Abstract
Background
Majority of patients of hepatocellular carcinoma (HCC) in India present in advanced stages, when curative treatment options are limited. We undertook this study to assess the cost-effectiveness of treating advanced HCC patients with sorafenib compared with best supportive care (BSC).
Methods
A Markov model was parameterized to model the lifetime costs and consequences of treating advanced HCC patients with sorafenib versus BSC using a societal perspective. Cost of routine care, diagnostics, management of complications in both the arms and management of adverse effects of sorafenib treatment were considered. A probabilistic sensitivity analysis was undertaken to assess the effect of parameter uncertainty.
Results
The incremental cost and benefit gained by treating HCC using sorafenib was Indian rupees 94,182 ($1459) and 0.19 quality adjusted life years (QALYs) per patient, implying an incremental cost of Indian rupees 507,520 ($7861) per QALY gained.
Conclusions
Sorafenib is not cost-effective for use in advanced hepatocellular carcinoma treatment in India.
Keywords: hepatocellular carcinoma, cost effectiveness analysis, cancer, sorafenib
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; BSC, Best Supportive Care; CEAC, Cost-Effectiveness Acceptability Curve; CGHS, Central Government Health Scheme; GDP, Gross Domestic Product; HBV, Hepatitis B Viral; HCC, Hepatocellular Carcinoma; ICER, Incremental Cost-Effectiveness Ratio; ICU, Intensive Care Unit; INASL, Indian National Association for Study of Liver; INR, Indian National Rupees; LY, Life Year; PD, Progressive Disease; PFS, Progression Free State; PSA, Probabilistic Sensitivity Analysis; QALY, Quality Adjusted Life Year; QOL, Quality of Life; RCC, Renal Cell Carcinoma; TTSP, Time to Symptomatic Progression; UGIE, Upper Gastrointestinal Endoscopy; USD, US Dollars
Hepatocellular carcinoma (HCC) is the primary malignant neoplasm of the liver. It is the fifth most common cancer in men worldwide, with one of the highest mortality rates among all cancers.1 In India, the annual age-adjusted incidence rate of HCC per 100,000 persons ranges from 0.7 to 7.5 for men and 0.2–2.2 for women.2
Majority (70%) of cases of HCC in India present at the advanced stage, Barcelona Clinic Liver Cancer (BCLC) stage C and D in which curative resection is not possible.3 For these unresectable, advanced HCC cases with extra-hepatic spread or vascular invasion, treatment options are limited. Targeted molecular therapy—sorafenib—is indicated for such advanced BCLC stage C patients of HCC.4 Sorafenib has been reported to have an increased median overall survival and time to progression by 3 months in advanced HCC.5 It is the only recommended standard of care in BCLC stage C patients of HCC. Patients progressing or not tolerating sorafenib are offered best supportive care (BSC). However, this modest increase in survival with sorafenib comes at a considerable cost.6 This added cost needs to be weighed in any decision making for treatment guidelines, especially in setting of low- and middle-income countries such as India.
There is limited evidence on cost-effectiveness of sorafenib.7, 8, 9 Broadly, the existing evidence indicates that sorafenib is not cost-effective. However, there are methodological gaps in these studies.7, 8, 9 First, a Chinese study which evaluated cost-effectiveness of sorafenib did not consider any cost for BSC arm, hence not giving a uniform ground for comparison.9 Second, most of these studies have used western data on efficacy for treatment in the cost-effectiveness models.7, 8 Whereas 70% of cases of HCC in the Asia-Pacific region coexist with chronic Hepatitis B infection, nearly three-fourths of the total HCC cases in the developed countries of West are attributable to chronic Hepatitis C infection.10 This means that the existing cost-effectiveness models of sorafenib may not be representative for India. Third, both the previous economic evaluations used the quality of life (QOL) of different health states which were derived from UK-based evidence synthesis for use of sorafenib in renal cell carcinoma (RCC) patients.11 However, this may not be appropriate, given that the QOL of HCC patients may be different from that of RCC patients. Finally, the cost of providing health services in India are significantly different from those reported in other countries, further limiting generalizability of existing evidence.
In view of this, we undertook this study to assess the incremental cost per quality adjusted life year (QALY) gained with use of sorafenib as compared with BSC among advanced, unresectable BCLC stage C HCC patients in India.
Materials and methods
Model overview
A Markov model with finite disease states was developed in MS Excel 2013®. A societal perspective incorporating both the health system costs and out-of-pocket expenditures was used. A societal perspective is considered more appropriate for the present analysis, as majority of treatment costs in India are borne out of pocket by households.12 Outcomes were valued in terms of life years (LYs) gained and QALYs. Future costs and consequences were discounted at 3%.13 The results are reported in terms of incremental cost per QALY gained with use of sorafenib.
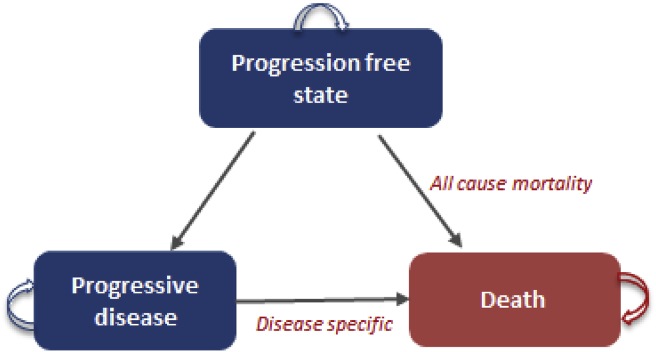
The three finite health transition states in the model include progression-free state (PFS), progressive disease (PD) and death (Figure 1). We modelled the overall costs and consequences of treating the advanced HCC patient cohort, starting at age 40 years14, with either of the two treatment options. We assumed that the cohort started with the PFS and then transitioned to other states. Cycle length of 1 month was considered based on available relevant literature on overall and progression free survival.15 Death due to disease happened after the PD stage only, whereas patients in PFS were modelled based on all-cause mortality. This was again based on the clinical trials data, which considered the patients who had died while in the PFS as censored, rather than disease-related mortality.8
Figure 1.
Markov model to assess cost-effectiveness of Sorafenib.
Intervention and control
The intervention arm consisted of patients on sorafenib 400 mg BD15 until disease progression or serious adverse effects requiring discontinuation of the drug. During the treatment, patients were followed up with laboratory and radiological investigations for assessing response, tolerance to treatment and liver functions. The data for adverse effects, progression free survival and overall survival as reported in Asia-Pacific trial were used.15 This trial results were considered appropriate for parameterization of our model, given the similarities in the profile of HCC cases reported in the trial and Indian patients. Majority (70.7% in the sorafenib arm and 77.6% in the placebo group) of these patients had chronic Hepatitis B Viral (HBV) infection, which is similar to Indian epidemiological profile. Second, 95.3% and 96.1% of patients in the sorafenib arm and the placebo arm, respectively, were in BCLC stage C. Owing to similarity in ethnicity, high incidence of HBV infection and inclusion of BCLC stage B advanced HCC in this study, it was considered to be the most representative for India. We estimated the overall survival probabilities using the reported median overall survival for the sorafenib group (6.5 months) and the BSC arm (4.2 months) from the trial.15
First, in the BSC arm, patients were assumed to be regularly followed up at the hospital and underwent the basic minimum laboratory and radiological investigations, as per requirement. Second, patients were assumed to be managed symptomatically using standard guidelines.16, 17 All life-threatening events (45.3%)15 or intervention procedures such as ascitic tapping were assumed to be managed under hospitalization.
Costing
For both the treatment and control group, cost of specialist consultation once a month was included. Patients in the intervention arm were treated with sorafenib 400 mg twice a day in the PFS, until disease progression or intolerance to treatment due to grade 3 or 4 adverse effects. Furthermore, it was assumed that the dose of sorafenib would be reduced for adverse effect management. Overall, based on dose reductions (30.9%) due to adverse effects and treatment discontinuation (19.5%), we assumed an overall dose of 568 mg a day per patient, for which cost was assessed (Table 1).15 Cost of laboratory and radiological investigations required for both the sorafenib and BSC groups was estimated based on standard treatment guidelines prescribed by the Indian National Association for Study of Liver (INASL)17 consensus on prevention, diagnosis and management of HCC in India (Table 2). Unit prices for the same were procured from Central Government Health Scheme and a published study.12, 18
Table 1.
Clinical Parameters for Assessing Cost-effectiveness of Sorafenib Versus BSC.
| Parameter | Base value | 95% confidence linterval |
Distribution | Source | |
|---|---|---|---|---|---|
| Lower value | Upper value | ||||
| Utilities | |||||
| PFS utility | 0.76 | 0.67 | 0.85 | Beta | 31 |
| PD utility | 0.68 | 0.60 | 0.76 | Beta | 31 |
| Transition probabilities | |||||
| Sorafenib | |||||
| PFS to PD | 0.179 | 0.158 | 0.199 | Beta | 15 [Author estimation from Cheng et al.] |
| PD to Deathdeath | 0.375 | 0.333 | 0.417 | Beta | 15 [Author estimation from Cheng et al.] |
| BSC | |||||
| PFS to PD | 0.357 | 0.317 | 0.398 | Beta | 15 [Author estimation from Cheng et al.] |
| PD to Deathdeath | 0.412 | 0.262 | 0.328 | Beta | 15 [Author estimation from Cheng et al.] |
| All-cause mortality | 0.004 | 0.003 | 0.004 | Beta | 32, 34 |
| Average length of stay (days) | |||||
| General ward | 8.14 | 12 | |||
| ICU | 13 | 24 | |||
| Discount rate (%) | 3.0 | 13 | |||
| Adverse effect requiring management in Sorafenib sorafenib (%) | |||||
| Hand foot syndrome | 10.7 | 15 | |||
| Diarrhoea | 6.0 | 15 | |||
| Fatigue | 3.4 | 15 | |||
| Hyper tension | 2.0 | 15 | |||
| Rash | 0.7 | 15 | |||
| Nausea | 0.7 | 15 | |||
| Pain | 68.0 | 20 | |||
| Management of complications | 47.7 | 15 | |||
| General ward | 70.0 | 15 | |||
| ICU | 30.0 | 15 | |||
| Complications requiring management in BSC (%) | |||||
| Pain | 68.0 | 20 | |||
| Nutritional Support | 55.0 | 20 | |||
| Ascites | 51.0 | 20 | |||
| UGIE varices | 70.0 | 20 | |||
| Anorexia | 74.0 | 20 | |||
| Nausea & and Vomitingvomiting | 50.0 | 20 | |||
| Jaundice & and Pruritispruritis | 35.0 | 20 | |||
| Management of complications | 45.3 | 15 | |||
| General ward | 70.0 | 15 | |||
| ICU | 30.0 | 15 | |||
| Treatment discontinuation in Sorafenib sorafenib (%) | 19.5 | 15 | |||
| Dose reduction in Sorafenib sorafenib (%) | 30.9 | 15 | |||
| Average Sorafenib sorafenib daily dose (mg) | 568 | 15 | |||
| Survival rates | |||||
| Sorafenib MOS (months) | 6.5 | 15 | |||
| Sorafenib MTTP (months) | 2.8 | 15 | |||
| BSC MOS (months) | 4.2 | 15 | |||
| BSC MTTP (months) | 1.4 | 15 | |||
PFS: progression free state, PD: progressive disease, ICU: intensive care unit, BSC: best supporting care, MOS: median overall survival, MTTP: median Time to progression, UGIE: upper gastrointestinal endoscopy.
Table 2.
Cost Parameters for Assessing Cost-effectiveness of Sorafenib Versus BSC.
| Parameter | Type of cost | Monthly cost INR (USD) | 95% confidence interval |
Distribution | Source | |
|---|---|---|---|---|---|---|
| Lower INR (USD) | Upper INR (USD) | |||||
| Sorafenib | ||||||
| Drug | OOP | 4730 (73.3) | 4396 (68.1) | 5064 (78.4) | gamma | Review of private chemist sources |
| Laboratory investigation | HS | 2757 (42.7) | 2416 (37.4) | 3098 (48.0) | gamma | 12, 18 |
| Management of adverse effects | ||||||
| Hand foot syndrome | OOP | 36 (0.6) | 30 (0.5) | 41 (0.6) | gamma | 21, 22 |
| Diarrhoea | OOP | 12 (0.2) | 9 (0.1) | 16 (0.2) | gamma | 21, 22, 23 |
| Hyper-tension | OOP | 50 (0.8) | 24 (0.4) | 77 (1.2) | gamma | 22, 23 |
| Rash | OOP | 231 (3.6) | 113 (1.8) | 349 (5.4) | gamma | Review of private chemist sources |
| Nausea | OOP | 1.07 (0.02) | 0.85 (0.01) | 1.3 (0.02) | gamma | 22, 23 |
| Pain | OOP | 28 (0.4) | 23 (0.5) | 33 (0.5) | gamma | 21, 22 |
| BSC | ||||||
| Laboratory investigation | HS | 884 (13.7) | 857 (13.3) | 910 (14.1) | gamma | 18 |
| CECTa | HS | 2025 (31.4) | 1567 (24.3) | 2483 (38.5) | gamma | 18 |
| CECTb | HS | 1350 (20.9) | 1044 (16.2) | 1656 (25.6) | gamma | 18 |
| Management of complications | ||||||
| Pain | OOP | 10,228 (158.4) | 7774 (120.4) | 12,682 (196.4) | gamma | Review of private chemist sources |
| Nutritional protein | OOP | 1310 (20.2) | 1094 (16.9) | 1526 (23.6) | gamma | Review of private chemist sources |
| Ascites | OOP | 6 (0.1) | 5 (0.1) | 7 (0.1) | gamma | 22 |
| UGIE varices | OOP | 11 (0.2) | 3 (0.1) | 18 (0.3) | gamma | 22, 23 |
| Nausea | OOP | 1.07 (0.02) | 0.85 (0.01) | 1.3 (0.02) | gamma | 22, 23 |
| Jaundice | OOP | 544 (8.4) | 387 (5.9) | 702 (10.8) | gamma | 22, 23 |
| Per outpatient consultation | HS | 259 (4.0) | 109 (1.7) | 408 (6.3) | gamma | 33 |
| Per patient bed days General Ward | OOP | 2219 (34.4) | 870 (13.5) | 3569 (55.3) | gamma | 12, 33 |
| Per patient bed days ICU | OOP | 12,801 (198.3) | 12,597 (195.1) | 13,005 (201.4) | gamma | 12, 24 |
1 USD = INR 64.56.
INR: Indian national rupee, USD: US dollar, OOP: out of pocket, HS: health system, BSC: best supporting care, ICU: intensive care unit, CECT: contrast enhanced computed tomography, UGIE: upper gastrointestinal endoscopy.
10% of patients requiring CECT.
20% of patients requiring CECT.
Management of the most common grade 3 and 4 adverse effects in sorafenib arm, as reported in the Asia-Pacific trial, which were considered for cost assessment included hand foot syndrome 10.7%, diarrhoea 6%, fatigue 3.4%, hypertension 2%, rashes 0.7% and nausea 0.7% (Table 1).15 These adverse effects were assumed to be managed as per standard recommendations.19 Besides, all patients in the intervention arm were also assumed to be managed symptomatically for the common symptoms such as pain, nutritional support, upper gastrointestinal endoscopy (UGIE) varices or vomiting. For all life-threatening disabilities (47.7%)15 in the sorafenib arm, cost of hospitalization was estimated.
In the BSC arm patients were assumed to be managed symptomatically—most common symptoms being pain (68%), nutritional support (55%), ascites (51%), UGIE varices (70%), anorexia (74%), nausea and vomiting (50%) and jaundice and pruritis (35%).20 These symptoms were managed as per the guidelines issued by the INASL.16, 17 All life-threatening disabilities (45.3%) in the BSC arm were assumed to require hospitalized care.15
Cost of antiviral treatment was not included in both the arms. In both the arms, duration and quantity of drugs, need for ward or Intensive Care Unit (ICU) hospitalization were done as per the guidelines individualized for patients based on expert opinion. Cost of the therapeutic management in each health states was estimated based on INASL guidelines and the unit prices of drugs as obtained from the medical services corporation from the states.21, 22, 23 In addition, the unit cost of other medical services such as outpatient consultation, hospitalization, intensive care and other procedures were estimated as reported in a recent study undertaken in a large tertiary care hospital in North India.12, 24
Valuation of consequences
The data on overall survival rate and median time to progression, as reported in the Asia-Pacific study, were used to derive transition probabilities.15 The median overall survival in the sorafenib and BSC arm were 6.5 months and 4.2 months, respectively15 (Table 1). Similarly, median time to progression was 2.8 months and 1.4 months, respectively. The primary effectiveness measure was QALY. QOL for PD and PFS, as reported in the previous cost-effectiveness analysis, were used in our base case.7, 9, 25, 26 The Asia-Pacific trial reports no difference in the QOL of different health states, evaluated based on radiological diagnosis. The authors claim that the QOL is determined by the symptomatic progression (rather than radiological progression), which does not differ much because the underlying liver disease is present in both the states.15 In view of this, we also undertook a scenario analysis, wherein we used same QOL (0.55, 0.55) for both the PFS and PD health states. This QOL was obtained by analysing data reported by Indian HCC patients from a large tertiary care centre in North India.27
Finally, incremental cost per QALY gained with use of sorafenib was calculated. Incremental cost-effectiveness ratio (ICER) was estimated and plotted (Supplementary Figure 1) in Indian National Rupees (INR), and converted to US Dollars (USD) based on the average currency exchange rate in 2017 (Table 3).28
Table 3.
Costs, Effects and Cost Effectiveness of Sorafenib as Compared with BSC.
| Finding | Sorafenib | BSC |
|---|---|---|
| Lifetime cost per patient | 2,93,978 | 1,99,796 |
| Health consequences per patient | ||
| LYs | 0.68 | 0.43 |
| QALYs | 0.50 | 0.31 |
| Incremental cost | 94,182 | |
| Incremental benefit | ||
| LY | 0.25 | |
| QALY | 0.19 | |
| Incremental Cost-effectiveness Ratio | ||
| INR per person LY gained | 3,82,796 | |
| INR per person QALY gained | 5,07,520 | |
BSC: best supporting care, QALY: quality adjusted life years, LY: life years, INR: Indian national rupee.
Sensitivity analysis
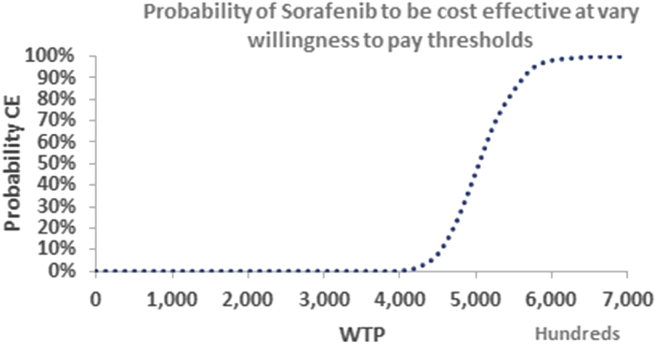
Probabilistic sensitivity analysis (PSA) using a Monte Carlo simulation was conducted to assess the impact of the joint uncertainty around the key parameters. Transition probabilities and utilities were varied by 10% around the base value. Gamma distribution was applied to all costs; and beta distribution was used for utilities and transition probabilities. The PSA was based on 1000 iterations. Probability for sorafenib to be cost-effective was plotted on a cost-effectiveness acceptability curve (Figure 2).
Figure 2.
Probability of sorafenib to be cost-effective at varying willingness to pay thresholds.
Results
Base case results
Overall, we found that the lifetime cost of treating advanced HCC patient was INR 293,978 (USD 4554) and INR 199,796 (USD 3095) per patient using sorafenib and BSC, respectively. Incremental cost of treatment using sorafenib was INR 94,182 (USD 1459) per patient (Table 3). Between both the sorafenib (83%) and BSC (96%) arms, predominant cost was on account of management of life-threatening disabilities. The cost of sorafenib drug and treatment of the adverse effects of drug was 9% and 0.1% of the total lifetime cost, respectively. The share of diagnostics cost increased from 3% in the BSC arm to 7% in the sorafenib arm in view of increase in type and intensity of diagnostic workup (Supplementary Figure 3 and Supplementary Figure 4).
Overall, LYs lived per patient on sorafenib and BSC treatments were found to be 0.68 and 0.43, respectively. Similarly, the number of QALYs lived per patient were 0.50 in the sorafenib arm and 0.31 in the BSC arm. The incremental health benefit of treatment with sorafenib was 0.25 LYs and 0.19 QALYs (69.35 quality adjusted life days) per patient (Table 3).
Based on these costs and health consequences, we found that the use of sorafenib incurs an incremental cost of INR 507,520 (USD 7861) per QALY gained (Table 3). The value of ICER is nearly 4.2 times the per capita gross domestic product (GDP) of India, and hence will not be considered as cost-effective for use.
Sensitivity analysis
First, the parameters which had maximum influence on the value of ICER were the values of disease stage utility, and cost of treatment (cost of intensive care, pain cost and diagnostics) (Supplementary Figure 2). However, in none of the instance, ICER was less than even three times GDP per capita of India. Second, we use constant Indian health state utilities of HCC for both PFS and PD health states; again, the ICER was cost-ineffective (INR 695,992 or USD 10,781 per QALY gained).
Finally, there is minimal probability for sorafenib to be cost-effective at a willingness to pay which equals the GDP per capita. Even at a willingness to pay of three times the GDP per capita, there is 0% probability for sorafenib to be cost-effective.
Discussion
Management of HCC poses a special challenge in India, where majority of the patients are detected in an advanced stage where no curative treatment can be provided.3 Whether to provide BSC or to resort to treatment with sorafenib is a major question.
Overall, we found that use of sorafenib incurs incremental cost of INR 507,520 (USD 7861) per QALY gained, which is much beyond the threshold of either 1-time or 3-times the GDP per capita in Indian context. Hence, based on economic argument, use of Sorafenib is not a good value for money in the Indian setup.
Findings in context of existing evidence
Previous attempts at evaluating cost-effectiveness of sorafenib have mainly been undertaken in developed country setting with clear differences in terms of patient profile and resource use for management.7, 8 The Chinese study also reported that sorafenib was not cost-effective.9 A major limitation of the Chinese study was lack of cost assessment for supportive services in the BSC arm.
First, in our study, we used the data on effectiveness and other clinical parameters from the Asia-Pacific study, which was considered as most representative of Indian disease epidemiology.15 Second, all our costing parameters were derived based on Indian studies.14, 20, 29, 30 Third, we also assessed the cost in the BSC arm for all supportive services, life -threatening disabilities requiring hospitalization and end-of-life care as given in the standard treatment guidelines,16, 17 besides expert opinions. Hence, the BSC arm in our study was appropriately costed for and not merely considered to be a home-based care or an equivalent of no treatment, giving a fair comparator to sorafenib for cost-effectiveness.
To the best of our knowledge, no Indian data exists on efficacy, tolerance or adverse effects of sorafenib in HCC. The Asia-Pacific study15 was used to derive clinical parameters, because the patients in this study comprised those from the Asian subcontinent, with more than 70% in both the arms having chronic HBV infection, and were predominantly of BCLC stage C (>95%) in both the arms. This seemed to be the most representative Indian disease epidemiology.
All the previous economic evaluations carried out have used QOL valuation which is different for both the PFS (0.76) and PD (0.68) health states.7, 8 The findings of the Asia Pacific study contradict this assumption on the ground that the classification of PFS and PD is based on radiological progression. However, QOL is likely to be determined based on symptomatic progression.15 Moreover, the time to symptomatic progression was similar in both the arms with no difference in the QOL in the two groups.15 This implies that because the underlying chronic liver disease in the population progresses at a similar rate in both the groups, irrespective of treatment for HCC, they have similar QOL. In view of this, we undertook a scenario analysis, in which we assumed a constant QOL for both the PFS and PD health states, and this QOL was derived from analysis of data collected from patients of HCC in a tertiary care hospital of North India. In this scenario analysis also, sorafenib remains cost-ineffective.
Additional cost of sorafenib drug and its related side effects or need for monitoring disease progression were marginal. Despite not so significant increase in the cost of overall management, directly attributable to drug, sorafenib still remains not cost-effective. This is more likely to be attributable to the lack of any clinically significant health gains in terms of either overall survival or disease progression or QOL.
Limitations
First, the major limitation of our study is lack of any Indian data on survival and efficacy of sorafenib in HCC. However, we feel that the results of Asia-Pacific study are representative of Indian population in view of the similarities mentioned earlier.15
Second, in the Indian setup, there is significant heterogeneity in health-care delivery system in different states of the country which could influence cost of services. Although we made deliberate attempt to incorporate cost of service in both public and private sector, these are much more representative of North India. This is likely to be generalizable at national level, because northern part of India is endemic to HBV and has a high incidence for HCC. Moreover, we varied the estimates of cost in our sensitivity analysis and found that overall conclusion remains robust to variation in price. Nonetheless, more representative costing of treatment for HCC would be an important future area of research.
Conclusion and policy implications
India is trying to universalize coverage of health-care services. The central and state governments have introduced several publicly financed health insurance schemes to meet the objectives of universal health coverage. Treatment for cancer is an integral component of the benefit package in most of these schemes. In addition, some states such as Punjab have introduced specific schemes for free treatment of cancer and Hepatitis C infection. The results of our study hold significant importance for setting standard treatment guidelines and purchasing of care in these health insurance schemes. More data needs to be analysed in the Indian setting with respect to the drug efficacy, tolerance, adverse effects and underlying liver functions, performance status and tumour load.
Author contributions
N.G. and R.K.V. were involved in the design of the study. N.G., R.K.V. and S.P. were involved in the acquisition, analysis or interpretation of data. N.G., R.K.V., S.P. and R.K.D. were involved in drafting the work or revising it critically for important intellectual content.
Conflicts of interest
The authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2018.10.003.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Canc. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.IARC–WHO . International Agency for Research on Cancer; 2017. (Cancer IAfRo). 2017. [Google Scholar]
- 3.Paul S.B., Chalamalasetty S.B., Vishnubhatla S. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology. 2009;77:162–171. doi: 10.1159/000231886. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Muszbek N., Shah S., Carroll S. Economic evaluation of sorafenib in the treatment of hepatocellular carcinoma in Canada. Curr Med Res Opin. 2008;24:3559–3569. doi: 10.1185/03007990802563706. [DOI] [PubMed] [Google Scholar]
- 7.Cammà C., Cabibbo G., Petta S. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046–1054. doi: 10.1002/hep.26221. [DOI] [PubMed] [Google Scholar]
- 8.Carr B.I., Carroll S., Muszbek N., Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1739–1746. doi: 10.1111/j.1440-1746.2010.06404.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P., Yang Y., Wen F. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27:853–859. doi: 10.1097/MEG.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 10.Llovet J.M., Burroughs A., Bruix J. Hepatocellular carcinoma. Lancet (London, England) 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence. Bevacizumab (First-line), sorafenib (First and second-line), sunitinib (second-line) and temsirolimus (First-line) for the treatment of Advanced And/or Metastatic Renal Cell Carcinoma. Vol 20172017.
- 12.Sangwan A., Prinja S., Aggarwal S., Jagnoor J., Bahuguna P., Ivers R. Cost of trauma care in secondary-and tertiary-care public sector hospitals in North India. Appl Health Econ Health Policy. 2017:1–12. doi: 10.1007/s40258-017-0329-7. [DOI] [PubMed] [Google Scholar]
- 13.Edejer T.T.-T., Baltussen R., Adam T. World Health Organization; Geneva: 2002. WHO Guide to Cost-effectiveness Analysis. [Google Scholar]
- 14.Mukherjee S., Dhar K., Datta S., Mukherjee A.K. Hepatocellular carcinoma in eastern India, a detail analytical report from a tertiary care hospital. Int J Sci Rep. 2015;1:69–73. [Google Scholar]
- 15.Cheng A.-L., Kang Y.-K., Chen Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 16.Kumar M., Panda D. Role of supportive care for terminal stage hepatocellular carcinoma. J clin exp hepatol. 2014;4:S130–S139. doi: 10.1016/j.jceh.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A., Acharya S.K., Singh S.P. The Indian National Association for Study of the Liver (INASL) consensus on prevention, diagnosis and management of hepatocellular carcinoma in India: the Puri recommendations. J clin exp hepatol. 2014;4:S3–S26. doi: 10.1016/j.jceh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Central Government Health Scheme . Ministry of Health & Family Welfare. Government of India; 2014. CGHS rate list. [Google Scholar]
- 19.Brose M.S., Frenette C.T., Keefe S.M., Stein S.M. vol. 41. Elsevier; 2014. pp. S1–S16. (Management of sorafenib-related Adverse Events: A Clinician's Perspective. Seminars in Oncology). [DOI] [PubMed] [Google Scholar]
- 20.Kumar R., Saraswat M.K., Sharma B.C., Sakhuja P., Sarin S. Characteristics of hepatocellular carcinoma in India: a retrospective analysis of 191 cases. QJM: Int J Med. 2008;101:479–485. doi: 10.1093/qjmed/hcn033. [DOI] [PubMed] [Google Scholar]
- 21.Department of Health & Family Welfare Goverment of Punjab . 2017. Essential Drugs List. [Google Scholar]
- 22.Haryana Medical Services Corporation Ltd Goverment of Haryana. Essential Drug List; 2017. [Google Scholar]
- 23.Tamil Nadu Medical Services Corporation Limited Government of Tamil Nadu. Essential Drug List; 2018. [Google Scholar]
- 24.Prinja S., Bahuguna P., Duseja A., Kaur M., Chawla Y.K. Cost of intensive care treatment for liver disorders at tertiary care level in India. PharmacoEconomics-Open. 2017:1–12. doi: 10.1007/s41669-017-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung H.W., Liu C.-F., Chan A.L. Cost-effectiveness of sorafenib versus SBRT for unresectable advanced hepatocellular carcinoma. Radiat Oncol. 2016;11:69. doi: 10.1186/s13014-016-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P., Wen F., Li Q. FOLFOX4 or sorafenib as the first-line treatments for advanced hepatocellular carcinoma: a cost-effectiveness analysis. Dig Liver Dis. 2016;48:1492–1497. doi: 10.1016/j.dld.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Prinja S., Kaur M., Duseja A., Chawla Y.K. Post Graduate Institute of Medical Education and Research; Chandigarh: 2016. Cost and outcome of treatment in liver ICU of a tertiary care hospital in North India. [Google Scholar]
- 28.Xenon Laboratories. XE Currency Converter: USD to INR. Vol 20172017.
- 29.Abraham A, Purushothaman C, Damien D, James J, Rodrigues PA, Singh G. Efficacy of sorafenib therapy in Patients with Advanced Hepatocellular Carcinoma in Indian Population.
- 30.Nandennavar M.I., Karpurmath S.V., Mandakalatur G., Prasad A.E. Clinical profile of hepatocellular carcinoma and experience with sorafenib from a tertiary cancer centre in Southern India. Int J Res Med Sci. 2017;5:379–383. [Google Scholar]
- 31.The National Institute for Health and Care Excellence. Sorafenib for treating Advanced Hepatocellular Carcinoma. Vol 20172017.
- 32.Office of the Registrar General & Census Commissioner I. SRS Stat Rep. 2015;2017 http://www.censusindia.gov.in/Vital_Statistics/SRS_Life_Table/Srs_life_Table_2012-16.html Available from. [Google Scholar]
- 33.Prinja S., Sharma Y.P., Kumar R. Indian Council of Medical Research; New Delhi: 2016. Economic Burden of Rheumatic Fever/Rheumatic Heart Disease and Cost Effectiveness of its prevention strategies in Punjab State, India. [Google Scholar]
- 34.Prinja S., Bahuguna P., Faujdar D.S. Cost effectiveness of HPV vaccination for adolescent girls in Punjab State: implications for India's UIP program. Cancer. 2017;123(17):3253–3260. doi: 10.1002/cncr.30734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.