence of cholangitis (OR = 0.24, 95% CI = 0.08–0.66, P = 0.009) were significant predictors of a successful KPE (Table 2). On logistic regression analysis, younger age of the infant at the time of KPE (adjusted OR = 0.97, 95% CI = 0.95–0.99, P = 0.038), use of steroids (adjusted OR = 5.1, 95% CI = 1.7–15.6, P = 0.004) and absence of cholangitis (adjusted OR = 4.2, 95% CI = 1.3–13.7, P = 0.017) were independent predictors of a successful KPE.
Table 2.
Predictors of Successful Kasai Portoenterostomy (Bilirubin < 2 mg/dl at 6 Months)—Univariate and Multivariate Analysis.
| Factors | Successful KPE (n = 29) | Unsuccessful KPE (n = 50) | Effect size (95% CI) | P value | Logistic Regression Analysis |
|---|---|---|---|---|---|
| Age of Presentation (days) | 69.7 ± 17.9 | 85.5 ± 32 | −16 (-27 to -5) | 0.006 | Not included in LRA |
| Age at KPE (days) | 78.9 ± 15.9 | 96.4 ± 33.7 | −17 (-29 to -6) | 0.003 | Adj OR 0.97 (95% CI: 0.95–0.99); P = 0.038 |
| Total bilirubin (mg/dl) | 9.9 ± 3.2 | 10.7 ± 3.6 | −0.9 (−2.5 to 0.7) | 0.287 | |
| Aspartate aminotransferase (IU/ml) | 285 ± 345 | 332 ± 212 | −47 (−185 to 95) | 0.507 | |
| Alanine aminotransferase (IU/ml) | 187 ± 152 | 238 ± 188 | −51 (−142 to 39) | 0.260 | |
| Serum albumin (G/dl) | 3.5 ± 0.4 | 3.3 ± 0.5 | 0.2 (−0.14 to 0.36) | 0.365 | |
| Transient elastography (kPa) | 34.6 ± 28.3 | 41.8 ± 26.2 | −7.2 (−21 to 6.5) | 0.297 | |
| Presence of BASM | 1 (3.4%) | 7 (14%) | 0.2 (0.03–1.8)a | 0.246 | |
| Presence of DPM | 5 (17.2%) | 7 (14%) | 1.2 (0.35–4.3)a | 0.756 | |
| Duct size (μ) | 166.4 ± 126.5 | 146 ± 136 | 20.4 (−71 to 113) | 0.657 | |
| Advanced fibrosis | 16 (55.2%) | 39 (78%) | 0.36 (0.13–0.96)a | 0.046 | NS |
| Use of steroidsafterKPE | 16 (55.2%) | 10 (20%) | 5.3 (1.9–14.8)a | 0.001 | Adj OR 5.1(95%CI: 1.7–15.6) P = 0.004 |
| Cholangitis | 15 (51.7%) | 41 (82%) | 0.24 (0.08–0.66)a | 0.009 | Adj OR 4.2 (95%CI: 1.3–13.7) P = 0.017 |
Adj OR, adjusted odds ratio; BASM, biliary atresia splenic malformation; CI, confidence interval; DPM, ductal plate malformation; kPa, kilo-Pascals; KPE, Kasai portoenterostomy; NS, not significant.
Effect size described in odds ratio.
Survival with native liver at 2 years of age
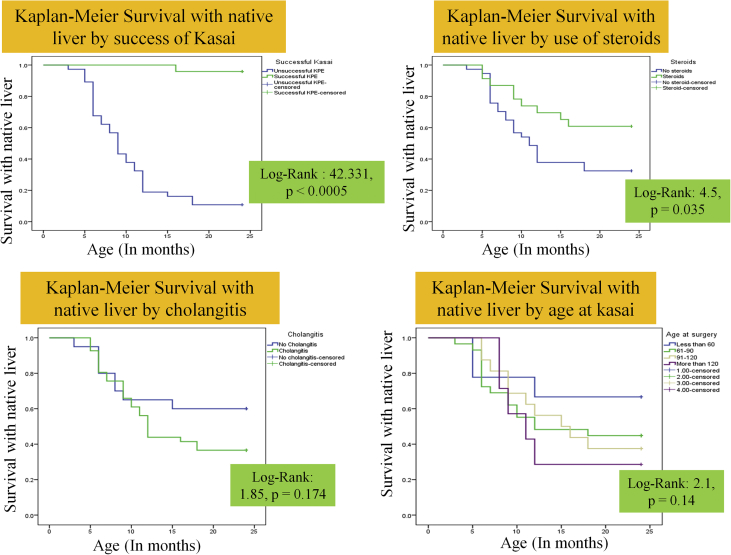
Of the 79 post-KPE children, 9 were lost to follow up after 6 months after KPE, and another 9 are still less than 2 years old. All those lost to follow up were from the unsuccessful KPE group. Of the remaining 61, 27 (44.3%) survived with their native liver at 2 years of age, 31 (50.8%) died and 3 (4.9%) received LT (Figure 1). The reason for no LT in 31 cases was due to declining the LT option and logistic constrains including nonavailability of donor and funds. The SNL at 2 years was 66.7%, 44.8%, 37.5% and 28.6% in children undergoing KPE at <60, 61–90, 91–120 and > 120 days, respectively. On univariate analysis, lower bilirubin at 3 months after KPE (mean difference = 9.3 mg/dL, 95% CI = 7.1–11.5, p < 0.0005), higher albumin at 3 months (mean difference = 0.6 g/dL, 95% CI = 0.2–1, P = 0.006), use of steroids (mean difference = 1.73, 95% CI = 0.99–3, P = 0.037) and successful KPE (OR = 21.4, 95% CI = 3.1–146, p < 0.0005) were significant predictors for SNL at 2 years of age (Table 3). On logistic regression, a successful KPE was the only independent predictor of SNL at 2 years of age (adjusted OR = 115.5, 95% CI = 11.9–1119, P < 0.0005). Figure 2 shows the Kaplan–Meier survival curve until 2 years of age based on successful KPE, age at KPE, usage of steroids and presence of cholangitis.
Table 3.
Predictors of Survival With Native LLiver at 2 Years (Univariate and Multivariate Analysis).
| Factors | SNL 2 years (n = 27) | No survival at 2 years (n = 34) | Effect size (95% CI) | P value | Logistic Regression Analysis |
|---|---|---|---|---|---|
| Age of presentation (days) | 71.5 ± 22.1 | 82.9 ± 31.7 | −11.4 (−25.7 to 3.1) | 0.120 | |
| Age at KPE (days) | 81.9 ± 24.7 | 92.7 ± 32.5 | −10.8 (−25.8 to 4.4) | 0.160 | |
| Bilirubin at 3 months (mg/dl) | 3.2 ± 3.1 | 12.5 ± 5.2 | −9.3 (-11.5 to -7.1) | < 0.0005 | NS |
| Albumin at 3 months (G/dl) | 2.9 ± 0.8 | 2.3 ± 0.5 | 0.6 (0.2–1) | 0.005 | NS |
| Transient elastography (kPa) | 40.7 ± 29.7 | 39.6 ± 27 | 1.1 (−14.1 to 16.4) | 0.967 | |
| Presence of BASM | 2 (7.4%) | 6 (17.6%) | 0.7 (0.44–1.1)a | 0.283 | |
| Presence of DPM | 3 (11.1%) | 5 (14.7%) | 0.88 (0.49–1.6)a | 1 | |
| Presence of advanced fibrosis | 17 (62.9%) | 24 (70.6%) | 0.85 (0.5–1.4)a | 0.590 | |
| Use ofsteroidsafterKPE | 14 (51.8%) | 9 (26.5%) | 1.73 (0.99–3)a | 0.037 | NS |
| Cholangitis | 15 (55.6%) | 26 (76.5%) | 0.63 (0.35–1.132)a | 0.105 | |
| Successful KPE | 23 (85.2%) | 1 (2.9%) | 21.4 (3.1–146)a | <0.0005 | Adjusted OR 115.5 (95% CI 11.9–1119), P < 0.0005 |
BASM, biliary atresia splenic malformation; CI, confidence interval; DPM, ductal plate malformation; kPa, kilo-Pascals; KPE, Kasai portoenterostomy; NS, not significant; OR, odds ratio.
Effect size described as odds ratio.
Figure 2.
Kaplan–Meier survival curve for survival with native liver stratified by success of Kasai, age at Kasai, use of steroids and occurrence of cholangitis.
Discussion
In the present study, successful KPE was seen in 29 of 79 (36.7%) of those operated, which is similar to that seen in previous studies from India.18, 19, 20 SNL at 2 years was seen in 27 of 61 patients (44.3%) which is again similar to another report from Western Indian metro.20 The present study reveals that age at KPE, use of steroids and absence of cholangitis are independent predictors of successful KPE. A successful KPE in this study was the single independent predictor of SNL at 2 years of age. The mean age at presentation and at KPE was 79.7 ± 28.6 days and 90 ± 29.6 days, respectively. The delayed age of KPE at our centre is similar to other studies from India.18, 19, 20, 26, 27 In contrast, the usual age at surgery is less in patients operated outside India. It has been observed in several studies that eventual outcome after KPE is better if KPE is performed before 45–60 days of age.2, 3, 4, 5, 6 In our study, age at KPE was an independent predictor of success of KPE (adjusted OR = 0.97, 95% CI = 0.95–0.99, P = 0.038). Although SNL at 2 years was better in children undergoing KPE at a younger age, the difference was not significant, probably because of a small sample size. Another factor that impacts the outcome of KPE is the surgical technique, which involves the meticulous dissection of the liver hilum and biliary remnant, and the outcome is better in centres that perform more than 5 surgeries per year.5, 9 There was no difference in the success of KPE between children operated at our centre and those operated at other centres as most of the patients were operated at specialized centres. The degree of fibrosis, especially advanced fibrosis, has been associated with a poor outcome of KPE in few studies.12, 13, 14, 15 In the present study, although the presence of advanced fibrosis was a predictor of unsuccessful KPE on univariate analysis, it did not attain statistical significance on multivariate analysis. TE has been used as a noninvasive method for assessing the fibrosis in children with biliary atresia.28, 29 In the present study, preoperative TE did not predict either the outcome after KPE or SNL at 2 years.
Steroids have been used as an adjunct therapy after KPE to increase the bile flow, with conflicting results in a systematic review30 and in a recent meta-analysis.16 In the present study, the use of steroids was associated with higher clearance of jaundice and was an independent predictive factor for successful KPE. However, steroid use did not directly influence the SNL at 2 years. Similar results were demonstrated by Davenport et al who showed that use of high-dose steroids (beginning with 5 mg/kg/day) led to higher clearance of jaundice than no steroids but did not affect the SNL.31 Davenport et al concluded that steroids may have a beneficial role in the first 6 months after KPE, especially in those operated at < 70 days of age.4 Despite the higher age at KPE, steroids have been beneficial in the cohort used in the present study also. This reveals the promise steroids hold in successful bile drainage after KPE.
Cholangitis is one of the most important determinants of success after KPE, and the incidence of cholangitis has been to be found to be as high as 40%–93% in various studies.32, 33 Similarly, 71% of children in the present study had at least one episode of cholangitis despite the use of prophylactic antibiotics. Moreover, the presence of cholangitis was an independent predictor of unsuccessful KPE. In a previous large Chinese study, the presence of recurrent cholangitis was the only independent predictor of a failed KPE.32 Three out of 4 studies including a randomized controlled trial analyzed in a systematic review published in 2016 concluded that the use of prophylactic antibiotics reduced the number of cholangitis, and hence, it may be advocated to routinely use postoperative prophylactic antibiotics in all children after KPE.34 In the present study, we have alternated the antibiotics cyclically to prevent the emergence of antibiotic resistance.
Decline in serum bilirubin, reflecting effective bile drainage, has been regarded as a clinical sign of successful KPE and is associated with improved native liver survival.2, 7 Various studies have found different cut-off for bilirubin for predicting the success of KPE.4, 15, 19 In Taiwanese infants, a postoperative bilirubin level <20 mmol/L (1.2 mg/dL) at any stage predicted a better prognosis;2 however, in another study from the United States, bilirubin levels <34 mmol/L (2 mg/dL) at 3 months was found to be associated with better native liver survival.7 In the present study, a successful KPE defined by clearance of jaundice within 6 months after KPE was the only independent predictor of SNL at 2 years. Most studies now define a successful KPE as clearance of jaundice within 6 months after KPE.2, 3, 4, 5
The present study is limited by the small sample size, retrospective nature of data collection and surgical cohort from multiple centres. To conclude, age at KPE, use of postoperative steroids and absence of cholangitis are independent predictors of successful KPE, and a successful KPE is an independent predictor of SNL at 2 years.
Conflicts of interest
The authors have none to declare.
Author contributions
S.A., B.B.L., Ru.K., R.K. and V.S. were involved in study conception and design. Ru.K., B.B.L. and V.S. were involved in acquisition of data. S.K. and K.G.S.B. were the operating surgeons. B.B.L., Ru.K. and S.A. were involved with analysis and interpretation of data. B.B.L. and Ru.K. prepared the first draft of the manuscript. S.A., S.K. and R.K. critically revised the manuscript. All authors approved the final manuscript.
References
- 1.Hartley J.L., Davenport M., Kelly D.A. Biliary atresia. Lancet. 2009;374(9702):1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 2.Hung P.Y., Chen C.C., Chen W.J. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr. 2006;42(2):190–195. doi: 10.1097/01.mpg.0000189339.92891.64. [DOI] [PubMed] [Google Scholar]
- 3.Chardot C., Carton M., Spire-Bendelac N., Le Pommelet C., Golmard J.L., Auvert B. Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology. 1999;30(3):606–611. doi: 10.1002/hep.510300330. [DOI] [PubMed] [Google Scholar]
- 4.Tyraskis A., Davenport M. Steroids after the Kasai procedure for biliary atresia: the effect of age at Kasai portoenterostomy. Pediatr Surg Int. 2016;32(3):193–200. doi: 10.1007/s00383-015-3836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davenport M., De Ville de Goyet J., Stringer M.D. Seamless management of biliary atresia in England and Wales (1999-2002) Lancet. 2004;363(9418):1354–1357. doi: 10.1016/S0140-6736(04)16045-5. [DOI] [PubMed] [Google Scholar]
- 6.Shneider B.L., Brown M.B., Haber B., Biliary Atresia Research Consortium A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148(4):467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Superina R., Magee J.C., Brandt M.L. Childhood Liver Disease Research and Education Network. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early post-operative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011;254(4):577–585. doi: 10.1097/SLA.0b013e3182300950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohhama Y., Shinkai M., Fujita S., Nishi T., Yamamoto H. Early prediction of long-term survival and the timing of liver transplantation after the Kasai operation. J Pediatr Surg. 2000;35(7):1031–1034. doi: 10.1053/jpsu.2000.7765. [DOI] [PubMed] [Google Scholar]
- 9.Davenport M., Ong E., Sharif K. Biliary atresia in England and Wales: results of centralization and new benchmark. J Pediatr Surg. 2011;46(9) doi: 10.1016/j.jpedsurg.2011.04.013. 1689-4. [DOI] [PubMed] [Google Scholar]
- 10.Davenport M., Tizzard S.A., Underhill J., Mieli-Vergani G., Portmann B., Hadzić N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr. 2006;149(3):393–400. doi: 10.1016/j.jpeds.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Falchetti D., de Carvalho F.B., Clapuyt P. Liver transplantation in children with biliary atresia and polysplenia syndrome. J Pediatr Surg. 1991;26(5):528–531. doi: 10.1016/0022-3468(91)90698-s. [DOI] [PubMed] [Google Scholar]
- 12.Shteyer E., Ramm G.A., Xu C., White F.V., Shepherd R.W. Outcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activation. J Pediatr Gastroenterol Nutr. 2006;42(1):93–99. doi: 10.1097/01.mpg.0000189324.80323.a6. [DOI] [PubMed] [Google Scholar]
- 13.Baruah R.R., Bhatnagar V., Agarwala S., Gupta S.D. Correlation of pre- and post-operative liver function, duct diameter at porta hepatis, and portal fibrosis with surgical outcomes in biliary atresia. J Indian Assoc Pediatr Surg. 2015;20(4):184–188. doi: 10.4103/0971-9261.161040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb N.L., Jiwane A., Ooi C.Y., Nightinghale S., Adams S.E., Krishnan U. Clinical significance of liver histology on outcomes in biliary atresia. J Paediatr Child Health. 2017;53(3):252–256. doi: 10.1111/jpc.13371. [DOI] [PubMed] [Google Scholar]
- 15.Roy P., Chatterjee U., Ganguli M., Banerjee S., Chatterjee S.K., Basu A.K. A histopathological study of liver and biliary remnants with clinical outcome in cases of extrahepatic biliary atresia. Indian J Pathol Microbiol. 2010;53(1):101–105. doi: 10.4103/0377-4929.59194. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M.Z., Xun P.C., He K., Cai W. Adjuvant steroid treatment following Kasai portoenterostomy and clinical outcomes of biliary atresia patients: an updated meta-analysis. World J Pediatr. 2017;13(1):20–26. doi: 10.1007/s12519-016-0052-8. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima H., Koga H., Okawada M., Nakamura H., Lane G.J., Yamataka A. Does time taken to achieve jaundice-clearance influence survival of the native liver in post-Kasai biliary atresia? World J Pediatr. 2018 Mar 26 doi: 10.1007/s12519-018-0139-5. [DOI] [PubMed] [Google Scholar]
- 18.Narsimhan K.L., Chowdhry S.K., Vaiphei K. Outcome of biliary atresia from Chandigarh: results of a prospective analysis. Indian Pediatr. 2001;38:1144–1148. [PubMed] [Google Scholar]
- 19.Sanghai S.R., Shah I., Bhatnagar S., Murthy A. Incidence and prognostic factors associated with biliary atresia in Western India. Ann Hepatol. 2009;8:120–122. [PubMed] [Google Scholar]
- 20.Redkar R., Karkera P.J., Raj V., Bangar A., Hathiramani V., Krishnan J. Outcome of biliary atresia after kasai's portoenterostomy: a 15-year experience. Indian Pediatr. 2017;54(4):291–294. doi: 10.1007/s13312-017-1091-5. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi A., Krishnani N., Yachha S.K., Khanna V., Poddar U., Lal R. Histopathological features and accuracy for diagnosing biliary atresia by prelaparotomy liver biopsy in developing countries. J Gastroenterol Hepatol. 2009;24(1):97–102. doi: 10.1111/j.1440-1746.2008.05737.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee W.S., Looi L.M. Usefulness of a scoring system in the interpretation of histology in neonatal cholestasis. World J Gastroenterol. 2009;15(42) doi: 10.3748/wjg.15.5326. 5326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphrey T.M., Stringer M.D. Biliary atresia: US diagnosis. Radiology. 2007;244:845–851. doi: 10.1148/radiol.2443061051. [DOI] [PubMed] [Google Scholar]
- 24.Lee M.S., Kim M.J., Lee M.J. Biliary atresia: color Doppler USG findings in neonates and infants. Radiology. 2009;252:282–289. doi: 10.1148/radiol.2522080923. [DOI] [PubMed] [Google Scholar]
- 25.Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2) doi: 10.1002/hep.510240201. 289-3. [DOI] [PubMed] [Google Scholar]
- 26.Gupta L., Bhatnagar V. A study of associated congenital anomalies with biliary atresia. J Indian Assoc Pediatr Surg. 2016;21:10–13. doi: 10.4103/0971-9261.158095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran P., Safwan M., Srinivas S., Shanmugam N., Vij M., Rela M. The extended Kasai portoenterostomy for biliary atresia: a preliminary report. J Indian Assoc Pediatr Surg. 2016;21:66–71. doi: 10.4103/0971-9261.176941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin N.Y., Kim M.J., Lee M.J. Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med. 2014;33(5):853–864. doi: 10.7863/ultra.33.5.853. [DOI] [PubMed] [Google Scholar]
- 29.Shen Q.L., Chen Y.J., Wang Z.M. Assessment of liver fibrosis by Fibroscan as compared to liver biopsy in biliary atresia. World J Gastroenterol. 2015;21(22):6931–6936. doi: 10.3748/wjg.v21.i22.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Nah S.A., Chiang L., Krishnaswamy G., Low Y. Post-operative steroid therapy for biliary atresia: systematic review and meta-analysis. J Pediatr Surg. 2015;50(9):1590–1594. doi: 10.1016/j.jpedsurg.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Davenport M., Parsons C., Tizzard S., Hadzic N. Steroids in biliary atresia: single surgeon, single centre, prospective study. J Hepatol. 2013;59(5):1054–1058. doi: 10.1016/j.jhep.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Chung P.H., Wong K.K., Tam P.K. Predictors for failure after Kasai operation. J Pediatr Surg. 2015;50(2):293–296. doi: 10.1016/j.jpedsurg.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Ohkohchi N., Chiba T., Ohi R., Mori S. Long-term follow-up study of patients with cholangitis after successful Kasai operation in biliary atresia: selection of recipients for liver transplantation. J Pediatr Gastroenterol Nutr. 1989;9(4):416–420. doi: 10.1097/00005176-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Decharun K., Leys C.M., West K.W., Finnell S.M. Prophylactic antibiotics for prevention of cholangitis in patients with biliary atresia status post-kasai portoenterostomy: a systematic review. Clin Pediatr (Phila) 2016;55(1):66–72. doi: 10.1177/0009922815594760. [DOI] [PubMed] [Google Scholar]