Abstract
Nonalcoholic fatty liver disease (NAFLD) is now the leading cause of chronic liver disease in industrialized economies. With no licensed treatment currently available, together with a growing prevalence that parallels global increases in obesity and type 2 diabetes, NAFLD will dominate the landscape of hepatology for the foreseeable future. A multifaceted etiopathogenesis, paucity of reproducible preclinical models that effectively recreate human NAFLD, and lack of robust surrogate trial endpoints have presented major hurdles in drug discovery and development. Smooth collaboration between bench scientists, biotechnology, pharmaceutical industries, and clinicians will be pivotal to target identification, development of effective therapies, biomarker discovery, and ultimately to bring pipeline drugs to market. This review examines the key challenges remaining in NAFLD drug development, outlines early and late phase clinical trials of candidate treatments, and discusses the journey toward biomarker discovery which may facilitate development of novel endpoints in NAFLD clinical trials, enabling meaningful response to be determined noninvasively.
Keywords: NAFLD, therapeutics, endpoints, preclinical models, targets
Abbreviation: BMI, Body Mass Index; DGAT, Diacyl Glycerol Acyl Transferase; ELF, Extended Liver Fibrosis Panel; FIB-4, Fibrosis 4; FXR, Farnesoid X Receptor; HbA1C, Hemoglobin A1C; HCC, Hepatocellular Carcinoma; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; HVPG, Hepatic Venous Pressure Gradient; MCD, Methionine-Choline–Deficient Diet; MRE, MR Elastography; MRI, Magnetic Resonance Imaging; MRS, Magnetic Resonance Spectroscopy; NAFLD, Nonalcoholic Fatty Liver Disease; NASH, Nonalcoholic Steatohepatitis; OCA, Obeticholic Acid; OGTT, Oral Glucose Tolerance Test; Pro-C3, Pro-collagen 3; TE, Transient Elastography
Nonalcoholic fatty liver disease (NAFLD) has rapidly emerged as a leading cause of chronic liver disease and liver transplantation in high-income economies.1 Closely associated with obesity, atherogenic dyslipidemia, and impaired glucose tolerance, it is broadly considered to be the hepatic corollary of the metabolic syndrome. In this context, global prevalence of NAFLD is expected to soar in coming decades in parallel with obesity and type 2 diabetes.2 This translates into a growing economic challenge, with an estimated direct annual cost attributable to NAFLD of $103 billion in the United States.3 As an independent risk factor for both liver-related and extrahepatic outcomes, including cirrhosis, liver cancer, cardiovascular events, and chronic kidney disease, reducing the global burden of NAFLD has become a public health priority.
Before considering emerging pharmacological intervention, it is worth highlighting the evidence base for established therapies. Data from multiple randomized control trials confirm the efficacy of pioglitazone, an agonist of the nuclear peroxisome proliferator-activated receptor gamma on fibrosis reduction NAFLD, in both type 2 diabetic and nondiabetic patient populations4, 5, 6, 7, 8; these findings were further consolidated in a recent meta-analysis.9 Given that advanced fibrosis in NAFLD is independently associated with liver-related outcomes, the importance of pioglitazone as a potentially efficacious disease-modifying therapy in NAFLD is clear.10 In large population-based cohorts, pioglitazone has been associated with a reduction in cardiovascular disease, heart failure, and all-cause mortality.11 Further, use of pioglitazone has been associated with a reduced incidence of hepatocellular carcinoma (HCC) in individuals with type 2 diabetes.12 Pioglitazone treatment in patients with advanced fibrosis in addition to lifestyle intervention has been shown to be cost-effective13; now off-patent, it is also inexpensive. It should be noted that both the National Institute of Clinical Excellence and the European Association for the Study of the Liver advocate consideration of pioglitazone in the treatment of selected patients with nonalcoholic steatohepatitis (NASH).14, 15 However, adverse effects associated with thiazolidinediones, including weight gain and osteonecrosis, together with apprehensions related to the circumstances of market withdrawal of rosiglitazone have resulted in underuse of pioglitazone in clinical practice as a treatment for NASH.
To date, no drug has been specifically approved for the treatment of NAFLD. By 2025, it is estimated that the drug market for NAFLD will be worth $20 billion to $35 billion per year, with over 190 products (from 150 companies) estimated to be in the pipeline of development.16 It is, therefore, unsurprising that there are over 300 on-going clinical trials investigating potential treatment for this condition. However, drug discovery and development in NAFLD pose several unique challenges limiting swift translation into clinical practice. The current landscape is that of a complex condition which is as exciting as it is challenging and as full of potential as it is of false promises.
Pitching to arrest progression
NAFLD is characterized by progressive accumulation of lipid species within the liver parenchyma, giving rise, over time, to necroinflammation, oxidative stress, and fibrogenesis. This results in achronic liver disease with a wide range of pathology, from simple steatosis (NAFL) through to NASH, fibrosis, cirrhosis, and HCC. The global prevalence of NAFLD is estimated to be 25.24%,17 rising to 66% and 90% in type 2 diabetic and obese populations, respectively.18 In a large retrospective cohort study involving 646 biopsy-proven patients with NAFLD, the entire group showed a trend towards higher risk for mortality than controls (HR = 1.14; 95% confidence interval [CI] = 0.99–1.32; P = 0.07).19 During follow-up, liver-related mortality was 7.9% in cases versus 1.4% in controls (P < 0.001); endocrine-related mortality including diabetes was significantly more common in NAFLD than controls (5.1% vs 2.7%; P = 0.02). NASH conferred a slight increase in overall mortality (HR = 1.22; 95% CI = 1.02–1.46; P = 0.03). The population prevalence of NAFLD means that the burden of morbidity and mortality, even when the minority progress to cirrhosis, still form a compelling case of need for the development of effective intervention. However, key factors distinguishing the progressive form of NAFLD among a substantial proportion of the affected population are not yet established.20
It is logical that drug development in NAFLD would look to focus on drugs which potentially influence several of the diverse pathophysiological processes implicated in disease progression, including insulin resistance, chronic inflammation, and fibrogenesis, reflecting the multidimensional etiopathogenesis of the disease.21 For example, obeticholic acid (OCA), one of the first drugs shown to demonstrate therapeutic potential in NAFLD, is an agonist of the nuclear transcription factor Farnesoid X receptor (FXR). OCA administration can therefore alter gene expression affecting multiple metabolic and cellular injury pathways, including lipid metabolism, insulin resistance, and oxidative stress. However, given the diversity of triggers for a NASH phenotype, effective long-term treatment strategies are likely to involve a combination of agents, each targeting different pathways in NAFLD pathogenesis, together with dietary and lifestyle measures.
The dilemma of preclinical models
Attempts to recapitulate the biology and natural history of human NAFLD in animal models with the aim of identifying specific pathways driving disease progression and therapeutic targets for drug development has resulted in a plethora of preclinical models to date. Animal models have been developed to recreate the systemic inflammatory, insulin resistant, and profibrotic milieu that defines human NASH; these are achieved either through dietary means or genetic modification. Examples of dietary interventions in mouse models include the methionine-choline–deficient diet (MCD) and choline-deficient, l-amino acid–supplemented, high-fat diet (CDAA). Both of these models involve high-fat feeding (10%), together with deficient dietary choline. These interventions recreate the histological phenotypes of NAFLD, but, animals do not gain weight or develop insulin resistance, thus diverging from the human metabolic milieu and rendering these models suboptimal for the study of metabolic profile in human NAFLD. The high-fat high-fructose diet has gained popularity in preclinical studies because of its physiological fidelity to human NASH, including hepatocellular inflammation, oxidative stress, and fibrogenesis in an insulin-resistant phenotype.22 However, lack of consistency in progression to fibrosis and HCC renders this model somewhat less reliable. The DIAMOND mouse model consists of a high-carbohydrate, high-fat diet with ad libitum glucose and fructose consumption in an isogenic inbred strain, which overcomes some of the limitations highlighted in previous models and shows good metabolic and histological concordance with human NASH.23
One typical example of dilemmas generated by experimental murine models involves genetic modification of mice to establish leptin receptor deficiency as in db/db mice. Development of liver fibrosis in db/db mice requires the MCD diet. Intriguingly, when pretreated with antisense oligonucleotide for diacyl glycerol acyl transferase (DGAT) in addition to MCD diet, db/db mice show resolution of hepatic steatosis while fibrosis progresses.24 While these results have generated an academic debate regarding the role of fat within the liver, they did not halt the development of drugs that interfere with DGAT with a view to attenuating hepatic steatosis. Interestingly, a small molecule with inhibitory properties toward this particular enzyme is currently undergoing evaluation in a clinical trial setting.25
The Innovations Medicine Initiative consortium “Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS)”, involving academic and European Federation of Pharmaceutical Industry and Associations partners, is taking a “reverse translation” route to identify an appropriate animal model for preclinical drug development in NAFLD. This approach aims to identify key noninvasive markers of NAFLD pathology through systematic review and robust evaluation in large cohorts to facilitate a choice of animal models which faithfully reflect similar associations between pathology and biomarkers for future preclinical development.
Experimental methodologies in NAFLD research should allow mechanistic evaluation of metabolic processes within the liver, muscle, and adipose tissue in vivo and their response to drugs. For example, dual step euglycemic, hyperinsulinemic clamping methods have long been established and have been used effectively in early studies of OCA.26 Magnetic resonance spectroscopy (MRS) has proven to be a robust and powerful tool in quantification of liver fat, glycogen stores, and phosphorus metabolites, each probing different metabolic processes key to the pathogenesis of NAFLD. This is of particular relevance to the study of hepatic and whole-body insulin resistance, given the ability to simultaneously quantify lipid and glucose metabolism as well as energy homeostasis in the liver and skeletal muscle, the major insulin target tissues, using 1H-MRS, 13C MRS, and 31P-MRS, respectively.27, 28
Novel MRS methodologies developed recently could bring about a step change in experimental medicine studies. In a study involving two animal models, investigators used labeled intravenous glycine together with 13C MRS to estimate glutathione flux in vivo.29 Another study combined intravenous acetate with 13C spectroscopy to directly measure hepatic mitochondrial β-oxidation.30 These methods can directly monitor metabolic steps related to lipid accumulation of fat and development of processes causing hepatocellular injury.
As higher MR field strengths (3T and 7T) become available in research settings, opportunities for evaluating substrate flux in vivo noninvasively continue to emerge. This will build a dynamic picture of liver metabolism, including energy homeostasis and oxidative stress. Use of these modalities in combination may differentiate stages of NAFLD, such that with time, magnetic resonance imaging (MRI)/MRS may become a good substitute to liver biopsy, with added benefits of providing real-time information on human liver metabolism.
Plethora of targets
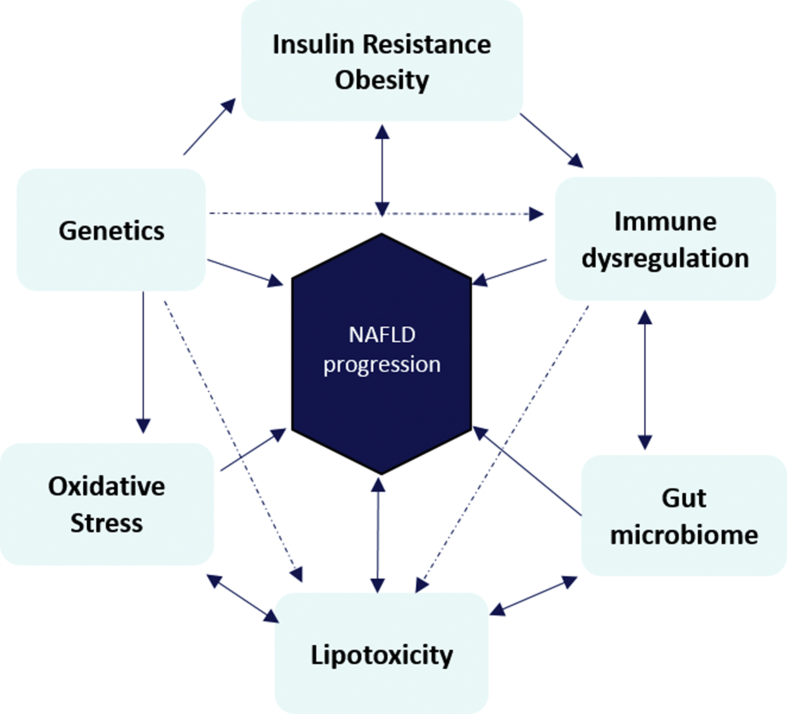
NAFLD is the result of multiple cellular and molecular derangements occurring in multiple organ systems, involving a combination of genetic, dietary, metabolic, and immunological triggers (Figure 1). Bi-directionality of its pathogenesis means that NAFLD is considered to be both the driver and the consequence of its many associations. Challenges to development of effective therapy include identification of a key step or process involved in disease progression and demonstration that effectively targeting that particular pathogenic step can change the natural history of the disease.
Figure 1.
Multifactorial etiopathogenesis of NAFLD. Some putative mechanisms, notably insulin resistance, are bidirectional, with growing evidence to suggest that NAFLD is a driver, as well as a consequence, of systemic insulin resistance and the metabolic syndrome. NAFLD, Nonalcoholic fatty liver disease.
Insulin resistance has been consistently shown to be a key pathogenic mechanism underlying the development and progression of NAFLD.31 However, medications that are primarily used in type 2 diabetes have had varied response in the treatment of NAFLD. For example, while metformin has been considered ineffective,32 pioglitazone has been shown to improve all histological features of NAFLD. The Chemokine receptor 2 and 5 inhibitor cenicriviroc did not demonstrate any efficacy on steatohepatitis (even when the effect of the drug on the target was evident in a recent phase 2 RCT), yet reduced liver fibrosis significantly.33 A study that evaluated a combination of selonsertib, a selective inhibitor of apoptosis signal-regulating kinase 1 and simtuzumab, a humanized monoclonal antibody directed against lysyl oxidase–like molecule 2, an enzyme that catalyzes the cross-linkage of collagen and elastin, demonstrated that selonsertib alone could reduce fibrosis while simtuzumab had no effect on liver histology.34
A wide range of therapeutic targets for NASH exists, including glucagon-like peptide 1 analogues, fibroblast growth factor 19 and 21 analogues, galectin-3 antagonists, acetyl-coenzyme A carboxylase inhibitors, and thyroid hormone receptor-β selective thyromimetics, all of which are currently under investigation. Irrespective of their varied mechanisms of action, resolution of histopathological changes in NASH is currently the outcome necessary for demonstration of efficacy of these agents. It remains to be seen as to which (if any) of these potential drugs may be developed as an effective treatment for NASH.
Trials and endpoints
Finally, selecting clinically relevant endpoints has been a major stumbling block in drug development and validation. NAFLD is a slowly progressive disease, with a gap of several years between onset and development of “hard” clinical outcomes, such as liver-related and all-cause mortality. For 10% of patients with NAFLD to develop cirrhosis, decompensation, or HCC, it would take 22–26 years for those with no or grade 1 fibrosis at baseline, 9.3 years for grade 2, and 2.3 years with grade 3 fibrosis.19 While phase 3 studies are still required to achieve these clinical endpoints, pivotal studies have been designed to focus on resolution of NASH and fibrosis regression as primary endpoints. Fibrosis progression itself is slow, with a recent systematic review demonstrating that the average time taken to progress by 1 fibrosis stage is 7.1 years in patients with NASH and 14.3 years in patients with NAFLD alone.35 Liver biopsy is inherently susceptible to sampling error and interobserver variability; its invasive nature also renders it a barrier for large clinical trials. Given these limitations, the “Holy Grail” in NAFLD research is the development of robust noninvasive endpoints which can accurately stage disease through quantitative analysis of inflammatory activity and amount of fibrosis (Table 1).
Table 1.
Overview of Completed Phase 2 Clinical Trials Evaluating Pharmacotherapy in NAFLD That Have Moved on to Phase 3 Evaluation.
| Drug | Mechanism | Clinical trial | Phase | Duration (weeks) | Primary outcome measure | Proportion achieving outcome in treatment group | Proportion achieving outcome in control group | Result |
|---|---|---|---|---|---|---|---|---|
| Obeticholic acid | FXR agonist | FLINT | 2 | 72 weeks | ≥2-point improvement in NAS, no worsening of fibrosis | 45% | 21% | P = 0.0002 |
| Elafibrinor | PPAR α/δ agonists | NCT01694849 | 2 | 52 weeks | NASH resolution, no worsening of fibrosis | 19%* | 12% | P = 0.045 |
| Cenicriviroc | CCR-2 inhibitor | CENTAUR | 2 | 52 weeks | ≥2-point improvement in NAS, no worsening of fibrosis | Improvement in hepatocellular injury: 16%Improvement in fibrosis: 20% | Hepatocellular injury: 19% Fibrosis: 10% | P = 0.49 P = 0.02 |
| Selonsertib ± simtuzumab | ASK-1 inhibitor | 2 | 24 weeks | ≥1 stage improvement in fibrosis | 18 mg: 43% 6 mg: 30% |
20% | ||
| ≥30% Reduction in MRI-PDFF measure of liver fat |
18 mg group: 26% 6 mg group: 13% |
10% |
||||||
| ≥15% reduction in MRE-derived measures of liver stiffness |
18 mg group: 15% 6 mg group: 32% |
0% |
PPAR, peroxisome-proliferator activated receptor; FXR, Farnesoid X receptor; MRI, magnetic resonance imaging; MRE, MR elastography; NAFLD, Nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PDFF, Proton Density Fat Fraction; ASK-1, Apoptosis signal-regulating kinase 1; CCR-2, C-C chemokine receptor type 2; NAS, NAFLD Activity Score.
*Subgroup analysis of patients assigned to receive 120 mg elafibrinor (no significant difference was seen in the group receiving 80 mg compared to placebo).
A combination of the slow nature of disease progression in NAFLD, heterogeneity of therapeutic targets, and well-established limitations of serial liver biopsy to evaluate effects of intervention have significantly hampered clinical trial design and development of effective therapies. These limitations have spawned huge research interest in the development of accurate, robust, and reproducible noninvasive surrogate endpoints which may ultimately supplant biopsy in trials, facilitating pursuit of effective therapies (Table 2)8, 36. Algorithms such as NAFLD fibrosis score and FIB-4 may be useful tools for prescreening, so as to enrich the patient group with a suitable spectrum of NASH and fibrosis for enrollment. Early clinical trials of the FXR agonist, OCA, utilized extended liver fibrosis panel markers to demonstrate potential effect of the drug on fibrosis within a 6-week period of the intervention26 Serum Pro-C3 levels have also been proposed as an indicator of active fibrogenesis; estimation of Pro-C3 levels may facilitate patient selection as well as help to accelerate antifibrotic drug development and validation.37 MRI and MRS, vibration controlled transient elastography, and MR elastography (MRE) can map hepatic anatomy, chemical composition, and stiffness, directly and accurately.28 Indeed, MRI and MRE were used as surrogate endpoints in a recent phase 2 trial evaluating the ASK-1 inhibitor selonsertib in patients with noncirrhotic NAFLD.34
Table 2.
Relevant Endpoints in NAFLD Clinical Trials and Proposed Strategies for Noninvasive Quantitative Assessment.
| Clinical endpoints | Surrogate markers | Noninvasive surrogate endpoints |
|---|---|---|
| Metabolic endpoints | Δ Liver fat (*) | MRI-PDFF†, 1H-MRS†, USS, TE with CAP |
| Δ Insulin resistance | Euglycemic hyperinsulinemic clamp, HOMA-IR, OGTT, HbA1C, fasting glucose | |
| Δ Glycometabolic profile | Lipid profile, HbA1C, OGTT, fasting glucose, change in weight/BMI | |
| Inflammatory endpoints | Δ Steatohepatitis* | Risk prediction tools: NASH resolution score, OxNASH Wet biomarkers: ALT Imaging: multiparametric MRI, MRS |
|
|
|
|
|
|
| Fibrosis endpoints | Δ fibrosis stage (*) | Risk prediction tools: NAFLD fibrosis score, FIB-4, Fibrosis improvement score, Fibrometer Imaging: TE, MRE, 31P MRS Wet biomarkers: ELF, Pro-C3 |
*Denotes parameters for which liver biopsy is currently the reference standard.
FIB-4, fibrosis 4; ELF, extended liver fibrosis panel; TE, transient elastography; MRE, MR elastography; MRS, magnetic resonance spectroscopy; HOMA-IR, homeostasis model assessment of insulin resistance; OGTT, oral glucose tolerance test; HbA1C, hemoglobin A1C; Pro-C3, Pro-collagen 3; HVPG, hepatic venous pressure gradient; BMI, body mass index; NAFLD, Nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; MRI, magnetic resonance imaging; USS, Ultrasound; ATP, Adenosine Triphosphate; ALT, Alanine Aminotransferase.
Denotes validated noninvasive markers.
Conclusion
The socioeconomic impact of rising NAFLD burden highlights a critical unmet need for broadly applicable and effective therapeutic interventions. Major challenges to drug development which require urgent attention include the multitude of therapeutic targets, paucity of robust preclinical models and difficulties in trial design to establish clinically meaningful outcomes. Given its complex etiopathogenesis, combination therapy with multiple mechanistic targets is likely to yield more success than monotherapy in NAFLD. Standardizing trial design and outcome measures which correlate to clinically significant outcomes may be achievable in the context of noninvasive endpoint validation. The advent of precision imaging and methods for quantitation of dynamic metabolic processes, including substrate oxidation, mitochondrial energetics, and oxidative stress, may enable more holistic evaluation of the effects of intervention on metabolic, inflammatory, and fibrosis indices in real time.
Compelling evidence that fibrosis progression is the strongest independent predictor of long-term liver-related outcomes will guide development of robust surrogate endpoints which quantify fibrosis change to establish whether trial interventions can have meaningful benefit in NAFLD. Establishing durable benefit of therapy on “hard” clinical outcomes will require detailed and long-term follow-up given the slow natural history of NAFLD and fibrosis progression. Ultimately, a coordinated effort from clinicians, scientists, and biotechnology and pharmaceutical industries is needed, with cooperation from regulators, to accelerate development and dissemination of safe, effective, and broadly applicable pharmacological agents to combat the growing burden of NAFLD worldwide.
Conflicts of interest
The authors have none to declare.
References
- 1.Noureddin M., Vipani A., Bresee C. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113(11):1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z.M., Blissett D., Blissett R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 4.Aithal G.P., Thomas J.A., Kaye P.V. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 5.Belfort R., Harrison S.A., Brown K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal A.J., Mofrad P.S., Contos M.J. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2(12):1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal A.J., Chalasani N., Kowdley K.V. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cusi K., Orsak B., Bril F. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 9.Musso G., Cassader M., Paschetta E., Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177(5):633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas J.A., Gupta R., Aithal G.P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis. Ann Intern Med. 2017;166(3):229–230. doi: 10.7326/L16-0628. [DOI] [PubMed] [Google Scholar]
- 11.Hippisley-Cox J., Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ. 2016;354:i3477. doi: 10.1136/bmj.i3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C.H., Lin J.W., Wu L.C., Lai M.S., Chuang L.M., Chan K.A. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55(5):1462–1472. doi: 10.1002/hep.25509. [DOI] [PubMed] [Google Scholar]
- 13.Mahady S.E., Wong G., Craig J.C., George J. Pioglitazone and vitamin E for nonalcoholic steatohepatitis: a cost utility analysis. Hepatology. 2012;56(6):2172–2179. doi: 10.1002/hep.25887. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2016. Non-alcoholic Fatty Liver Disease (NAFLD): Assessment and Management [NG49]. [Online]https://www.nice.org.uk/guidance/ng49/chapter/recommendations Available from: [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016 Jun;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Drew L. Development pipeline review 2018. Nature. 2017;551(7681):S86–S89. doi: 10.1038/d41586-017-06926-1. https://www.nature.com/articles/d41586-017-06926-1 [Online]. Accessed 19/11/2018. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 18.Bellentani S., Scaglioni F., Marino M., Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 19.Hagstrom H., Nasr P., Ekstedt M. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 20.DeWeerdt S. Disease progression: divergent paths. Nature. 2017;551(7681) doi: 10.1038/d41586-017-06925-2. [DOI] [PubMed] [Google Scholar]
- 21.Drew L. Drug development: sprint finish. Nature. 2017;551:s89. doi: 10.1038/d41586-017-06926-1. [DOI] [PubMed] [Google Scholar]
- 22.Charlton M., Krishnan A., Viker K. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G825–G834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santhekadur P.K., Kumar D.P., Sanyal A.J. Preclinical models of non-alcoholic fatty liver disease. J Hepatol. 2018;68(2):230–237. doi: 10.1016/j.jhep.2017.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi K., Yang L., McCall S. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 25.Ionis Pharmaceuticals Safety, Tolerability, and Pharmacodynamics of IONIS-DGAT2Rx in Adult Patients With Type 2 Diabetes [Online] https://clinicaltrials.gov/ct2/show/NCT03334214 Available from:
- 26.Mudaliar S., Henry R.R., Sanyal A.J. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 27.Bawden S., Stephenson M., Falcone Y. Increased liver fat and glycogen stores following high compared with low glycaemic index foods: a randomized cross over study. Diabetes Obes Metab. 2017;19(1):70–77. doi: 10.1111/dom.12784. [DOI] [PubMed] [Google Scholar]
- 28.Bawden S.J., Scott R.A., Aithal G.P. Current and future magnetic resonance technologies for assessing liver disease in clinical and experimental medicine. Dig Dis. 2017;35(4):314–322. doi: 10.1159/000456582. [DOI] [PubMed] [Google Scholar]
- 29.Skamarauskas J.T., Oakley F., Smith F.E., Bawn C., Dunn M., Vidler D.S. Noninvasive in vivo magnetic resonance measures of glutathione synthesis in human and rat liver as an oxidative stress biomarker. Hepatology. 2014;59(6):2321–2330. doi: 10.1002/hep.26925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Befroy D.E., Perry R.J., Jain N. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat Med. 2014;20(1):98–102. doi: 10.1038/nm.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birkenfeld A.L., Shulman G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres D.M., Jones F.J., Shaw J.C., Williams C.D., Ward J.A., Harrison S.A. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54(5):1631–1639. doi: 10.1002/hep.24558. [DOI] [PubMed] [Google Scholar]
- 33.Friedman S.L., Ratziu V., Harrison S.A. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loomba R., Lawitz E., Mantry P.S. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67:549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–654.e1-9. doi: 10.1016/j.cgh.2014.04.014. quiz e39-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannah W.N., Torres D.M., Harrison S.A. Nonalcoholic steatohepatitis and endpoints in clinical trials. Gastroenterol Hepatol. 2016;12(12):756–763. [PMC free article] [PubMed] [Google Scholar]
- 37.Karsdal M.A., Henriksen K., Nielsen M.J. Fibrogenesis assessed by serological type III collagen formation identifies patients with progressive liver fibrosis and responders to a potential antifibrotic therapy. Am J Physiol Gastrointest Liver Physiol. 2016;311(6) doi: 10.1152/ajpgi.00283.2016. G1009-g17. [DOI] [PubMed] [Google Scholar]